Constant Light in Critical Postnatal Days Affects Circadian Rhythms in Locomotion and Gene Expression in the Suprachiasmatic Nucleus, Retina, and Pineal Gland Later in Life
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Locomotor Activity Measurement
2.3. Quantitative Real-Time RT–PCR
2.4. Data Analysis and Statistical Procedures
3. Results
3.1. Effects of Postnatal LL Condition on Locomotor Activity of Adult Male Rats
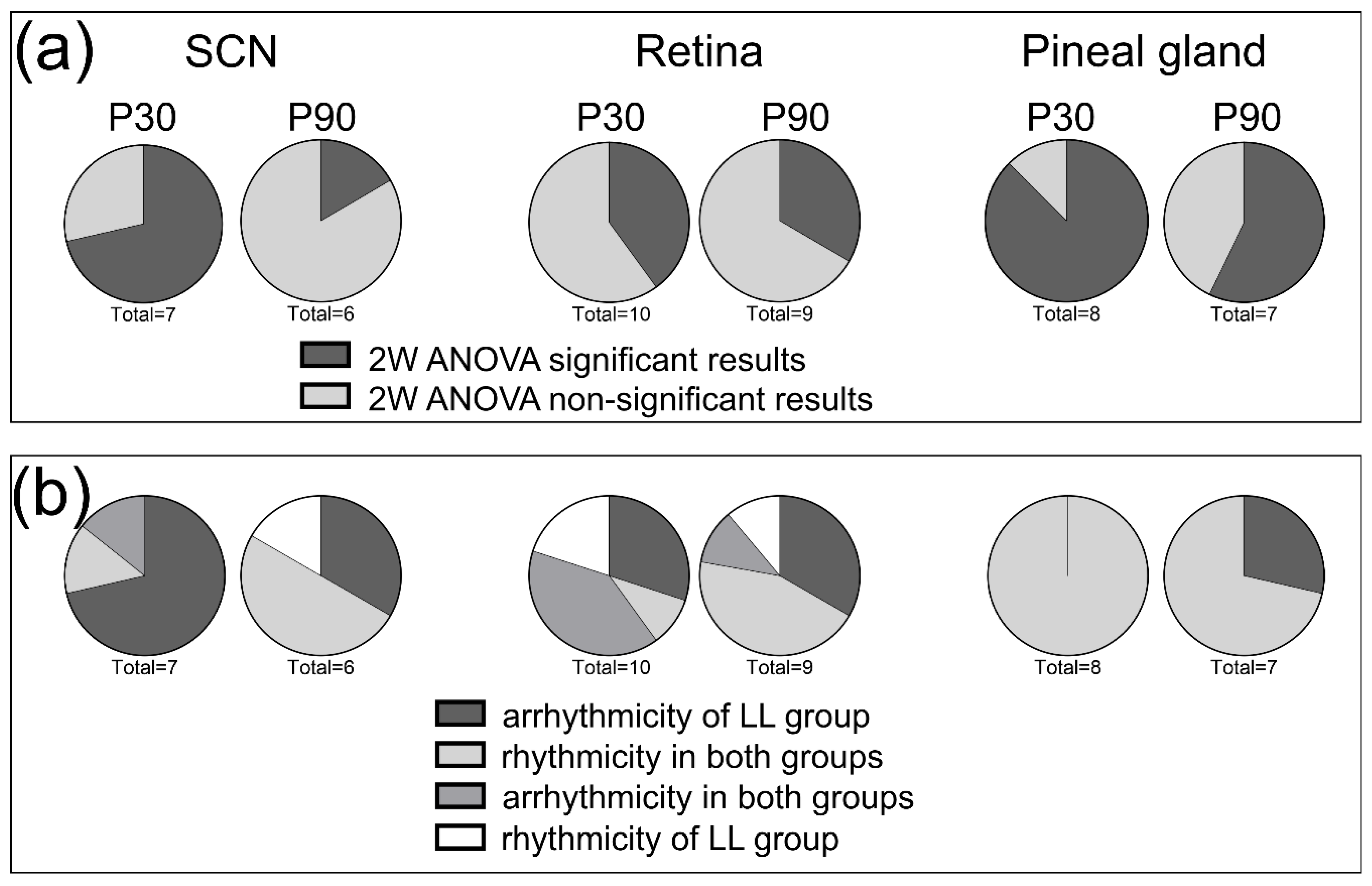
3.2. Effects of Postnatal LL Condition on Gene Expression in the SCN
3.3. Effects of Postnatal LL Condition on Gene Expression in the Retina
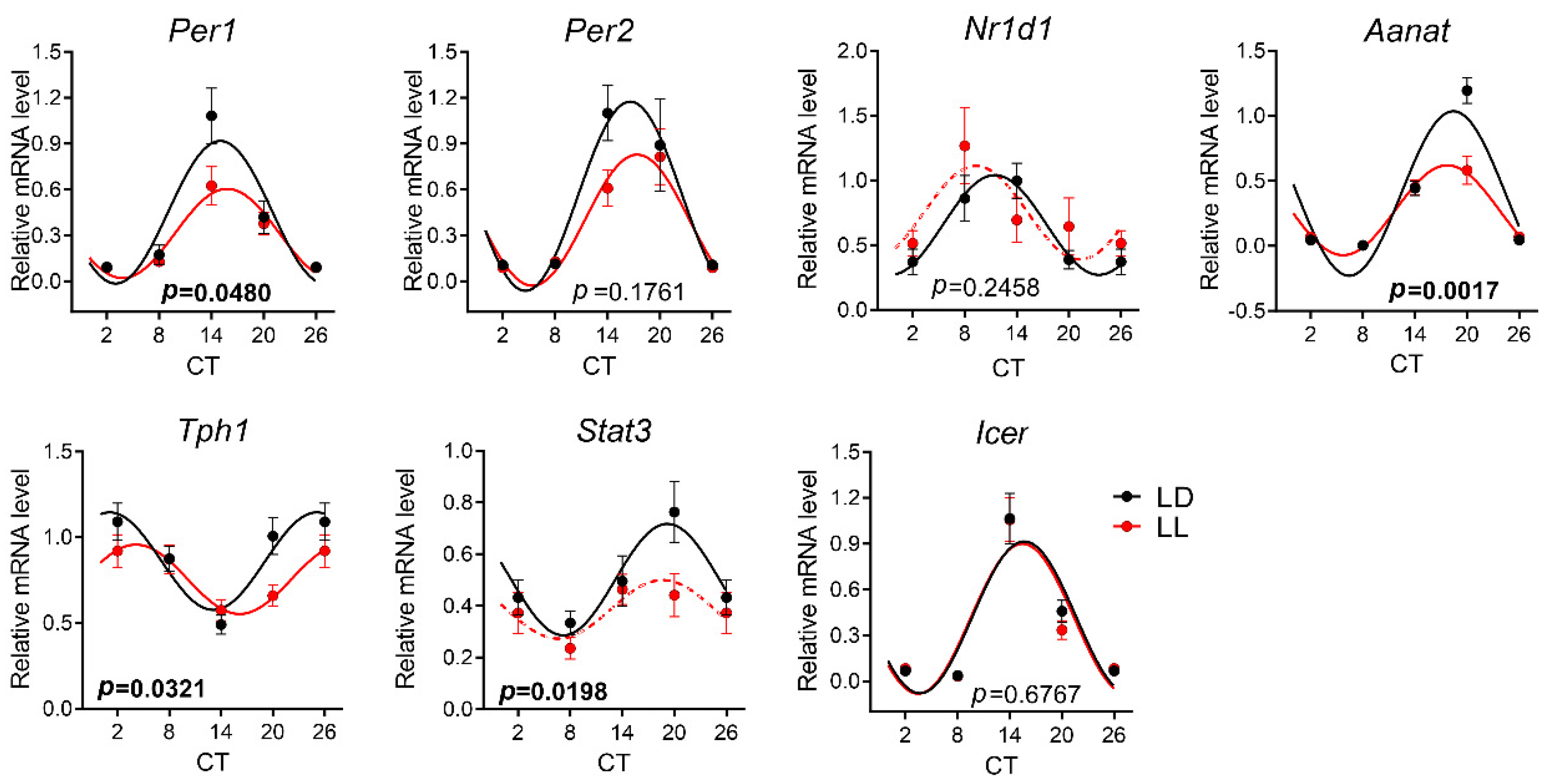
3.4. Effects of Postnatal LL Condition on Gene Expression in the Pineal Gland
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dibner, C.; Schibler, U.; Albrecht, U. The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 2010, 72, 517–549. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.S. Molecular Architecture of the Circadian Clock in Mammals. In A Time for Metabolism and Hormones; Sassone-Corsi, P., Christen, Y., Eds.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Hattar, S.; Liao, H.W.; Takao, M.; Berson, D.M.; Yau, K.W. Melanopsin-containing retinal ganglion cells: Architecture, projections, and intrinsic photosensitivity. Science 2002, 295, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.; Jagannath, A.; Hankins, M.W.; Foster, R.G.; Peirson, S.N. Photic regulation of clock systems. Methods Enzymol. 2015, 552, 125–143. [Google Scholar] [CrossRef] [PubMed]
- Nieto, P.S.; Valdez, D.J.; Acosta-Rodriguez, V.A.; Guido, M.E. Expression of novel opsins and intrinsic light responses in the mammalian retinal ganglion cell line RGC-5. Presence of OPN5 in the rat retina. PLoS ONE 2011, 6, e26417. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.; Rodgers, J.; Hickey, D.; Foster, R.G.; Peirson, S.N.; Hankins, M.W. Characterisation of light responses in the retina of mice lacking principle components of rod, cone and melanopsin phototransduction signalling pathways. Sci. Rep. 2016, 6, 28086. [Google Scholar] [CrossRef] [PubMed]
- Klein, D.C.; Moore, R.Y. Pineal N-acetyltransferase and hydroxyindole-O-methyltransferase: Control by the retinohypothalamic tract and the suprachiasmatic nucleus. Brain Res. 1979, 174, 245–262. [Google Scholar] [CrossRef]
- Stehle, J.H.; Foulkes, N.S.; Molina, C.A.; Simonneaux, V.; Pevet, P.; Sassone-Corsi, P. Adrenergic signals direct rhythmic expression of transcriptional re-pressor CREM in the pineal gland. Nature 1993, 365, 314–3204. [Google Scholar] [CrossRef]
- Foulkes, N.S.; Borjigin, J.; Snyder, S.H.; Sassone-Corsi, P. Transcriptionalcontrol of circadian hormone synthesis via the CREM feedback loop. Proc. Natl. Acad. Sci. USA 1996, 93, 14140–141455. [Google Scholar] [CrossRef]
- Maronde, E.; Pfeffer, M.; Olcese, J.; Molina, C.A.; Schlotter, F.; Dehghani, F.; Korf, H.W.; Stehle, J.H. Transcription factors in neuroendocrine regulation: Rhythmic changes in pCREB and ICER levels frame melatonin synthesis. J. Neurosci. 1999, 19, 3326–3336. [Google Scholar] [CrossRef]
- Ho, A.K.; Terriff, D.L.; Price, D.M.; Wloka, M.T.; Chik, C.L. The Role of Inducible Repressor Proteins in the Adrenergic Induction of Arylalkylamine-N-Acetyltransferase and Mitogen-Activated Protein Kinase Phosphatase-1 in Rat Pinealocytes. Endocrinology 2007, 148, 743–751. [Google Scholar] [CrossRef]
- Martinez-Nicolas, A.; Juan, A.M.; Rol, M.A. Day–night contrast as source of health for the human circadian system. Chronobiol. Int. 2014, 31, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.S.; Kuchenbecker, J.A.; Anderson, J.R.; Neitz, M.; Neitz, J. A Color Vision Circuit for Non-Image-Forming Vision in the Primate Retina. Curr. Biol. 2020, 30, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Ohta, H.; Yamazaki, S.; McMahon, D.G. Constant light desynchronizes mammalian clock neurons. Nat. Neurosci. 2005, 8, 267–926. [Google Scholar] [CrossRef] [PubMed]
- Coomans, C.P.; van den Berg, S.A.; Houben, T.; van Klinken, J.B.; van den Berg, R.; Pronk, A.C.; Havekes, L.M.; Romijn, J.A.; van Dijk, K.W.; Biermasz, N.R.; et al. Detrimental effects of constant light exposure and high-fat diet on circadian energy metabolism and insulin sensitivity. FASEB J. 2013, 27, 1721–1732. [Google Scholar] [CrossRef] [PubMed]
- Molcan, L.; Sutovska, H.; Okuliarova, M.; Senko, T.; Krskova, L.; Zeman, M. Dim light at night attenuates circadian rhythms in the cardiovascular system and suppresses melatonin in rats. Life Sci. 2019, 231, 116568. [Google Scholar] [CrossRef] [PubMed]
- Rumanova, V.S.; Okuliarova, M.; Zeman, M. Differential Effects of Constant Light and Dim Light at Night on the Circadian Control of Metabolism and Behavior. Int. J. Mol. Sci. 2020, 21, 5478. [Google Scholar] [CrossRef]
- Ono, D.; Honma, S.; Honma, K. Postnatal constant light compensates Cryptochrome1 and 2 double deficiency for disruption of circadian behavioral rhythms in mice under constant dark. PLoS ONE 2013, 8, e80615. [Google Scholar] [CrossRef] [PubMed]
- Sulli, G.; Manoogian, E.N.C.; Taub, P.R.; Panda, S. Training the Circadian Clock, Clocking the Drugs, and Drugging the Clock to Prevent, Manage, and Treat Chronic Diseases. Trends Pharmacol. Sci. 2018, 39, 812–827. [Google Scholar] [CrossRef]
- Sládek, M.; Sumová, A.; Kováčiková, Z.; Bendová, Z.; Laurinová, K.; Illnerová, H. Insight into molecular core clock mechanism of embryonic and early postnatal rat suprachiasmatic nucleus. Proc. Natl. Acad. Sci. USA 2004, 101, 6231–6236. [Google Scholar] [CrossRef]
- Carmona-Alcocer, V.; Abel, J.H.; Sun, T.C.; Petzold, L.R.; Doyle, F.J., 3rd; Simms, C.L.; Herzog, E.D. Ontogeny of Circadian Rhythms and Synchrony in the Suprachiasmatic Nucleus. J. Neurosci. 2018, 38, 1326–1334. [Google Scholar] [CrossRef]
- Bendová, Z.; Sumová, A.; Illnerová, H. Development of circadian rhythmicity and photoperiodic response in subdivisions of the rat suprachiasmatic nucleus. Brain Res. Dev. Brain Res. 2004, 148, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Kováčiková, Z.; Sládek, M.; Bendová, Z.; Illnerová, H.; Sumová, A. Expression of clock and clock-driven genes in the rat suprachiasmatic nucleus during late fetal and early postnatal development. J. Biol. Rhythms 2006, 21, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Hahnová, K.; Pačesová, D.; Volfová, B.; Červená, K.; Kašparová, D.; Žurmanová, J.; Bendová, Z. Circadian Dexras1 in rats: Development, location and responsiveness to light. Chronobiol. Int. 2016, 33, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Canal-Corretger, M.M.; Cambras, T.; Vilaplana, J.; Díez-Noguera, A. Bright light during lactation alters the functioning of the circadian system of adult rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 278, R201–R208. [Google Scholar] [CrossRef] [PubMed]
- Canal-Corretger, M.M.; Vilaplana, J.; Cambras, T.; Díez-Noguera, A. Functioning of the rat circadian system is modified by light applied in critical postnatal days. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, R1023–R1030. [Google Scholar] [CrossRef] [PubMed]
- Brooks, E.; Canal, M.M. Development of circadian rhythms: Role of postnatal light environment. Neurosci. Biobehav. Rev. 2013, 37, 551–560. [Google Scholar] [CrossRef]
- Canal-Corretger, M.M.; Cambras, T.; Díez-Noguera, A. Effect of light during lactation on the phasic and tonic responses of the rat pacemaker. Chronobiol. Int. 2003, 20, 21–35. [Google Scholar] [CrossRef]
- Brooks, E.; Patel, D.; Canal, M.M. Programming of mice circadian photic responses by postnatal light environment. PLoS ONE 2014, 9, e97160. [Google Scholar] [CrossRef]
- Madahi, P.G.; Ivan, O.; Adriana, B.; Diana, O.; Carolina, E. Constant light during lactation programs circadian and metabolic systems. Chronobiol. Int. 2018, 35, 1153–1167. [Google Scholar] [CrossRef]
- Cambras, T.; López, L.; Arias, J.L.; Díez-Noguera, A. Quantitative changes in neuronal and glial cells in the suprachiasmatic nucleus as a function of the lighting conditions during weaning. Brain Res. Dev. Brain Res. 2005, 157, 27–33. [Google Scholar] [CrossRef]
- Canal, M.M.; Mohammed, N.M.; Rodríguez, J.J. Early programming of astrocyte organization in the mouse suprachiasmatic nuclei by light. Chronobiol. Int. 2009, 26, 1545–1558. [Google Scholar] [CrossRef] [PubMed]
- Brooks, E.; Waters, E.; Farrington, L.; Canal, M.M. Differential hypothalamic tyrosine hydroxylase distribution and activation by light in adult mice reared under different light conditions during the suckling period. Brain Struct. Funct. 2011, 216, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Vilches, N.; Spichiger, C.; Mendez, N.; Abarzua-Catalan, L.; Galdames, H.A.; Hazlerigg, D.G.; Richter, H.G.; Torres-Farfan, C. Gestational chronodisruption impairs hippocampal expression of NMDA receptor subunits Grin1b/Grin3a and spatial memory in the adult offspring. PLoS ONE 2014, 9, e91313. [Google Scholar] [CrossRef] [PubMed]
- Coleman, G.; Gigg, J.; Canal, M.M. Postnatal light alters hypothalamic-pituitary-adrenal axis function and induces a depressive-like phenotype in adult mice. Eur. J. Neurosci. 2016, 44, 2807–2817. [Google Scholar] [CrossRef]
- Noell, W.K.; Walker, V.S.; Kang, B.S.; Berman, S. Retinal damage by light in rats. Investig. Ophthalmol. 1966, 5, 450–473. [Google Scholar]
- Luik, A.I.; Zuurbier, L.A.; Hofman, A.; Van Someren, E.J.; Tiemeier, H. Stability and fragmentation of the activity rhythm across the sleep-wake cycle: The importance of age, lifestyle, and mental health. Chronobiol. Int. 2013, 30, 1223–1230. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Deprés-Brummer, P.; Lévi, F.; Metzger, G.; Touitou, Y. Light-induced suppression of the rat circadian system. Am. J. Physiol. 1995, 268 Pt 2, R1111–R1116. [Google Scholar] [CrossRef]
- Honma, S.; Kanematsu, N.; Katsuno, Y.; Honma, K. Persistence of circadian oscillation while locomotor activity and plasma melatonin levels became aperiodic under prolonged continuous light in the rat. Neurosci. Lett. 1996, 216, 49–52. [Google Scholar] [CrossRef]
- Wideman, C.H.; Murphy, H.M. Constant light induces alterations in melatonin levels, food intake, feed efficiency, visceral adiposity, and circadian rhythms in rats. Nutr. Neurosci. 2009, 12, 233–240. [Google Scholar] [CrossRef]
- Diatroptov, M.E.; Diatroptova, M.A.; Kosyreva, A.M.; Dzhalilova, D.S.; Mkhitarov, V.A.; Mikhailova, L.P.; Makarova, O.V. Ultradian Rhythms of Body Temperatures in Male Wistar Rats Maintained under Conditions of Constant Illumination. Bull. Exp. Biol. Med. 2019, 167, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Block, G.D.; Colwell, C.S.; Matveyenko, A.V. Consequences of exposure to light at night on the pancreatic islet circadian clock and function in rats. Diabetes 2013, 62, 3469–3478. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Vargas, N.N.; Navarro-Espíndola, R.; Guzmán-Ruíz, M.A.; Basualdo, M.D.C.; Espitia-Bautista, E.; López-Bago, A.; Lascurain, R.; Córdoba-Manilla, C.; Buijs, R.M.; Escobar, C. Circadian disruption promotes tumor growth by anabolic host metabolism; experimental evidence in a rat model. BMC Cancer 2017, 17, 625. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Osorio, A.; Salgado-Delgado, R.; Angeles-Castellanos, M.; Escobar, C. Disruption of circadian rhythms due to chronic constant light leads to depressive and anxiety-like behaviors in the rat. Behav. Brain Res. 2013, 252, 1–9. [Google Scholar] [CrossRef]
- Cambras, T.; Canal, M.M.; Torres, A.; Vilaplana, J.; Díez-Noguera, A. Manifestation of circadian rhythm under constant light depends on lighting conditions during lactation. Am. J. Physiol. 1997, 272 Pt 2, R1039–R1046. [Google Scholar] [CrossRef]
- Canal-Corretger, M.M.; Vilaplana, J.; Cambras, T.; Díez-Noguera, A. Effect of light on the development of the circadian rhythm of motor activity in the mouse. Chronobiol. Int. 2001, 18, 683–696. [Google Scholar] [CrossRef]
- Aschoff, J. Circadian rhythms: Influences of internal and external factors on the period measured in constant conditions. Zeitschrift fur Tierpsychologie 1979, 49, 225–249. [Google Scholar] [CrossRef]
- Cambras, T.; Vilaplana, J.; Torres, A.; Canal, M.M.; Casamitjana, N.; Campuzano, A.; Díez-Noguera, A. Constant bright light (LL) during lactation in rats prevents arrhythmicity due to LL. Physiol. Behav. 1998, 63, 875–882. [Google Scholar] [CrossRef]
- Bendová, Z.; Sumová, A.; Mikkelsen, J.D. Circadian and developmental regulation of N-methyl-d-aspartate-receptor 1 mRNA splice variants and N-methyl-d-aspartate-receptor 3 subunit expression within the rat suprachiasmatic nucleus. Neuroscience 2009, 159, 599–609. [Google Scholar] [CrossRef]
- Bendová, Z.; Sládek, M.; Svobodová, I. The expression of NR2B subunit of NMDA receptor in the suprachiasmatic nucleus of Wistar rats and its role in glutamate-induced CREB and ERK1/2 phosphorylation. Neurochem. Int. 2012, 61, 43–47. [Google Scholar] [CrossRef]
- Wang, L.M.; Schroeder, A.; Loh, D.; Smith, D.; Lin, K.; Han, J.H.; Michel, S.; Hummer, D.L.; Ehlen, J.C.; Albers, H.E.; et al. Role for the NR2B subunit of the N-methyl-D-aspartate receptor in mediating light input to the circadian system. Eur. J. Neurosci. 2008, 27, 1771–1779. [Google Scholar] [CrossRef]
- Kubová, H.; Bendová, Z.; Moravcová, S.; Pačesová, D.; Rocha, L.L.; Mareš, P. Neonatal Clonazepam Administration Induces Long-Lasting Changes in Glutamate Receptors. Front. Mol. Neurosci. 2018, 11, 382. [Google Scholar] [CrossRef] [PubMed]
- Honma, S.; Katsuno, Y.; Shinohara, K.; Abe, H.; Honma, K. Circadian rhythm and response to light of extracellular glutamate and aspartate in rat suprachiasmatic nucleus. Am. J. Physiol. 1996, 271 Pt 2, R579–R585. [Google Scholar] [CrossRef]
- Tosini, G.; Menaker, M. Circadian rhythms in cultured mammalian retina. Science 1996, 272, 419–421. [Google Scholar] [CrossRef] [PubMed]
- Ruan, G.X.; Zhang, D.Q.; Zhou, T.; Yamazaki, S.; McMahon, D.G. Circadian organization of the mammalian retina. Proc. Natl. Acad. Sci. USA 2006, 103, 9703–9708. [Google Scholar] [CrossRef]
- Tosini, G.; Davidson, A.J.; Fukuhara, C.; Kasamatsu, M.; Castanon-Cervantes, O. Localization of a circadian clock in mammalian photoreceptors. FASEB J. 2007, 21, 3866–3871. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, C.; Sandu, C.; Malan, A.; Mellac, K.; Hicks, D.; Felder-Schmittbuhl, M.P. Circadian organization of the rodent retina involves strongly coupled, layer-specific oscillators. FASEB J. 2015, 29, 1493–1504. [Google Scholar] [CrossRef] [PubMed]
- Kamphuis, W.; Cailotto, C.; Dijk, F.; Bergen, A.; Buijs, R.M. Circadian expression of clock genes and clock-controlled genes in the rat retina. Biochem. Biophys. Res. Commun. 2005, 330, 18–26. [Google Scholar] [CrossRef] [PubMed]
- González-Menéndez, I.; Contreras, F.; Cernuda-Cernuda, R.; García-Fernández, J.M. Daily rhythm of melanopsin-expressing cells in the mouse retina. Front. Cell Neurosci. 2009, 3, 3. [Google Scholar] [CrossRef]
- González-Menéndez, I.; Contreras, F.; Cernuda-Cernuda, R.; García-Fernández, J.M. No loss of melanopsin-expressing ganglion cells detected during postnatal development of the mouse retina. Histol. Histopathol. 2010, 25, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Liu, C.; Tosini, G. Classical photoreceptors regulate melanopsin mRNA levels in the rat retina. J. Neurosci. 2004, 24, 9693–9697. [Google Scholar] [CrossRef] [PubMed]
- Hannibal, J.; Georg, B.; Fahrenkrug, J. Differential expression of melanopsin mRNA and protein in Brown Norwegian rats. Exp. Eye Res. 2013, 106, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Nieto, P.S.; Acosta-Rodriguez, V.A.; Valdez, D.J.; Guido, M.E. Differential responses of the mammalian retinal ganglion cell line RGC-5 to physiological stimuli and trophic factors. Neurochem. Int. 2010, 57, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Benedetto, M.M.; Guido, M.E.; Contin, M.A. Non-Visual Photopigments Effects of Constant Light-Emitting Diode Light Exposure on the Inner Retina of Wistar Rats. Front. Neurol. 2017, 8, 417. [Google Scholar] [CrossRef] [PubMed]
- Peichl, L. Diversity of mammalian photoreceptor properties: Adaptations to habitat and lifestyle? Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2005, 287, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Gastel, J.A.; Roseboom, P.H.; Rinaldi, P.A.; Weller, J.L.; Klein, D.C. Melatonin production: Proteasomal proteolysisin serotoninN-acetyltransferase regulation. Science 1998, 279, 1358–1360. [Google Scholar] [CrossRef]
- Fukuhara, C.; Dirden, J.C.; Tosini, G. Circadian expression of period 1, period 2, and arylalkylamine N-acetyltransferase mRNA in the rat pineal gland under different light conditions. Neurosci. Lett. 2000, 286, 167–170. [Google Scholar] [CrossRef]
- Simonneaux, V.; Poirel, V.J.; Garidou, M.L.; Nguyen, D.; Diaz-Rodriguez, E.; Pévet, P. Daily rhythm and regulation of clock gene expression in the rat pineal gland. Brain Res. Mol. Brain Res. 2004, 120, 164–172. [Google Scholar] [CrossRef]
- Karolczak, M.; Burbach, G.J.; Sties, G.; Korf, H.W.; Stehle, J.H. Clock gene mRNA and protein rhythms in the pineal gland of mice. Eur. J. Neurosci. 2004, 19, 3382–3388. [Google Scholar] [CrossRef]
- Wongchitrat, P.; Felder-Schmittbuhl, M.P.; Phansuwan-Pujito, P.; Pévet, P.; Simonneaux, V. Endogenous rhythmicity of Bmal1 and Rev-erb alpha in the hamster pineal gland is not driven by norepinephrine. Eur. J. Neurosci. 2009, 29, 2009–2016. [Google Scholar] [CrossRef]
- Simonneaux, V.; Ribelayga, C. Generation of the melatonin endocrine message in mammals: A review of the complex regulation of melatonin synthesis by norepinephrine, peptides, and other pineal transmitters. Pharmacol. Rev. 2003, 55, 325–395. [Google Scholar] [CrossRef] [PubMed]
- Bellavía, S.L.; Gallará, R.V. Modification of the beta- and alpha2-adrenergic sensitivity of rat submandibular glands by environmental stimuli and stress. Arch. Oral Biol. 1998, 43, 933–939. [Google Scholar] [CrossRef]


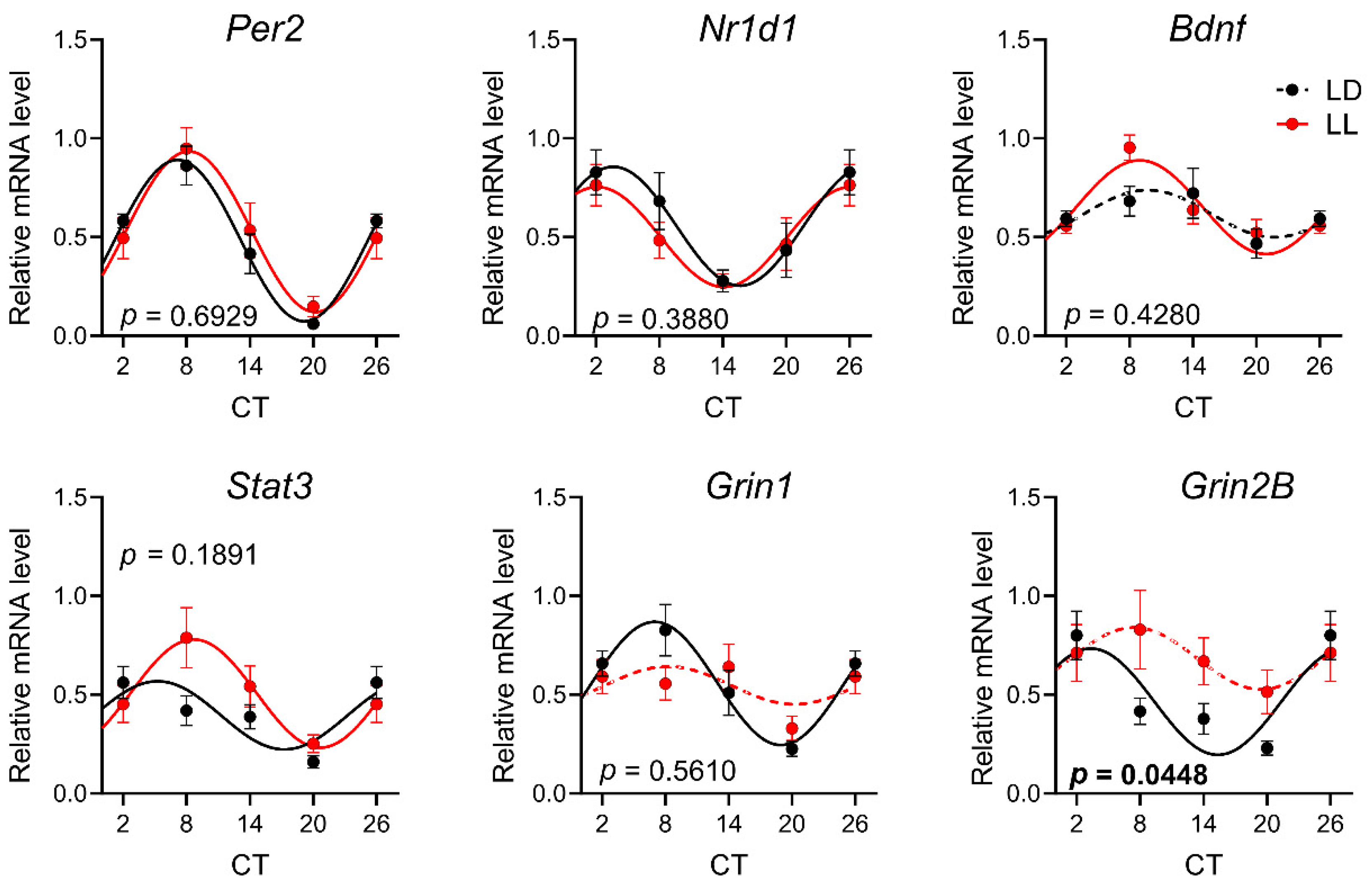
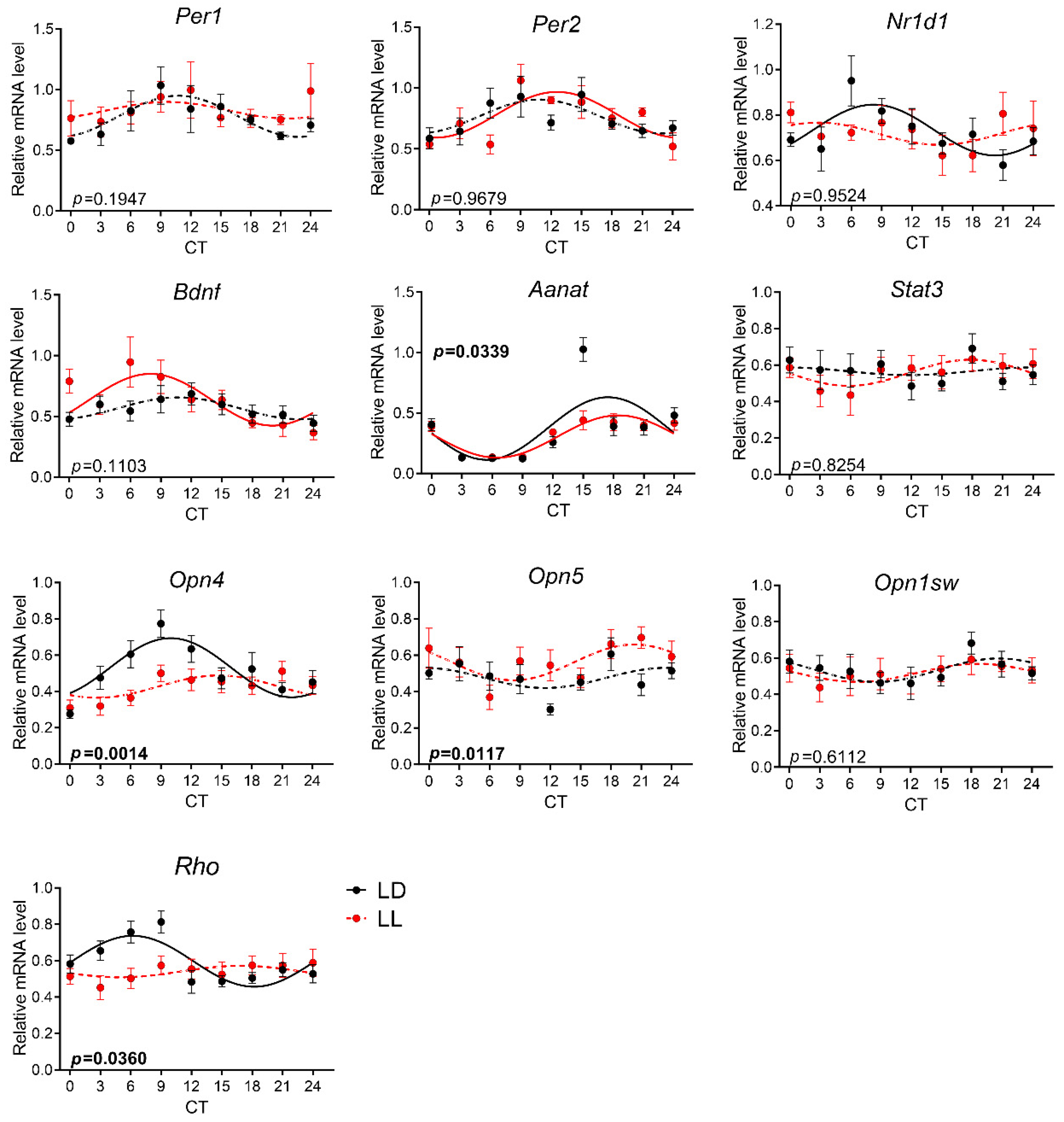
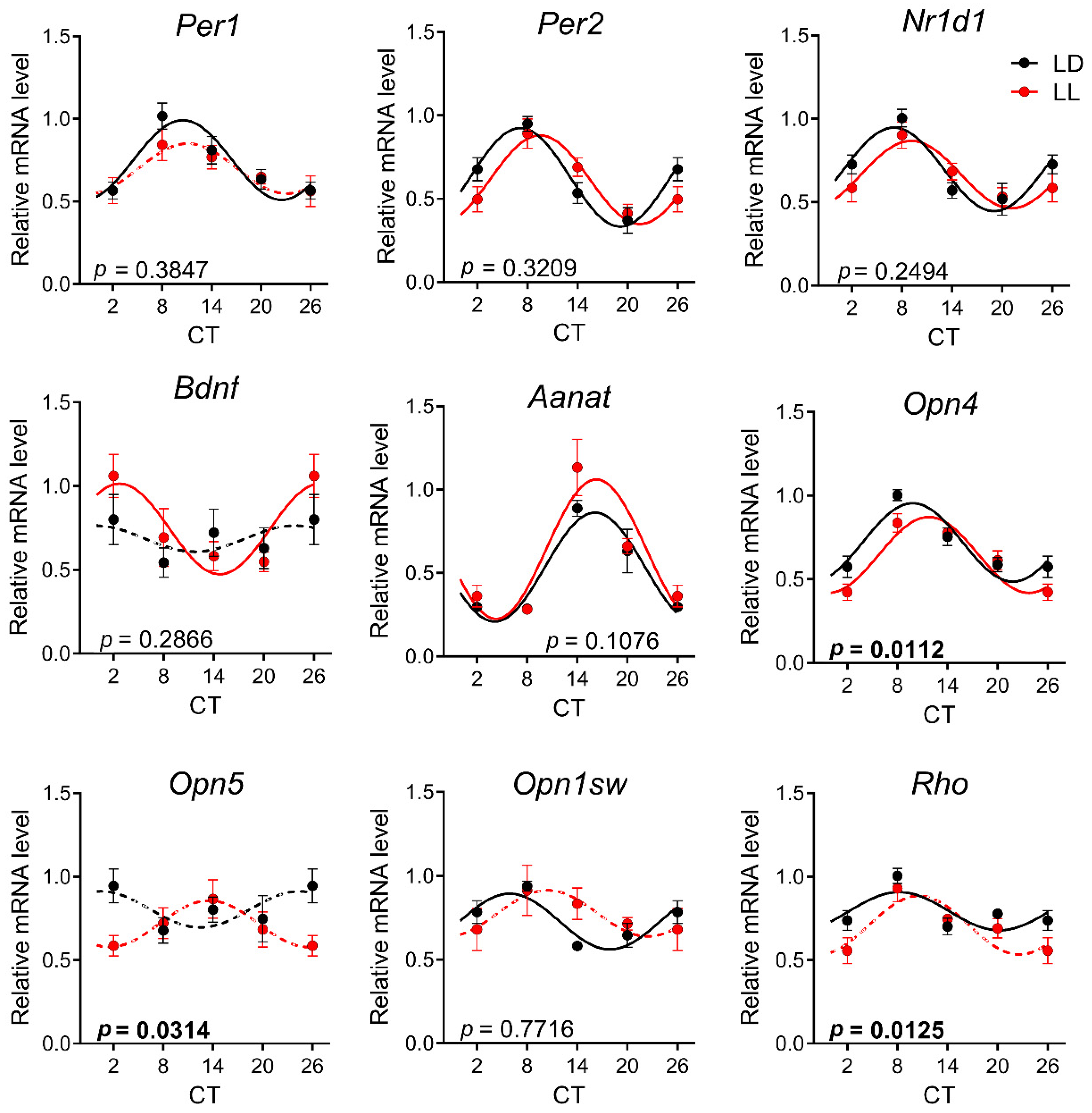
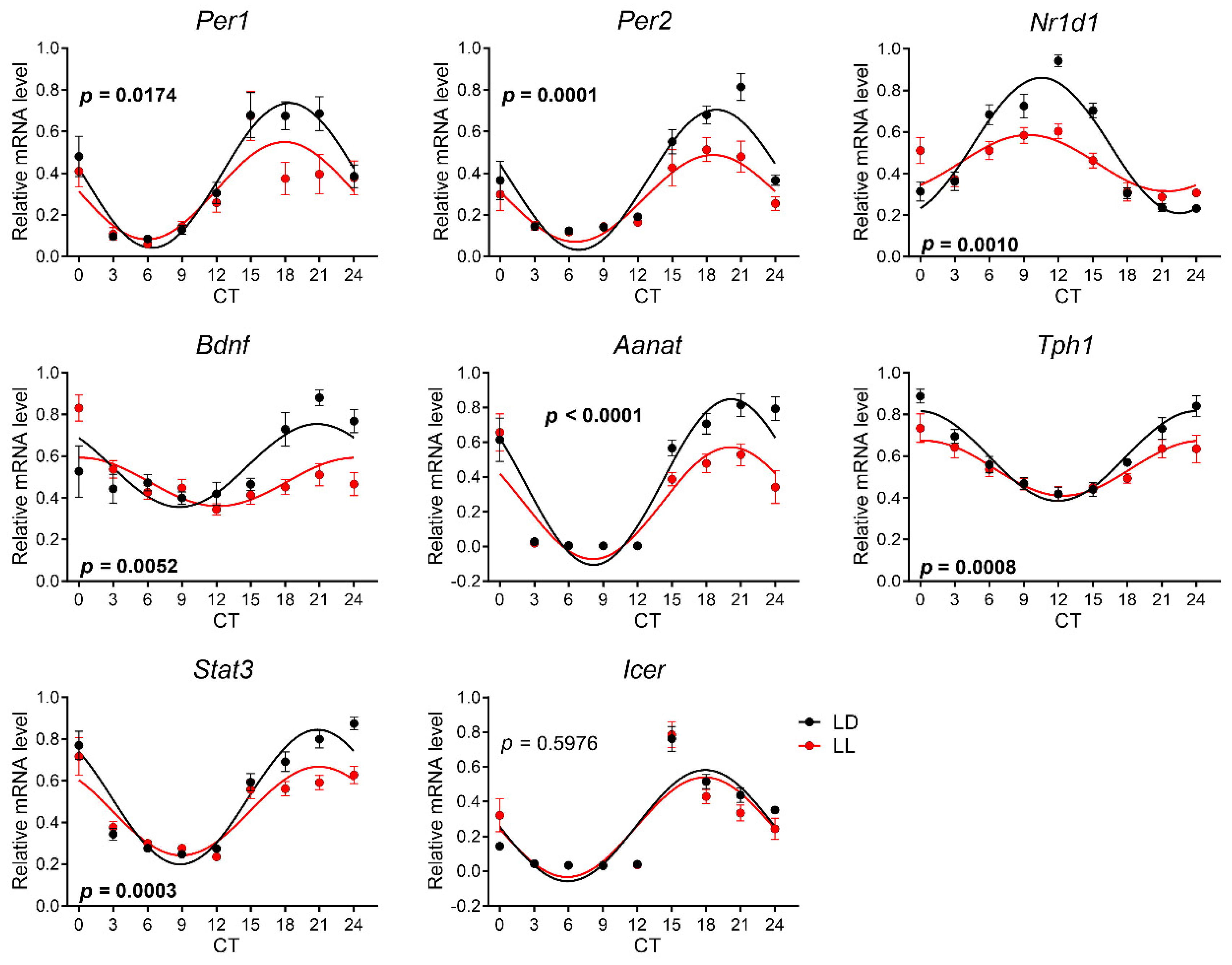
| Gene Symbol | Ref. No. | Gene Name |
|---|---|---|
| Aanat | Rn01461110_m2 | arylalkylamine N-acetyltransferase |
| Bdnf | Rn02531967_s1 | brain-derived neurotrophic factor |
| Gapdh | Rn01775763_g1 | glyceraldehyde 3-phosphate dehydrogenase |
| Grin1 | Rn01436034_m1 | glutamate receptor, ionotropic N-methyl D-aspartate 1 |
| Grin2b | Rn00680474_m1 | glutamate receptor, ionotropic N-methyl D-aspartate 2B |
| Hprt | Rn01527840_m1 | hypoxanthine phosphoribosyltransferase 1 |
| Icer | Rn04338541_m1 | inducible cAMP early repressor |
| Nr1d1 | Rn01460662_m1 | Rev-Erbα |
| Opn1sw | Rn00578824_m1 | opsin 1, short wavelength Sensitive |
| Opn4 | Rn00593931_m1 | melanopsin |
| Opn5 | Rn00710997_m1 | opsin 5 |
| Per1 | Rn01325256_m1 | period 1 |
| Per2 | Rn01427704_m1 | period 2 |
| Rho | Rn00583728_m1 | rhodopsin |
| Stat3 | Rn00680715_m1 | signal transducer and activator of transcription 3 |
| Tph1 | Rn01476867_m1 | tryptophan hydroxylase 1 |
| SCN | P30 | P90 | Retina | P30 | P90 | Pineal Gland | P30 | P90 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F= | p Value | F= | p Value | F= | p Value | F= | p Value | F= | p Value | F= | p Value | ||||||
| Per1 | mesor | NA | - | - | Per1 | mesor | NA | NA | Per1 | mesor | 12.240 | =0.0006 | 5.230 | =0.0257 | |||
| amplitude | - | - | amplitude | amplitude | 11.560 | =0.0009 | 4.521 | =0.0376 | |||||||||
| acrophase | - | - | acrophase | acrophase | 0.305 | =0.5816 | 0.656 | =0.4210 | |||||||||
| Per2 | mesor | 12.840 | =0.0005 | 0.573 | =0.4522 | Per2 | mesor | NA | 0.103 | =0.7495 | Per2 | mesor | 4.526 | =0.0352 | 3.322 | =0.0733 | |
| amplitude | 7.028 | =0.0091 | 0.001 | =0.9825 | amplitude | 0.189 | =0.6655 | amplitude | 4.903 | =0.0285 | 2.427 | =0.1246 | |||||
| acrophase | 1.063 | =0.3047 | 2.187 | =0.1449 | acrophase | 9.218 | =0.0035 | acrophase | 0.450 | =0.5033 | 0.743 | =0.3921 | |||||
| Nr1d1 | mesor | NA | 0.611 | =0.4375 | Nr1d1 | mesor | NA | 0.486 | =0.4881 | Nr1d1 | mesor | 16.250 | <0.0001 | 0.699 | =0.4066 | ||
| amplitude | 0.282 | =0.5973 | amplitude | 0.476 | =0.4926 | amplitude | 42.960 | <0.0001 | 0.021 | =0.8846 | |||||||
| acrophase | 1.007 | =0.3198 | acrophase | 4.370 | =0.0406 | acrophase | 2.807 | =0.0962 | 1.772 | =0.1881 | |||||||
| Bdnf | mesor | NA | NA | Bdnf | mesor | NA | NA | Bdnf | mesor | 6.881 | =0.0097 | - | - | ||||
| amplitude | amplitude | amplitude | 4.087 | =0.0452 | - | - | |||||||||||
| acrophase | acrophase | acrophase | 7.194 | =0.0082 | - | - | |||||||||||
| Stat3 | mesor | NA | 3.265 | =0.0762 | Stat3 | mesor | NA | - | - | Stat3 | mesor | 9.273 | =0.0028 | 5.793 | =0.0193 | ||
| amplitude | 1.236 | =0.2710 | amplitude | - | - | amplitude | 12.770 | =0.0005 | 1.980 | =0.1648 | |||||||
| acrophase | 4.795 | =0.0328 | acrophase | - | - | acrophase | 0.047 | =0.8286 | 0.112 | =0.7391 | |||||||
| Grin1 | mesor | NA | NA | Aanat | mesor | 3.344 | =0.0698 | 3.734 | =0.0580 | Tph1 | mesor | 9.218 | =0.0029 | 3.562 | =0.0639 | ||
| amplitude | amplitude | 2.554 | =0.1125 | 1.407 | =0.2402 | amplitude | 10.290 | =0.0017 | 1.157 | =0.2864 | |||||||
| acrophase | acrophase | 1.214 | =0.2727 | 0.036 | =0.8507 | acrophase | 0.407 | =0.5244 | 4.460 | =0.0389 | |||||||
| Grin2b | mesor | NA | NA | OPN4 | mesor | NA | 4.445 | =0.0390 | Aanat | mesor | 14.270 | =0.0002 | 7.947 | =0.0065 | |||
| amplitude | amplitude | 0.025 | =0.8759 | amplitude | 11.190 | =0.0011 | 18.040 | <0.0001 | |||||||||
| acrophase | acrophase | 5.149 | =0.0267 | acrophase | 0.008 | =0.9299 | 1.662 | =0.2024 | |||||||||
| OPN5 | mesor | NA | NA | Icer | mesor | 0.088 | =0.7672 | 0.016 | =0.8983 | ||||||||
| amplitude | amplitude | 0.506 | =0.4782 | 0.005 | =0.9461 | ||||||||||||
| acrophase | acrophase | 0.010 | =0.9184 | 0.075 | =0.7849 | ||||||||||||
| OPN1SW | mesor | NA | NA | ||||||||||||||
| amplitude | |||||||||||||||||
| acrophase | |||||||||||||||||
| Rho | mesor | NA | NA | ||||||||||||||
| amplitude | |||||||||||||||||
| acrophase | |||||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubištová, A.; Spišská, V.; Petrželková, L.; Hrubcová, L.; Moravcová, S.; Maierová, L.; Bendová, Z. Constant Light in Critical Postnatal Days Affects Circadian Rhythms in Locomotion and Gene Expression in the Suprachiasmatic Nucleus, Retina, and Pineal Gland Later in Life. Biomedicines 2020, 8, 579. https://doi.org/10.3390/biomedicines8120579
Kubištová A, Spišská V, Petrželková L, Hrubcová L, Moravcová S, Maierová L, Bendová Z. Constant Light in Critical Postnatal Days Affects Circadian Rhythms in Locomotion and Gene Expression in the Suprachiasmatic Nucleus, Retina, and Pineal Gland Later in Life. Biomedicines. 2020; 8(12):579. https://doi.org/10.3390/biomedicines8120579
Chicago/Turabian StyleKubištová, Aneta, Veronika Spišská, Lucie Petrželková, Leona Hrubcová, Simona Moravcová, Lenka Maierová, and Zdeňka Bendová. 2020. "Constant Light in Critical Postnatal Days Affects Circadian Rhythms in Locomotion and Gene Expression in the Suprachiasmatic Nucleus, Retina, and Pineal Gland Later in Life" Biomedicines 8, no. 12: 579. https://doi.org/10.3390/biomedicines8120579
APA StyleKubištová, A., Spišská, V., Petrželková, L., Hrubcová, L., Moravcová, S., Maierová, L., & Bendová, Z. (2020). Constant Light in Critical Postnatal Days Affects Circadian Rhythms in Locomotion and Gene Expression in the Suprachiasmatic Nucleus, Retina, and Pineal Gland Later in Life. Biomedicines, 8(12), 579. https://doi.org/10.3390/biomedicines8120579






