Aerobic Exercise Training Inhibits Neointimal Formation via Reduction of PCSK9 and LOX-1 in Atherosclerosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Balloon Injury Model
2.3. Exercise Protocol
2.4. Experimental Animals
2.5. Hematoxylin and Eosin (H & E) Staining
2.6. Western Blotting
2.7. Statistical Analysis
3. Results
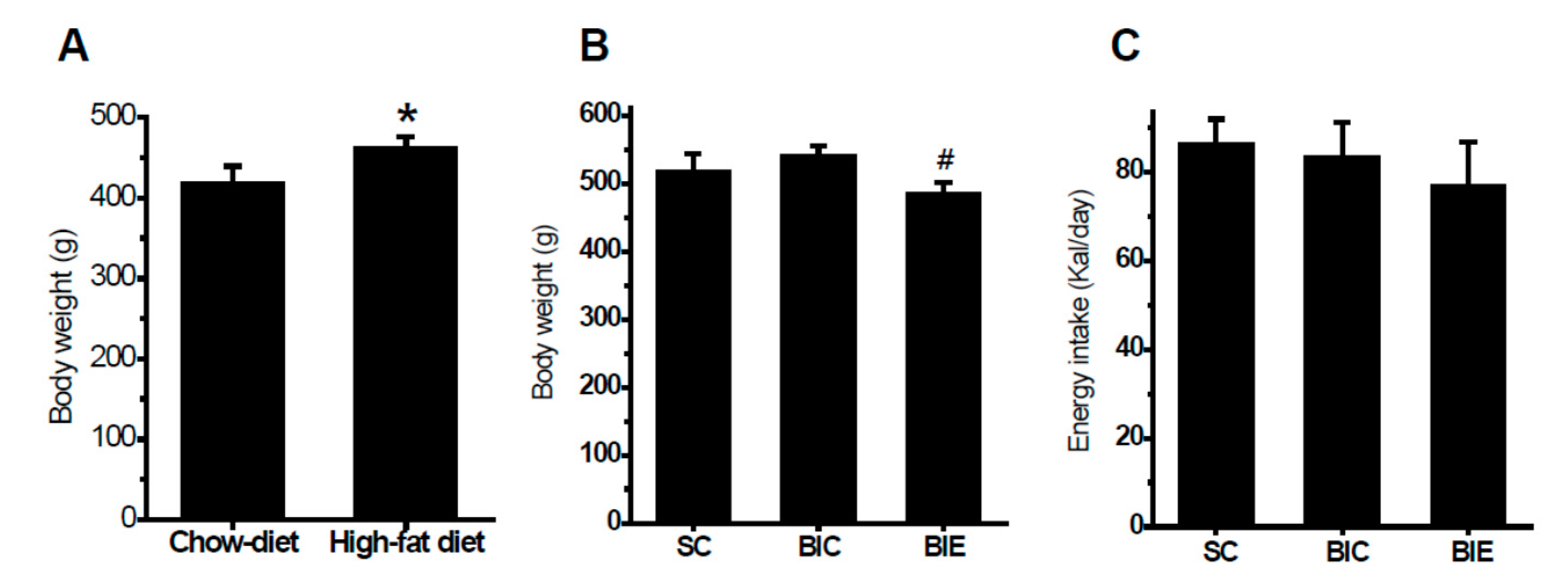
3.1. Aerobic Exercise Training Inhibited Body Weight Gain
3.2. Aerobic Exercise Inhibited Neointimal Formation
3.3. Aerobic Exercise Increased LDLr Expression in Liver of Rats, but Did not Affect PCSK9 Expression
3.4. Aerobic Exercise Suppressed Expression of PCSK9, LOX-1 and VCAM-1 in CCA
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ramli, J.; CalderonArtero, P.; Block, R.C.; Mousa, S.A. Novel therapeutic targets for preserving a healthy endothelium: Strategies for reducing the risk of vascular and cardiovascular disease. Cardiol. J. 2011, 18, 352–363. [Google Scholar]
- Zhang, W.L.; Yan, W.J.; Sun, B.; Zou, Z.P. Synergistic effects of atorvastatin and rosiglitazone on endothelium protection in rats with dyslipidemia. Lipids Health Dis. 2014, 13, 168. [Google Scholar] [CrossRef] [PubMed]
- Noeman, S.A.; Hamooda, H.E.; Baalash, A.A. Biochemical Study of Oxidative Stress Markers in the Liver, Kidney and Heart of High Fat Diet Induced Obesity in Rats. Diabetol. Metab. Syndr. 2011, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Morawietz, H. LOX-1 and atherosclerosis: Proof of concept in LOX-1-knockout mice. Circ. Res. 2007, 100, 1534–1536. [Google Scholar] [CrossRef] [PubMed]
- Colavitti, R.; Pani, G.; Bedogni, B.; Anzevino, R.; Borrello, S.; Waltenberger, J.; Galeotti, T. Reactive oxygen species as downstream mediators of angiogenic signaling by vascular endothelial growth factor receptor-2/KDR. J. Biol. Chem. 2002, 277, 3101–3108. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.F.; Liu, S.J.; Wang, X.W.; Dai, Y.; Khaidakov, M.; Romeo, F.; Mehta, J.L. LOX-1, oxidant stress, mtDNA damage, autophagy, and immune response in atherosclerosis. Can. J. Physiol. Pharmacol. 2014, 92, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Chen, X.N.; Xu, X.H.; Liu, J.Z.; Zhang, Z.P.; Wang, M.X.; Li, X.Z.; Chen, H.; Zhao, D.Q.; Wang, J.; et al. Active polypeptides from Hirudo inhibit endothelial cell inflammation and macrophage foam cell formation by regulating the LOX-1/LXR-alpha/ABCA1 pathway. Biomed. Pharmacother. 2019, 115, 108840. [Google Scholar] [CrossRef]
- Abifadel, M.; Bernier, L.; Dubuc, G.; Nuel, G.; Rabes, J.P.; Bonneau, J.; Marques, A.; Marduel, M.; Devillers, M.; Munnich, A.; et al. A PCSK9 variant and familial combined hyperlipidaemia. J. Med Genet. 2008, 45, 780–786. [Google Scholar] [CrossRef]
- Cohen, J.C.; Boerwinkle, E.; Mosley, T.H., Jr.; Hobbs, H.H. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N. Engl. J. Med. 2006, 354, 1264–1272. [Google Scholar] [CrossRef]
- Cunningham, D.; Danley, D.E.; Geoghegan, K.F.; Griffor, M.C.; Hawkins, J.L.; Subashi, T.A.; Varghese, A.H.; Ammirati, M.J.; Culp, J.S.; Hoth, L.R.; et al. Structural and biophysical studies of PCSK9 and its mutants linked to familial hypercholesterolemia. Nat. Struct. Mol. Biol. 2007, 14, 413–419. [Google Scholar] [CrossRef]
- Poirier, S.; Mayer, G.; Benjannet, S.; Bergeron, E.; Marcinkiewicz, J.; Nassoury, N.; Mayer, H.; Nimpf, J.; Prat, A.; Seidah, N.G. The proprotein convertase PCSK9 induces the degradation of low density lipoprotein receptor (LDLR) and its closest family members VLDLR and ApoER2. J. Biol. Chem. 2008, 283, 2363–2372. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.-W.; Schmidt, R.J.; Zhang, Y.; Chu, S.; Lin, A.; Wang, H.; Wang, X.; Beyer, T.P.; Bensch, W.R.; Li, W.; et al. Secreted PCSK9 downregulates low density lipoprotein receptor through receptor-mediated endocytosis. J. Lipid Res. 2007, 48, 1488–1498. [Google Scholar] [CrossRef] [PubMed]
- Fisher, T.S.; Lo Surdo, P.; Pandit, S.; Mattu, M.; Santoro, J.C.; Wisniewski, D.; Cummings, R.T.; Calzetta, A.; Cubbon, R.M.; Fischer, P.A.; et al. Effects of pH and low density lipoprotein (LDL) on PCSK9-dependent LDL receptor regulation. J. Biol. Chem. 2007, 282, 20502–20512. [Google Scholar] [CrossRef] [PubMed]
- Burke, A.C.; Dron, J.S.; Hegele, R.A.; Huff, M.W. PCSK9: Regulation and Target for Drug Development for Dyslipidemia. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 223–244. [Google Scholar] [CrossRef]
- Norata, G.D.; Ballantyne, C.M.; Catapano, A.L. New therapeutic principles in dyslipidaemia: Focus on LDL and Lp(a) lowering drugs. Eur. Heart J. 2013, 34, 1783–1789. [Google Scholar] [CrossRef]
- Ferri, N.; Tibolla, G.; Pirillo, A.; Cipollone, F.; Mezzetti, A.; Pacia, S.; Corsini, A.; Catapano, A.L. Proprotein convertase subtilisin kexin type 9 (PCSK9) secreted by cultured smooth muscle cells reduces macrophages LDLR levels. Atherosclerosis 2012, 220, 381–386. [Google Scholar] [CrossRef]
- Indolfi, C.; Torella, D.; Coppola, C.; Curcio, A.; Rodriguez, F.; Bilancio, A.; Leccia, A.; Arcucci, O.; Falco, M.; Leosco, D.; et al. Physical training increases eNOS vascular expression and activity and reduces restenosis after balloon angioplasty or arterial stenting in rats. Circ. Res. 2002, 91, 1190–1197. [Google Scholar] [CrossRef]
- Li, W.; Jeong, J.H.; Park, H.G.; Lee, Y.R.; Li, M.; Lee, S.K. Endurance exercise training inhibits neointimal formation via enhancement of FOXOs expression in balloon-induced atherosclerosis rat model. J. Exerc. Nutr. Biochem. 2014, 18, 105–110. [Google Scholar] [CrossRef]
- Laufs, U.; Werner, N.; Link, A.; Endres, M.; Wassmann, S.; Jurgens, K.; Miche, E.; Bohm, M.; Nickenig, G. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation 2004, 109, 220–226. [Google Scholar] [CrossRef]
- Kannan, U.; Vasudevan, K.; Balasubramaniam, K.; Yerrabelli, D.; Shanmugavel, K.; John, N.A. Effect of exercise intensity on lipid profile in sedentary obese adults. J. Clin. Diagn. Res. 2014, 8, BC08–BC10. [Google Scholar] [CrossRef]
- Tjonna, A.E.; Leinan, I.M.; Bartnes, A.T.; Jenssen, B.M.; Gibala, M.J.; Winett, R.A.; Wisloff, U. Low- and High-Volume of Intensive Endurance Training Significantly Improves Maximal Oxygen Uptake after 10-Weeks of Training in Healthy Men. PLoS ONE 2013, 8, e65382. [Google Scholar] [CrossRef] [PubMed]
- Padilla, J.; Jenkins, N.T.; Roberts, M.D.; Arce-Esquivel, A.A.; Martin, J.S.; Laughlin, M.H.; Booth, F.W. Differential changes in vascular mRNA levels between rat iliac and renal arteries produced by cessation of voluntary running. Exp. Physiol. 2013, 98, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Jadhav, K.S.; Williamson, D.L.; Rideout, T.C. Treadmill Exercise Training Modulates Hepatic Cholesterol Metabolism and Circulating PCSK9 Concentration in High-Fat-Fed Mice. J. Lipids 2013, 2013, 908048. [Google Scholar] [CrossRef] [PubMed]
- Sock, E.T.N.; Chapados, N.A.; Lavoie, J.M. LDL Receptor and Pcsk9 Transcripts are Decreased in Liver of Ovariectomized Rats: Eff ects of Exercise Training. Horm. Metab. Res. 2014, 46, 550–555. [Google Scholar]
- Sock, E.T.N.; Mayer, G.; Lavoie, J.M. Combined effects of rosuvastatin and exercise on gene expression of key molecules involved in cholesterol metabolism in ovariectomized rats. PLoS ONE 2016, 11, e0159550. [Google Scholar]
- Farahnak, Z.; Chapados, N.; Lavoie, J.M. Exercise training increased gene expression of LDL-R and PCSK9 in intestine: Link to transintestinal cholesterol excretion. Gen. Physiol. Biophys. 2018, 37, 309–317. [Google Scholar] [CrossRef]
- Careskey, H.E.; Davis, R.A.; Alborn, W.E.; Troutt, J.S.; Cao, G.Q.; Konrad, R.J. Atorvastatin increases human serum levels of proprotein convertase subtilisin/kexin type 9. J. Lipid Res. 2008, 49, 394–398. [Google Scholar] [CrossRef]
- Welder, G.; Zineh, I.; Pacanowski, M.A.; Troutt, J.S.; Cao, G.Q.; Konrad, R.J. High-dose atorvastatin causes a rapid sustained increase in human serum PCSK9 and disrupts its correlation with LDL cholesterol. J. Lipid Res. 2010, 51, 2714–2721. [Google Scholar] [CrossRef]
- Lee, H.M.; Jeon, B.H.; Won, K.J.; Lee, C.K.; Park, T.K.; Choi, W.S.; Bae, Y.M.; Kim, H.S.; Lee, S.K.; Park, S.H.; et al. Gene Transfer of Redox Factor-1 Inhibits Neointimal Formation Involvement of Platelet-Derived Growth Factor-beta Receptor Signaling via the Inhibition of the Reactive Oxygen Species-Mediated Syk Pathway. Circ. Res. 2009, 104, 219–227. [Google Scholar] [CrossRef]
- Blair, S.N.; Archer, E.; Hand, G.A. Commentary: Luke and Cooper are wrong: Physical activity has a crucial role in weight management and determinants of obesity. Int. J. Epidemiol. 2013, 42, 1836–1838. [Google Scholar] [CrossRef]
- Pynn, M.; Schafer, K.; Konstantinides, S.; Halle, M. Exercise training reduces neointimal growth and stabilizes vascular lesions developing after injury in apolipoprotein e-deficient mice. Circulation 2004, 109, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.Q.; Li, J.J. PCSK9 gene mutations and low-density lipoprotein cholesterol. Clin. Chim. Acta 2014, 431, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.N.; Breslow, J.L. Adenoviral-mediated expression of Pcsk9 in mice results in a low-density lipoprotein receptor knockout phenotype. Proc. Natl. Acad. Sci. USA 2004, 101, 7100–7105. [Google Scholar] [CrossRef] [PubMed]
- Kosenko, T.; Golder, M.; Leblond, G.; Weng, W.; Lagace, T.A. Low density lipoprotein binds to proprotein convertase subtilisin/kexin type-9 (PCSK9) in human plasma and inhibits PCSK9-mediated low density lipoprotein receptor degradation. J. Biol. Chem. 2013, 288, 8279–8288. [Google Scholar] [CrossRef]
- Pinto, P.R.; Rocco, D.D.; Okuda, L.S.; Machado-Lima, A.; Castilho, G.; da Silva, K.S.; Gomes, D.J.; Pinto, R.S.; Iborra, R.T.; Ferreira, G.S.; et al. Aerobic exercise training enhances the in vivo cholesterol trafficking from macrophages to the liver independently of changes in the expression of genes involved in lipid flux in macrophages and aorta. Lipids Health Dis. 2015, 14, 109. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Brown, M.S. The LDL Receptor. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 431–438. [Google Scholar] [CrossRef]
- Lambert, G.; Charlton, F.; Rye, K.A.; Piper, D.E. Molecular basis of PCSK9 function. Atherosclerosis 2009, 203, 1–7. [Google Scholar] [CrossRef]
- Horton, J.D.; Cohen, J.C.; Hobbs, H.H. PCSK9: A convertase that coordinates LDL catabolism. J. Lipid Res. 2009, 50, S172–S177. [Google Scholar] [CrossRef]
- Teodoro, B.G.; Natali, A.J.; Fernandes, S.A.T.; da Silva, L.A.; de Pinho, R.A.; da Matta, S.L.P.; Peluzio, M.D.G. Improvements of Atherosclerosis and Hepatic Oxidative Stress are Independent of Exercise Intensity in LDLr-/- Mice. J. Atheroscler. Thromb. 2012, 19, 904–911. [Google Scholar] [CrossRef]
- Guizoni, D.M.; Dorighello, G.G.; Oliveira, H.C.F.; Delbin, M.A.; Krieger, M.H.; Davel, A.P. Aerobic exercise training protects against endothelial dysfunction by increasing nitric oxide and hydrogen peroxide production in LDL receptor-deficient mice. J. Transl. Med. 2016, 14, 213. [Google Scholar] [CrossRef]
- Mehta, J.L.; Chen, J.; Hermonat, P.L.; Romeo, F.; Novelli, G. Lectin-like, oxidized low-density lipoprotein receptor-1 (LOX-1): A critical player in the development of atherosclerosis and related disorders. Cardiovasc. Res. 2006, 69, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Riahi, S.; Mohammadi, M.T.; Sobhani, V.; Soleimany, M. Chronic effects of aerobic exercise on gene expression of LOX-1 receptor in the heart of rats fed with high fat diet. Iran. J. Basic Med Sci. 2015, 18, 805–812. [Google Scholar] [PubMed]
- Ding, Z.F.; Liu, S.J.; Wang, X.W.; Deng, X.Y.; Fan, Y.B.; Shahanawaz, J.; Reis, R.J.S.; Varughese, K.I.; Sawamura, T.; Mehta, J.L. Cross-talk between LOX-1 and PCSK9 in vascular tissues. Cardiovasc. Res. 2015, 107, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Tang, Z.H.; Jiang, L.; Li, X.F.; Jiang, Z.S.; Liu, L.S. PCSK9 siRNA inhibits HUVEC apoptosis induced by ox-LDL via Bcl/Bax-caspase9-caspase3 pathway. Mol. Cell. Biochem. 2012, 359, 347–358. [Google Scholar] [CrossRef]
- Ding, Z.F.; Liu, S.J.; Wang, X.W.; Deng, X.Y.; Fan, Y.B.; Sun, C.Q.; Wang, Y.N.; Mehta, J.L. Hemodynamic Shear Stress via ROS Modulates PCSK9 Expression in Human Vascular Endothelial and Smooth Muscle Cells and Along the Mouse Aorta. Antioxid. Redox Signal. 2015, 22, 760–771. [Google Scholar] [CrossRef] [PubMed]
- Balcells, M.; Martorell, J.; Olive, C.; Santacana, M.; Chitalia, V.; Cardoso, A.A.; Edelman, E.R. Smooth Muscle Cells Orchestrate the Endothelial Cell Response to Flow and Injury. Circulation 2010, 121, 2192–2199. [Google Scholar] [CrossRef][Green Version]
- Byrkjeland, R.; Njerve, I.U.; Arnesen, H.; Seljeflot, I.; Solheim, S. Reduced endothelial activation after exercise is associated with improved HbA(1c) in patients with type 2 diabetes and coronary artery disease. Diabetes Vasc. Dis. Res. 2017, 14, 94–103. [Google Scholar] [CrossRef]
- Faulkner, J.; Lambrick, D.; Woolley, B.; Stoner, L.; Wong, L.K.; McGonigal, G. Effects of Early Exercise Engagement on Vascular Risk in Patients with Transient Ischemic Attack and Nondisabling Stroke. J. Stroke Cerebrovasc. Dis. 2013, 22, E388–E396. [Google Scholar] [CrossRef]
- Chow, C.K.; Jolly, S.; Rao-Melacini, P.; Fox, K.A.A.; Anand, S.S.; Yusuf, S. Association of Diet, Exercise, and Smoking Modification With Risk of Early Cardiovascular Events After Acute Coronary Syndromes. Circulation 2010, 121, 750–758. [Google Scholar] [CrossRef]
- Berk, B.C.; Min, W.; Yan, C.; Surapisitchat, J.; Liu, Y.; Hoefen, R. Atheroprotective mechanisms activated by fluid shear stress in endothelial cells. Drug. News. Perspect. 2002, 15, 133–139. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Park, H.; Guo, E.; Jo, W.; Sim, K.M.; Lee, S.K. Aerobic Exercise Training Inhibits Neointimal Formation via Reduction of PCSK9 and LOX-1 in Atherosclerosis. Biomedicines 2020, 8, 92. https://doi.org/10.3390/biomedicines8040092
Li W, Park H, Guo E, Jo W, Sim KM, Lee SK. Aerobic Exercise Training Inhibits Neointimal Formation via Reduction of PCSK9 and LOX-1 in Atherosclerosis. Biomedicines. 2020; 8(4):92. https://doi.org/10.3390/biomedicines8040092
Chicago/Turabian StyleLi, Wei, Heegeun Park, Erling Guo, Wooyeon Jo, Kyu Min Sim, and Sang Ki Lee. 2020. "Aerobic Exercise Training Inhibits Neointimal Formation via Reduction of PCSK9 and LOX-1 in Atherosclerosis" Biomedicines 8, no. 4: 92. https://doi.org/10.3390/biomedicines8040092
APA StyleLi, W., Park, H., Guo, E., Jo, W., Sim, K. M., & Lee, S. K. (2020). Aerobic Exercise Training Inhibits Neointimal Formation via Reduction of PCSK9 and LOX-1 in Atherosclerosis. Biomedicines, 8(4), 92. https://doi.org/10.3390/biomedicines8040092




