RNA Sequencing-Based Identification of Ganglioside GD2-Positive Cancer Phenotype
Abstract
1. Introduction
2. Materials and Methods
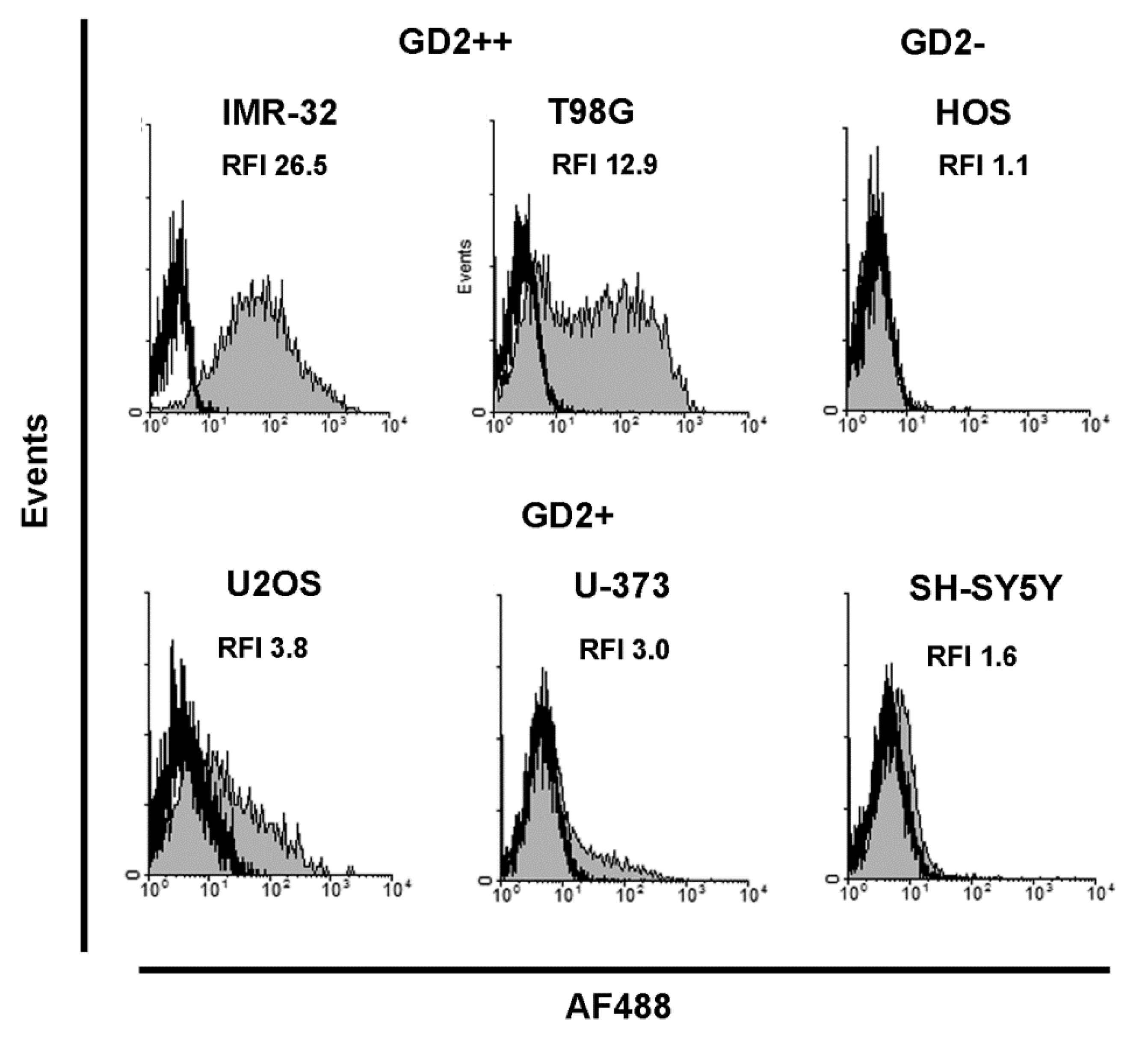
2.1. Cell Lines and Flow Cytometry
2.2. Biosamples
2.3. Library Preparation and RNA Sequencing
2.4. Extraction and Processing of Publicly Available Data
2.5. Data Extraction Normalization
2.6. Statistical Analysis
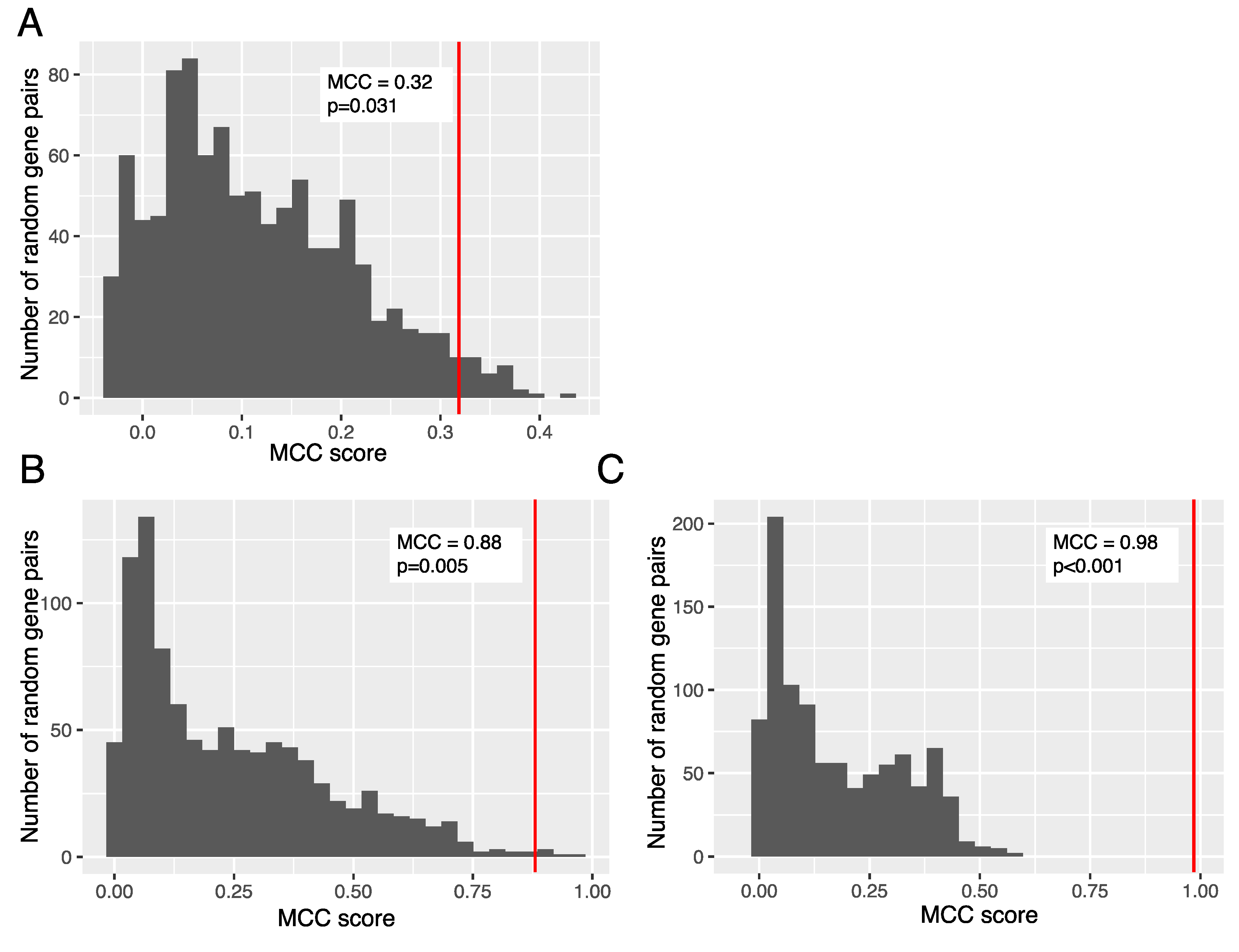
2.7. Gene Ontology and Random Testing of 2-Gene Signatures
3. Results
3.1. Characterization of Cancer Cell Lines by GD2 Expression
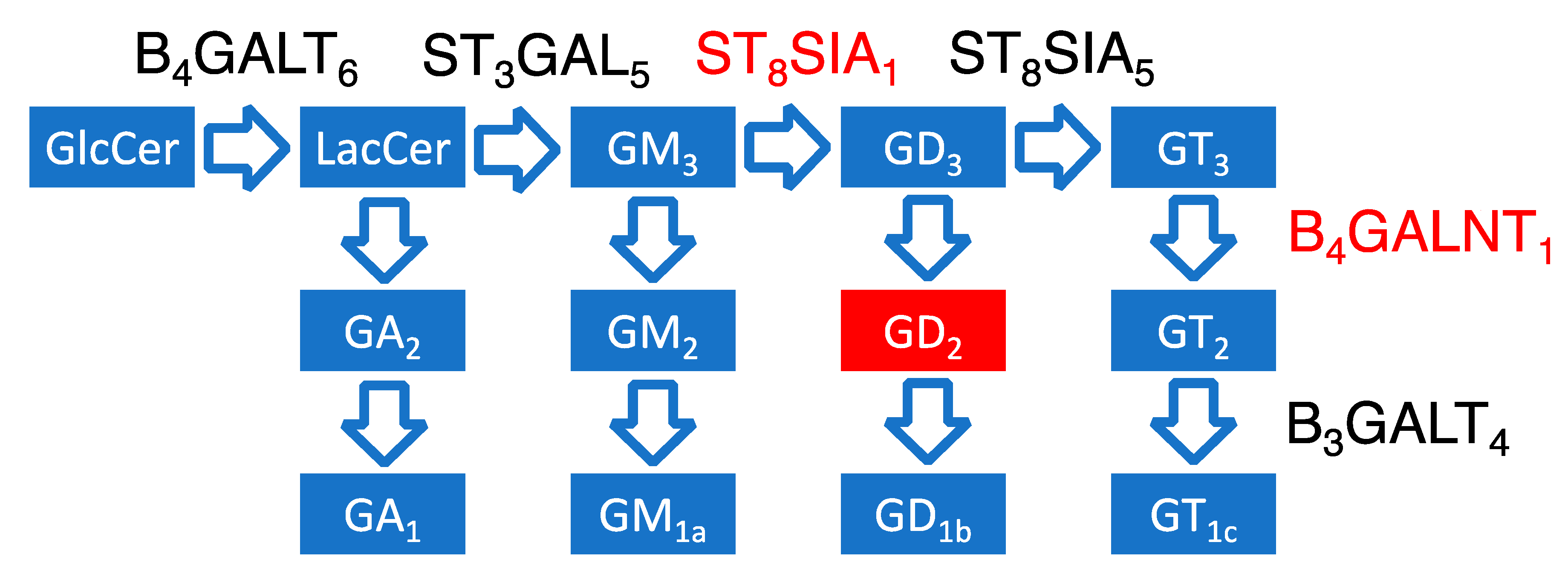
3.2. Association between Expression of Ganglioside Biosynthesis Enzymes and GD2-Positive Phenotype
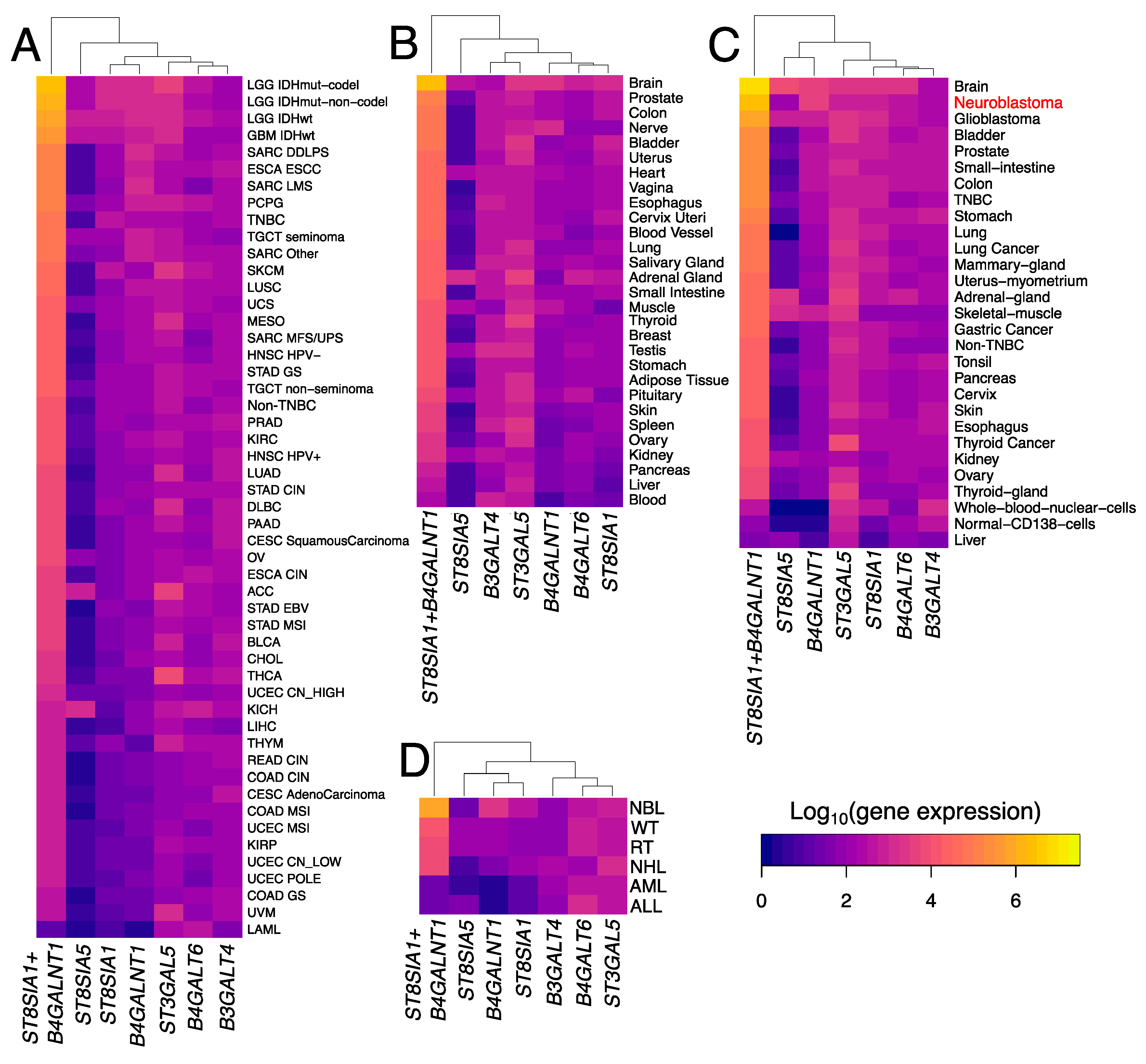
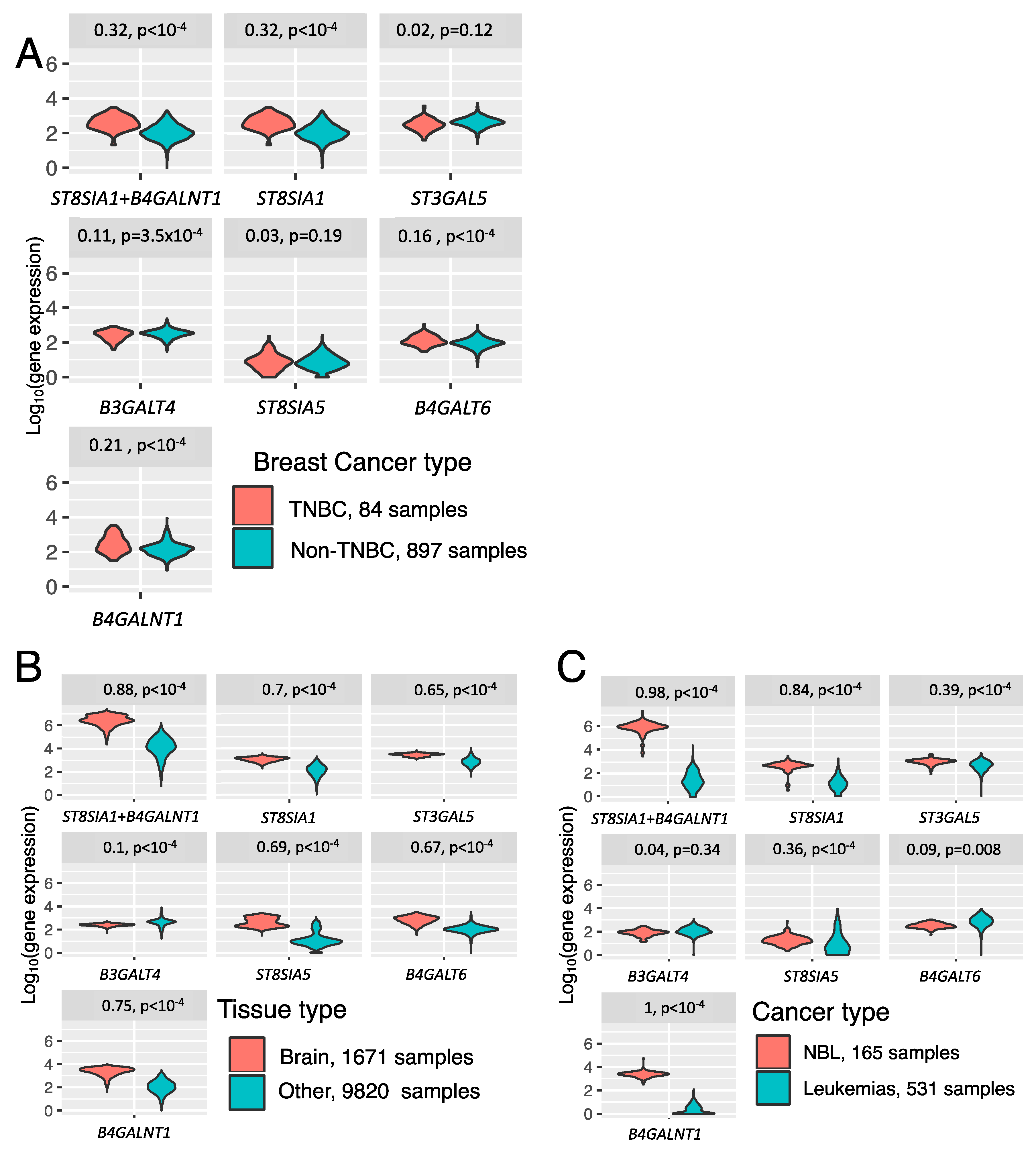
3.3. Trends in Ganglioside Biosynthesis Enzyme Expressions in Public Gene Expression Databases
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethics statement
References
- Cheresh, D.A.; Pierschbacher, M.D.; Herzig, M.A.; Mujoo, K. Disialogangliosides GD2 and GD3 are involved in the attachment of human melanoma and neuroblastoma cells to extracellular matrix proteins. J. Cell Biol. 1986, 102, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Doronin, I.I.; Vishnyakova, P.A.; Kholodenko, I.V.; Ponomarev, E.D.; Ryazantsev, D.Y.; Molotkovskaya, I.M.; Kholodenko, R.V. Ganglioside GD2 in reception and transduction of cell death signal in tumor cells. BMC Cancer 2014, 14, 295. [Google Scholar] [CrossRef] [PubMed]
- Vishnyakova, P.A.; Doronin, I.I.; Kholodenko, I.V.; Ryazantsev, D.Y.; Molotkovskaya, I.M.; Kholodenko, R.V. Caspases participation in cell death induced by the GD2-specific antibodies. Russ. J. Bioorganic Chem. 2014, 40, 279–287. [Google Scholar] [CrossRef]
- Battula, V.L.; Shi, Y.; Evans, K.W.; Wang, R.-Y.; Spaeth, E.L.; Jacamo, R.O.; Guerra, R.; Sahin, A.A.; Marini, F.C.; Hortobagyi, G.; et al. Ganglioside GD2 identifies breast cancer stem cells and promotes tumorigenesis. J. Clin. Investig. 2012, 122, 2066–2078. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-J.; Ding, Y.; Levery, S.B.; Lobaton, M.; Handa, K.; Hakomori, S. Differential expression profiles of glycosphingolipids in human breast cancer stem cells vs. cancer non-stem cells. Proc. Natl. Acad. Sci. USA 2013, 110, 4968–4973. [Google Scholar] [CrossRef] [PubMed]
- Julien, S.; Bobowski, M.; Steenackers, A.; Le Bourhis, X.; Delannoy, P. How Do Gangliosides Regulate RTKs Signaling? Cells 2013, 2, 751–756. [Google Scholar] [CrossRef]
- Lloyd, K.O.; Furukawa, K. Biosynthesis and functions of gangliosides: Recent advances. Glycoconj. J. 1998, 15, 627–636. [Google Scholar] [CrossRef]
- Pastor, E.R.; Mousa, S.A. Current management of neuroblastoma and future direction. Crit. Rev. Oncol. Hematol. 2019, 138, 38–43. [Google Scholar] [CrossRef]
- Kramer, K.; Gerald, W.L.; Kushner, B.H.; Larson, S.M.; Hameed, M.; Cheung, N.K. Disaloganglioside GD2 loss following monoclonal antibody therapy is rare in neuroblastoma. Med. Pediatr. Oncol. 2001, 36, 194–196. [Google Scholar] [CrossRef]
- Terzic, T.; Cordeau, M.; Herblot, S.; Teira, P.; Cournoyer, S.; Beaunoyer, M.; Peuchmaur, M.; Duval, M.; Sartelet, H. Expression of Disialoganglioside (GD2) in Neuroblastic Tumors: A Prognostic Value for Patients Treated with Anti-GD2 Immunotherapy. Pediatr. Dev. Pathol. 2018, 21, 355–362. [Google Scholar] [CrossRef]
- Hamilton, W.B.; Helling, F.; Lloyd, K.O.; Livingston, P.O. Ganglioside expression on human malignant melanoma assessed by quantitative immune thin-layer chromatography. Int. J. Cancer 1993, 53, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Fredman, P.; von Holst, H.; Collins, V.P.; Ammar, A.; Dellheden, B.; Wahren, B.; Granholm, L.; Svennerholm, L. Potential Ganglioside Antigens Associated with Human Gliomas. Neurol. Res. 1986, 8, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Arbit, E.; Cheung, N.K.; Yeh, S.D.; Daghighian, F.; Zhang, J.J.; Cordon-Cardo, C.; Pentlow, K.; Canete, A.; Finn, R.; Larson, S.M. Quantitative Studies of Monoclonal Antibody Targeting to Disialoganglioside GD2 in Human Brain Tumors. Eur. J. Nucl. Med. 1995, 22, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Longee, D.C.; Wikstrand, C.J.; Månsson, J.E.; He, X.; Fuller, G.N.; Bigner, S.H.; Fredman, P.; Svennerholm, L.; Bigner, D.D. Disialoganglioside GD2 in human neuroectodermal tumor cell lines and gliomas. Acta Neuropathol. 1991, 82, 45–54. [Google Scholar] [CrossRef]
- Chang, H.R.; Cordon-Cardo, C.; Houghton, A.N.; Cheung, N.K.; Brennan, M.F. Expression of disialogangliosides GD2 and GD3 on human soft tissue sarcomas. Cancer 1992, 70, 633–638. [Google Scholar] [CrossRef]
- Heiner, J.P.; Miraldi, F.; Kallick, S.; Makley, J.T.; Neely, J.G.; Smith-Mensah, W.H.; Cheung, N.-K.V. Localization of GD2-specificMonoclonal Antibody 3F8 in Human Osteosarcoma. Cancer Res. 1987, 47, 5377–5381. [Google Scholar]
- Chantada, G.L.; Rossi, J.; Casco, F.; Fandiño, A.; Scopinaro, M.; de Dávila, M.T.G.; Abramson, D.H. An aggressive bone marrow evaluation including immunocytology with GD2 for advanced retinoblastoma. J. Pediatr. Hematol. Oncol. 2006, 28, 369–373. [Google Scholar] [CrossRef]
- Cheresh, D.A.; Rosenberg, J.; Mujoo, K.; Hirschowitz, L.; Reisfeld, R.A. Biosynthesis and expression of the disialoganglioside GD2, a relevant target antigen on small cell lung carcinoma for monoclonal antibody-mediated cytolysis. Cancer Res. 1986, 46, 5112–5118. [Google Scholar]
- Nakamura, K.; Hanibuchi, M.; Yano, S.; Tanaka, Y.; Fujino, I.; Inoue, M.; Takezawa, T.; Shitara, K.; Sone, S.; Hanai, N. Apoptosis induction of human lung cancer cell line in multicellular heterospheroids with humanized antiganglioside GM2 monoclonal antibody. Cancer Res. 1999, 59, 5323–5330. [Google Scholar]
- Vantaku, V.; Donepudi, S.R.; Ambati, C.R.; Jin, F.; Putluri, V.; Nguyen, K.; Rajapakshe, K.; Coarfa, C.; Battula, V.L.; Lotan, Y.; et al. Expression of ganglioside GD2, reprogram the lipid metabolism and EMT phenotype in bladder cancer. Oncotarget 2017, 8, 95620–95631. [Google Scholar] [CrossRef]
- Lupatov, A.Y.; Gisina, A.M.; Kim, Y.S.; Bykasov, S.A.; Volchenko, N.N.; Sidorov, D.V.; Yarygin, K.N.; Kholodenko, R.V. [Expression of Ganglioside GD2 on Colorectal Adenocarcinoma Cells]. Biomed. Khim. 2020, 66, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Orsi, G.; Barbolini, M.; Ficarra, G.; Tazzioli, G.; Manni, P.; Petrachi, T.; Mastrolia, I.; Orvieto, E.; Spano, C.; Prapa, M.; et al. GD2 expression in breast cancer. Oncotarget 2017, 8, 31592–31600. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, M.; Roudi, R.; Abbasi, A.; Abolhasani, M.; Abdi Rad, I.; Shariftabrizi, A.; Madjd, Z. High GD2 expression defines breast cancer cells with enhanced invasiveness. Exp. Mol. Pathol. 2019, 109, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Perez Horta, Z.; Goldberg, J.L.; Sondel, P.M. Anti-GD2 mAbs and Next-Generation mAb-based Agents for Cancer Therapy. Immunotherapy 2016, 8, 1097–1117. [Google Scholar] [CrossRef]
- Rizk, E.M.; Gartrell, R.D.; Barker, L.W.; Esancy, C.L.; Finkel, G.G.; Bordbar, D.D.; Saenger, Y.M. Prognostic and Predictive Immunohistochemistry-Based Biomarkers in Cancer and Immunotherapy. Hematol. Oncol. Clin. N. Am. 2019, 33, 291–299. [Google Scholar] [CrossRef]
- Werner, M.; Chott, A.; Fabiano, A.; Battifora, H. Effect of Formalin Tissue Fixation and Processing on Immunohistochemistry. Am. J. Surg. Pathol. 2000, 24, 1016–1019. [Google Scholar] [CrossRef]
- Zhu, Q.; Izumchenko, E.; Aliper, A.M.; Makarev, E.; Paz, K.; Buzdin, A.; Zhavoronkov, A.; Sidransky, D. Pathway Activation Strength Is a Novel Independent Prognostic Biomarker for Cetuximab Sensitivity in Colorectal Cancer Patients. Hum. Genome Var. 2015, 2. [Google Scholar] [CrossRef]
- Makarev, E.; Izumchenko, E.; Aihara, F.; Wysocki, P.T.; Zhu, Q.; Buzdin, A.; Sidransky, D.; Zhavoronkov, A.; Atala, A. Common pathway signature in lung and liver fibrosis. Cell Cycle 2016, 15, 1667–1673. [Google Scholar] [CrossRef]
- Buzdin, A.; Sorokin, M.; Garazha, A.; Sekacheva, M.; Kim, E.; Zhukov, N.; Wang, Y.; Li, X.; Kar, S.; Hartmann, C.; et al. Molecular Pathway Activation—New Type of Biomarkers for Tumor Morphology and Personalized Selection of Target Drugs. Semin. Cancer Biol. 2018, 53, 110–124. [Google Scholar] [CrossRef]
- Shepelin, D.; Korzinkin, M.; Vanyushina, A.; Aliper, A.; Borisov, N.; Vasilov, R.; Zhukov, N.; Sokov, D.; Prassolov, V.; Gaifullin, N.; et al. Molecular Pathway Activation Features Linked With Transition From Normal Skin to Primary and Metastatic Melanomas in Human. Oncotarget 2016, 7, 656. [Google Scholar] [CrossRef]
- Venkova, L.; Aliper, A.; Suntsova, M.; Kholodenko, R.; Shepelin, D.; Borisov, N.; Malakhova, G.; Vasilov, R.; Roumiantsev, S.; Zhavoronkov, A.; et al. Combinatorial high-throughput experimental and bioinformatic approach identifies molecular pathways linked with the sensitivity to anticancer target drugs. Oncotarget 2015, 6, 27227–27238. [Google Scholar] [CrossRef] [PubMed]
- Doronin, I.; Kholodenko, I.; Molotkovskaya, I.; Kholodenko, R. Preparation of Fab-fragments of GD2-specific Antibodies and Analysis of Their Antitumor Activity in Vitro. Bull. Exp. Biol. Med. 2013, 154. [Google Scholar] [CrossRef] [PubMed]
- Home|HUGO Gene Nomenclature Committee. Available online: https://www.genenames.org/ (accessed on 22 April 2020).
- Hutter, C.; Zenklusen, J.C. The Cancer Genome Atlas: Creating Lasting Value Beyond Its Data. Cell 2018, 173, 283–285. [Google Scholar] [CrossRef] [PubMed]
- GTEx Consortium The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 2013, 45, 580–585. [CrossRef] [PubMed]
- Gal, O.; Auslander, N.; Fan, Y.; Meerzaman, D. Predicting Complete Remission of Acute Myeloid Leukemia: Machine Learning Applied to Gene Expression. Cancer Inform. 2019, 18, 1176935119835544. [Google Scholar] [CrossRef]
- Suntsova, M.; Gaifullin, N.; Allina, D.; Reshetun, A.; Li, X.; Mendeleeva, L.; Surin, V.; Sergeeva, A.; Spirin, P.; Prassolov, V.; et al. Atlas of RNA sequencing profiles for normal human tissues. Sci. Data 2019, 6, 36. [Google Scholar] [CrossRef]
- Sorokin, M.; Poddubskaya, E.; Baranova, M.; Glusker, A.; Kogoniya, L.; Markarova, E.; Allina, D.; Suntsova, M.; Tkachev, V.; Garazha, A.; et al. RNA sequencing profiles and diagnostic signatures linked with response to ramucirumab in gastric cancer. Cold Spring Harb. Mol. Case Stud. 2020, 6, a004945. [Google Scholar] [CrossRef]
- Chicco, D.; Jurman, G. The advantages of the Matthews correlation coefficient (MCC) over F1 score and accuracy in binary classification evaluation. BMC Genomics 2020, 21, 6. [Google Scholar] [CrossRef]
- Mcc: Compute a Mathews Correlation Coefficient in bhklab/PharmacoGx: Analysis of Large-Scale Pharmacogenomic Data. Available online: https://rdrr.io/github/bhklab/PharmacoGx/man/mcc.html (accessed on 23 April 2020).
- AmiGO 2: Term Details for & Quot; Lipid Metabolic Process & Quot; (GO:0006629). Available online: http://amigo.geneontology.org/amigo/term/GO:0006629 (accessed on 18 May 2020).
- Zeng, G.; Yu, R.K. Cloning and transcriptional regulation of genes responsible for synthesis of gangliosides. Curr. Drug Targets 2008, 9, 317–324. [Google Scholar] [CrossRef]
- Anh-Tuan, N.; Pick, J.; Mód, A.; Hollán, S.R. Gangliosides in acute myeloid leukaemia (AML) and non-Hodgkin’s lymphoma (NHL). Eur. J. Cancer Clin. Oncol. 1986, 22, 1003–1007. [Google Scholar] [CrossRef]
- Macher, B.A.; Lee, W.M.; Westrick, M.A. Glycosphingolipids of normal and leukemic human leukocytes. Mol. Cell. Biochem. 1982, 47, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Ando, S.; Chang, N.C.; Yu, R.K. High-performance thin-layer chromatography and densitometric determination of brain ganglioside compositions of several species. Anal. Biochem. 1978, 89, 437–450. [Google Scholar] [CrossRef]
- Marconi, S.; De Toni, L.; Lovato, L.; Tedeschi, E.; Gaetti, L.; Acler, M.; Bonetti, B. Expression of gangliosides on glial and neuronal cells in normal and pathological adult human brain. J. Neuroimmunol. 2005, 170, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Furman, W.L.; Federico, S.M.; McCarville, M.B.; Shulkin, B.L.; Davidoff, A.M.; Krasin, M.J.; Sahr, N.; Sykes, A.; Wu, J.; Brennan, R.C.; et al. A Phase II Trial of Hu14.18K322A in Combination with Induction Chemotherapy in Children with Newly Diagnosed High-Risk Neuroblastoma. Clin. Cancer Res. 2019, 25, 6320–6328. [Google Scholar] [CrossRef] [PubMed]
- Kholodenko, R.V.; Kalinovsky, D.V.; Doronin, I.I.; Ponomarev, E.D.; Kholodenko, I.V. Antibody Fragments as Potential Biopharmaceuticals for Cancer Therapy: Success and Limitations. Curr. Med. Chem. 2019, 26. [Google Scholar] [CrossRef] [PubMed]
- Kholodenko, I.V.; Kalinovsky, D.V.; Svirshchevskaya, E.V.; Doronin, I.I.; Konovalova, M.V.; Kibardin, A.V.; Shamanskaya, T.V.; Larin, S.S.; Deyev, S.M.; Kholodenko, R.V. Multimerization through Pegylation Improves Pharmacokinetic Properties of scFv Fragments of GD2-Specific Antibodies. Molecules 2019, 24, 3835. [Google Scholar] [CrossRef]
- Mueller, I.; Ehlert, K.; Endres, S.; Pill, L.; Siebert, N.; Kietz, S.; Brock, P.; Garaventa, A.; Valteau-Couanet, D.; Janzek, E.; et al. Tolerability, Response and Outcome of High-Risk Neuroblastoma Patients Treated with Long-Term Infusion of anti-GD 2 Antibody ch14.18/CHO. MAbs 2018, 10, 55–61. [Google Scholar] [CrossRef]
- Matthay, K.K. Interleukin 2 plus anti-GD2 immunotherapy: Helpful or harmful? Lancet Oncol. 2018, 19, 1549–1551. [Google Scholar] [CrossRef]
- Sorokin, M.; Kholodenko, R.; Suntsova, M.; Malakhova, G.; Garazha, A.; Kholodenko, I.; Poddubskaya, E.; Lantsov, D.; Stilidi, I.; Arhiri, P.; et al. Oncobox Bioinformatical Platform for Selecting Potentially Effective Combinations of Target Cancer Drugs Using High-Throughput Gene Expression Data. Cancers 2018, 10, 365. [Google Scholar] [CrossRef]
- Horwacik, I.; Rokita, H. Targeting of Tumor-Associated Gangliosides With Antibodies Affects Signaling Pathways and Leads to Cell Death Including Apoptosis. Apoptosis 2015, 20. [Google Scholar] [CrossRef]
- Doronin, I.I.; Kholodenko, I.V.; Zubareva, A.A.; Yarygin, K.N.; Deev, S.M.; Kholodenko, R.V. Involvement of Actin Filaments in the Cytotoxic Effect of GD2-Specific Antibodies. Bull. Exp. Biol. Med. 2019, 166, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Durbas, M.; Horwacik, I.; Boratyn, E.; Kamycka, E.; Rokita, H. GD2 Ganglioside Specific Antibody Treatment Downregulates PI3K/Akt/mTOR Signaling Network in Human Neuroblastoma Cell Lines. Int. J. Oncol. 2015, 47. [Google Scholar] [CrossRef] [PubMed]
- Doronin, I.I.; Kholodenko, A.A.; Buzdin, M.I.; Sorokin, T.V.; Shamanskaya, S.M.; Deyev, R.V.; Kholodenko, I.I. The approach for selection optimal combinations of chemotherapy drugs with GD2-specific antibodies for the effective elimination of GD2-positive tumor cells. In Febs Open Bio; Wiley: Hoboken, NJ, USA, 2019; p. 339. [Google Scholar]
- Pugh, T.J.; Morozova, O.; Attiyeh, E.F.; Asgharzadeh, S.; Wei, J.S.; Auclair, D.; Carter, S.L.; Cibulskis, K.; Hanna, M.; Kiezun, A.; et al. The genetic landscape of high-risk neuroblastoma. Nat. Genet. 2013, 45, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Cheung, N.K. Engineering anti-GD2 Monoclonal Antibodies for Cancer Immunotherapy. FEBS Lett. 2014, 588. [Google Scholar] [CrossRef]
- Kholodenko, I.V.; Kalinovsky, D.V.; Doronin, I.I.; Deyev, S.M.; Kholodenko, R.V. Neuroblastoma Origin and Therapeutic Targets for Immunotherapy. J. Immunol. Res. 2018, 2018, 7394268. [Google Scholar] [CrossRef]
- Suzuki, M.; Cheung, N.K. Disialoganglioside GD2 as a Therapeutic Target for Human Diseases. Expert Opin. Ther. Targets 2015, 19. [Google Scholar] [CrossRef]
- Woo, S.; Oh, Y.; An, J.; Kang, B.; Nam, D.; Joo, K. Glioblastoma Specific Antigens, GD2 and CD90, Are Not Involved in Cancer Stemness. Anat. Cell Biol. 2015, 48. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorokin, M.; Kholodenko, I.; Kalinovsky, D.; Shamanskaya, T.; Doronin, I.; Konovalov, D.; Mironov, A.; Kuzmin, D.; Nikitin, D.; Deyev, S.; et al. RNA Sequencing-Based Identification of Ganglioside GD2-Positive Cancer Phenotype. Biomedicines 2020, 8, 142. https://doi.org/10.3390/biomedicines8060142
Sorokin M, Kholodenko I, Kalinovsky D, Shamanskaya T, Doronin I, Konovalov D, Mironov A, Kuzmin D, Nikitin D, Deyev S, et al. RNA Sequencing-Based Identification of Ganglioside GD2-Positive Cancer Phenotype. Biomedicines. 2020; 8(6):142. https://doi.org/10.3390/biomedicines8060142
Chicago/Turabian StyleSorokin, Maxim, Irina Kholodenko, Daniel Kalinovsky, Tatyana Shamanskaya, Igor Doronin, Dmitry Konovalov, Aleksei Mironov, Denis Kuzmin, Daniil Nikitin, Sergey Deyev, and et al. 2020. "RNA Sequencing-Based Identification of Ganglioside GD2-Positive Cancer Phenotype" Biomedicines 8, no. 6: 142. https://doi.org/10.3390/biomedicines8060142
APA StyleSorokin, M., Kholodenko, I., Kalinovsky, D., Shamanskaya, T., Doronin, I., Konovalov, D., Mironov, A., Kuzmin, D., Nikitin, D., Deyev, S., Buzdin, A., & Kholodenko, R. (2020). RNA Sequencing-Based Identification of Ganglioside GD2-Positive Cancer Phenotype. Biomedicines, 8(6), 142. https://doi.org/10.3390/biomedicines8060142






