Sex Differences in Biological Processes and Nitrergic Signaling in Mouse Brain
Abstract
1. Introduction
2. Material and Methods
2.1. Materials and Reagents
2.2. Sample Preparation for MS
2.3. MS Analysis
2.4. MS Data Extraction and Processing
2.5. Systems Biology Analysis
2.6. Ethic Items
3. Results
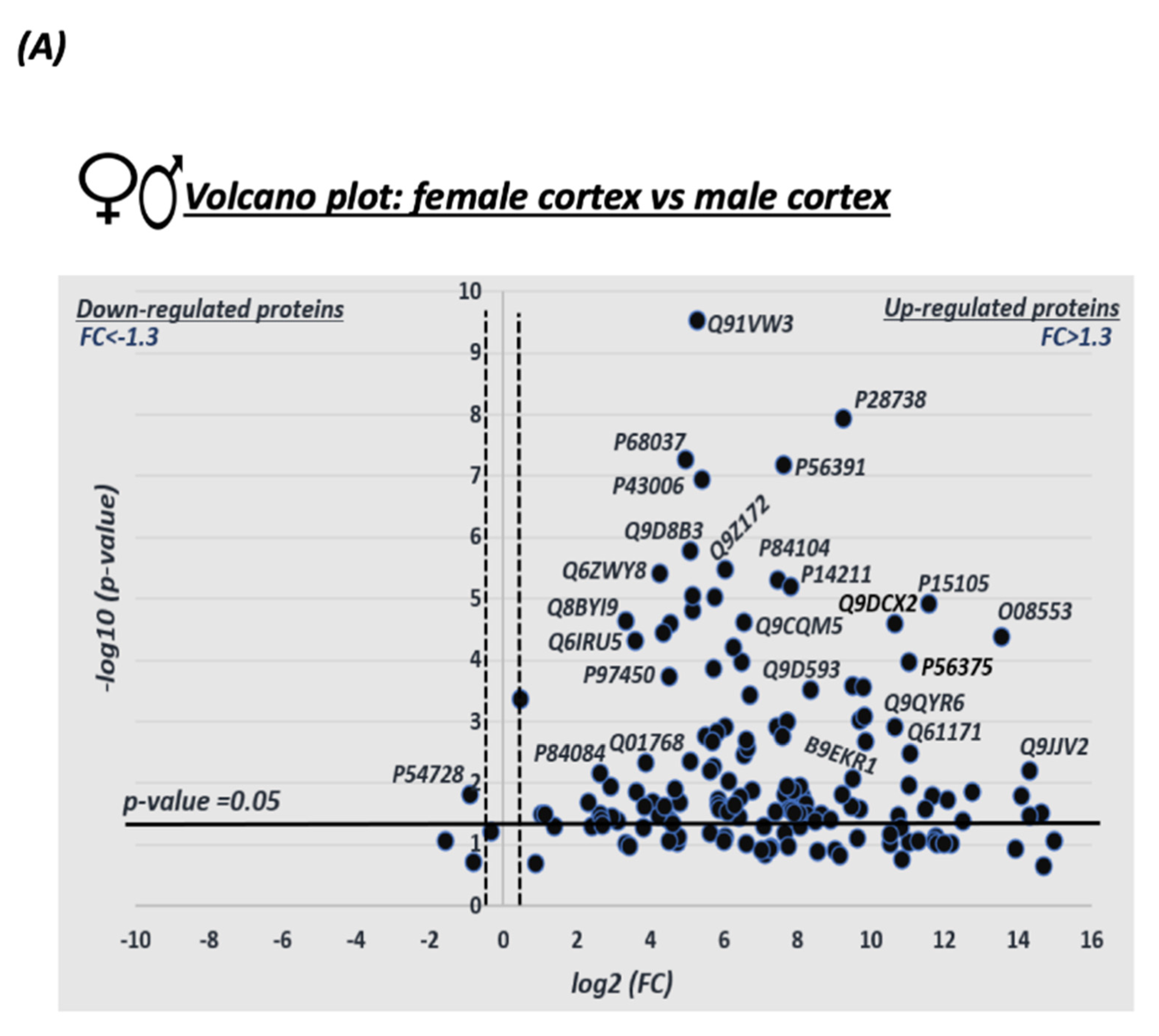
3.1. Sex Differences in S-Nitrosylation in the Mouse Cortex
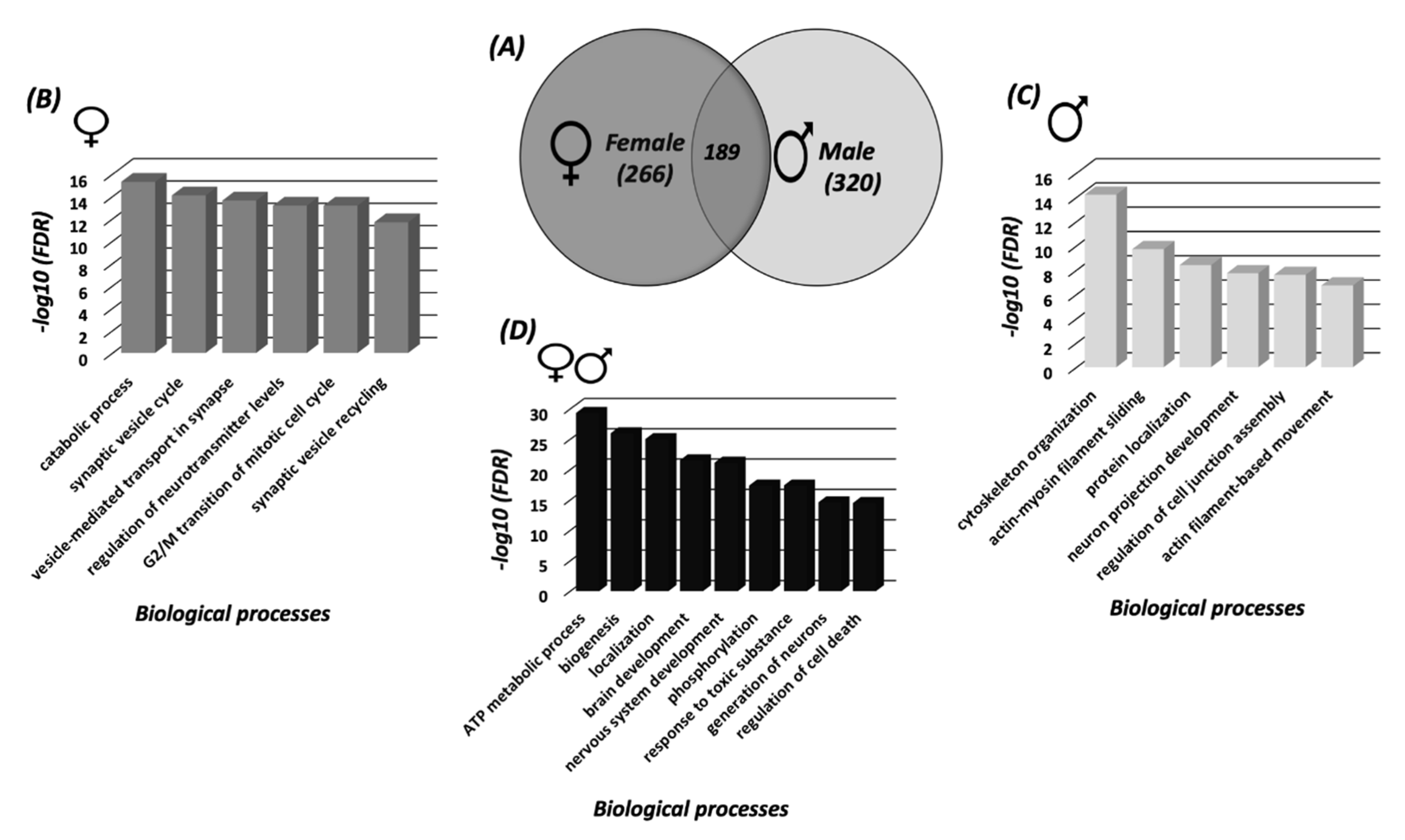
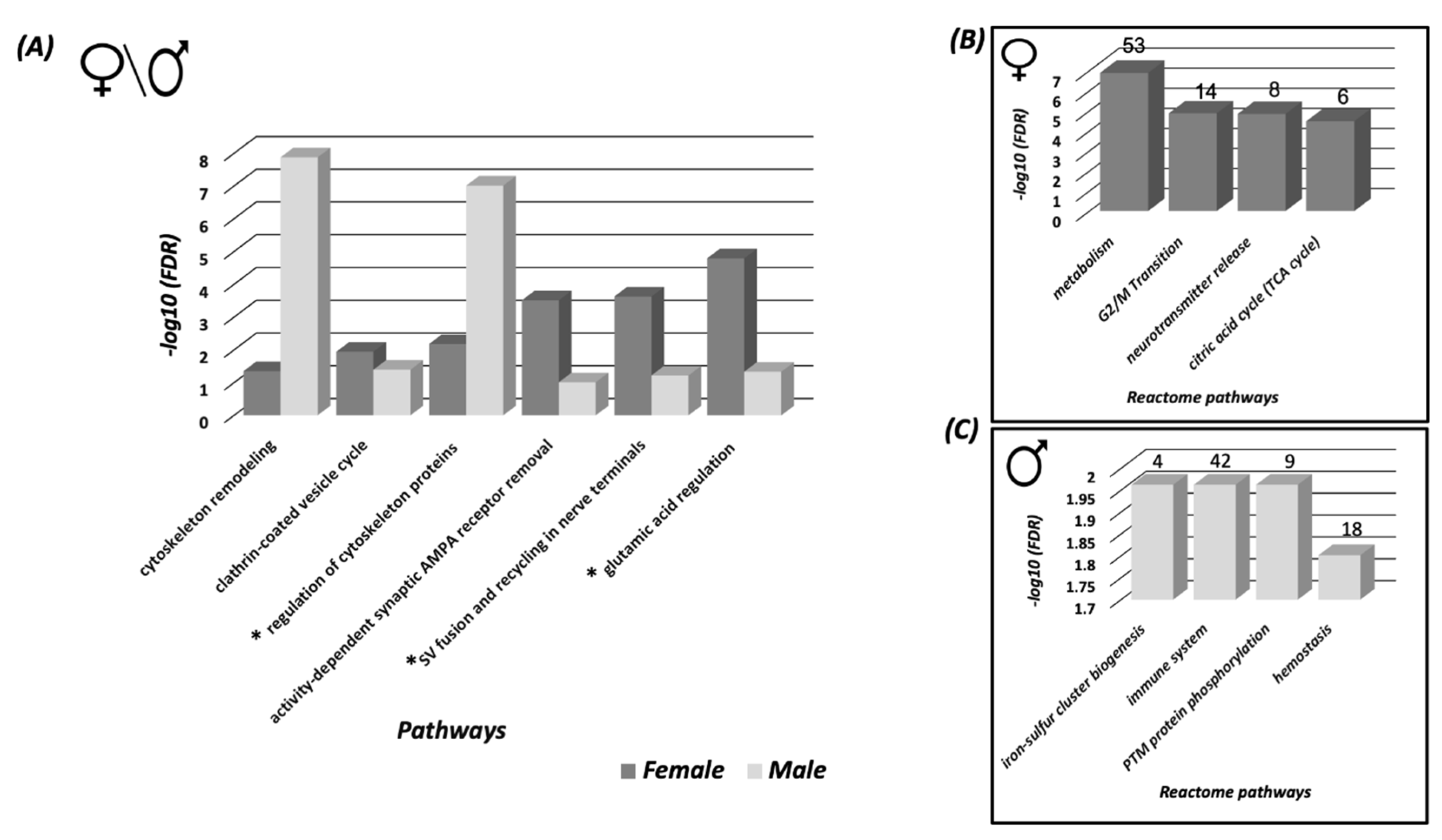
3.2. Systems Biology Analysis of the SNO Proteins in the Cortices of Female and Male Mice
3.3. Protein Classification Analysis of the SNO Proteins in Female and Male Cortices
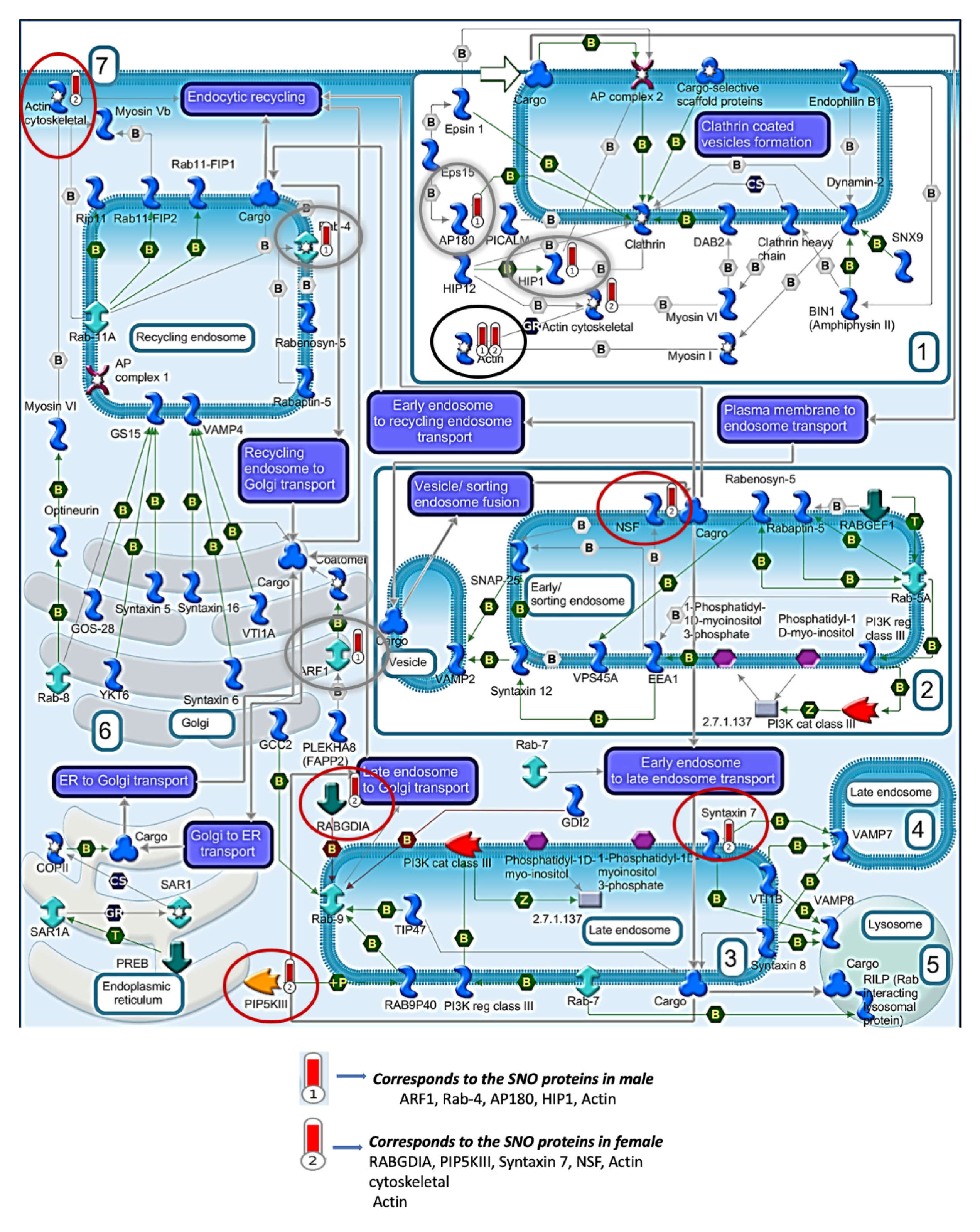
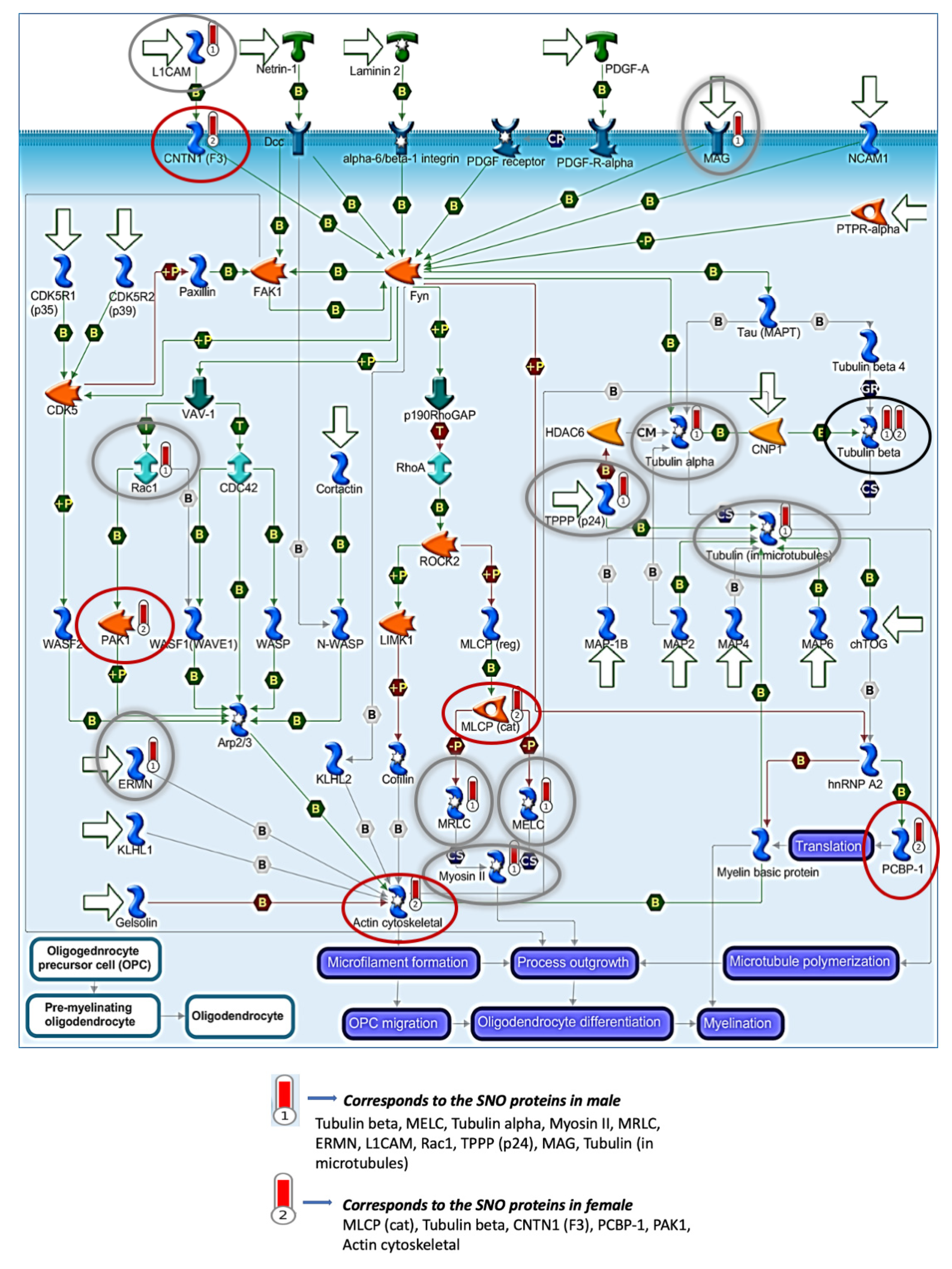
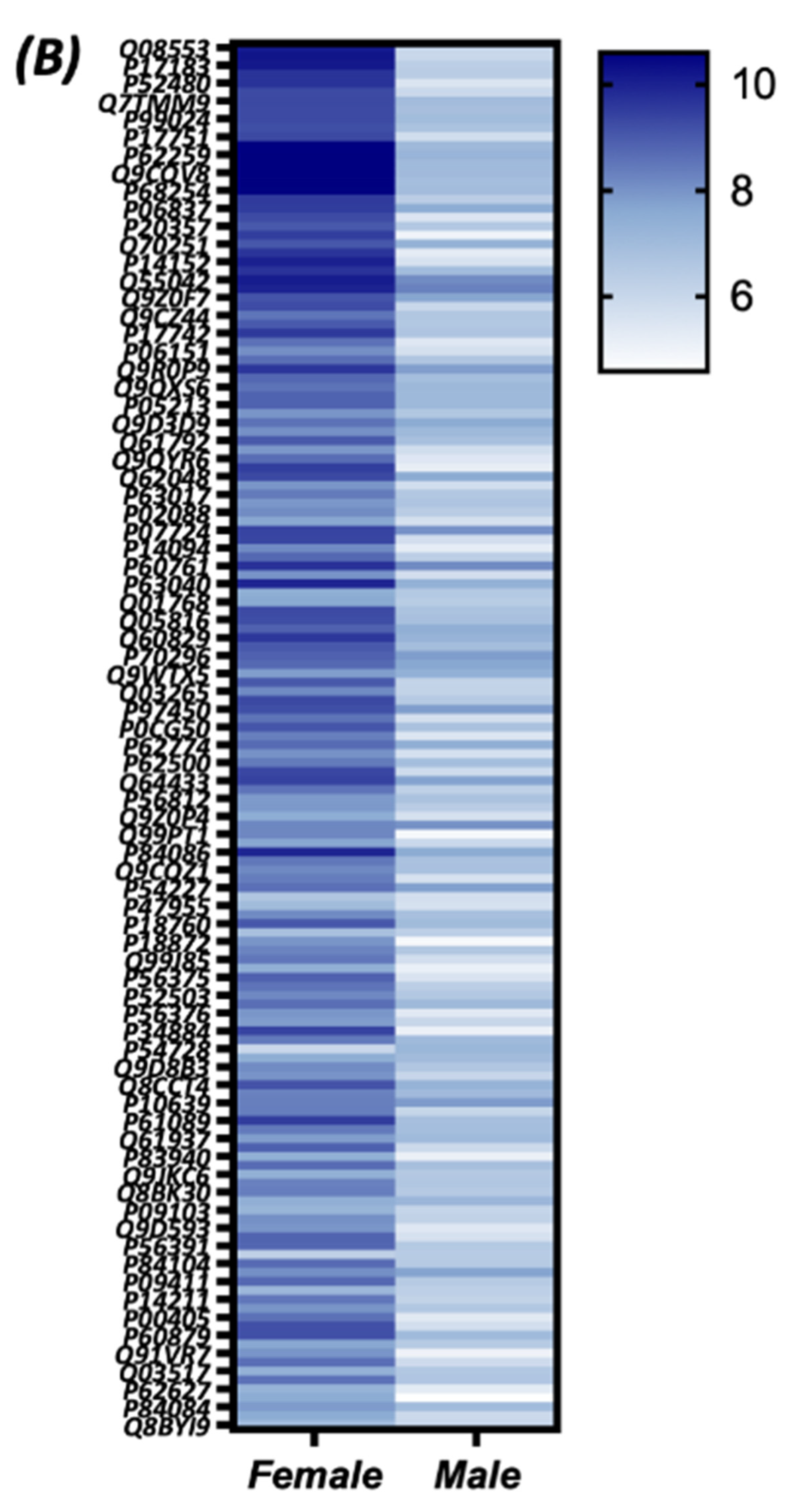
3.4. Quantitative Analysis of the S-Nitroso-Proteome in Both Sexes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bredt, D.S.; Snyder, S.H. Nitric oxide: A physiologic messenger molecule. Annu. Rev. Biochem. 1994, 63, 175–195. [Google Scholar] [CrossRef]
- Bredt, D.S.; Hwang, P.M. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature 1991, 351, 714. [Google Scholar] [CrossRef]
- Förstermann, U.; Schmidt, H.H.; Pollock, J.S.; Sheng, H.; Mitchell, J.A.; Warner, T.D.; Nakane, M.; Murad, F. Isoforms of nitric oxide synthase characterization and purification from different cell types. Biochemical. Pharmacol. 1991, 42, 1849–1857. [Google Scholar] [CrossRef]
- Muller, U.; Bicker, G. Calcium-activated release of nitric oxide and cellular distribution of nitric oxide-synthesizing neurons in the nervous system of the locust. J. Neurosci. 1994, 14, 7521–7528. [Google Scholar] [CrossRef]
- Lopez-Collazo, E.; Mateo, J.; Miras-Portugal, M.T.; Bosca, L. Requirement of nitric oxide and calcium mobilization for the induction of apoptosis in adrenal vascular endothelial cells. FEBS Lett. 1997, 413, 124–128. [Google Scholar] [CrossRef]
- Brenman, J.E.; Chao, D.S.; Gee, S.H.; McGee, A.W.; Craven, S.E.; Santillano, D.R.; Wu, Z.; Huang, F.; Xia, H.; Peters, M.F.; et al. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell 1996, 84, 757–767. [Google Scholar] [CrossRef]
- Chachlaki, K.; Garthwaite, J.; Prevot, V. The gentle art of saying NO: How nitric oxide gets things done in the hypothalamus. Nat. Rev. Endocrinol. 2017, 13, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Hess, D.T.; Matsumoto, A.; Kim, S.O.; Marshall, H.E.; Stamler, J.S. Protein S-nitrosylation: Purview and parameters. Nat. Rev. Mol. Cell Biol. 2005, 6, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Doulias, P.T.; Greene, J.L.; Greco, T.M.; Tenopoulou, M.; Seeholzer, S.H.; Dunbrack, R.L.; Ischiropoulos, H. Structural profiling of endogenous S-nitrosocysteine residues reveals unique features that accommodate diverse mechanisms for protein S-nitrosylation. Proc. Natl. Acad. Sci. USA 2010, 107, 16958–16963. [Google Scholar] [CrossRef]
- Stamler, J.S.; Toone, E.J.; Lipton, S.A.; Sucher, N.J. (S) NO signals: Translocation, regulation, and a consensus motif. Neuron 1997, 18, 691–696. [Google Scholar] [CrossRef]
- Horenberg, A.L.; Houghton, A.M.; Pandey, S.; Seshadri, V.; Guilford, W.H. S-nitrosylation of cytoskeletal proteins. Cytoskeleton 2019, 76, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Tu, S.; Akhtar, M.W.; Sunico, C.R.; Okamoto, S.; Lipton, S.A. Aberrant protein s-nitrosylation in neurodegenerative diseases. Neuron 2013, 78, 596–614. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Prikhodko, O.A.; Pirie, E.; Nagar, S.; Akhtar, M.W.; Oh, C.K.; McKercher, S.R.; Ambasudhan, R.; Okamoto, S.; Lipton, S.A. Aberrant protein S-nitrosylation contributes to the pathophysiology of neurodegenerative diseases. Neurobiol. Dis. 2015, 84, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Deckel, A.W. Nitric oxide and nitric oxide synthase in Huntington’s disease. J. Neurosci. Res. 2001, 64, 99–107. [Google Scholar] [CrossRef]
- Jaffrey, S.R.; Erdjument-Bromage, H.; Ferris, C.D.; Tempst, P.; Snyder, S.H. Protein S-nitrosylation: A physiological signal for neuronal nitric oxide. Nat. Cell Biol. 2001, 3, 193–197. [Google Scholar] [CrossRef]
- Smith, B.C.; Marletta, M.A. Mechanisms of S-nitrosothiol formation and selectivity in nitric oxide signaling. Curr. Opin. Chem. Biol. 2012, 16, 498–506. [Google Scholar] [CrossRef]
- Stamler, J.S.; Lamas, S.; Fang, F.C. Nitrosylation: The prototypic redox-based signaling mechanism. Cell 2001, 106, 675–683. [Google Scholar] [CrossRef]
- Stamler, J.S.; Simon, D.I.; Osborne, J.A.; Mullins, M.E.; Jaraki, O.; Michel, T.; Singel, D.J.; Loscalzo, J. S-nitrosylation of proteins with nitric oxide: Synthesis and characterization of biologically active compounds. Proc. Natl. Acad. Sci. USA 1992, 89, 444–448. [Google Scholar] [CrossRef]
- Edelmann, M.; Wolfe, C.; Scordalakes, E.M.; Rissman, E.F.; Tobet, S. Neuronal nitric oxide synthase and calbindin delineate sex differences in the developing hypothalamus and preoptic area. Dev. Neurobiol. 2007, 67, 1371–1381. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, D.-L.; Luo, C.-X.; Zhu, L.-J.; Zhang, J.; Wu, H.-Y.; Zhu, D.-Y. Hippocampal nitric oxide contributes to sex difference in affective behaviors. Proc. Natl. Acad. Sci. USA 2012, 109, 14224–14229. [Google Scholar] [CrossRef]
- Ishihara, T.; Orikasa, C.; Araki, T.; Sakuma, Y. Sex difference in the expression and regulation of nitric oxide synthase gene in the rat preoptic area. Neurosci. Res. 2002, 43, 147–154. [Google Scholar] [CrossRef]
- Scordalakes, E.M.; Shetty, S.J.; Rissman, E. Roles of estrogen receptor ? and androgen receptor in the regulation of neuronal nitric oxide synthase. J. Comp. Neurol. 2002, 453, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, F.; Cerón, S.N.; Fenoy, F.J.; López, B.; Hernández, I.; Martinez, R.R.; Soriano, M.J.G.; Salom, M.G. Sex differences in nitrosative stress during renal ischemia. Am. J. Physiol. Integr. Comp. Physiol. 2010, 299, R1387–R1395. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Bayır, H.; Lai, Y.-C.; Graham, S.H.; Clark, R.S.; Zhang, X.; Kochanek, P.M.; Watkins, S.C. Innate Gender-based Proclivity in Response to Cytotoxicity and Programmed Cell Death Pathway. J. Boil. Chem. 2004, 279, 38563–38570. [Google Scholar] [CrossRef] [PubMed]
- Carruth, L.L.; Reisert, I.; Arnold, A.P. Sex chromosome genes directly affect brain sexual differentiation. Nat. Neurosci. 2002, 5, 933–934. [Google Scholar] [CrossRef] [PubMed]
- Rafikov, R.; James, J.; McClain, N.; Tofovic, S.P.; Rafikova, O. Role of Gender in Regulation of Redox Homeostasis in Pulmonary Arterial Hypertension. Antioxidants 2019, 8, 135. [Google Scholar] [CrossRef]
- Ko, E.; Choi, H.; Kim, B.; Kim, M.; Park, K.-N.; Bae, I.-H.; Sung, Y.K.; Lee, T.R.; Shin, D.W.; Bae, Y.S. Testosterone stimulates Duox1 activity through GPRC6A in skin keratinocytes. J. Boil. Chem. 2014, 289, 28835–28845. [Google Scholar] [CrossRef]
- Lopes, R.A.M.; Neves, K.B.; Pestana, C.R.; Queiroz, A.L.; Zanotto, C.Z.; Chignalia, A.Z.; Valim, Y.M.; Silveira, L.R.; Curti, C.; Tostes, R.C. Testosterone induces apoptosis in vascular smooth muscle cells via extrinsic apoptotic pathway with mitochondria-generated reactive oxygen species involvement. Am. J. Physiol. Circ. Physiol. 2014, 306, H1485–H1494. [Google Scholar] [CrossRef]
- Di Domenico, F.; Casalena, G.; Jia, J.; Sultana, R.; Barone, E.; Cai, J.; Pierce, W.M.; Cini, C.; Mancuso, C.; Perluigi, M. Sex differences in brain proteomes of neuron-specific STAT3-null mice after cerebral ischemia/reperfusion. J. Neurochem. 2012, 121, 680–692. [Google Scholar] [CrossRef]
- Nilsen, J.; Brinton, R.D. Mitochondria as therapeutic targets of estrogen action in the central nervous system. Curr. Drug Target -CNS Neurol. Disord. 2004, 3, 297–313. [Google Scholar] [CrossRef]
- Guneykaya, D.; Ivanov, A.; Hernandez, D.P.; Haage, V.; Wojtas, B.; Meyer, N.; Maricos, M.; Jordan, P.; Buonfiglioli, A.; Gielniewski, B. Transcriptional and translational differences of microglia from male and female brains. Cell Rep. 2018, 24, 2773–2783. [Google Scholar] [CrossRef] [PubMed]
- Bundy, J.L.; Vied, C.; Nowakowski, R.S. Sex differences in the molecular signature of the developing mouse hippocampus. BMC Genomics 2017, 18, 237. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, U.; Nott, A.; Bhat, V.B.; Ravindra, K.C.; Wishnok, J.S.; Tsai, L.H.; Tannenbaum, S.R. S-nitrosation of proteins relevant to Alzheimer’s disease during early stages of neurodegeneration. Proc. Natl Acad Sci USA 2016, 113, 4152–4157. [Google Scholar] [CrossRef]
- Rappsilber, J.; Mann, M.; Ishihama, Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc. 2007, 2, 1896–1906. [Google Scholar] [CrossRef]
- Huang da, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Statistical Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef]
- Tierney, A.L.; Nelson III, C.A. Brain Development and the Role of Experience in the Early Years. Zero Three 2009, 30, 9–13. [Google Scholar]
- Cline, H.; Spring, C. Dendrite Development, Synapse Formation and Elimination. Dev. Neurobiol. 2009, 456. [Google Scholar]
- Cossenza, M.; Socodato, R.; Portugal, C.C.; Domith, I.C.; Gladulich, L.F.; Encarnação, T.G.; Calaza, K.C.; Mendonça, H.R.; Campello-Costa, P.; Paes-de-Carvalho, R. Nitric oxide in the nervous system: Biochemical, developmental, and neurobiological aspects. Vitam. Horm. 2014, 96, 79–125. [Google Scholar] [PubMed]
- Ishide, T.; Nauli, S.M.; Maher, T.J.; Ally, A. Cardiovascular responses and neurotransmitter changes following blockade of nNOS within the ventrolateral medulla during static muscle contraction. Brain Res. 2003, 977, 80–89. [Google Scholar] [CrossRef]
- Wang, S.; Teschemacher, A.G.; Paton, J.F.; Kasparov, S.; Wang, S.; Teschemacher, A.G.; Paton, J.F.; Kasparov, S. Mechanism of nitric oxide action on inhibitory GABAergic signaling within the nucleus tractus solitarii. FASEB J. 2006, 20, 1537–1539. [Google Scholar] [CrossRef] [PubMed]
- Segieth, J.; Getting, S.J.; Biggs, C.S.; Whitton, P.S. Nitric oxide regulates excitatory amino acid release in a biphasic manner in freely moving rats. Neurosci. Lett. 1995, 200, 101–104. [Google Scholar] [CrossRef]
- Lawrence, A.J.; Jarrott, B. Nitric oxide increases interstitial excitatory amino acid release in the rat dorsomedial medulla oblongata. Neurosci. Lett. 1993, 151, 126–129. [Google Scholar] [CrossRef]
- Nei, K.; Matsuyama, S.; Shuntoh, H.; Tanaka, C. NMDA receptor activation induces glutamate release through nitric oxide synthesis in guinea pig dentate gyrus. Brain Res. 1996, 728, 105–110. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, S.-R.; Li, D.-P.; Pan, H.-L. Kv1. 1/1.2 channels are downstream effectors of nitric oxide on synaptic GABA release to preautonomic neurons in the paraventricular nucleus. Neuroscience 2007, 149, 315–327. [Google Scholar] [CrossRef]
- Halmos, G.; Horvath, T.; Polony, G.; Fekete, A.; Kittel, A.; Vizi, E.; van der Laan, B.; Zelles, T.; Lendvai, B. The role of N-methyl-d-aspartate receptors and nitric oxide in cochlear dopamine release. Neuroscience 2008, 154, 796–803. [Google Scholar] [CrossRef]
- Hull, E.M.; Dominguez, J.M. Getting his act together: Roles of glutamate, nitric oxide, and dopamine in the medial preoptic area. Brain Res. 2006, 1126, 66–75. [Google Scholar] [CrossRef]
- Ho, G.P.; Selvakumar, B.; Mukai, J.; Hester, L.D.; Wang, Y.; Gogos, J.A.; Snyder, S.H. S-nitrosylation and S-palmitoylation reciprocally regulate synaptic targeting of PSD-95. Neuron 2011, 71, 131–141. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Z.; Leylek, T.; Tan, H.; Sun, Y.; Parkinson, F.; Wang, J.-F. Protein cysteine S-nitrosylation inhibits vesicular uptake of neurotransmitters. Neuroscience 2015, 311, 374–381. [Google Scholar] [CrossRef]
- McCARTHY, M.M.; TODD, B.J.; AMATEAU, S.K. Estradiol modulation of astrocytes and the establishment of sex differences in the brain. Ann. N. Y. Acad. Sci. 2003, 1007, 283–297. [Google Scholar] [CrossRef]
- Kramár, E.A.; Chen, L.Y.; Brandon, N.J.; Rex, C.S.; Liu, F.; Gall, C.M.; Lynch, G. Cytoskeletal changes underlie estrogen’s acute effects on synaptic transmission and plasticity. J. 2009, 29, 12982–12993. [Google Scholar] [CrossRef] [PubMed]
- Smejkalova, T.; Woolley, C.S. Estradiol acutely potentiates hippocampal excitatory synaptic transmission through a presynaptic mechanism. J. Neurosci. 2010, 30, 16137–16148. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Huang, G.Z.; Woolley, C.S. Latent sex differences in molecular signaling that underlies excitatory synaptic potentiation in the hippocampus. J. Neurosci. 2019, 39, 1552–1565. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.Z.; Woolley, C.S. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron 2012, 74, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Day, M.; Muniz, L.C.; Bitran, D.; Arias, R.; Revilla-Sanchez, R.; Grauer, S.; Zhang, G.; Kelley, C.; Pulito, V. Activation of estrogen receptor-β regulates hippocampal synaptic plasticity and improves memory. Nat. Neurosci. 2008, 11, 334–343. [Google Scholar] [CrossRef]
- Romeo, R.D.; Waters, E.M.; MCEWEN, B.S. Steroid-induced hippocampal synaptic plasticity: Sex differences and similarities. Neuron Glia Biol. 2004, 1, 219–229. [Google Scholar] [CrossRef]
- Mizuno, K.; Giese, K.P. Towards a molecular understanding of sex differences in memory formation. Trends Neurosci. 2010, 33, 285–291. [Google Scholar] [CrossRef]
- Bi, R.; Broutman, G.; Foy, M.R.; Thompson, R.F.; Baudry, M. The tyrosine kinase and mitogen-activated protein kinase pathways mediate multiple effects of estrogen in hippocampus. Proc. Natl. Acad. Sci. USA 2000, 97, 3602–3607. [Google Scholar] [CrossRef]
- Hao, J.; Rapp, P.R.; Janssen, W.G.; Lou, W.; Lasley, B.L.; Hof, P.R.; Morrison, J.H. Interactive effects of age and estrogen on cognition and pyramidal neurons in monkey prefrontal cortex. Proc. Natl. Acad. Sci. USA 2007, 104, 11465–11470. [Google Scholar] [CrossRef]
- Marrocco, J.; McEwen, B.S. Sex in the brain: Hormones and sex differences. Dialogues Clin. Neurosci. 2016, 18, 373–383. [Google Scholar] [PubMed]
- Chen, J.-R.; Yan, Y.-T.; Wang, T.-J.; Chen, L.-J.; Wang, Y.-J.; Tseng, G.-F. Gonadal hormones modulate the dendritic spine densities of primary cortical pyramidal neurons in adult female rat. Cerebral. Cortex 2009, 19, 2719–2727. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, S.; Lipton, S.A. S-Nitrosylation in neurogenesis and neuronal development. Biochim. Biophys. Acta 2015, 1850, 1588–1593. [Google Scholar] [CrossRef]
- Gordon-Weeks, P.R.; Fischer, I. MAP1B expression and microtubule stability in growing and regenerating axons. Microsc. Res. Tech. 2000, 48, 63–74. [Google Scholar] [CrossRef]
- Stroissnigg, H.; Trancikova, A.; Descovich, L.; Fuhrmann, J.; Kutschera, W.; Kostan, J.; Meixner, A.; Nothias, F.; Propst, F. S-nitrosylation of microtubule-associated protein 1B mediates nitric-oxide-induced axon retraction. Nat. Cell Biol. 2007, 9, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Dalle-donne, I.; Milzani, A.; Giustarini, D.; Di Simplicio, P.; Colombo, R.; Rossi, R. S-NO-actin: S-nitrosylation kinetics and the effect on isolated vascular smooth muscle. J. Muscle Res. Cell Motil. 2000, 21, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ortiz, A.; Martin-Cofreces, N.B.; Sales Ibiza, Á.O.; Izquierdo-Álvarez, A.; Trullo, A.; Victor, V.M.; Calvo, E.; Sot, B.; Martínez-Ruiz, A.; Vázquez, J. eNOS S-nitrosylates β-actin on Cys374 and regulates PKC-θ at the immune synapse by impairing actin binding to profilin-1. PLoS Biol. 2017, 15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.h.; Wang, W.; Feng, L.; Yang, Y.; Zheng, J.; Huang, L.; Chen, D.b. S-nitrosylation of Cofilin-1 serves as a novel pathway for VEGF-stimulated endothelial cell migration. J. Cell. Physiol. 2015, 230, 406–417. [Google Scholar] [CrossRef]
- Baba, S.P.; Wetzelberger, K.; Hoetker, J.D.; Bhatnagar, A. Posttranslational glutathiolation of aldose reductase (AKR1B1): A possible mechanism of protein recovery from S-nitrosylation. Chem. Biol. Interact. 2009, 178, 250–258. [Google Scholar] [CrossRef]
- Grau, M.; Pauly, S.; Ali, J.; Walpurgis, K.; Thevis, M.; Bloch, W.; Suhr, F. RBC-NOS-dependent S-nitrosylation of cytoskeletal proteins improves RBC deformability. PLoS ONE 2013, 8, e56759. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.; Derakhshan, B.; Shi, L.; Campagne, F.; Gross, S.S. SNOSID, a proteomic method for identification of cysteine S-nitrosylation sites in complex protein mixtures. Proc. Natl. Acad. Sci. USA 2006, 103, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Kamnev, A.; Muhar, M.; Preinreich, M.; Ammer, H.; Propst, F. Difficulties in generating specific antibodies for immunohistochemical detection of nitrosylated tubulins. PLoS ONE 2013, 8, e68168. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abat, J.K.; Saigal, P.; Deswal, R. S-Nitrosylation—Another biological switch like phosphorylation? Physiol. Mol. Boil. Plants 2008, 14, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, C.; Quilici, D.R.; Schlauch, K.A.; Buxton, I.L. The human uterine smooth muscle S-nitrosoproteome fingerprint in pregnancy, labor, and preterm labor. Am. J. Physiol. Physiol. 2013, 305, C803–C816. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, A.M.; Rao, V.S.; Filo, A.R.; Marozkina, N.V.; Doctor, A.; Jones, D.R.; Gaston, B.; Guilford, W.H. Direct regulation of striated muscle myosins by nitric oxide and endogenous nitrosothiols. PLoS ONE 2010, 5, e11209. [Google Scholar] [CrossRef]
- Hansberg-Pastor, V.; González-Arenas, A.; Piña-Medina, A.G.; Camacho-Arroyo, I. Sex Hormones Regulate Cytoskeletal Proteins Involved in Brain Plasticity. Front. Psychiatry 2015, 6, 165. [Google Scholar] [CrossRef]
- Yankova, M.; Hart, S.A.; Woolley, C.S. Estrogen increases synaptic connectivity between single presynaptic inputs and multiple postsynaptic CA1 pyramidal cells: A serial electron-microscopic study. Proc. Natl. Acad. Sci. USA 2001, 98, 3525–3530. [Google Scholar] [CrossRef]
- Goldstein, L.A.; Kurz, E.; Sengelaub, D.R. Androgen regulation of dendritic growth and retraction in the development of a sexually dimorphic spinal nucleus. J. Neurosci. 1990, 10, 935–946. [Google Scholar] [CrossRef]
- Lubischer, J.L.; Arnold, A.P. Axotomy of developing rat spinal motoneurons: Cell survival, soma size, muscle recovery, and the influence of testosterone. J. Neurosci. 1995, 26, 225–240. [Google Scholar] [CrossRef]
- Butler, R.; Leigh, P.N.; Gallo, J.M. Androgen-induced up-regulation of tubulin isoforms in neuroblastoma cells. J. Neurosci. 2001, 78, 854–861. [Google Scholar] [CrossRef]
- Hess, D.T.; Stamler, J.S. Regulation by S-nitrosylation of protein post-translational modification. J. Biol. Chem. 2012, 287, 4411–4418. [Google Scholar] [CrossRef]
- Numajiri, N.; Takasawa, K.; Nishiya, T.; Tanaka, H.; Ohno, K.; Hayakawa, W.; Asada, M.; Matsuda, H.; Azumi, K.; Kamata, H. On–off system for PI3-kinase–Akt signaling through S-nitrosylation of phosphatase with sequence homology to tensin (PTEN). Proc. Natl. Acad. Sci. USA 2011, 108, 10349–10354. [Google Scholar] [CrossRef] [PubMed]
- Ghafourifar, P.; Parihar, M.S.; Nazarewicz, R.; Zenebe, W.J.; Parihar, A. Detection assays for determination of mitochondrial nitric oxide synthase activity; advantages and limitations. Methods Enzymol. 2008, 440, 317–334. [Google Scholar] [PubMed]
- Lancaster Jr, J. A tutorial on the diffusibility and reactivity of free nitric oxide. Nitric Oxide 1997, 1, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Suliman, H.B.; Babiker, A.; Withers, C.M.; Sweeney, T.E.; Carraway, M.S.; Tatro, L.G.; Bartz, R.R.; Welty-Wolf, K.E.; Piantadosi, C.A. Nitric oxide synthase-2 regulates mitochondrial Hsp60 chaperone function during bacterial peritonitis in mice. Free Radic. Biol. Med. 2010, 48, 736–746. [Google Scholar] [CrossRef]
- Sun, J.; Morgan, M.; Shen, R.-F.; Steenbergen, C.; Murphy, E. Preconditioning results in S-nitrosylation of proteins involved in regulation of mitochondrial energetics and calcium transport. Circ. Res. 2007, 101, 1155–1163. [Google Scholar] [CrossRef]
- Chacko, B.K.; Reily, C.; Srivastava, A.; Johnson, M.S.; Ye, Y.; Ulasova, E.; Agarwal, A.; Zinn, K.R.; Murphy, M.P.; Kalyanaraman, B. Prevention of diabetic nephropathy in Ins2+/− AkitaJ mice by the mitochondria-targeted therapy MitoQ. Biochem. J. 2010, 432, 9–19. [Google Scholar] [CrossRef]
- Deocaris, C.C.; Kaul, S.C.; Wadhwa, R. On the brotherhood of the mitochondrial chaperones mortalin and heat shock protein 60. Cell Stress Chaperones 2006, 11, 116. [Google Scholar] [CrossRef]
- Piantadosi, C.A. Regulation of mitochondrial processes by protein S-nitrosylation. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2012, 1820, 712–721. [Google Scholar] [CrossRef]
- Garry, P.S.; Ezra, M.; Rowland, M.J.; Westbrook, J.; Pattinson, K.T.S. The role of the nitric oxide pathway in brain injury and its treatment—From bench to bedside. Exp. Neurol. 2015, 263, 235–243. [Google Scholar] [CrossRef]
- Amal, H.; Barak, B.; Bhat, V.; Gong, G.; Joughin, B.A.; Wang, X.; Wishnok, J.S.; Feng, G.; Tannenbaum, S.R. Shank3 mutation in a mouse model of autism leads to changes in the S-nitroso-proteome and affects key proteins involved in vesicle release and synaptic function. Mol. Psychiatry 2018. [Google Scholar] [CrossRef] [PubMed]
- Trifonova, E.; Khlebodarova, T.; Gruntenko, N. Molecular mechanisms of autism as a form of synaptic dysfunction. Russ. J. Genet. Appl. Res. 2017, 7, 869–877. [Google Scholar] [CrossRef]
- Fombonne, E. Epidemiological surveys of autism and other pervasive developmental disorders: An update. J. Autism Dev. Disord. 2003, 33, 365–382. [Google Scholar] [CrossRef] [PubMed]
- Weller, J.; Budson, A. Current understanding of Alzheimer’s disease diagnosis and treatment. F1000 Res. 2018, 7, 1161. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Nakamura, T.; Cao, G.; Holland, E.A.; McKercher, S.R.; Lipton, S.A. S-Nitrosylation activates Cdk5 and contributes to synaptic spine loss induced by beta-amyloid peptide. Proc. Natl Acad Sci U S A 2011, 108, 14330–14335. [Google Scholar] [CrossRef]
- Wang, X.; Su, B.; Lee, H.G.; Li, X.; Perry, G.; Smith, M.A.; Zhu, X. Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J. Neurosci. 2009, 29, 9090–9103. [Google Scholar] [CrossRef]
- Wang, S.; Song, J.; Tan, M.; Albers, K.M.; Jia, J. Mitochondrial fission proteins in peripheral blood lymphocytes are potential biomarkers for Alzheimer’s disease. Eur. J. Neurol. 2012, 19, 1015–1022. [Google Scholar] [CrossRef]
- Zahid, S.; Khan, R.; Oellerich, M.; Ahmed, N.; Asif, A.R. Differential S-nitrosylation of proteins in Alzheimer’s disease. Neuroscience 2014, 256, 126–136. [Google Scholar] [CrossRef]
- Uehara, T.; Nakamura, T.; Yao, D.; Shi, Z.Q.; Gu, Z.; Ma, Y.; Masliah, E.; Nomura, Y.; Lipton, S.A. S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature 2006, 441, 513–517. [Google Scholar] [CrossRef]
- Irvine, K.; Laws, K.R.; Gale, T.M.; Kondel, T.K. Greater cognitive deterioration in women than men with Alzheimer’s disease: A meta analysis. J. Clin. Exp. Neuropsychol. 2012, 34, 989–998. [Google Scholar] [CrossRef]
- Vina, J.; Lloret, A. Why women have more Alzheimer’s disease than men: Gender and mitochondrial toxicity of amyloid-β peptide. J. Alzheimer’s Dis. 2010, 20, S527–S533. [Google Scholar] [CrossRef] [PubMed]
- McCullough, L.D.; Zeng, Z.; Blizzard, K.K.; Debchoudhury, I.; Hurn, P.D. Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: Male toxicity, female protection. J. Cereb. Blood Flow Metab. 2005, 25, 502–512. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaliulin, I.; Kartawy, M.; Amal, H. Sex Differences in Biological Processes and Nitrergic Signaling in Mouse Brain. Biomedicines 2020, 8, 124. https://doi.org/10.3390/biomedicines8050124
Khaliulin I, Kartawy M, Amal H. Sex Differences in Biological Processes and Nitrergic Signaling in Mouse Brain. Biomedicines. 2020; 8(5):124. https://doi.org/10.3390/biomedicines8050124
Chicago/Turabian StyleKhaliulin, Igor, Maryam Kartawy, and Haitham Amal. 2020. "Sex Differences in Biological Processes and Nitrergic Signaling in Mouse Brain" Biomedicines 8, no. 5: 124. https://doi.org/10.3390/biomedicines8050124
APA StyleKhaliulin, I., Kartawy, M., & Amal, H. (2020). Sex Differences in Biological Processes and Nitrergic Signaling in Mouse Brain. Biomedicines, 8(5), 124. https://doi.org/10.3390/biomedicines8050124





