Fecal Recovery of Probiotics Administered as a Multi-Strain Formulation during Antibiotic Treatment
Abstract
1. Introduction
2. Materials and Methods
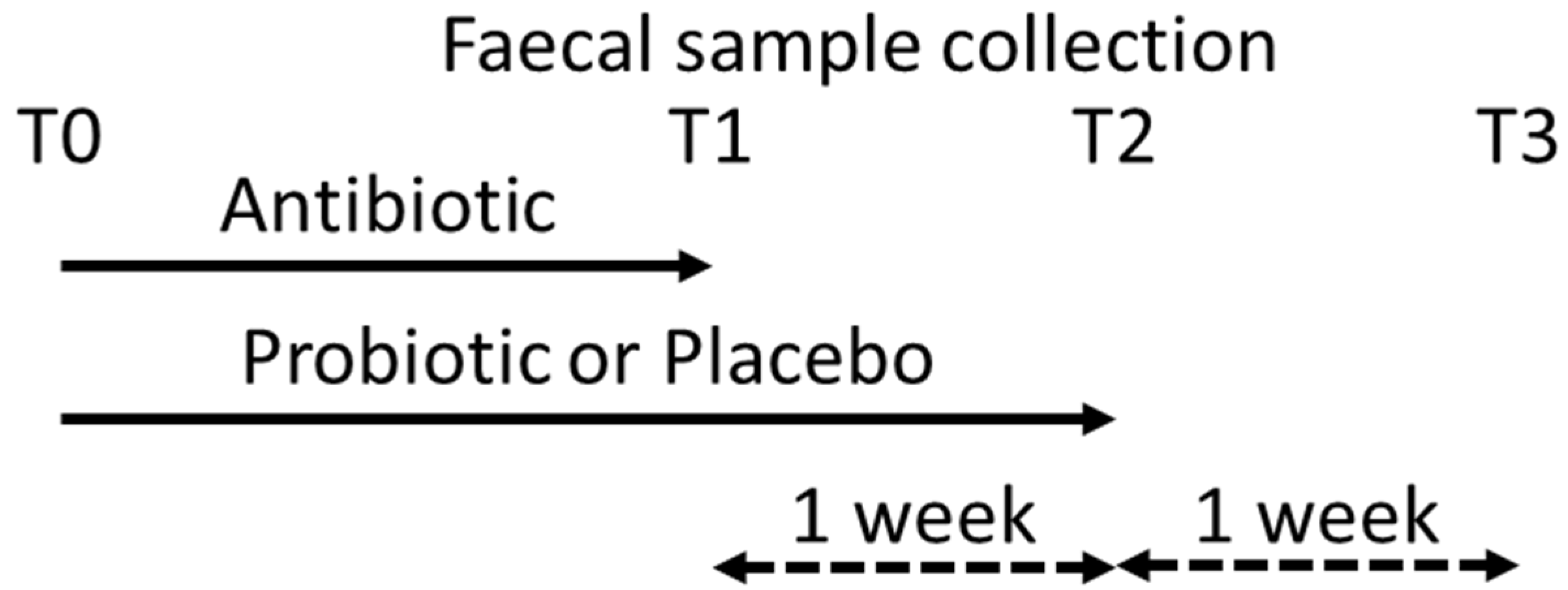
2.1. Study Population
2.2. Sampling
2.3. Extraction and Quantification of Bacterial DNA
2.4. Statistical Analysis
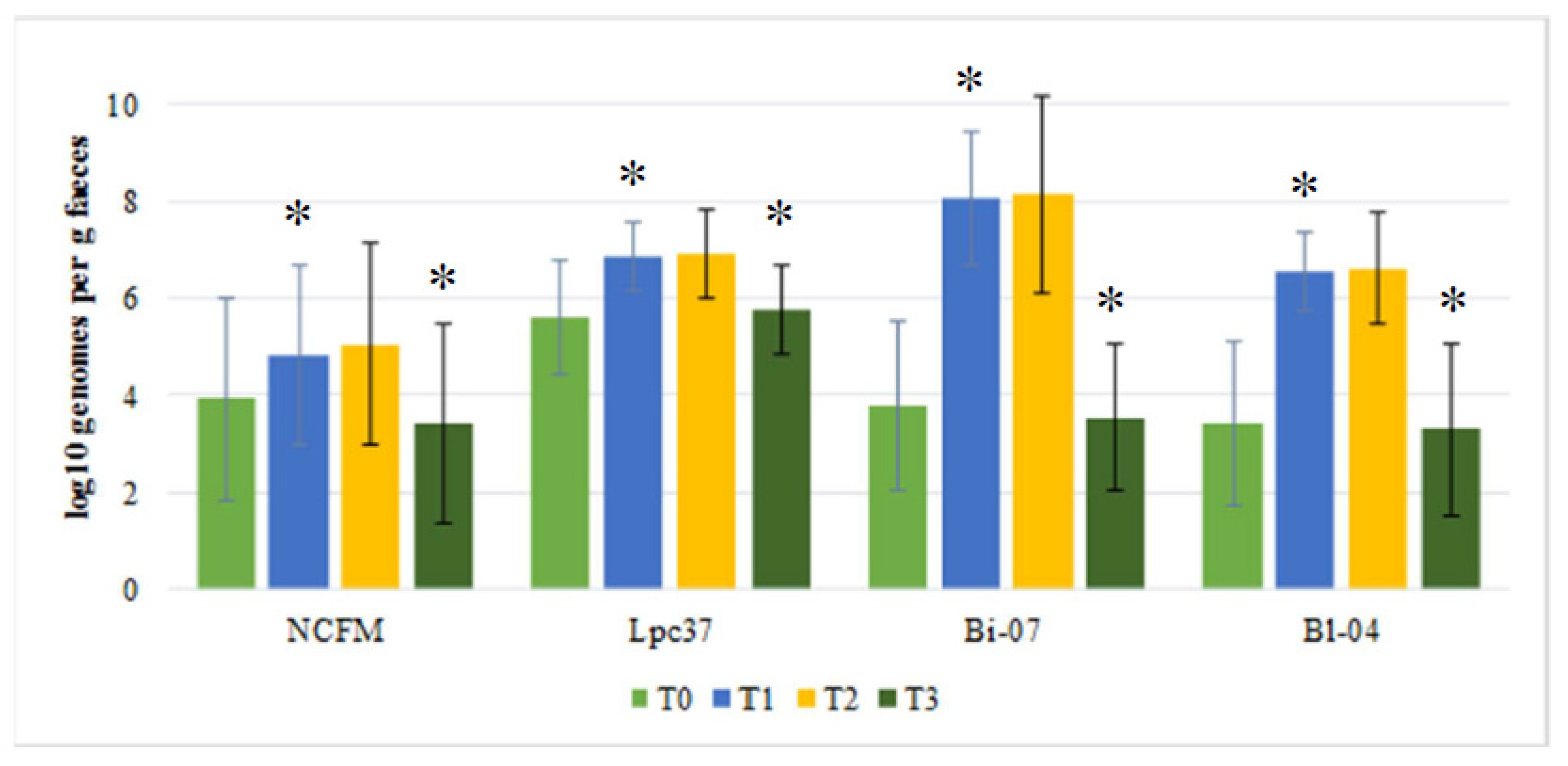
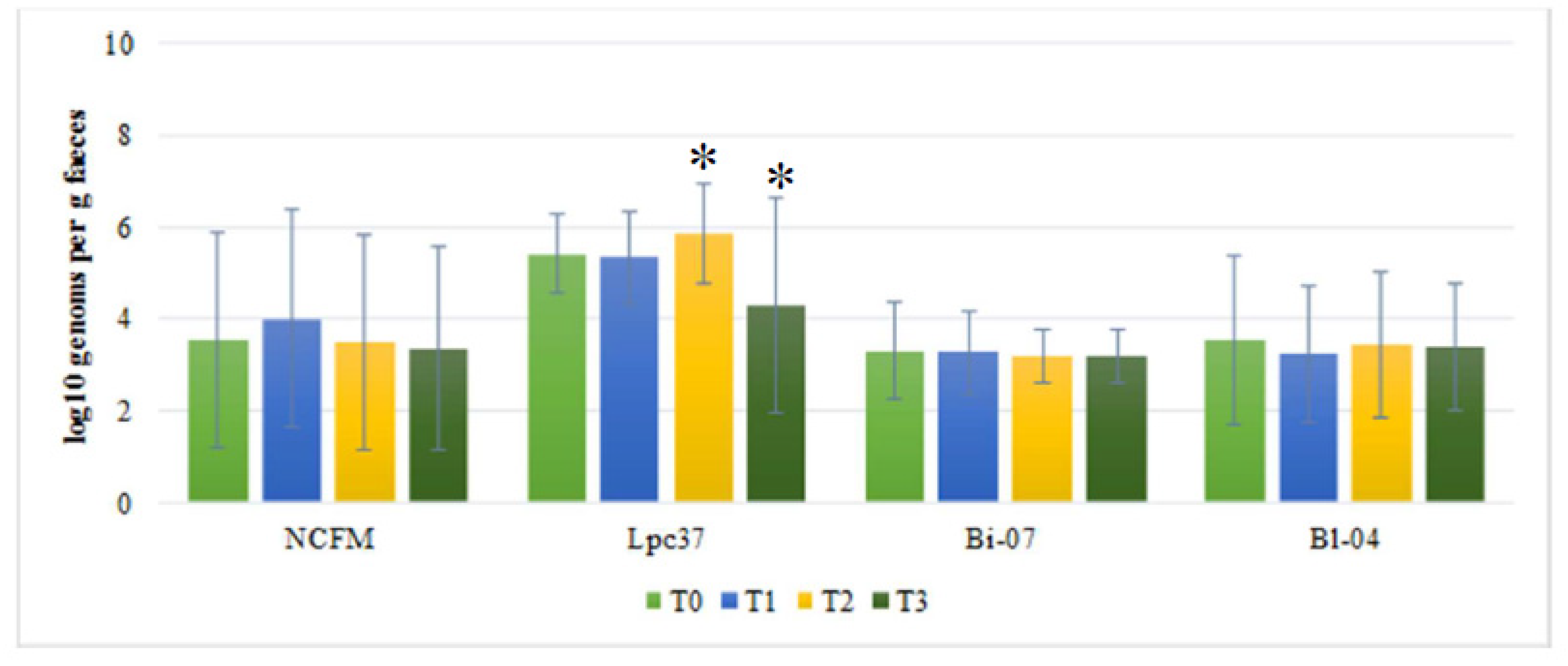
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AAD | Antibiotic associated diarrhea |
| CFU | Colony forming units |
| SE | Standard error |
References
- Wiström, J.; Norrby, S.R.; Myhre, E.B.; Eriksson, S.; Granström, G.; Lagergren, L.; Englund, G.; Nord, C.E.; Svenungsson, B. Frequency of antibiotic-associated diarrhoea in 2462 antibiotic-treated hospitalized patients: A prospective study. J. Antimicrob. Chemother. 2001, 47, 43–50. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L.V. Epidemiology, risk factors and treatments for antibiotic-associated diarrhea. Dig. Dis. 1998, 16, 292–307. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L.V. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am. J. Gastroenterol. 2006, 101, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Haran, J.P.; Hayward, G.; Skinner, S.; Merritt, C.; Hoaglin, D.C.; Hibberd, P.L.; Lu, S.; Boyer, E.W. Factors influencing the development of antibiotic associated diarrhea in ED patients discharged home: Risk of administering IV antibiotics. Am. J. Emerg. Med. 2014, 32, 1195–1199. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blaabjerg, S.; Artzi, D.M.; Aabenhus, R. Probiotics for the Prevention of Antibiotic-Associated Diarrhea in Outpatients-A Systematic Review and Meta-Analysis. Antibiotics 2017, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, A.C.; DongLian, C.; Weijian, X.; Stewart, M.; Ni, J.; Stewart, T.; Miller, L.E. Probiotics reduce symptoms of antibiotic use in a hospital setting: A randomized dose response study. Vaccine 2014, 32, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Forssten, S.D.; Ouwehand, A.C. Simulating colonic survival of probiotics in single-strain products compared to multi-strain products. Microb. Ecol. Health Dis. 2017, 28, 1378061. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.J.Z.; Morovic, W.; DeMeules, M.; Stahl, B.; Sindelar, C.W. Absolute Enumeration of Probiotic Strains Lactobacillus acidophilus NCFM® and Bifidobacterium animalis subsp. lactis Bl-04® via Chip-Based Digital PCR. Front. Microbiol. 2018, 9, 704. [Google Scholar] [CrossRef] [PubMed]
- Airaksinen, K.; Yeung, N.; Lyra, A.; Lahtinen, S.J.; Huttunen, T.; Shanahan, F.; Ouwehand, A.C. The effect of a probiotic blend on gastrointestinal symptoms in constipated patients: A double blind, randomised, placebo controlled 2-week trial. Benef. Microbes 2019, 10, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Haarman, M.; Knol, J. Quantitative real-time PCR analysis of fecal Lactobacillus species in infants receiving a prebiotic infant formula. Appl. Environ. Microbiol. 2006, 72, 2359–2365. [Google Scholar] [CrossRef] [PubMed]
- Poutsiaka, D.D.; Mahoney, I.J.; McDermott, L.A.; Stern, L.L.; Thorpe, C.M.; Kane, A.V.; Baez-Giangreco, C.; McKinney, J.; Davidson, L.E.; Leyva, R.; et al. Selective method for identification and quantification of Bifidobacterium animalis subspecies lactis BB-12 (BB-12) from the gastrointestinal tract of healthy volunteers ingesting a combination probiotic of BB-12 and Lactobacillus rhamnosus GG. J. Appl. Microbiol. 2017, 122, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, A.C.; Nermes, M.; Collado, M.C.; Rautonen, N.; Salminen, S.; Isolauri, E. Specific probiotics alleviate allergic rhinitis during the birch pollen season. World J. Gastroenterol. 2009, 15, 3261–3268. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, A.C.; ten Bruggencate, S.J.; Schonewille, A.J.; Alhoniemi, E.; Forssten, S.D.; Bovee-Oudenhoven, I.M. Lactobacillus acidophilus supplementation in human subjects and their resistance to enterotoxigenic Escherichia coli infection. Br. J. Nutr. 2014, 111, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Hemalatha, R.; Ouwehand, A.C.; Forssten, S.D.; Babu Geddan, J.J.; Sriswan Mamidi, R.; Bhaskar, V.; Radhakrishna, K.V. A Community-based Randomized Double Blind Controlled Trial of Lactobacillus paracasei and Bifidobacterium lactis on Reducing Risk for Diarrhea and Fever in Preschool Children in an Urban Slum in India. Eur. J. Nutr. Food Saf. 2014, 4, 325–342. [Google Scholar] [CrossRef] [PubMed]
- Bettler, J.; Mitchell, D.K.; Kullen, M.J. Administration of Bifidobacterium lactis with fructo-oligosaccharides to toddlers is safe and results in transient colonization. Int. J. Probiotics Prebiotics 2006, 1, 193–202. [Google Scholar]
- Forssten, S.; Evans, M.; Wilson, D.; Ouwehand, A.C. Influence of a probiotic mixture on antibiotic induced microbiota disturbances. World J. Gastroenterol. 2014, 20, 11878–11885. [Google Scholar] [CrossRef] [PubMed]
- Morovic, W.; Roper, J.M.; Smith, A.B.; Mukerji, P.; Stahl, B.; Rae, J.C.; Ouwehand, A.C. Safety evaluation of HOWARU Restore® (Lactobacillus acidophilus NCFM, Lactobacillus paracasei Lpc-37, Bifidobacterium animalis subsp. lactis Bl-04 and B. lactis Bi-07) for antibiotic resistance, genomic risk factors, and acute toxicity. Food Chem. Toxicol. 2017, 110, 316–324. [Google Scholar] [CrossRef] [PubMed]
| Species | Primer Name | Sequence | Reference |
|---|---|---|---|
| Bifidobacterium animalis subsp. lactis Bl-04 | Bl04_for | CTTCCCAGAAGGCCGGGT | [8] |
| Bl04_rev | CGAGGCCACGGTGCTCATATAGA | ||
| Bifidobacterium animalis subsp. lactis Bi-07 | Blac_CRins_qF | CGCCGCTGATTGACCTGTT | this manuscript |
| Blac_CRins_qP | 5FAM-ACGTGACGAATCATGGGCCGAGGGAT-2BHQ | ||
| Blac_CRins_qR | TGAGATTGATACCCGTGGCG | ||
| Lactobacillus acidophilus NCFM | Laci_NCFMMJ_RTfwd | CCACGACCAGATGTAACCAA | [9] |
| Laci_NCFM_Rtrev | TTAGAAGATGCCAACGTCGAG | ||
| Laci_NCFM_probe | 5’HEX TAA GCC GAA-ZEN- CAA TGC TGA AAC GAT 3’IABkFQ | ||
| Lactobacillus paracasei | F_paca_IS | ACATCAGTGTATTGCTTGTCAGTGAATAC | [10] |
| R_paca_IS | CCTGCGGGTACTGAGATGTTTC | ||
| P_paca_IS | 5’ FAM TGCCGCCGGCCAG 3’ IBQ |
| Intervention Group | Probiotic (n = 46) | Placebo (n = 50) | ||||||
|---|---|---|---|---|---|---|---|---|
| NCFM | Lpc-37 | Bi-07 | Bl-04 | NCFM | Lpc-37 | Bi-07 | Bl-04 | |
| T0 | 4 | 20 | 7 | 5 | 7 | 18 | 2 | 2 |
| T1 | 7 | 42a** | 43a** | 44a** | 9 | 15a | 2 a | 4a |
| T2 | 10 | 42a | 40a | 37a* | 16 | 25a* | 1 a | 5a |
| T3 | 3 | 23** | 3** | 4** | 6 | 24 | 1 | 4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forssten, S.D.; Yeung, N.; Ouwehand, A.C. Fecal Recovery of Probiotics Administered as a Multi-Strain Formulation during Antibiotic Treatment. Biomedicines 2020, 8, 83. https://doi.org/10.3390/biomedicines8040083
Forssten SD, Yeung N, Ouwehand AC. Fecal Recovery of Probiotics Administered as a Multi-Strain Formulation during Antibiotic Treatment. Biomedicines. 2020; 8(4):83. https://doi.org/10.3390/biomedicines8040083
Chicago/Turabian StyleForssten, Sofia D., Nicolas Yeung, and Arthur C. Ouwehand. 2020. "Fecal Recovery of Probiotics Administered as a Multi-Strain Formulation during Antibiotic Treatment" Biomedicines 8, no. 4: 83. https://doi.org/10.3390/biomedicines8040083
APA StyleForssten, S. D., Yeung, N., & Ouwehand, A. C. (2020). Fecal Recovery of Probiotics Administered as a Multi-Strain Formulation during Antibiotic Treatment. Biomedicines, 8(4), 83. https://doi.org/10.3390/biomedicines8040083





