Analgesic Tolerance Development during Repetitive Electric Stimulations Is Associated with Changes in the Expression of Activated Microglia in Rats with Osteoarthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Induction of OA
2.3. TENS Application
2.4. Intrathecal Catheterization
2.5. Drug Delivery
2.6. Behavioral Analyses
2.7. Tissue Processing and Immunohistochemistry
2.8. Quantitative Image Analysis
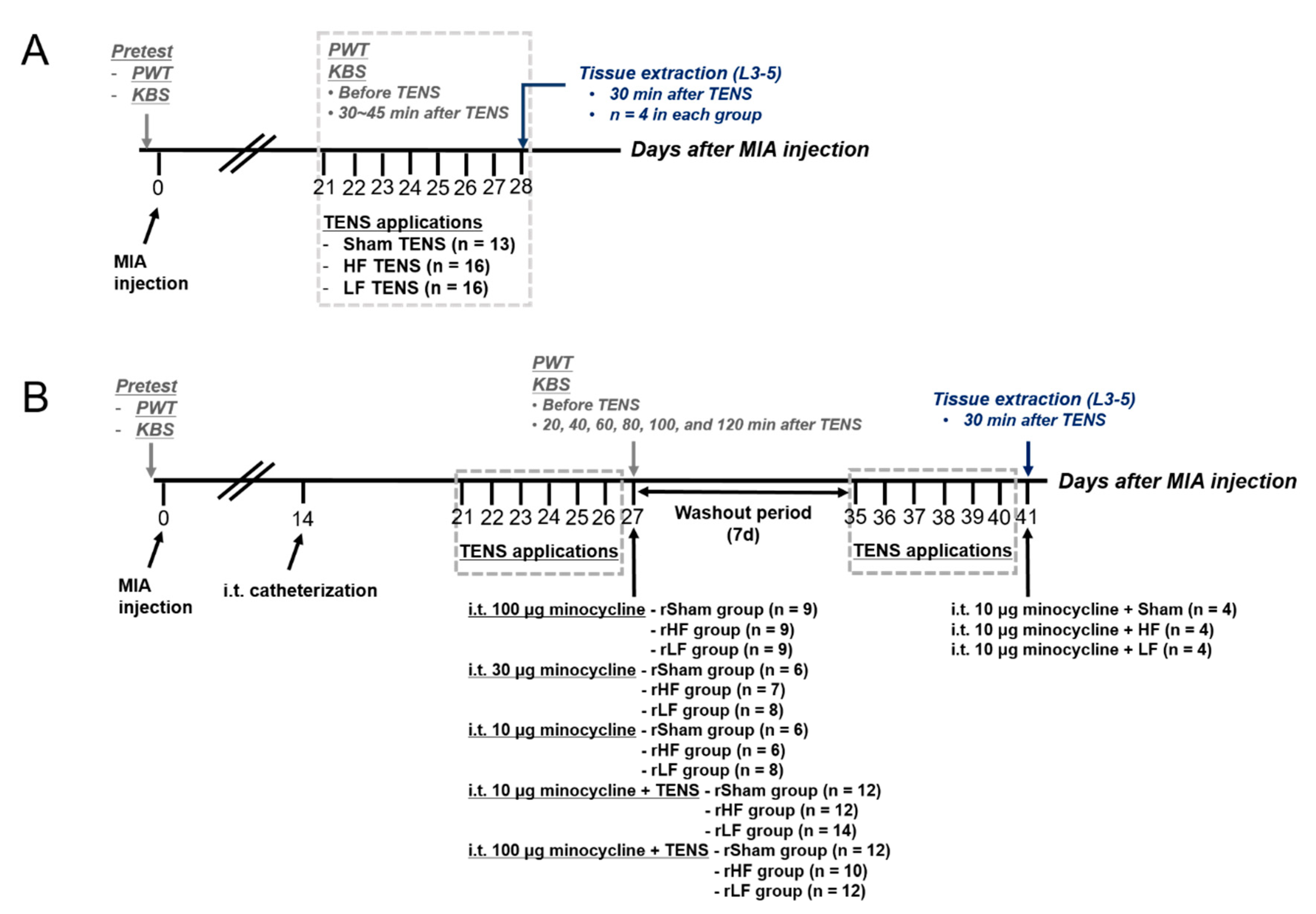
2.9. Experimental Procedure
2.10. Statistics
3. Results
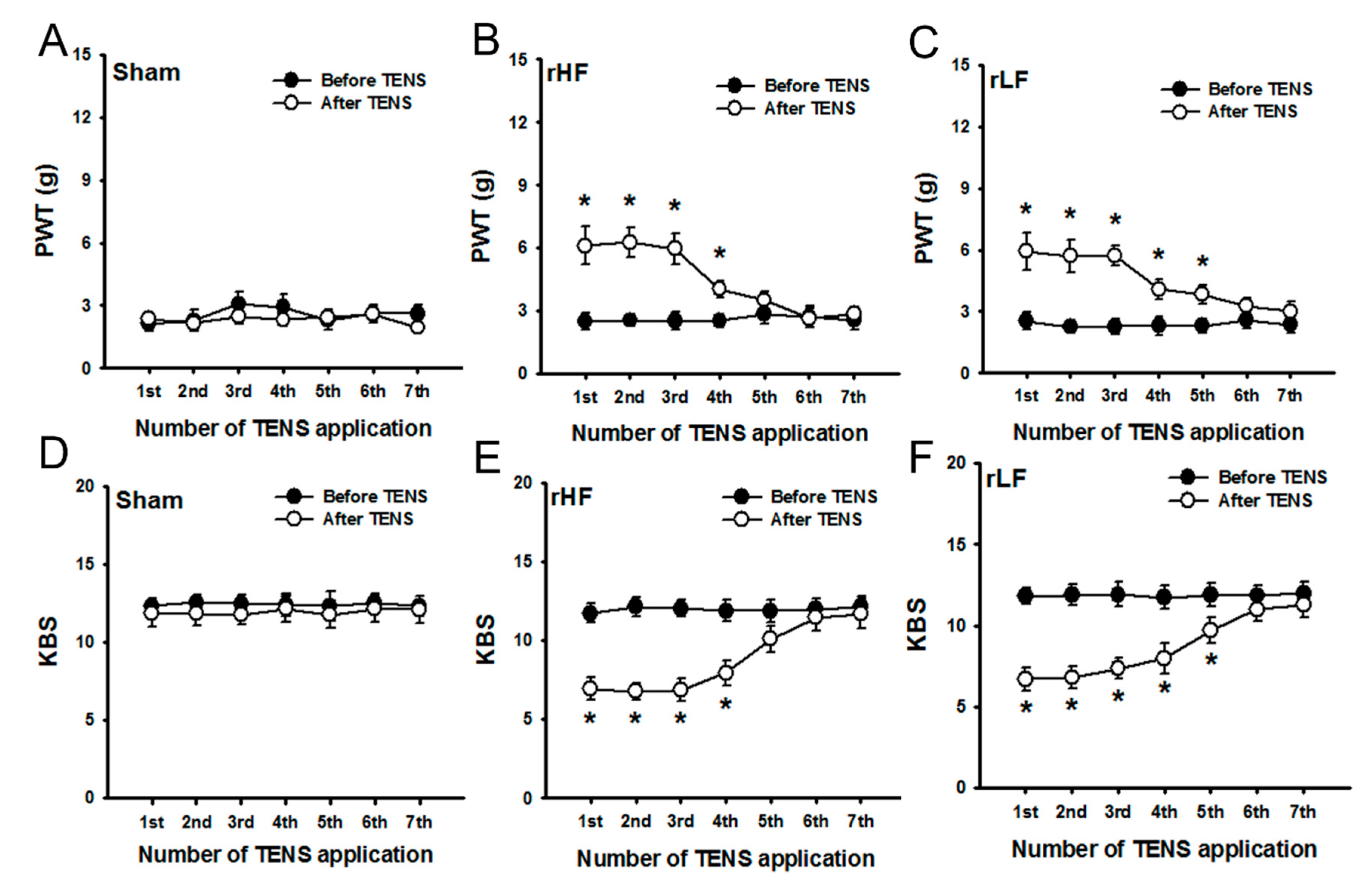
3.1. Changes of OA Pain by Repeated TENS in Rats with OA
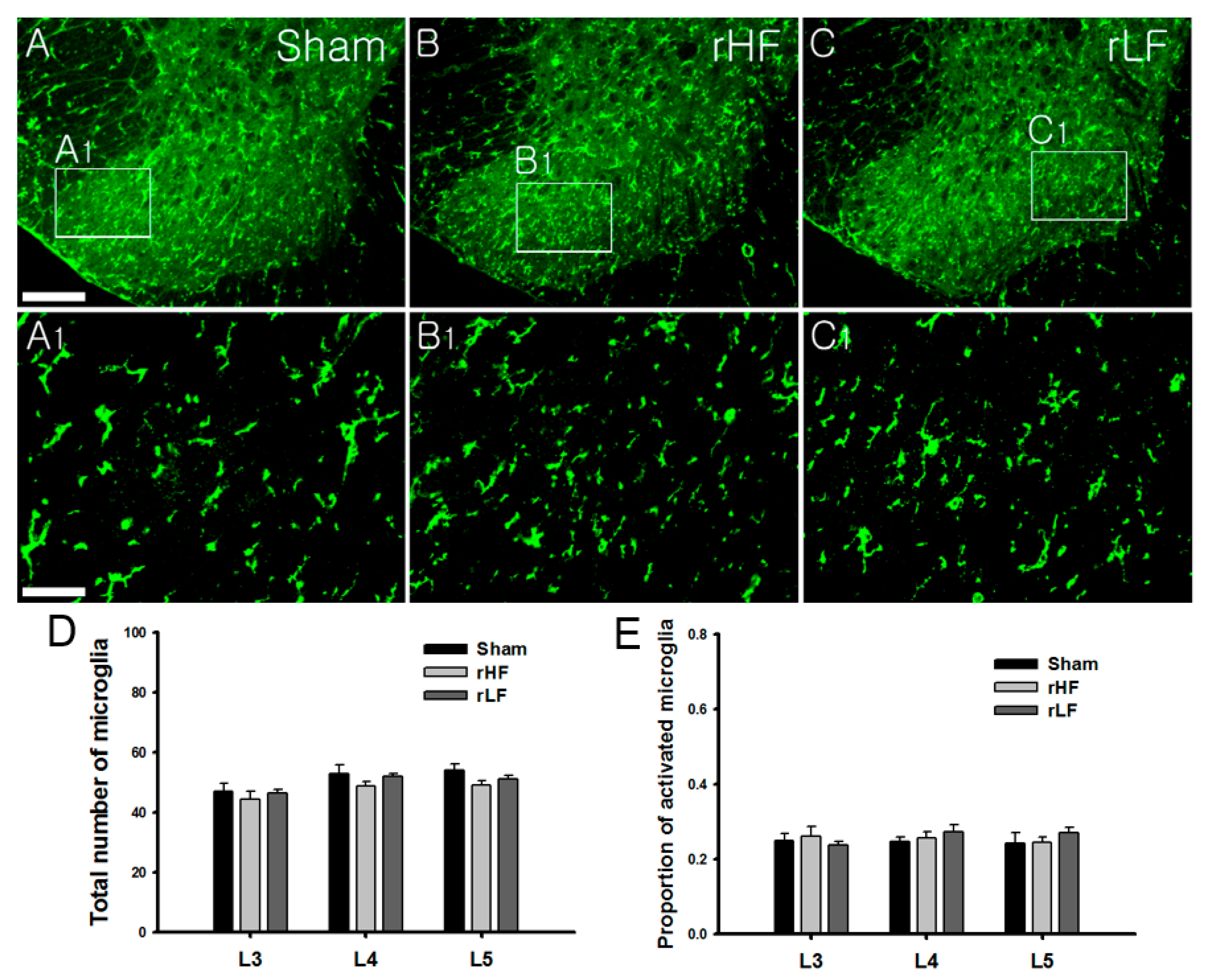
3.2. Changes in Spinal Microglia due to Repeated TENS Applications in Rats with OA
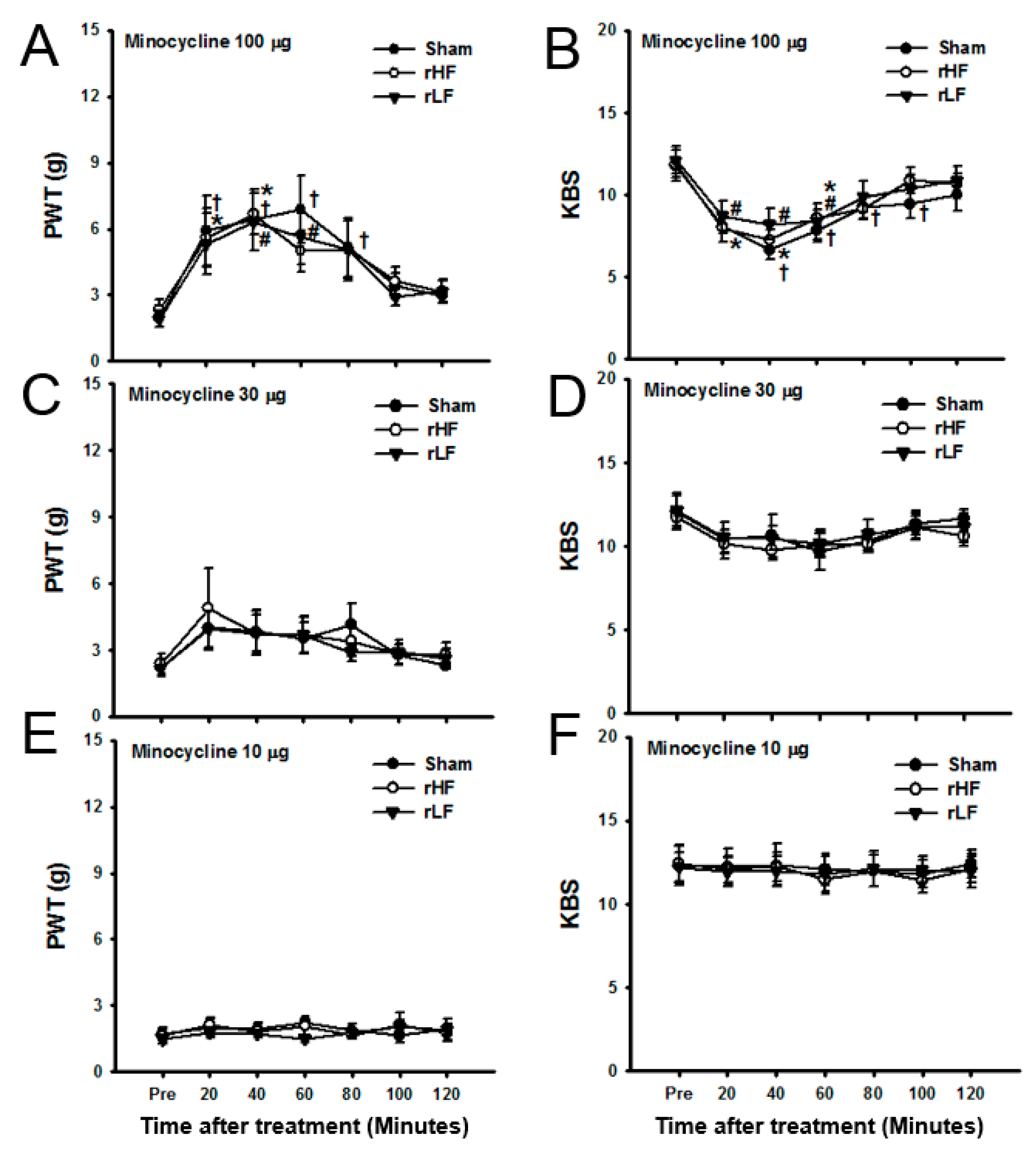
3.3. Effects of Microglial Inhibition on OA Pain in TENS-Tolerant Rats
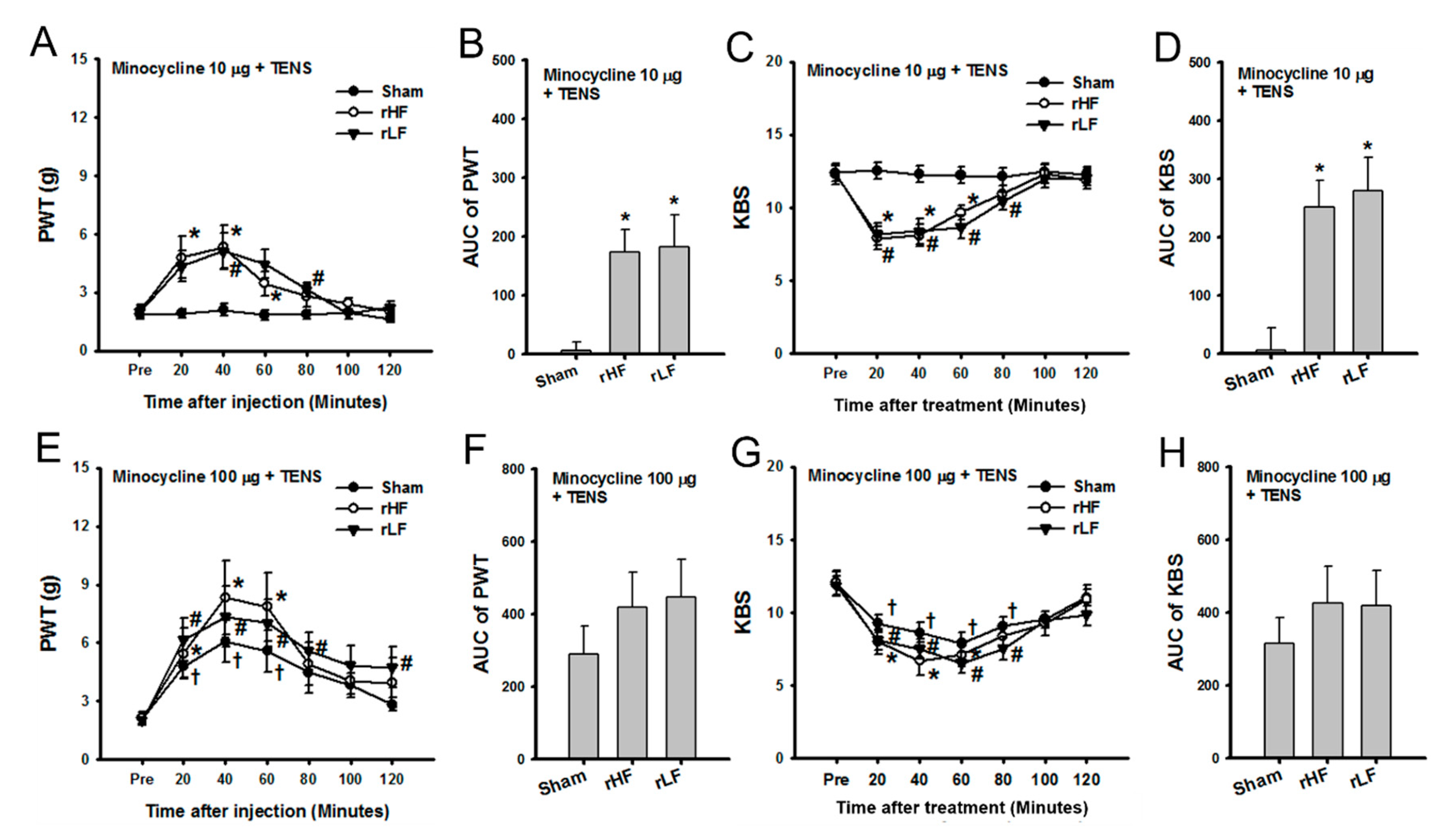
3.4. Effects of TENS with Microglia Inhibition on OA Pain in TENS-Tolerant Rats
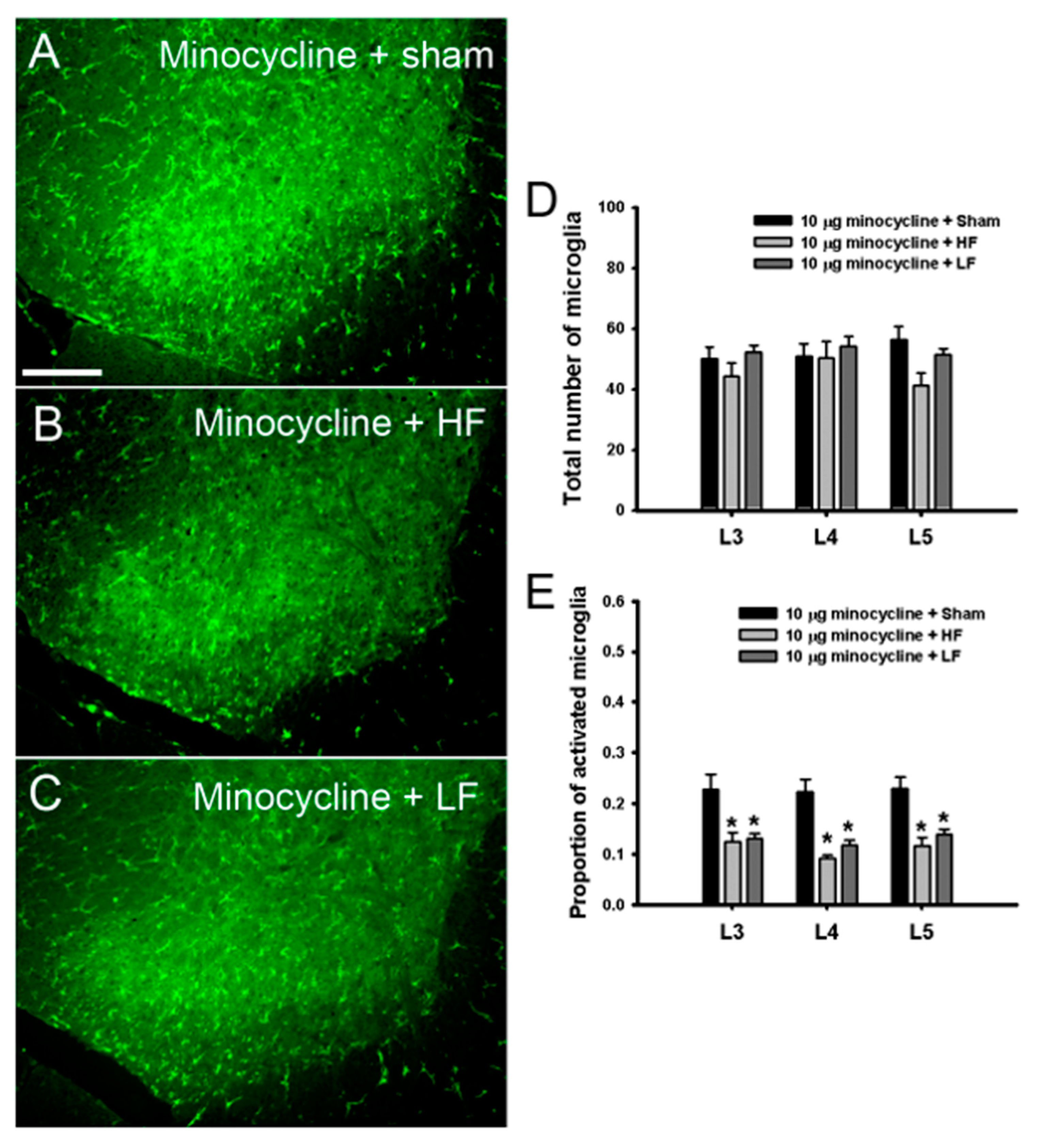
3.5. Spinal Glial Changes Induced by TENS Treatment with 10-μg Minocycline in TENS-Tolerant Rats with OA
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Neogi, T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage 2013, 21, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Elboim-Gabyzon, M.; Rozen, N.; Laufer, Y. Does neuromuscular electrical stimulation enhance the effectiveness of an exercise programme in subjects with knee osteoarthritis? A randomized controlled trial. Clin. Rehabil. 2013, 27, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Vance, C.G.; Rakel, B.A.; Blodgett, N.P.; DeSantana, J.M.; Amendola, A.; Zimmerman, M.B.; Walsh, D.M.; Sluka, K.A. Effects of transcutaneous electrical nerve stimulation on pain, pain sensitivity, and function in people with knee osteoarthritis: A randomized controlled trial. Phys. Ther. 2012, 92, 898–910. [Google Scholar] [CrossRef]
- Gundog, M.; Atamaz, F.; Kanyilmaz, S.; Kirazli, Y.; Celepoglu, G. Interferential current therapy in patients with knee osteoarthritis: Comparison of the effectiveness of different amplitude-modulated frequencies. Am. J. Phys. Med. Rehabil. 2012, 91, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Pietrosimone, B.G.; Saliba, S.A.; Hart, J.M.; Hertel, J.; Kerrigan, D.C.; Ingersoll, C.D. Effects of transcutaneous electrical nerve stimulation and therapeutic exercise on quadriceps activation in people with tibiofemoral osteoarthritis. J. Orthop. Sports Phys. Ther. 2011, 41, 4–12. [Google Scholar] [CrossRef]
- Hahm, S.C.; Song, E.; Jeon, H.; Yoon, Y.W.; Kim, J. Transcutaneous Electrical Nerve Stimulation Reduces Knee Osteoarthritic Pain by Inhibiting Spinal Glial Cells in Rats. Phys. Ther. 2019, 99, 1211–1223. [Google Scholar] [CrossRef]
- Kalra, A.; Urban, M.O.; Sluka, K.A. Blockade of opioid receptors in rostral ventral medulla prevents antihyperalgesia produced by transcutaneous electrical nerve stimulation (TENS). J. Pharmacol. Exp. Ther. 2001, 298, 257–263. [Google Scholar]
- Sluka, K.A.; Deacon, M.; Stibal, A.; Strissel, S.; Terpstra, A. Spinal blockade of opioid receptors prevents the analgesia produced by TENS in arthritic rats. J. Pharmacol. Exp. Ther. 1999, 289, 840–846. [Google Scholar]
- Han, J.S.; Chen, X.H.; Sun, S.L.; Xu, X.J.; Yuan, Y.; Yan, S.C.; Hao, J.X.; Terenius, L. Effect of low- and high-frequency TENS on Met-enkephalin-Arg-Phe and dynorphin A immunoreactivity in human lumbar CSF. Pain 1991, 47, 295–298. [Google Scholar] [CrossRef]
- Sluka, K.A.; Vance, C.G.; Lisi, T.L. High-frequency, but not low-frequency, transcutaneous electrical nerve stimulation reduces aspartate and glutamate release in the spinal cord dorsal horn. J. Neurochem. 2005, 95, 1794–1801. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; King, E.W.; Dickman, J.K.; Herold, C.A.; Johnston, N.F.; Spurgin, M.L.; Sluka, K.A. Spinal 5-HT(2) and 5-HT(3) receptors mediate low, but not high, frequency TENS-induced antihyperalgesia in rats. Pain 2003, 105, 205–213. [Google Scholar] [CrossRef]
- Sluka, K.A.; Lisi, T.L.; Westlund, K.N. Increased release of serotonin in the spinal cord during low, but not high, frequency transcutaneous electric nerve stimulation in rats with joint inflammation. Arch. Phys. Med. Rehabil. 2006, 87, 1137–1140. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Lisi, T.L.; Vance, C.G.; Sluka, K.A. Release of GABA and activation of GABA(A) in the spinal cord mediates the effects of TENS in rats. Brain Res. 2007, 1136, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.I.; Ashton, C.H.; Thompson, J.W. An in-depth study of long-term users of transcutaneous electrical nerve stimulation (TENS). Implications for clinical use of TENS. Pain 1991, 44, 221–229. [Google Scholar] [CrossRef]
- Liebano, R.E.; Rakel, B.; Vance, C.G.; Walsh, D.M.; Sluka, K.A. An investigation of the development of analgesic tolerance to TENS in humans. Pain 2011, 152, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Maguma, H.T.; Thayne, K.; Davis, B.; Taylor, D.A. Correlation of the time course of development and decay of tolerance to morphine with alterations in sodium pump protein isoform abundance. Biochem. Pharmacol. 2010, 79, 1015–1024. [Google Scholar] [CrossRef]
- Hutchinson, M.R.; Lewis, S.S.; Coats, B.D.; Skyba, D.A.; Crysdale, N.Y.; Berkelhammer, D.L.; Brzeski, A.; Northcutt, A.; Vietz, C.M.; Judd, C.M.; et al. Reduction of opioid withdrawal and potentiation of acute opioid analgesia by systemic AV411 (ibudilast). Brain Behav. Immun. 2009, 23, 240–250. [Google Scholar] [CrossRef]
- Cui, Y.; Liao, X.X.; Liu, W.; Guo, R.X.; Wu, Z.Z.; Zhao, C.M.; Chen, P.X.; Feng, J.Q. A novel role of minocycline: Attenuating morphine antinociceptive tolerance by inhibition of p38 MAPK in the activated spinal microglia. Brain Behav. Immun. 2008, 22, 114–123. [Google Scholar] [CrossRef]
- Song, P.; Zhao, Z.Q. The involvement of glial cells in the development of morphine tolerance. Neurosci. Res. 2001, 39, 281–286. [Google Scholar] [CrossRef]
- Gao, Y.J.; Zhang, L.; Samad, O.A.; Suter, M.R.; Yasuhiko, K.; Xu, Z.Z.; Park, J.Y.; Lind, A.L.; Ma, Q.; Ji, R.R. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J. Neurosci. 2009, 29, 4096–4108. [Google Scholar] [CrossRef]
- Zhuang, Z.Y.; Wen, Y.R.; Zhang, D.R.; Borsello, T.; Bonny, C.; Strichartz, G.R.; Decosterd, I.; Ji, R.R. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: Respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J. Neurosci. 2006, 26, 3551–3560. [Google Scholar] [CrossRef] [PubMed]
- Hains, B.C.; Waxman, S.G. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J. Neurosci. 2006, 26, 4308–4317. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, M.R.; Bland, S.T.; Johnson, K.W.; Rice, K.C.; Maier, S.F.; Watkins, L.R. Opioid-induced glial activation: Mechanisms of activation and implications for opioid analgesia, dependence, and reward. Sci. World J. 2007, 7, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Ueda, M. Mechanisms underlying morphine analgesic tolerance and dependence. Front. Biosci. (Landmark Ed.) 2009, 14, 5260–5272. [Google Scholar] [CrossRef] [PubMed]
- Solomon, R.A.; Viernstein, M.C.; Long, D.M. Reduction of postoperative pain and narcotic use by transcutaneous electrical nerve stimulation. Surgery 1980, 87, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Q.; Liu, D.Q.; Chen, S.P.; Sun, J.; Wang, X.M.; Tian, Y.K.; Wu, W.; Ye, D.W. Minocycline as a promising therapeutic strategy for chronic pain. Pharmacol. Res. 2018, 134, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Sagar, D.R.; Burston, J.J.; Hathway, G.J.; Woodhams, S.G.; Pearson, R.G.; Bennett, A.J.; Kendall, D.A.; Scammell, B.E.; Chapman, V. The contribution of spinal glial cells to chronic pain behaviour in the monosodium iodoacetate model of osteoarthritic pain. Mol. Pain 2011, 7, 88. [Google Scholar] [CrossRef]
- Combe, R.; Bramwell, S.; Field, M.J. The monosodium iodoacetate model of osteoarthritis: A model of chronic nociceptive pain in rats? Neurosci. Lett. 2004, 370, 236–240. [Google Scholar] [CrossRef]
- Liu, P.; Okun, A.; Ren, J.; Guo, R.C.; Ossipov, M.H.; Xie, J.; King, T.; Porreca, F. Ongoing pain in the MIA model of osteoarthritis. Neurosci. Lett. 2011, 493, 72–75. [Google Scholar] [CrossRef]
- Nwosu, L.N.; Mapp, P.I.; Chapman, V.; Walsh, D.A. Relationship between structural pathology and pain behaviour in a model of osteoarthritis (OA). Osteoarthritis Cartilage 2016, 24, 1910–1917. [Google Scholar] [CrossRef]
- Kim, T.; Seol, D.R.; Hahm, S.C.; Ko, C.; Kim, E.H.; Chun, K.; Kim, J.; Lim, T.H. Analgesic Effect of Intra-Articular Injection of Temperature-Responsive Hydrogel Containing Bupivacaine on Osteoarthritic Pain in Rats. BioMed Res. Int. 2015, 2015, 812949. [Google Scholar] [CrossRef] [PubMed]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Dixon, W.J. Staircase bioassay: The up-and-down method. Neurosci. Biobehav. Rev. 1991, 15, 47–50. [Google Scholar] [CrossRef]
- Ferreira-Gomes, J.; Adães, S.; Castro-Lopes, J.M. Assessment of movement-evoked pain in osteoarthritis by the knee-bend and CatWalk tests: A clinically relevant study. J. Pain 2008, 9, 945–954. [Google Scholar] [CrossRef]
- Hahm, S.C.; Yoon, Y.W.; Kim, J. High-frequency transcutaneous electrical nerve stimulation alleviates spasticity after spinal contusion by inhibiting activated microglia in rats. Neurorehabil. Neural Repair. 2015, 29, 370–381. [Google Scholar] [CrossRef]
- Im, H.J.; Kim, J.S.; Li, X.; Kotwal, N.; Sumner, D.R.; van Wijnen, A.J.; Davis, F.J.; Yan, D.; Levine, B.; Henry, J.L.; et al. Alteration of sensory neurons and spinal response to an experimental osteoarthritis pain model. Arthritis Rheum. 2010, 62, 2995–3005. [Google Scholar] [CrossRef]
- Thakur, M.; Dickenson, A.H.; Baron, R. Osteoarthritis pain: Nociceptive or neuropathic? Nat. Rev. Rheumatol. 2014, 10, 374–380. [Google Scholar] [CrossRef]
- Lee, Y.; Pai, M.; Brederson, J.D.; Wilcox, D.; Hsieh, G.; Jarvis, M.F.; Bitner, R.S. Monosodium iodoacetate-induced joint pain is associated with increased phosphorylation of mitogen activated protein kinases in the rat spinal cord. Mol. Pain 2011, 7, 39. [Google Scholar] [CrossRef]
- Matsushita, Y.; Ueda, H. Curcumin blocks chronic morphine analgesic tolerance and brain-derived neurotrophic factor upregulation. Neuroreport 2009, 20, 63–68. [Google Scholar] [CrossRef]
- Takayama, N.; Ueda, H. Morphine-induced chemotaxis and brain-derived neurotrophic factor expression in microglia. J. Neurosci. 2005, 25, 430–435. [Google Scholar] [CrossRef][Green Version]
- Small, D.L.; Murray, C.L.; Mealing, G.A.; Poulter, M.O.; Buchan, A.M.; Morley, P. Brain derived neurotrophic factor induction of N-methyl-D-aspartate receptor subunit NR2A expression in cultured rat cortical neurons. Neurosci. Lett. 1998, 252, 211–214. [Google Scholar] [CrossRef]
- Mao, J.; Sung, B.; Ji, R.R.; Lim, G. Chronic morphine induces downregulation of spinal glutamate transporters: Implications in morphine tolerance and abnormal pain sensitivity. J. Neurosci. 2002, 22, 8312–8323. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, T.; Nakagawa, T.; Shige, K.; Minami, M.; Satoh, M. Changes in the expression of glial glutamate transporters in the rat brain accompanied with morphine dependence and naloxone-precipitated withdrawal. Brain Res. 2001, 905, 254–258. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hahm, S.-C.; Lee, J.S.; Yoon, Y.W.; Kim, J. Analgesic Tolerance Development during Repetitive Electric Stimulations Is Associated with Changes in the Expression of Activated Microglia in Rats with Osteoarthritis. Biomedicines 2020, 8, 575. https://doi.org/10.3390/biomedicines8120575
Hahm S-C, Lee JS, Yoon YW, Kim J. Analgesic Tolerance Development during Repetitive Electric Stimulations Is Associated with Changes in the Expression of Activated Microglia in Rats with Osteoarthritis. Biomedicines. 2020; 8(12):575. https://doi.org/10.3390/biomedicines8120575
Chicago/Turabian StyleHahm, Suk-Chan, Jin Seung Lee, Young Wook Yoon, and Junesun Kim. 2020. "Analgesic Tolerance Development during Repetitive Electric Stimulations Is Associated with Changes in the Expression of Activated Microglia in Rats with Osteoarthritis" Biomedicines 8, no. 12: 575. https://doi.org/10.3390/biomedicines8120575
APA StyleHahm, S.-C., Lee, J. S., Yoon, Y. W., & Kim, J. (2020). Analgesic Tolerance Development during Repetitive Electric Stimulations Is Associated with Changes in the Expression of Activated Microglia in Rats with Osteoarthritis. Biomedicines, 8(12), 575. https://doi.org/10.3390/biomedicines8120575





