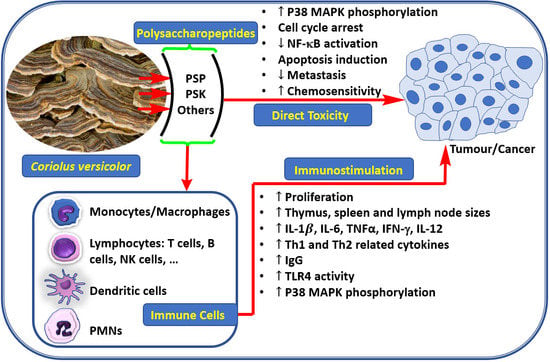
Trametes versicolor (Synn. Coriolus versicolor) Polysaccharides in Cancer Therapy: Targets and Efficacy
Abstract
1. Introduction
2. Overview of Chemistry
2.1. Small Molecular Weight Compounds
2.2. Polysaccharides
3. Anticancer Effect through Direct Toxicity to Cancer Cells
3.1. Evidence of Efficacy through In Vitro Studies
3.2. Evidence of Efficacy through Animal Models
3.3. Evidence of Efficacy through Clinical Trials
4. Anti-Cancer Effect Via Immunostimulation
4.1. Evidence of Immunotherapy Potential through In Vitro Studies
4.2. Evidence of Immunotherapy Potential through In Vivo Studies
4.3. Evidence of Immunotherapy Potential through Human Studies
5. Other Benefits of C. versicolor Polysaccharides
6. General Summary and Conclusions
Funding
Conflicts of Interest
References
- WHO. Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 1 May 2020).
- Habtemariam, S. The chemistry, pharmacology and therapeutic potential of the edible mushroom Dictyophora indusiata (Vent ex. Pers.) Fischer (Synn. Phallus indusiatus). Biomedicines 2019, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- NBNatlas. Trametes Versicolor (L.) Lloyd: Turkeytail. 2020. Available online: https://species.nbnatlas.org/species/nhmsys0001499939 (accessed on 1 May 2020).
- Shen-Nong. Herbal Glossary—Chinese Herb List—Coriolus Versicolor. 2020. Available online: http://www.shen-nong.com/eng/herbal/yunzhi.html (accessed on 1 May 2020).
- Wang, S.R.; Zhang, L.; Chen, H.P.; Li, Z.H.; Dong, Z.J.; Wei, K.; Liu, J.K. Four new spiroaxane sesquiterpenes and one new rosenonolactone derivative from cultures of Basidiomycete Trametes versicolor. Fitoterapia 2015, 105, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Janjušević, L.; Karaman, M.; Šibul, F.; Tommonaro, G.; Iodice, C.; Jakovljevic, D.M.; Pejin, B. The lignicolous fungus Trametes versicolor (L.) Lloyd (1920): A promising natural source of antiradical and AChE inhibitory agents. J. Enzyme Inhib. Med. Chem. 2017, 32, 355–362. [Google Scholar] [CrossRef]
- Rau, U.; Kuenz, A.; Wray, V.; Nimtz, M.; Wrenger, J.; Cicek, H. Production and structural analysis of the polysaccharide secreted by Trametes (Coriolus) versicolor ATCC 200801. Appl. Microbiol. Biotechnol. 2009, 81, 827–837. [Google Scholar] [CrossRef]
- Cui, J.; Goh, K.K.; Archer, R.; Singh, H. Characterisation and bioactivity of protein-bound polysaccharides from submerged-culture fermentation of Coriolus versicolor Wr-74 and ATCC-20545 strains. J. Ind. Microbiol. Biotechnol. 2007, 34, 393–402. [Google Scholar] [CrossRef]
- Ng, T.B. A review of research on the protein-bound polysaccharide (polysaccharopeptide, PSP) from the mushroom Coriolus versicolor (Basidiomycetes: Polyporaceae). Gen. Pharmacol. 1998, 30, 1–4. [Google Scholar] [CrossRef]
- Wang, K.L.; Lu, Z.M.; Mao, X.; Chen, L.; Gong, J.S.; Ren, Y.; Geng, Y.; Li, H.; Xu, H.Y.; Xu, G.H.; et al. Structural characterization and anti-alcoholic liver injury activity of a polysaccharide from Coriolus versicolor mycelia. Int. J. Biol. Macromol. 2019, 137, 1102–1111. [Google Scholar] [CrossRef]
- Awadasseid, A.; Hou, J.; Gamallat, Y.; Xueqi, S.; Eugene, K.D.; Musa Hago, A.; Bamba, D.; Meyiah, A.; Gift, C.; Xin, Y. Purification, characterization, and antitumor activity of a novel glucan from the fruiting bodies of Coriolus versicolor. PLoS ONE 2017, 12, e0171270. [Google Scholar] [CrossRef]
- Zhang, J.S.; Han, W.W.; Pan, Y.J. Studies on chemical structure of polysaccharide from fruit body of Coriolus versicolor. Yao Xue Xue Bao 2001, 36, 664–667. [Google Scholar]
- Nakajima, T.; Ichikawa, S.; Uchida, S.; Komada, T. Effects of a protein-bound polysaccharide from a basidiomycetes against hepatocarcinogenesis induced by 3′-methyl-4-dimethylaminoazobenzene in rats. Clin. Ther. 1990, 12, 385–392. [Google Scholar]
- Hirose, K.; Hakozaki, M.; Matsunaga, K.; Yoshikumi, C.; Hotta, T.; Yanagisawa, M.; Yamamoto, M.; Endo, H. Cloning of sequences induced and suppressed by administration of PSK, antitumor protein-bound polysaccharide. Biochem. Biophys. Res. Commun. 1985, 126, 884–892. [Google Scholar] [CrossRef]
- Miyaji, C.; Ogawa, Y.; Imajo, Y.; Imanaka, K.; Kimura, S. Combination therapy of radiation and immunomodulators in the treatment of MM46 tumor transplanted in C3H/He mice. Oncology 1983, 40, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Hidaka, H.; Sugiura, M. Effects of coriolan, an antitumor polysaccharide, produced by Coriolus versicolor Iwade. Jpn. J. Pharmacol. 1979, 29, 953–957. [Google Scholar] [CrossRef] [PubMed]
- Pawlikowska, M.; Piotrowski, J.; Jedrzejewski, T.; Kozak, W.; Slominski, A.T.; Brozyna, A.A. Coriolus versicolor-derived protein-bound polysaccharides trigger the caspase-independent cell death pathway in amelanotic but not melanotic melanoma cells. Phytother. Res. 2020, 34, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.S.; Kumarasamy, M.; Sosnik, A.; Danino, D. Enhanced thermostability and anticancer Activity in breast cancer cells of laccase immobilized on pluronic-stabilized nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 39436–39448. [Google Scholar] [CrossRef] [PubMed]
- Roca-Lema, D.; Martinez-Iglesias, O.; Fernandez de Ana Portela, C.; Rodriguez-Blanco, A.; Valladares-Ayerbes, M.; Diaz-Diaz, A.; Casas-Pais, A.; Prego, C.; Figueroa, A. In vitro anti-proliferative and anti-invasive effect of polysaccharide-rich extracts from Trametes versicolor and Grifola frondosa in colon cancer cells. Int. J. Med. Sci. 2019, 16, 231–240. [Google Scholar] [CrossRef]
- Shnyreva, A.V.; Shnyreva, A.A.; Espinoza, C.; Padron, J.M.; Trigos, A. Antiproliferative Activity and Cytotoxicity of some medicinal wood-destroying mushrooms from Russia. Int. J. Med. Mushrooms 2018, 20, 1–11. [Google Scholar] [CrossRef]
- Knezevic, A.; Stajic, M.; Sofrenic, I.; Stanojkovic, T.; Milovanovic, I.; Tesevic, V.; Vukojevic, J. Antioxidative, antifungal, cytotoxic and antineurodegenerative activity of selected Trametes species from Serbia. PLoS ONE 2018, 13, e0203064. [Google Scholar] [CrossRef]
- Ricciardi, M.R.; Licchetta, R.; Mirabilii, S.; Scarpari, M.; Parroni, A.; Fabbri, A.A.; Cescutti, P.; Reverberi, M.; Fanelli, C.; Tafuri, A. Preclinical Antileukemia Activity of Tramesan: A Newly Identified Bioactive Fungal Metabolite. Oxid. Med. Cell Longev. 2017, 2017, 5061639. [Google Scholar] [CrossRef]
- Luo, K.W.; Yue, G.G.; Ko, C.H.; Lee, J.K.; Gao, S.; Li, L.F.; Li, G.; Fung, K.P.; Leung, P.C.; Lau, C.B. In vivo and in vitro anti-tumor and anti-metastasis effects of Coriolus versicolor aqueous extract on mouse mammary 4T1 carcinoma. Phytomedicine 2014, 21, 1078–1087. [Google Scholar] [CrossRef]
- Hirahara, N.; Edamatsu, T.; Fujieda, A.; Fujioka, M.; Wada, T.; Tajima, Y. Protein-bound polysaccharide-K induces apoptosis via mitochondria and p38 mitogen-activated protein kinase-dependent pathways in HL-60 promyelomonocytic leukemia cells. Oncol. Rep. 2013, 30, 99–104. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hirahara, N.; Edamatsu, T.; Fujieda, A.; Fujioka, M.; Wada, T.; Tajima, Y. Protein-bound polysaccharide-K (PSK) induces apoptosis via p38 mitogen-activated protein kinase pathway in promyelomonocytic leukemia HL-60 cells. Anticancer Res. 2012, 32, 2631–2637. [Google Scholar] [PubMed]
- Hirahara, N.; Fujioka, M.; Edamatsu, T.; Fujieda, A.; Sekine, F.; Wada, T.; Tanaka, T. Protein-bound polysaccharide-K (PSK) induces apoptosis and inhibits proliferation of promyelomonocytic leukemia HL-60 cells. Anticancer Res. 2011, 31, 2733–2738. [Google Scholar] [PubMed]
- Hsieh, T.C.; Wu, J.M. Regulation of cell cycle transition and induction of apoptosis in HL-60 leukemia cells by the combination of Coriolus versicolor and Ganoderma lucidum. Int. J. Mol. Med. 2013, 32, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.F.; Lou, N.; Li, X.D. Effect of Coriolus versicolor polysaccharide-B on the biological characteristics of human esophageal carcinoma cell line eca109. Cancer Biol. Med. 2012, 9, 164–167. [Google Scholar]
- Luk, S.U.; Lee, T.K.; Liu, J.; Lee, D.T.; Chiu, Y.T.; Ma, S.; Ng, I.O.; Wong, Y.C.; Chan, F.L.; Ling, M.T. Chemopreventive effect of PSP through targeting of prostate cancer stem cell-like population. PLoS ONE 2011, 6, e19804. [Google Scholar] [CrossRef]
- Wan, J.M.; Sit, W.H.; Yang, X.; Jiang, P.; Wong, L.L. Polysaccharopeptides derived from Coriolus versicolor potentiate the S-phase specific cytotoxicity of Camptothecin (CPT) on human leukemia HL-60 cells. Chin. Med. 2010, 5, 16. [Google Scholar] [CrossRef]
- Harhaji, L.; Mijatovic, S.; Maksimovic-Ivanic, D.; Stojanovic, I.; Momcilovic, M.; Maksimovic, V.; Tufegdzic, S.; Marjanovic, Z.; Mostarica-Stojkovic, M.; Vucinic, Z.; et al. Anti-tumor effect of Coriolus versicolor methanol extract against mouse B16 melanoma cells: In vitro and in vivo study. Food Chem. Toxicol. 2008, 46, 1825–1833. [Google Scholar] [CrossRef]
- Wan, J.M.; Sit, W.H.; Louie, J.C. Polysaccharopeptide enhances the anticancer activity of doxorubicin and etoposide on human breast cancer cells ZR-75-30. Int. J. Oncol. 2008, 32, 689–699. [Google Scholar] [CrossRef]
- Jimenez-Medina, E.; Berruguilla, E.; Romero, I.; Algarra, I.; Collado, A.; Garrido, F.; Garcia-Lora, A. The immunomodulator PSK induces in vitro cytotoxic activity in tumour cell lines via arrest of cell cycle and induction of apoptosis. BMC Cancer 2008, 8, 78. [Google Scholar] [CrossRef]
- Chan, S.L.; Yeung, J.H. Effects of polysaccharide peptide (PSP) from Coriolus versicolor on the pharmacokinetics of cyclophosphamide in the rat and cytotoxicity in HepG2 cells. Food Chem. Toxicol. 2006, 44, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.Y.; Kim, C.F.; Leung, K.N.; Fung, K.P.; Tse, T.F.; Chan, H.; Lau, C.B. Coriolus versicolor (Yunzhi) extract attenuates growth of human leukemia xenografts and induces apoptosis through the mitochondrial pathway. Oncol. Rep. 2006, 16, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.C.; Wu, P.; Park, S.; Wu, J.M. Induction of cell cycle changes and modulation of apoptogenic/anti-apoptotic and extracellular signaling regulatory protein expression by water extracts of I’m-Yunity (PSP). BMC Complement. Altern. Med. 2006, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.Y.; Kim, C.F.; Leung, K.N.; Fung, K.P.; Tse, T.F.; Chan, H.; Lau, C.B. Differential anti-tumor activity of Coriolus versicolor (Yunzhi) extract through p53- and/or Bcl-2-dependent apoptotic pathway in human breast cancer cells. Cancer Biol. Ther. 2005, 4, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Hui, K.P.; Sit, W.H.; Wan, J.M. Induction of S phase cell arrest and caspase activation by polysaccharide peptide isolated from Coriolus versicolor enhanced the cell cycle dependent activity and apoptotic cell death of doxorubicin and etoposide, but not cytarabine in HL-60 cells. Oncol. Rep. 2005, 14, 145–155. [Google Scholar]
- Yang, X.; Sit, W.H.; Chan, D.K.; Wan, J.M. The cell death process of the anticancer agent polysaccharide-peptide (PSP) in human promyelocytic leukemic HL-60 cells. Oncol. Rep. 2005, 13, 1201–1210. [Google Scholar] [CrossRef]
- Zeng, F.; Hon, C.C.; Sit, W.H.; Chow, K.Y.; Hui, R.K.; Law, I.K.; Ng, V.W.; Yang, X.T.; Leung, F.C.; Wan, J.M. Molecular characterization of Coriolus versicolor PSP-induced apoptosis in human promyelotic leukemic HL-60 cells using cDNA microarray. Int. J. Oncol. 2005, 27, 513–523. [Google Scholar] [CrossRef]
- Lau, C.B.; Ho, C.Y.; Kim, C.F.; Leung, K.N.; Fung, K.P.; Tse, T.F.; Chan, H.H.; Chow, M.S. Cytotoxic activities of Coriolus versicolor (Yunzhi) extract on human leukemia and lymphoma cells by induction of apoptosis. Life Sci. 2004, 75, 797–808. [Google Scholar] [CrossRef]
- Mao, X.W.; Green, L.M.; Gridley, D.S. Evaluation of polysaccharopeptide effects against C6 glioma in combination with radiation. Oncology 2001, 61, 243–253. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Wu, J.M. Cell growth and gene modulatory activities of Yunzhi (Windsor Wunxi) from mushroom Trametes versicolor in androgen-dependent and androgen-insensitive human prostate cancer cells. Int. J. Oncol. 2001, 18, 81–88. [Google Scholar] [CrossRef]
- Aoyagi, H.; Iino, Y.; Takeo, T.; Horii, Y.; Morishita, Y.; Horiuchi, R. Effects of OK-432 (picibanil) on the estrogen receptors of MCF-7 cells and potentiation of antiproliferative effects of tamoxifen in combination with OK-432. Oncology 1997, 54, 414–423. [Google Scholar] [CrossRef]
- Dong, Y.; Kwan, C.Y.; Chen, Z.N.; Yang, M.M. Antitumor effects of a refined polysaccharide peptide fraction isolated from Coriolus versicolor: In vitro and in vivo studies. Res. Commun. Mol. Pathol Pharmacol. 1996, 92, 140–148. [Google Scholar] [PubMed]
- Kobayashi, Y.; Kariya, K.; Saigenji, K.; Nakamura, K. Enhancement of anti-cancer activity of cisdiaminedichloroplatinum by the protein-bound polysaccharide of Coriolus versicolor QUEL (PS-K) in vitro. Cancer Biother. 1994, 9, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Kariya, K.; Saigenji, K.; Nakamura, K. Suppression of cancer cell growth in vitro by the protein-bound polysaccharide of Coriolus versicolor QUEL (PS-K) with SOD mimicking activity. Cancer Biother. 1994, 9, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.M.; Chen, Z.; Kwok, J.S. The anti-tumor effect of a small polypeptide from Coriolus versicolor (SPCV). Am. J. Chin. Med. 1992, 20, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Kim, F.; Sakagami, H.; Tanuma, S.; Konno, K. Stimulation of interferon-gamma-induced human myelogenous leukemic cell differentiation by high molecular weight PSK subfraction. Anticancer Res. 1990, 10, 55–58. [Google Scholar]
- Ko, C.H.; Yue, G.G.; Gao, S.; Luo, K.W.; Siu, W.S.; Shum, W.T.; Shiu, H.T.; Lee, J.K.; Li, G.; Leung, P.C.; et al. Evaluation of the combined use of metronomic zoledronic acid and Coriolus versicolor in intratibial breast cancer mouse model. J. Ethnopharmacol. 2017, 204, 77–85. [Google Scholar] [CrossRef]
- Wenner, C.A.; Martzen, M.R.; Lu, H.; Verneris, M.R.; Wang, H.; Slaton, J.W. Polysaccharide-K augments docetaxel-induced tumor suppression and antitumor immune response in an immunocompetent murine model of human prostate cancer. Int. J. Oncol. 2012, 40, 905–913. [Google Scholar] [CrossRef]
- Jiang, J.; Thyagarajan-Sahu, A.; Loganathan, J.; Eliaz, I.; Terry, C.; Sandusky, G.E.; Sliva, D. BreastDefend prevents breast-to-lung cancer metastases in an orthotopic animal model of triple-negative human breast cancer. Oncol. Rep. 2012, 28, 1139–1145. [Google Scholar] [CrossRef]
- Coles, M.; Toth, B. Lack of prevention of large intestinal cancer by VPS, an extract of Coriolus versicolor mushroom. In Vivo 2005, 19, 867–871. [Google Scholar]
- Ho, J.C.; Konerding, M.A.; Gaumann, A.; Groth, M.; Liu, W.K. Fungal polysaccharopeptide inhibits tumor angiogenesis and tumor growth in mice. Life Sci. 2004, 75, 1343–1356. [Google Scholar] [CrossRef]
- Fujii, T.; Saito, K.; Matsunaga, K.; Oguchi, Y.; Ikuzawa, M.; Furusho, T.; Taguchi, T. Prolongation of the survival period with the biological response modifier PSK in rats bearing N-methyl-N-nitrosourea-induced mammary gland tumors. In Vivo 1995, 9, 55–57. [Google Scholar]
- Chay, W.Y.; Tham, C.K.; Toh, H.C.; Lim, H.Y.; Tan, C.K.; Lim, C.; Wang, W.W.; Choo, S.P. Coriolus versicolor (Yunzhi) Use as Therapy in Advanced Hepatocellular Carcinoma Patients with Poor Liver Function or Who Are Unfit for Standard Therapy. J. Altern. Complement. Med. 2017, 23, 648–652. [Google Scholar] [CrossRef]
- Brown, D.C.; Reetz, J. Single agent polysaccharopeptide delays metastases and improves survival in naturally occurring hemangiosarcoma. Evid. Based Complement Alternat. Med. 2012, 2012, 384301. [Google Scholar] [CrossRef]
- Eliza, W.L.; Fai, C.K.; Chung, L.P. Efficacy of Yun Zhi (Coriolus versicolor) on survival in cancer patients: Systematic review and meta-analysis. Recent Pat. Inflamm. Allergy Drug Discov. 2012, 6, 78–87. [Google Scholar] [CrossRef]
- Kataoka, T.; Oh-hashi, F.; Tsukagoshi, S.; Sakurai, Y. Enhanced induction of immune resistance by concanavalin A-bound L1210 vaccine and an immunopotentiator prepared from Coriolus versicolor. Cancer Res. 1977, 37, 4416–4419. [Google Scholar]
- Mori, H.; Mihara, M.; Teshima, K.; Uesugi, U.; Xu, Q.; Sakamoto, O.; Koda, A. Effect of immunostimulants and antitumor agents on tumor necrosis factor (TNF) production. Int. J. Immunopharmacol. 1987, 9, 881–892. [Google Scholar] [CrossRef]
- Qian, Z.M.; Xu, M.F.; Tang, P.L. Polysaccharide peptide (PSP) restores immunosuppression induced by cyclophosphamide in rats. Am. J. Chin. Med. 1997, 25, 27–35. [Google Scholar] [CrossRef]
- Kohgo, Y.; Hirayama, Y.; Sakamaki, S.; Matsunaga, T.; Ohi, S.; Kuga, T.; Kato, J.; Niitsu, Y. Improved recovery of myelosuppression following chemotherapy in mice by combined administration of PSK and various cytokines. Acta Haematol. 1994, 92, 130–135. [Google Scholar] [CrossRef]
- Fujii, T.; Kano, T.; Saito, K.; Kobayashi, Y.; Iijima, H.; Matsumoto, T.; Yoshikumi, C.; Taguchi, T. Effect of PSK on prohibited immunity of splenectomized mice. Anticancer Res. 1987, 7, 845–848. [Google Scholar]
- Matsunaga, K.; Morita, I.; Oguchi, Y.; Fujii, T.; Yoshikumi, C.; Nomoto, K. Restoration of immune responsiveness by a biological response modifier, PSK, in aged mice bearing syngeneic transplantable tumor. J. Clin. Lab. Immunol. 1987, 24, 143–149. [Google Scholar]
- Matsunaga, K.; Morita, I.; Oguchi, Y.; Fujii, T.; Yoshikumi, C.; Nomoto, K. Competitive effect of PSK against the immunosuppressive effect induced in the sera of mice bearing syngeneic tumors. Gan To Kagaku Ryoho 1986, 13, 3461–3467. [Google Scholar] [PubMed]
- Matsunaga, K.; Morita, I.; Oguchi, Y.; Fujii, T.; Yoshikumi, C.; Nomoto, K. Restoration of immunologic responsiveness by PSK in tumor-bearing animals. Gan To Kagaku Ryoho 1986, 13, 3468–3475. [Google Scholar]
- Matsunaga, K.; Morita, I.; Oguchi, Y.; Fujii, T.; Yoshikumi, C.; Nomoto, K. Restoration of depressed immune responses by PSK in C3H/He mice bearing the syngeneic X5563 tumor. Gan To Kagaku Ryoho 1986, 13, 3453–3460. [Google Scholar]
- Hattori, T.; Hamai, Y.; Ikeda, T.; Takiyama, W.; Hirai, T.; Miyoshi, Y. Survival time of tumor-bearing rats as related to operative stress and immunopotentiators. Jpn. J. Surg. 1982, 12, 143–147. [Google Scholar] [CrossRef]
- Mayer, P.; Drews, J. The effect of a protein-bound polysaccharide from Coriolus versicolor on immunological parameters and experimental infections in mice. Infection 1980, 8, 13–21. [Google Scholar] [CrossRef]
- Li, X.Y.; Wang, J.F.; Zhu, P.P.; Liu, L.; Ge, J.B.; Yang, S.X. Immune enhancement of a polysaccharides peptides isolated from Coriolus versicolor. Zhongguo Yao Li Xue Bao 1990, 11, 542–545. [Google Scholar]
- Kanoh, T.; Saito, K.; Matsunaga, K.; Oguchi, Y.; Taniguchi, N.; Endoh, H.; Yoshimura, M.; Fujii, T.; Yoshikumi, C. Enhancement of the antitumor effect by the concurrent use of a monoclonal antibody and the protein-bound polysaccharide PSK in mice bearing a human cancer cell line. In Vivo 1994, 8, 241–245. [Google Scholar] [PubMed]
- Matsunaga, K.; Iijima, H.; Aota, M.; Oguchi, Y.; Fujii, T.; Yoshikumi, C.; Nomoto, K. Enhancement of effector cell activities in mice bearing syngeneic plasmacytoma X5563 by a biological response modifier, PSK. J. Clin. Lab. Immunol. 1992, 37, 21–37. [Google Scholar]
- Matsunaga, K.; Morita, I.; Iijima, H.; Endoh, H.; Oguchi, Y.; Yoshimura, M.; Fujii, T.; Yoshikumi, C.; Nomoto, K. Effects of biological response modifiers with different modes of action used separately and together on immune responses in mice with syngeneic tumours. J. Int. Med. Res. 1992, 20, 406–421. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, K.; Morita, I.; Iijima, H.; Endo, H.; Oguchi, Y.; Yoshimura, M.; Fujii, T.; Yoshikumi, C.; Nomoto, K. Competitive action of a biological response modifier, PSK, on a humoral immunosuppressive factor produced in tumor-bearing hosts. J. Clin. Lab. Immunol. 1990, 31, 127–136. [Google Scholar]
- Liu, W.K.; Ng, T.B.; Sze, S.F.; Tsui, K.W. Activation of peritoneal macrophages by polysaccharopeptide from the mushroom, Coriolus versicolor. Immunopharmacology 1993, 26, 139–146. [Google Scholar] [CrossRef]
- Sakagami, H.; Sugaya, K.; Utsumi, A.; Fujinaga, S.; Sato, T.; Takeda, M. Stimulation by PSK of interleukin-1 production by human peripheral blood mononuclear cells. Anticancer Res. 1993, 13, 671–675. [Google Scholar]
- Jedrzejewski, T.; Pawlikowska, M.; Piotrowski, J.; Kozak, W. Protein-bound polysaccharides from Coriolus versicolor attenuate LPS-induced synthesis of pro-inflammatory cytokines and stimulate PBMCs proliferation. Immunol. Lett. 2016, 178, 140–147. [Google Scholar] [CrossRef]
- Pawlikowska, M.; Jędrzejewski, T.; Piotrowski, J.; Kozak, W. Fever-range hyperthermia inhibits cells immune response to protein-bound polysaccharides derived from Coriolus versicolor extract. Mol. Immunol. 2016, 80, 50–57. [Google Scholar] [CrossRef]
- Kowalczewska, M.; Piotrowski, J.; Jedrzejewski, T.; Kozak, W. Polysaccharide peptides from Coriolus versicolor exert differential immunomodulatory effects on blood lymphocytes and breast cancer cell line MCF-7 in vitro. Immunol. Lett. 2016, 174, 37–44. [Google Scholar] [CrossRef]
- Quayle, K.; Coy, C.; Ztandish, L.; Lu, H. The TLR2 agonist in polysaccharide-K is a structurally distinct lipid which acts synergistically with the protein-bound beta-glucan. J. Nat. Med. 2015, 69, 198–208. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, B.; Feng, Z.; Yu, S.; Bao, Y. A study on immunomodulatory mechanism of Polysaccharopeptide mediated by TLR4 signaling pathway. BMC Immunol. 2015, 16, 34. [Google Scholar] [CrossRef]
- Yang, S.F.; Zhuang, T.F.; Si, Y.M.; Qi, K.Y.; Zhao, J. Coriolus versicolor mushroom polysaccharides exert immunoregulatory effects on mouse B cells via membrane Ig and TLR-4 to activate the MAPK and NF-κB signaling pathways. Mol. Immunol. 2015, 64, 144–151. [Google Scholar] [CrossRef]
- Koido, S.; Homma, S.; Okamoto, M.; Namiki, Y.; Takakura, K.; Takahara, A.; Odahara, S.; Tsukinaga, S.; Yukawa, T.; Mitobe, J.; et al. Combined TLR2/4-activated dendritic/tumor cell fusions induce augmented cytotoxic T lymphocytes. PLoS ONE 2013, 8, e59280. [Google Scholar] [CrossRef]
- Engel, A.L.; Sun, G.C.; Gad, E.; Rastetter, L.R.; Strobe, K.; Yang, Y.; Dang, Y.; Disis, M.L.; Lu, H. Protein-bound polysaccharide activates dendritic cells and enhances OVA-specific T cell response as vaccine adjuvant. Immunobiology 2013, 218, 1468–1476. [Google Scholar] [CrossRef]
- Sekhon, B.K.; Sze, D.M.; Chan, W.K.; Fan, K.; Li, G.Q.; Moore, D.E.; Roubin, R.H. PSP activates monocytes in resting human peripheral blood mononuclear cells: Immunomodulatory implications for cancer treatment. Food Chem. 2013, 138, 2201–2209. [Google Scholar] [CrossRef]
- Kuan, Y.C.; Wu, Y.J.; Hung, C.L.; Sheu, F. Trametes versicolor protein YZP activates regulatory B lymphocytes—Gene identification through de novo assembly and function analysis in a murine acute colitis model. PLoS ONE 2013, 8, e72422. [Google Scholar] [CrossRef]
- Lu, H.; Yang, Y.; Gad, E.; Inatsuka, C.; Wenner, C.A.; Disis, M.L.; Standish, L.J. TLR2 agonist PSK activates human NK cells and enhances the antitumor effect of HER2-targeted monoclonal antibody therapy. Clin. Cancer Res. 2011, 17, 6742–6753. [Google Scholar] [CrossRef]
- Price, L.A.; Wenner, C.A.; Sloper, D.T.; Slaton, J.W.; Novack, J.P. Role for toll-like receptor 4 in TNF-alpha secretion by murine macrophages in response to polysaccharide Krestin, a Trametes versicolor mushroom extract. Fitoterapia 2010, 81, 914–919. [Google Scholar] [CrossRef]
- Li, W.; Liu, M.; Lai, S.; Xu, C.; Lu, F.; Xiao, X.; Bao, Y. Immunomodulatory effects of polysaccharopeptide (PSP) in human PBMC through regulation of TRAF6/TLR immunosignal-transduction pathways. Immunopharmacol. Immunotoxicol. 2010, 32, 576–584. [Google Scholar] [CrossRef]
- Maruyama, S.; Akasaka, T.; Yamada, K.; Tachibana, H. Protein-bound polysaccharide-K (PSK) directly enhanced IgM production in the human B cell line BALL-1. Biomed. Pharmacother. 2009, 63, 409–412. [Google Scholar] [CrossRef]
- Lee, C.L.; Sit, W.H.; Jiang, P.P.; So, I.W.; Wan, J.M. Polysaccharopeptide mimics ciclosporin-mediated Th1/Th2 cytokine balance for suppression of activated human T cell proliferation by MAPKp38 and STAT5 pathways. J. Pharm. Pharmacol. 2008, 60, 1491–1499. [Google Scholar] [CrossRef]
- Ho, C.Y.; Lau, C.B.; Kim, C.F.; Leung, K.N.; Fung, K.P.; Tse, T.F.; Chan, H.H.; Chow, M.S. Differential effect of Coriolus versicolor (Yunzhi) extract on cytokine production by murine lymphocytes in vitro. Int. Immunopharmacol. 2004, 4, 1549–1557. [Google Scholar] [CrossRef]
- Kanazawa, M.; Mori, Y.; Yoshihara, K.; Iwadate, M.; Suzuki, S.; Endoh, Y.; Ohki, S.; Takita, K.; Sekikawa, K.; Takenoshita, S. Effect of PSK on the maturation of dendritic cells derived from human peripheral blood monocytes. Immunol. Lett. 2004, 91, 229–238. [Google Scholar] [CrossRef]
- Asai, K.; Kato, H.; Hirose, K.; Akaogi, K.; Kimura, S.; Mukai, S.; Inoue, M.; Yamamura, Y.; Sano, H.; Sugino, S.; et al. PSK and OK-432-induced immunomodulation of inducible nitric oxide (NO) synthase gene expression in mouse peritoneal polymorphonuclear leukocytes and NO-mediated cytotoxicity. Immunopharmacol. Immunotoxicol. 2000, 22, 221–235. [Google Scholar] [CrossRef]
- Jedrzejewski, T.; Piotrowski, J.; Pawlikowska, M.; Wrotek, S.; Kozak, W. Extract from Coriolus versicolor fungus partially prevents endotoxin tolerance development by maintaining febrile response and increasing IL-6 generation. J. Therm. Biol. 2019, 83, 69–79. [Google Scholar] [CrossRef]
- Awadasseid, A.; Eugene, K.; Jamal, M.; Hou, J.; Musa Hago, A.; Gamallat, Y.; Meyiah, A.; Bamba, D.; Gift, C.; Abdalla, M.; et al. Effect of Coriolus versicolor glucan on the stimulation of cytokine production in sarcoma-180-bearing mice. Biomed. Rep. 2017, 7, 567–572. [Google Scholar] [CrossRef][Green Version]
- Sekhon, B.K.; Roubin, R.H.; Li, Y.; Devi, P.B.; Nammi, S.; Fan, K.; Sze, D.M. Evaluation of selected immunomodulatory glycoproteins as an adjunct to cancer immunotherapy. PLoS ONE 2016, 11, e0146881. [Google Scholar] [CrossRef]
- Jedrzejewski, T.; Piotrowski, J.; Kowalczewska, M.; Wrotek, S.; Kozak, W. Polysaccharide peptide from Coriolus versicolor induces interleukin 6-related extension of endotoxin fever in rats. Int. J. Hyperth. 2015, 31, 626–634. [Google Scholar] [CrossRef]
- Jedrzejewski, T.; Piotrowski, J.; Wrotek, S.; Kozak, W. Polysaccharide peptide induces a tumor necrosis factor-alpha-dependent drop of body temperature in rats. J. Therm. Biol. 2014, 44, 1–4. [Google Scholar] [CrossRef]
- Chan, S.L.; Yeung, J.H. Polysaccharide peptides from COV-1 strain of Coriolus versicolor induce hyperalgesia via inflammatory mediator release in the mouse. Life Sci. 2006, 78, 2463–2470. [Google Scholar] [CrossRef]
- Jeong, S.C.; Yang, B.K.; Kim, G.N.; Jeong, H.; Wilson, M.A.; Cho, Y.; Rao, K.S.; Song, C.H. Macrophage-stimulating activity of polysaccharides extracted from fruiting bodies of Coriolus versicolor (Turkey Tail Mushroom). J. Med. Food 2006, 9, 175–181. [Google Scholar] [CrossRef]
- Mao, X.W.; Archambeau, J.O.; Gridley, D.S. Immunotherapy with low-dose interleukin-2 and a polysaccharopeptide derived from Coriolus versicolor. Cancer Biother. Radiopharm. 1996, 11, 393–403. [Google Scholar] [CrossRef]
- Wu, D.; Han, S.N.; Bronson, R.T.; Smith, D.E.; Meydani, S.N. Dietary supplementation with mushroom-derived protein-bound glucan does not enhance immune function in young and old mice. J. Nutr. 1998, 128, 193–197. [Google Scholar] [CrossRef]
- Wang, J.; Dong, B.; Tan, Y.; Yu, S.; Bao, Y.X. A study on the immunomodulation of polysaccharopeptide through the TLR4-TIRAP/MAL-MyD88 signaling pathway in PBMCs from breast cancer patients. Immunopharmacol. Immunotoxicol. 2013, 35, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Ito, G.; Tanaka, H.; Ohira, M.; Yoshii, M.; Muguruma, K.; Kubo, N.; Yashiro, M.; Yamada, N.; Maeda, K.; Sawada, T.; et al. Correlation between efficacy of PSK postoperative adjuvant immunochemotherapy for gastric cancer and expression of MHC class I. Exp. Ther. Med. 2012, 3, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Torkelson, C.J.; Sweet, E.; Martzen, M.R.; Sasagawa, M.; Wenner, C.A.; Gay, J.; Putiri, A.; Standish, L.J. Phase 1 Clinical trial of Trametes versicolor in women with breast cancer. ISRN Oncol. 2012, 2012, 251632. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Muguruma, K.; Kubo, N.; Amano, R.; Noda, E.; Yamada, N.; Yashiro, M.; Maeda, K.; Sawada, T.; Ohira, M.; et al. Effect of PSK on recurrence of stage II/III gastric cancer. Gan To Kagaku Ryoho 2010, 37, 2258–2260. [Google Scholar] [PubMed]
- Tsang, K.W.; Lam, C.L.; Yan, C.; Mak, J.C.; Ooi, G.C.; Ho, J.C.; Lam, B.; Man, R.; Sham, J.S.; Lam, W.K. Coriolus versicolor polysaccharide peptide slows progression of advanced non-small cell lung cancer. Respir. Med. 2003, 97, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Yan, P.; Lam, W.C.; Yao, L.; Bian, Z. Coriolus versicolor and Ganoderma lucidum related natural products as an adjunct therapy for cancers: A systematic review and meta-analysis of randomized controlled trials. Front. Pharmacol. 2019, 10, 703. [Google Scholar] [CrossRef]
- Wong, C.K.; Bao, Y.X.; Wong, E.L.; Leung, P.C.; Fung, K.P.; Lam, C.W. Immunomodulatory activities of Yunzhi and Danshen in post-treatment breast cancer patients. Am. J. Chin. Med. 2005, 33, 381–395. [Google Scholar] [CrossRef]
- Wong, C.K.; Tse, P.S.; Wong, E.L.; Leung, P.C.; Fung, K.P.; Lam, C.W. Immunomodulatory effects of yun zhi and danshen capsules in health subjects--a randomized, double-blind, placebo-controlled, crossover study. Int. Immunopharmacol. 2004, 4, 201–211. [Google Scholar] [CrossRef]
- Kariya, K.; Nakamura, K.; Nomoto, K.; Matama, S.; Saigenji, K. Mimicking of superoxide dismutase activity by protein-bound polysaccharide of Coriolus versicolor QUEL, and oxidative stress relief for cancer patients. Mol. Biother. 1992, 4, 40–46. [Google Scholar] [PubMed]
- Maehara, Y.; Inutsuka, S.; Takeuchi, H.; Baba, H.; Kusumoto, H.; Sugimachi, K. Postoperative PSK and OK-432 immunochemotherapy for patients with gastric cancer. Cancer Chemother. Pharmacol. 1993, 33, 171–175. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kariya, K.; Saigenji, K.; Nakamura, K. Oxidative stress relief for cancer-bearing hosts by the protein-bound polysaccharide of Coriolus versicolor QUEL with SOD mimicking activity. Cancer Biother. 1994, 9, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.S.; Tan, J.Q.; Guo, F.; Ghen, H.S.; Zhou, Z.Y.; Zhang, Z.H.; Gui, L. Effects of Coriolus versicolor polysaccharides on superoxide dismutase activities in mice. Zhongguo Yao Li Xue Bao 1996, 17, 174–178. [Google Scholar] [PubMed]
- Li, X.; Chen, P.; Zhang, P.; Chang, Y.; Cui, M.; Duan, J. Protein-Bound beta-glucan from Coriolus versicolor has potential for use against obesity. Mol. Nutr. Food Res. 2019, 63, e1801231. [Google Scholar] [CrossRef] [PubMed]
- Xian, H.M.; Che, H.; Qin, Y.; Yang, F.; Meng, S.Y.; Li, X.G.; Bai, Y.L.; Wang, L.H. Coriolus versicolor aqueous extract ameliorates insulin resistance with PI3K/Akt and p38 MAPK signaling pathways involved in diabetic skeletal muscle. Phytother. Res. 2018, 32, 551–560. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Z.; Cui, R.; Chu, H. Polysaccharopeptide from Trametes versicolor blocks inflammatory osteoarthritis pain-morphine tolerance effects via activating cannabinoid type 2 receptor. Int. J. Biol. Macromol. 2019, 126, 805–810. [Google Scholar] [CrossRef]
- Lim, B.O. Coriolus versicolor suppresses inflammatory bowel disease by Inhibiting the expression of STAT1 and STAT6 associated with IFN-gamma and IL-4 expression. Phytother. Res. 2011, 25, 1257–1261. [Google Scholar] [CrossRef]
- Gong, S.; Zhang, H.Q.; Yin, W.P.; Yin, Q.Z.; Zhang, Y.; Gu, Z.L.; Qian, Z.M.; Tang, P.L. Involvement of interleukin-2 in analgesia produced by Coriolus versicolor polysaccharide peptides. Zhongguo Yao Li Xue Bao 1998, 19, 67–70. [Google Scholar]
- Wang, Y.; Li, H.; Li, Y.; Zhao, Y.; Xiong, F.; Liu, Y.; Xue, H.; Yang, Z.; Ni, S.; Sahil, A.; et al. Coriolus versicolor alleviates diabetic cardiomyopathy by inhibiting cardiac fibrosis and NLRP3 inflammasome activation. Phytother. Res. 2019, 33, 2737–2748. [Google Scholar] [CrossRef]
- Pramudya, M.; Wahyuningsih, S.P.A. Immunomodulatory potential of polysaccharides from Coriolus versicolor against intracellular bacteria Neisseria gonorrhoeae. Vet. World 2019, 12, 735–739. [Google Scholar] [CrossRef]
- Hor, S.Y.; Ahmad, M.; Farsi, E.; Lim, C.P.; Asmawi, M.Z.; Yam, M.F. Acute and subchronic oral toxicity of Coriolus versicolor standardized water extract in Sprague-Dawley rats. J. Ethnopharmacol. 2011, 137, 1067–1076. [Google Scholar] [CrossRef]
- Hoshi, H.; Saito, H.; Iijima, H.; Uchida, M.; Wada, T.; Ito, G.; Tanaka, H.; Sawada, T.; Hirakawa, K. Anti-protein-bound polysaccharide-K monoclonal antibody binds the active structure and neutralizes direct antitumor action of the compound. Oncol. Rep. 2011, 25, 905–913. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cao, Y.; Xu, X.; Liu, S.; Huang, L.; Gu, J. Ganoderma: A Cancer Immunotherapy Review. Front Pharmacol. 2018, 9, 1217. [Google Scholar] [CrossRef] [PubMed]
- Verstak, B.; Stack, J.; Ve, T.; Mangan, M.; Hjerrild, K.; Jeon, J.; Stahl, R.; Latz, E.; Gay, N.; Kobe, B.; et al. The TLR signaling adaptor TRAM interacts with TRAF6 to mediate activation of the inflammatory response by TLR4. J. Leukoc. Biol. 2014, 96, 427–436. [Google Scholar] [CrossRef] [PubMed]


| Preparation | Experimental Model | Key Findings | References |
|---|---|---|---|
| Protein-bound polysaccharides | Human SKMel-188 melanoma cells—100 and 200 μg/mL | Induces caspase-independent cytotoxicity; increases the intracellular level of ROS—effect inhibited by SP600125 (JNK inhibitor); cytotoxic effect abolished by receptor-interacting serine/threonine-protein kinase 1 inhibitor. | Pawlikowska et al. 2020 [17] |
| Immobilised fungal laccase on pH-responsive (and charge-switchable) Pluronic-stabilised silver nanoparticles (AgNPsTrp) | MCF-7 breast cancer cells | Inhibits cell proliferation through β-estradiol degradation and cell apoptosis; decreases in the mRNA levels of anti-apoptotic genes (BCL-2 and NF-kβ); increases the mRNA level of proapoptotic genes (p53). | Chauhan et al. 2019 [18] |
| Polysaccharide-rich extracts | Human colon carcinoma LoVo and HT-29 cells—proliferation; wound healing and invasion assays—10 or 100 µg/mL | Inhibits human colon cell proliferation and induces cytotoxicity; inhibits oncogenic potential, cell migration and invasion in colon cancer cells; suppresses MMP-2 enzyme activity; increases the expression of the E-cadherin. | Roca-Lema et al. 2019 [19] |
| Water extracts from mycelial biomass (strain It-1)—Russian origin—water and methanol extracts | Leukemia cell lines (Jukart, K562, and THP-1); solid tumors (A-549 and SWi573 (lung), HBL-100 and T-47D (breast), HeLa (cervix), and WiDr (colon)) cells—50 μg/mL | IC50 between 0.7–3.6 μg/mL—antiproliferative effect against lung and cervix tumors. | Shnyreva et al. 2018 [20] |
| Dried mycelia of Serbian origin—96% ethanol extract | Human cervix adenocarcinoma (HeLa), human colon carcinoma (LS174) and human lung adenocarcinoma (A549) cell lines | Cytotoxic activity with IC50 value between 60–90 μg/mL. | Knezevic et al. 2018 [21] |
| Polysaccharidic fraction, Tramesan (Patent number RM2012A000573)—extracted exopolysaccahride from fungal culture filtrate | Leukemic cell lines (human myeloid (OCI-AML3) and lymphoid (Jurkat) cell lines) and primary cells from AML patients—0.5–2 mg/mL | No cytotoxic effect on mononuclear cells from healthy donors; dose-dependent increase in G0 phase of cancer cells; decreases in both G1 and S phases; time- and dose-dependent induction of apoptosis in cancer cells | Ricciardi et al. 2017 [22] |
| Aqueous extract | Mouse mammary carcinoma 4T1 cells—0.125–2 mg/mL | No direct toxicity but inhibits cell migration and invasion; suppresses enzyme activities and protein levels of MMP-9 | Luo et al. 2014 [23] |
| PSK | Human malignant cell lines (WiDr, HT29, SW480, KATOIII, AGS, HL-60 and U937)—30–100 μg/mL | Antiproliferative—most potent against HL-60 cells; activates caspase-3 and induces p38 MAPK phosphorylation; co-treatment with SB203580 (A p38 MAPK inhibitor) blocked apoptosis induction, caspase-3 activation and growth inhibition; apoptosis induction via mitochondrial pathway (effect on mitochondrial depolarization reversed by SB203580). | Hirahara et al. 2011, 2012, 2013 [24,25,26] |
| Ethanolic extracts | Human promyelocytic HL-60 cells | Suppresses cell growth; induces apoptosis; downregulates the phosphorylation of Rb; increases PARP cleavage; better effect in combination with Ganoderma lucidum. | Hsieh et al. 2013 [27] |
| PSK | HL-60 cells—100 μg/mL | Induces apoptosis without inducing cell differentiation; induces p38 MAPK phosphorylation; effect on induction of apoptosis, caspase-3 activation and growth inhibition abolished by SB203580 (p38 MAPK inhibitor). | Wang et al. 2012 [28] |
| PSP—Commercial source | Prostate cancer cell line PC-3—250 or 500 µg/mL | Suppresses PC-3 cell growth and in spheroid formation assay; see Table 2 for in vivo effect. | Luk et al. 2011 [29] |
| Polysaccharopeptide (PSP)—Commercial source—Winsor Health Products Ltd, Hong Kong | HL-60–25 μg/mL | Reduces cell proliferation; inhibits cell progression through both S and G2 phase; reduces 3H-thymidine uptake and prolonged DNA synthesis time; enhances the cytotoxicity of camptothecin; no effect on normal human peripheral blood mononuclear cells. | Wan et al. 2010 [30] |
| Methanol extract of fruiting body of Serbian origin | B16 mouse melanoma cells—200 µg/mL | Induces cell cycle arrest in the G0/G1 phase, followed by both apoptotic and secondary necrotic cell death; see Table 2 for in vivo effect. | Harhaji et al. 2008 [31] |
| PSP | Human breast cancer (ZR-75-30) cells—50 μg/mL or with 5 μM of doxorubicin, etoposide or cytarabine | Enhances the cytotoxicity of doxorubicin and etoposide but not cytarabine; effect associated with S-phase trap; reduces the ratio of protein expression of Bcl-xL/Bax. | Wan et al. 2008 [32] |
| PSK | B16, A549, Hela, AGS, Jurkat, B9 and Ando-2 tumour cell lines—50 or 100 μg/mL | Inhibits cell growth; induces cell cycle arrest, with cell accumulation in G0/G1 phase; induces apoptosis and increases caspase-3 expression. | Jimenez-Medina et al. 2008 [33] |
| PSP | HepG2 cells | Non-toxic dose of PSP enhanced the cytotoxicity of cyclophosphamide; decreased cell viability by 22% at 10 µg/mL | Chan and Yeung 2006 [34] |
| Standardised aqueous ethanol extract | HL-60 cells | Suppresses cell proliferation in a dose-dependent manner (IC50 = 150.6 µg/mL); increases nucleosome production from apoptotic cells; increases Bax and downregulates Bcl-2 or increases Bax/Bcl-2 proteins ratio; increases the release of cytochrome-c from mitochondria to cytosol; other effects, see Table 2 | Ho et al. 2006 [35] |
| PSP | Human leukemia HL-60 and U-937 cells—0.1–1 mg/mL | Inhibits cell proliferation and induces apoptosis; cell type-dependent disruption of the G1/S and G2/M phases of cell cycle progression; more cytotoxic to HL-60 cells; suppresses the expression of bcl-2 and survivin while increasing Bax and cytochrome-c; enhances cleavage of PARP from its native 112-kDa form to the 89-kDa truncated product; decreases in p65 and to a lesser degree p50 forms of NF-κB; reduces the expression of COX-2. | Hsieh et al. 2006 [36] |
| Standardised aqueous ethanol extract—commercial source, Hong Kong | MDA-MB-231, MCF-7 and T-47D cells—400 or 600 µg/mL | Suppresses cell proliferation—IC50 values in ascending order of T-47D, MCF-7, MDA-MB-231, and BT-20 least affected; increases nucleosome productions in apoptotic cells; downregulates Bcl-2 protein expression (MCF-7 and T-47D cells, but not in MDA-MB-231 cells); upregulates p53 protein only in T-47D cells | Ho et al. 2005 [37] |
| Polysaccharide peptide (PSP) | Human promyelocytic leukemia HL-60 cells—25–100 µg/mL | Dose-dependently enhances cell apoptosis induced by doxorubicin and etoposide, but not cytarabine (Ara-C); enhances the apoptotic machinery of Doxo and VP-16 in a cell cycle-dependent manner; modulates the regulatory checkpoint cyclin E and caspase 3. | Hui et al. 2005 [38] |
| Polysaccharide peptide (PSP) | HL-60 cells | Induces apoptosis HL-60 cells but not of normal human T-lymphocytes; decrease in Bcl-2/Bax ratio, drop in mitochondrial transmembrane potential, cytochrome c release, and activation of caspase −3, −8 and −9 | Yang et al. 2005 [39] |
| Proteins and peptide bound polysaccharides (PSP) | HL-60 cells—400 µg/mL | Induces apoptosis; phosphorylated regulation of early transcription factors (AP-1, EGR1, IER2 and IER5) and downregulates NF-κB pathways; increases apoptotic or anti-proliferation genes (GADD45A/B and TUSC2) and the decrease of a batch of phosphatase and kinase genes; alters carcinogenesis-related gene transcripts (SAT, DCT, Melan-A, uPA and cyclin E1). | Zeng et al. 2005 [40] |
| Ethanol–water extract—commercial source (Hong Konk) | Raji, NB-4, and HL-60 cells—50 to 800 µg/mL | Suppresses cell proliferation; no cytotoxic effect on normal liver cell line WRL (IC50 > 800 µg/mL); increases nucleosome productions in cancer but not in normal cells. | Lau et al. 2004 [41] |
| Polysaccharopeptide (PSP) | C6 rat glioma cells exposed to radiation (4 Gy)—1 mg/mL | Inhibits 3H-thymidine uptake; augments radiation-induced cancer cell damage though radiation efficacy did not increase. | Mao et al. 2001 [42] |
| Yunzhi (Windsor Wunxi)—a proprietary dietary supplement—ethanolic extracts (70%) | Hormone-responsive LNCaP and androgen-refractory JCA-1, PC-3, and DU-145 prostate cancer cells—0.5 mg/mL | Increases the levels STAT1 and STAT3 in JCA-1 but not LNCaP cells; reduces LNCaP cell growth, downregulates the levels of secreted but not intracellular prostate-specific antigen; no effect on level of the androgen receptor; less antiproliferative effect on PC-3 and DU-145 cells than LNCaP, and no effect on JCA-1 cells. | Hsieh and Wo 2001 [43] |
| PSK | MCF-7 cells—200 µg/mL | Inhibited DNA synthesis with IC50 value of 200 µg/mL. | Aoyagi et al. 1997 [44] |
| RPSP, a refined polysaccharide peptide fraction isolated by fast performance liquid chromatography (FPLC) from the crude powder of total peptide-bound polysaccharides of cultivated Coriolus versicolor Cov-1 | Human hepatoma cell line (HepG2) | IC50 of 243 µg/mL for 3-day assay; no effect on normal human foetal hepatocytes. | Dong et al. 1996 [45] |
| PSK | NRK-49F (normal rat kidney) and H4-II-E ovarian cancer cells—100 µg/mL | Prevented cytotoxicity due to cisplatin toward NRK-49F, but enhanced the cytotoxicity on H4-II-E and human ovarian cancer cells; modulates cell-dependent effect on cisplatin-induced alteration in lipid peroxide and SOD activity. | Kobayashi et al. 1994 [46] |
| PSK | Walker 256 (fibrosarcoma) NRK-49F (rat normal kidney fibroblast), H4-II-E (rat hepatoma) and H4-II-E-C3 (rat hepatoma) cell lines—500 µg/mL | More pronounced antiproliferative effect in Walker 256 cells, which have more SOD activity; increased SOD activity in Walker 256 by 3.6 times and H2O2 by 2.56 times; no effect on CAT and GPx activity. | Kobayashi et al. 1994 [47] |
| Small polypeptide of about 10 Kd | HL-60 (leukaemia), LS174-T (colon), SMMU-7721 (hepatoma), and SCG-7901 (stomach) | Cytotoxicity against HL-60 (most sensitive cell line) with IC50 value of 30 µg/mL; more cytotoxic to leukemia and SCG-7901 cells than PSP or PSK. | Yang et al. 1992 [48] |
| PSK and four PSK subfractions | TNF-induced cytotoxicity in mouse L-929 fibroblast; interferon-γ-induced differentiation of human myelogenous leukemic U-937 and THP-1 cells. | Enhances the TNF-induced cytotoxicity against L-929 cells; induces cell differentiation; induces the expression of NBT-reducing and α-naphthyl acetate esterase activity; polysaccharides of over 200 kDa had the most potent stimulating activity. | Kim et al. 1990 [49] |
| Preparation | Experimental Model | Key Findings | References |
|---|---|---|---|
| Water extract of commercial source | Nude mice inoculated with human breast cancer cells - aqueous extract, metronomic zoledronic acid, or the combination of both for 4 week—1g/kg extract, p.o. daily), metronomic zoledronic acid group (0.0125 mg/kg, i.p. injected twice a week), or in combination. | Combination with metronomic zoledronic acid diminished tumor growth without increasing the incidence of lung and liver metastasis; combination therapy reserved the integrity of bones. | Ko et al. 2017 [50] |
| Aqueous extract | Mouse mammary carcinoma 4T1 tumour-bearing mice—1 g/kg, p.o. for 4 weeks | Decreased tumor weight by 36%, lung metastasis by 70.8%; protects bones from cancer-induced bone loss | Luo et al. 2014 [23] |
| PSK | Combination with taxanes for prostate transgenic adenocarcinoma of the mouse prostate (TRAMP)—C2-bearing mice—PSK with docetaxel—Mouse prostate tumor (TRAMP-C2) cells injected orthotopically—docetaxel (5 mg/kg, i.p. twice weekly); PSK (300 mg/kg daily p.o.) or in combination for 11–13 days | The combination increased more tumour suppression than either treatment alone—reduced tumor proliferation and enhanced apoptosis; other effects on immunomodulation (see Table 4). | Wenner et al. 2012 [51] |
| BreastDefend (BD)—extract that also contains several other mushrooms and herbal products | MDA-MB-231 cells implanted in female nude mice—100 mg/kg, ig., for 33 days. | Reduces tumour volume and anti-metastatic activity to the lungs; downregulates the expression of PLAU (uPA protein) and CXCR4 genes in breast tumors; no effect on genes associated with breast-to-lung cancer metastasis: ezrin (EZR), HRAS, S100A4, CDKN1A (protein p21) and HTATIP2 (protein TIP30). | Jiang et al. 2012 [52] |
| PSP | Transgenic mice (TgMAP) mice that spontaneously develop prostate tumors—200 or 300 mg/kg p.o. 5 days per week for 20 weeks | Suppress tumourogenicity–chemopreventive property; see Table 1 for in vitro effect. | Luk et al. 2011 [29] |
| Methanol extract of fruiting body of Serbian origin | C57BL/6 mice inoculated with syngeneic B16 tumor cells—50 mg/kg, i.p. for 14 days | Inhibits tumor growth; peritoneal macrophages collected 21 days after tumor implantation; see Table 1 and Table 4 for other effects. | Harhaji et al. 2008 [31] |
| Standardised aqueous ethanol extract | Athymic nude mouse with HL-60 leukaemic xenograft model—100 mg/kg, p.o. for 28 days | Inhibits tumour growth; see Table 1 for in vitro effect. | Ho et al. 2006 [35] |
| VPS, a hot water extract | Swiss mice—as a 2% dose in the powdered diet for life and 1,2-dimethylhydrazine dihydrochloride (1,2-DMH) injection | No inhibitory effect on the development of large intestinal cancers; intestinal tumours and the total number of these tumors in the intestine not significantly different. | Coles et al. 2005 [53] |
| PSP | S180 tumor-bearing mouse model—murine sarcoma S180 cells implanted in subcutaneously in the back of each mouse—PSP solution in drinking water (35 μg/day/mouse) for 20 days | Suppress the expression of VEGF and angiogenesis and tumour markers. | Ho et al. 2004 [54] |
| PSP | Tumour bearing mice—radiation (8 Gy/mouse) or with PSP, i.p. 5 days before implantation and for 10 days after | Increase natural killer cell, lymphocyte and granulocyte counts in blood and spleen; no direct tumor reducing effect; see Table 1 for direct cytotoxic effect. | Mao et al. 2001 [42] |
| RPSP, a refined polysaccharide peptide fraction isolated by fast performance liquid chromatography from the crude powder of total peptide-bound polysaccharides of cultivated Coriolus versicolor Cov-1 | Sarcoma 180 inoculated nude mice—1 mg, i.p. for 15 days | Reduces incidences of tumor growth; suppresses tumor mass; no pathological lesions in vital organs of animals such as heart, liver, spleen, lung and kidney. | Dong et al. 1996 [45] |
| PSK | N-methyl-N-nitrosourea-induced mammary gland tumors in rats—250 mg/kg twice a week for 3 weeks after tumour development | Inhibits tumour size and carcinogenesis | Fujii et al. 1995 [55] |
| PSK | Rat ascites hepatoma cell line (AH66) inoculated i.p. in rats—250 mg/kg, i.p. for 5 days before inoculation and 7 days after. | Direct effect on the transcription and translation of genens (pPIC1, pPIC2 and pPDC1). | Hirose et al. 1985 [14] |
| Preparation | Experimental Model | Key Findings | References |
|---|---|---|---|
| PSP | Normal and LPS-stimulated rat peripheral blood mononuclear cells (PBMCs—5–300 μg/mL | Enhances mitogenic activity and attenuates the induced cytokines (interleukin (IL)-1β and IL-6) production in stimulated macrophages; increases cell proliferation and pro-inflammatory cytokines release in unstimulated (LPS-free) macrophages. | Jedrzejewski et al. 2016 [77] |
| Protein-bound polysaccharides (PBP) | Blood lymphocytes and breast cancer cells (MCF-7)—100 and 300 μg/mL | Induces proliferative response on blood lymphocytes, as well as IL-1β and IL-6 mRNA expression; temperature of 39.5 °C blocks the PBP-induced cytotoxicity against MCF-7 cells, which correlates with reduction in TNFα level; see Table 4 for in vivo effect. | Pawlikowska et al. 2016 [78] |
| PSP | Breast cancer (MCF-7) cells and blood lymphocytes—100 μg/mL | Reduces cell growth; upregulates TNF-α- expression but not IL-1β and IL-6; enhances the proliferative response of blood lymphocytes associated with IL-6 and IL-1β mRNA upregulation. | Kowalczewska et al. 2016 [79] |
| PSK—isolation of TLR2 agonist activity from soluble β-glucan fraction—labeled the soluble β-glucan with fluorescein | Uptake of the labeled β-glucan in J774A macrophages and JAWSII dendritic cells—10–1000 µg/mL | Uptake inhibited by anti-Dectin-1 antibody but not by anti-TLR2 antibody; Dectin-1 is the receptor for β-glucan; lipid fraction enhances the uptake of the soluble β-glucan. | Quayle et al. 2015 [80] |
| PSP | Peritoneal macrophages from mice—25 μg/mL | Stimulates the expressions of cytokines, as well as TLR4, TRAF6, phosphorylation of NF-κB p65 and phosphorylation of c-Jun (a component of the transcription factor AP-1) in peritoneal macrophages from C57BL/10J (TLR4+/+) mice but not from C57BL/10ScCr (TLR4−/−) mice; see Table 4 for in vivo effect. | Wang et al. 2015 [81] |
| Polysaccharides—hot water extraction in house | Mouse splenocytes—high dose of 30 mg/mL | Stimulates splenocytes proliferation; fluorescence-labeled polysaccharides selectively stained mouse B cells but not T-cells; induces the production of IgM and IgG1 with or without exogenously added IL-4; membrane Ig (B cell antigen-receptor) acts as the polysaccharide binding protein; induces B-cell proliferation (inhibited by anti-mouse immunoglobulin (Ig) blocking antibody or in cells from TLR4-mutant mice; increases the phosphorylation of ERK-1/2 and p38 MAPK; enhances the nuclear translocation of the cytosolic NF-κB p65 subunit. | Yang et al. 2015 [82] |
| PSK as TLR2 agonist | PBMCs from healthy human donors—monocyte-derived DCs and tumor fusion cells | Upregulates MHC (class II and CD86) expression on DC/tumor; increases fusion efficiency; increases production of fusions derived IL-12p70; activates CD4+ and CD8+ T-cells to induce IFN-γ production; enhances induction of CTL activity specific for Mucin 1. | Koido et al. 2013 [83] |
| PSK | Mouse bone marrow-derived dendritic cells (DC)—5, 10, 20, 40, and 80 µg/mL) | Induces DC maturation—dose-dependent increase in the expression of CD80, CD86, MHCII, and CD40; induces the production (mRNA and protein levels) of IL-12, TNF-α, and IL-6. | Engel et al. 2013 [84] |
| PSP | PBMCs—10 and 100 μg/mL | Increases monocytes counts (CD14+/CD16−) compared to controls—confirmed by CD14 and MHCII antibodies; no significant effect on proliferation of T-cells, NK, and B-cells. | Sekhon et al. 2013 [85] |
| Purified new protein—YZP is a 12-kDa non-glycosylated protein comprising 139 amino acids, including an 18-amino acids signal peptide | Mice lymphocyte proliferation—20 μg/mL | Induced a greater than 60-fold increase in IL-10 secretion in mice B lymphocytes; specifically triggers the differentiation of CD1d+ B cells into IL-10-producing regulatory B cells (Bregs); enhances the expression of CD1d; activates Breg function via interaction with TLR2 and TLR4 and upregulation of the TLR-mediated signaling pathway. | Kuan et al. 2013 [86] |
| PSK | Human peripheral blood mononuclear cells—12–100 µg/mL | Activates NK cells to produce IFN-γ and to lyse K562 target cells; enhances trastuzumab-mediated antibody-dependent cell-mediated cytotoxicity ADCC against SKBR3 and MDA-MB-231 breast cancer cells; effect related to both direct and IL-12-dependent (indirect) mechanism. | Lu et al. 2011 [87] |
| PSK | J774A.1 cells and primary splenocytes—125 μg/mL | Induces TNF-α and IL-6 secretion by wild-type but not by TLR4-deficient peritoneal macrophages; TNFα secretion by J774A.1 cells and primary splenocytes effect inhibited by TLR4 blocking antibody. | Price et al. 2010 [88] |
| PSP | Human PBMCs—25 μg/mL | Upregulates the expression of (e.g., IFN-γ, CXCL10, TLR4, TLR5) while downregulating (e.g., TLR9, TLR10, SARM1, TOLLIP) other genes related with TLR signaling pathway; upregulated some cytokines (GCSF, GM-CSF, IL-1α, IL-6, IFN-γ) by more than 1.3 times; increases the mRNA levels of TRAM, TRIF, and TRAF6; increases the protein level of TRAF6. | Li et al. 2010 [89] |
| PSK | B-cells—human B-cell line BALL-1—1–100 µg/mL | Enhances IgM production in B-cells. | Maruyama et al. 2009 [90] |
| Polysaccharides from New Zealand isolate (Wr-74) and a patented strain (ATCC-20545) of C. versicolor—culture medium isolates | Murine splenocytes—extracellular polysaccharide (1150 µg/mL), and intracellular polysaccharide (IPS) (100 µg/mL) | Induces cytokine production (interleukin 12 and gamma interferon) in murine splenocytes. | Cui et al. 2007 [8] |
| PSP | Human T lymphocyte proliferation—100 or 500 µg/mL | Exhibits similar and additive inhibitory effects to ciclosporin to suppress activated T-cell proliferation, Th1 cytokines; reduces CD3+/CD25+ cell expression but not Th2 cytokine expression. | Lee et al. 2008 [91] |
| Ethanol–water extract—commercial source | Proliferation of murine (BALB/c mice) splenic lymphocytes—12.5–400 μg/mL | Enhances cell proliferation by up to 2.4-fold in a time- and dose-dependent manner; upregulates Th1-related cytokines (IL-2 and IL-12); enhanced the level of Th1-related cytokines (IFN-γ and IL-18) transiently (24 h, but not at 48 and 72 h) while Th2-(IL-4 and IL-6). | Ho et al. 2004 [92] |
| PSK | Dendritic cells derived from CD14-positive cells obtained from human peripheral blood monocytes | Increases the expression of HLA (class II antigen) and CD40; increases the number and expression of CD80-, CD86- and CD83-positive cells; decreases FITC-dextran uptake; augments IL-12 production and allogeneic mixed lymphocyte reaction; induces antigen-specific cytotoxicity. | Kanazawa et al. 2004 [93] |
| PSK | Mouse peritoneal PMNs—500 µg/ml | In combination with IFN-γ, increases NO production. | Asai et al. 2000 [94] |
| PSK and fractions (F1 <50 kDa; F2 50–100 kDa; F3 100–200 kDa; F4 >200 kDa) | U937 and THP-1 cells differentiation; TNF-induced cytotoxicity in L929 cells—5–500 µg/mL | In combination with IFN-γ, increases NO production and cell differentiation; enhances cytotoxicity in L929 cells; fraction F4 is the most active. | Kim et al. 1990 [49] |
| Preparation | Experimental Model | Key Findings | References |
|---|---|---|---|
| Extract from Coriolus versicolor (Cov 1 strain) | Pre-injection in LPS-treated rats and PBMCs isolated—100 mg/kg, i.p. | Partially prevents endotoxin tolerance through maintaining febrile response; increases IL-6 and greater NF-κB activation in response to LPS stimulation ex vivo; enhances mitogenic effect of LPS and increases ROS generation. | Jedrzejewski et al. 2019 [95] |
| Glucan—home-made purification—[→6)-α-D- Glcp-(1→]n. | Sarcoma 180-bearing mice—100 or 200 mg/kg for nine days, subcutaneously | Promotes the secretion of IL-2, −4, −6, −10, −17A and IFN-α and -γ; enhances cytokine production associated with T-helper Th2 and Th17 cells; effect dependent on IL-10. | Awadasseid et al. 2017 [96] |
| PSP | C57BL/6 male mice—50 mg/kg, p.o. | When combined with acacia gum, increased total IgG titre levels (day 4) while decreasing IgM titre had no effect on IgA or IgE titre levels. | Sekhon et al. 2016 [97] |
| Protein-bound polysaccharides (PBP) | Fever-range hyperthermia (FRH) combined with PBP in rats—100 mg/kg i.p. | Combination treatment of (FRH + PBP) decrease IL-1β, IL-6 and TNF-α mRNA expression in peripheral blood mononuclear cells; see Table 3 for in vitro effect. | Pawlikowska et al. 2016 [78] |
| PSP | Male Wistar rats—100 mg/kg, i.p. 2 h before LPS | Increases the duration of endotoxin fever; increases the blood level of IL-6 (3 or 14 h post-injection); effect inhibited by anti-IL-6 antibody (30 µg/rat). | Jedrzejewski et al. 2015 [98] |
| PSP | 500 mg/kg/d by p.o. in mice for 25 days | Decreases the mean weights of tumors; increases thymus index and spleen index relative in tumour-bearing C57BL/10J (TLR4+/+) mice but not in C57BL/10ScCr (TLR4−) mice; see Table 3 for in vitro effect. | Wang et al. 2015 [81] |
| PSP | Male Wistar rats—50, 100 and 200 mg/kg, i.p. | Induces a rapid reduction in temperature; elevates TNF-α level; anti-TNF-α antibody abolish effect on temperature. | Jedrzejewski et al. 2014 [99] |
| Aqueous extract | Mouse mammary carcinoma 4T1 tumor bearing mice—1 g/kg, p.o. for 4 weeks | Increases IL-2, 6, 12, TNF-α and IFN-γ productions from the spleen lymphocytes; see Table 1 and Table 2 for other effects | Luo et al. 2014 [23] |
| PSK | As an adjuvant to OVAp323-339 vaccine in vivo—DC activation 1000 µg—one injection by intradermal route | Enlarges draining lymph nodes with higher number of activated DC; stimulates the proliferation of OVA-specific T-cells, and induces T-cells that produce multiple cytokines (IFN-γ, IL-2, and TNF-α; see Table 3 for in vitro effect. | Engel et al. 2013 [84] |
| PSK | PSK with docetaxel- mouse prostate tumor (TRAMP-C2) cells injected orthotopically—docetaxel (5 mg/kg) injected i.p. twice weekly; PSK (300 mg/kg) daily by oral gavage or combination for 11–13 days | Lower level of decrease in number of white blood cells than docetaxel alone; increases numbers of tumor-infiltrating CD4+ and CD8+ T-cells; PSK with or without docetaxel enhance mRNA expression of IFN-γ—no effect on T-regulatory FoxP3 mRNA expression in tumors; augments the docetaxel-induced splenic natural killer cell cytolytic activity against YAC-1 target cells. | Wenner et al. 2012 [51] |
| PSK | Neu transgenic mice received subcutaneous implant of 1 million MMC cells—100 mg/kg, p.o. 3 times per week for up to 4 weeks | Potentiates the anti-tumour effect of anti-HER2/neu mAb therapy in neu-T mice; see Table 3 for in vitro effect. | Lu et al. 2011 [87] |
| Methanol extract of fruiting body of Serbian origin | C57BL/6 mice inoculated with syngeneic B16 tumor cells—50 mg/kg, i.p. for 14 days | Peritoneal macrophages collected 21 days after tumor implantation possess stronger tumouristatic activity ex vivo than those from untreated animals; see Table 1 and Table 2 for other effects. | Harhaji et al. 2008 [31] |
| PSP—composed of 90% polysaccharides (74.6% glucose, 2.7% galactose, 1.5% mannose, 2.4% fucose and 4.8% xylose) and 10% peptides (18 different amino acids, mostly aspartic acid and glutamic acid) | Acetic acid-induced writhing model—0.2–2 μmol/kg, i.p. in hot-plate test; 2–4 μmol/kg, i.p. in acetic acid-induced writhing response; 0.05–4 μmol/kg, i.p. induction of writhing response by itself. | Decreased the number of acetic acid-induced writhing by 92.9%; PSP itself induces a dose-dependent writhing response; increased the release of PGE2, TNF-α, IL-1β, and histamine in mouse peritoneal macrophages and mast cells both in vivo and in vitro (1–100 μM). | Chan et al. 2006 [100] |
| Purified polysaccharide (CV-S2-Fr.I) of C. versicolor obtained by Sepharose CL-6B gel chromatography | Mouse peritoneal macrophage—100 µg/mL | Enhanced macrophage lysosomal enzyme activity by 250%; enhances the induction of NO production by interferon-γ (no effect by its own). | Jeong et al. 2006 [101] |
| PSP | Tumour bearing mice—radiation (8 Gy/mouse) or with PSP, i.p. 5 days before implantation and for 10 days after | Increases natural killer cell, lymphocyte and granulocyte counts in blood and spleen; no direct tumor reducing effect; see Table 1 for direct cytotoxic effect. | Mao et al. 2001 [102] |
| PSP | C57BL/6NIA mice—diets containing 0.1, 0.5 or 1.0% PSP for 1 month | No effect on mitogenic response to Con A, PHA or LPS, or on production of IL-1, IL-2, IL- 4 and PGE2; induced higher delayed-type hypersensitivity response (1.0% PSP) in old but not in young mice. | Wu et al. 1998 [103] |
| Small polypeptide of about 10 Kd | Human tumour cells (SMMU-7721 or LS174-T) inculated into nude mice—2 mg, i.p. for 2 weeks. | Increases WBC and IgG levels; decreases the incidence of tumor mass. | Yang et al. 1992 [48] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habtemariam, S. Trametes versicolor (Synn. Coriolus versicolor) Polysaccharides in Cancer Therapy: Targets and Efficacy. Biomedicines 2020, 8, 135. https://doi.org/10.3390/biomedicines8050135
Habtemariam S. Trametes versicolor (Synn. Coriolus versicolor) Polysaccharides in Cancer Therapy: Targets and Efficacy. Biomedicines. 2020; 8(5):135. https://doi.org/10.3390/biomedicines8050135
Chicago/Turabian StyleHabtemariam, Solomon. 2020. "Trametes versicolor (Synn. Coriolus versicolor) Polysaccharides in Cancer Therapy: Targets and Efficacy" Biomedicines 8, no. 5: 135. https://doi.org/10.3390/biomedicines8050135
APA StyleHabtemariam, S. (2020). Trametes versicolor (Synn. Coriolus versicolor) Polysaccharides in Cancer Therapy: Targets and Efficacy. Biomedicines, 8(5), 135. https://doi.org/10.3390/biomedicines8050135