TNF-α Priming Elicits Robust Immunomodulatory Potential of Human Tonsil-Derived Mesenchymal Stem Cells to Alleviate Murine Colitis
Abstract
1. Introduction
2. Materials and Methods
2.1. TMSCs Culture and Cytokine Pre-Treatment
2.2. Cell Proliferation
2.3. Osteogenic and Adipogenic Differentiation
2.4. Cell Surface Marker Expression
2.5. Quantitative Real-Time PCR (qRT-PCR)
2.6. Mixed Lymphocyte Reaction
2.7. In Vitro Immune Cell Differentiation
2.7.1. T Cell Differentiation
2.7.2. THP-l-Derived Macrophage-Like Cell Differentiation
2.8. Experimental Murine Colitis Induction and TMSCs Administration
2.9. Statistical Analysis
3. Results
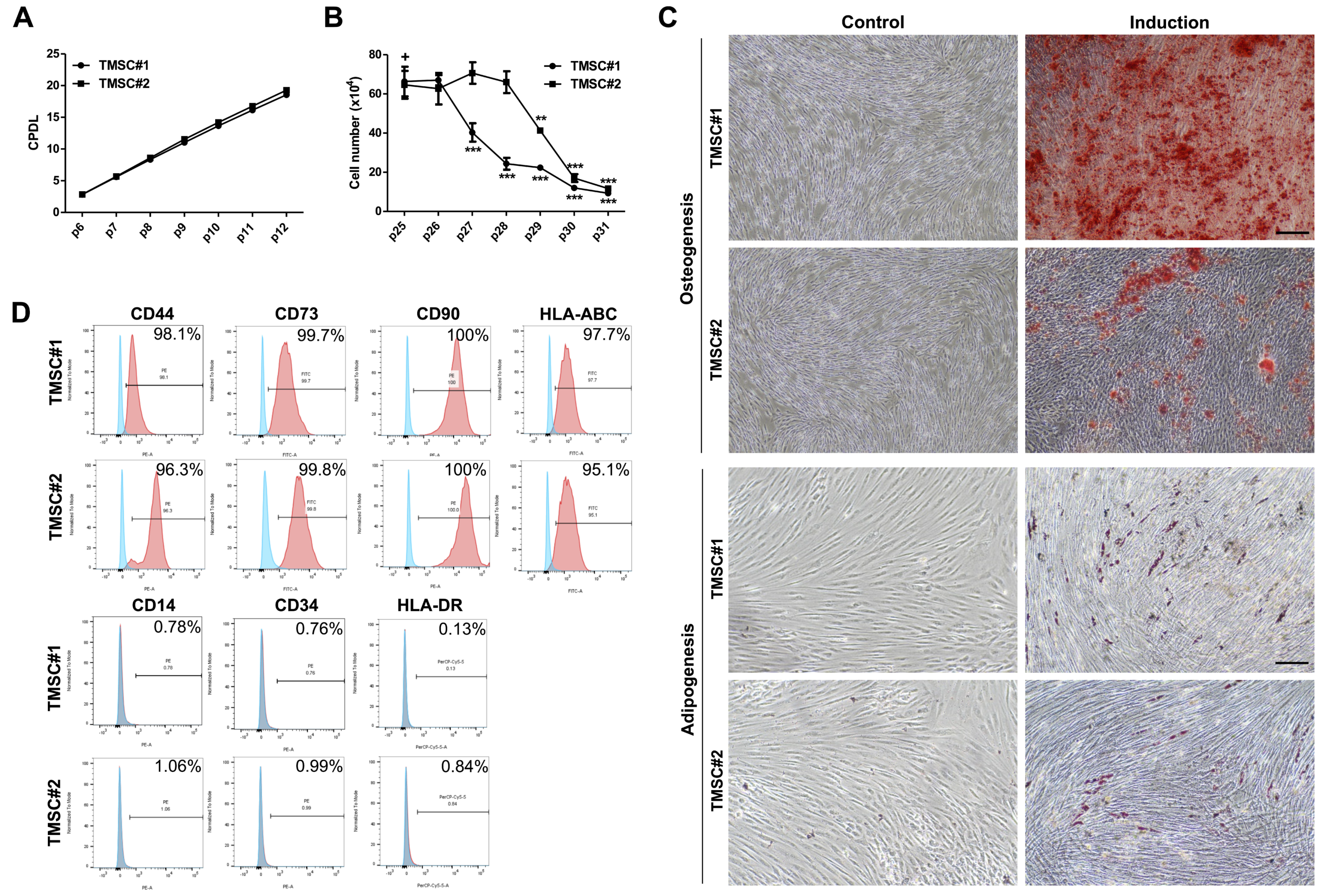
3.1. TMSCs Possess Genuine MSCs Features and Retain Them until Relatively Late Passages
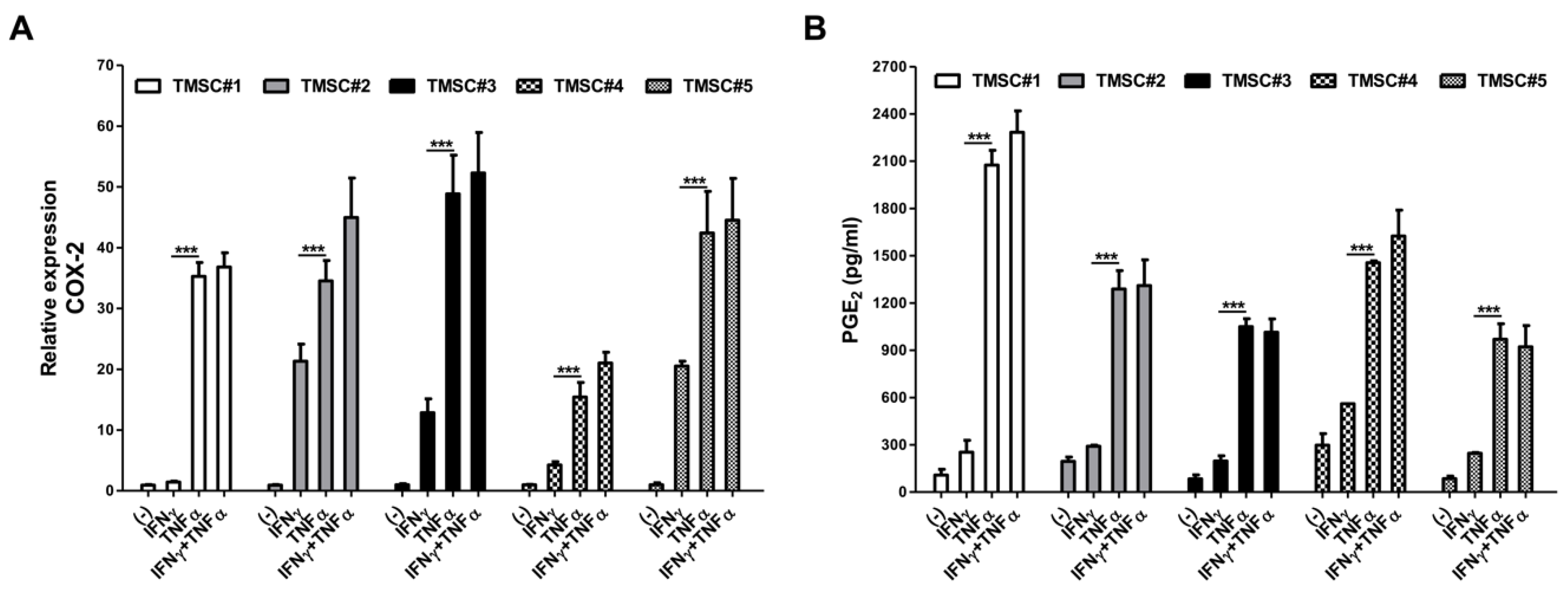
3.2. TNF-α Pretreatment Remarkably Augments Cyclooxygenase-2 (COX-2)/PGE2 Pathway in TMSCs
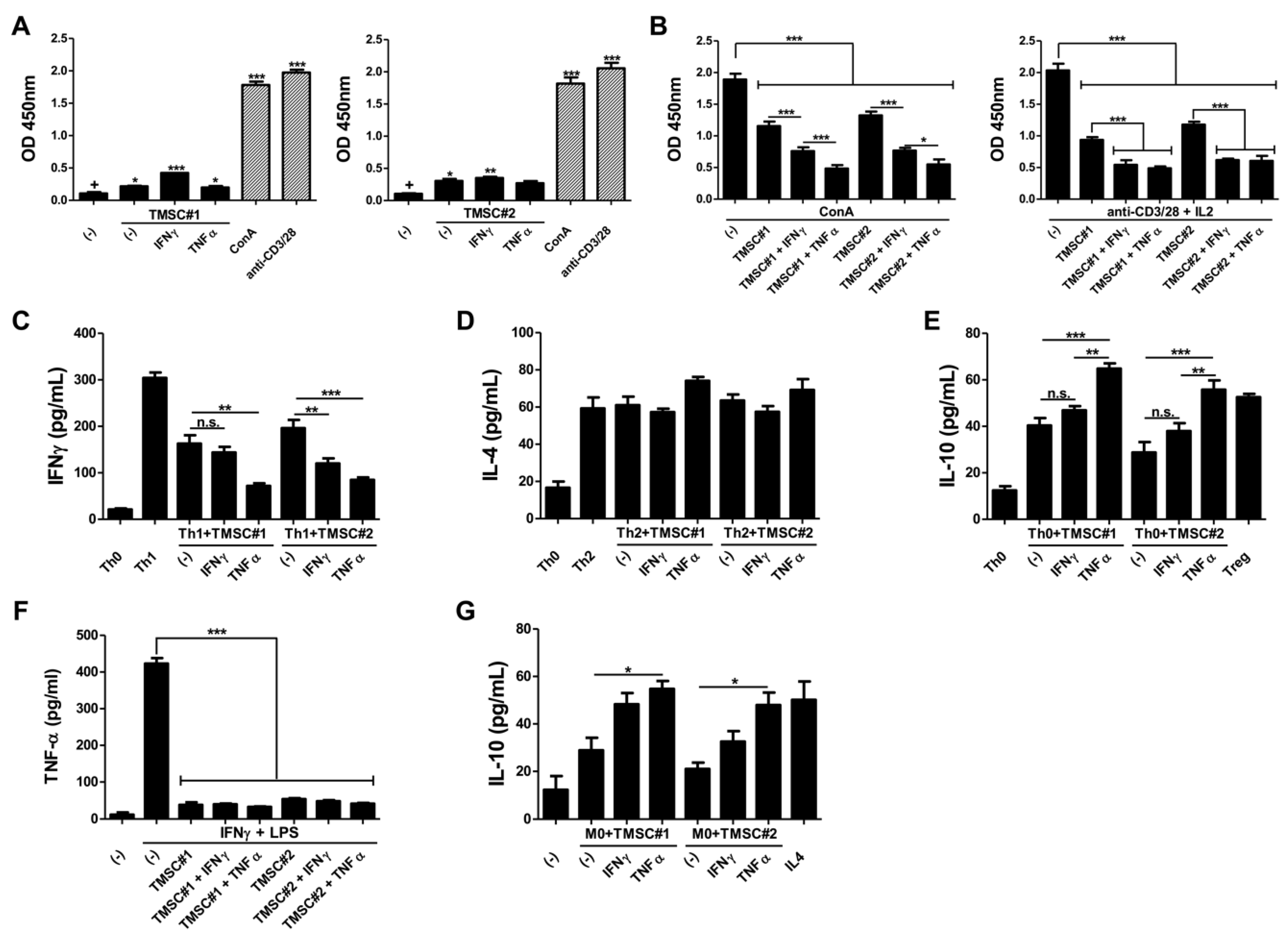
3.3. TMSCs Exhibit the Limited Immunogenicity but Exert the Significant Suppressive Effects on Immune Cell Proliferation after TNF-α Pre-Treatment
3.4. TMSCs Activated by TNF-α Repress the Th1 Cell Differentiation and Induce Anti-Inflammatory M2 Type Macrophages
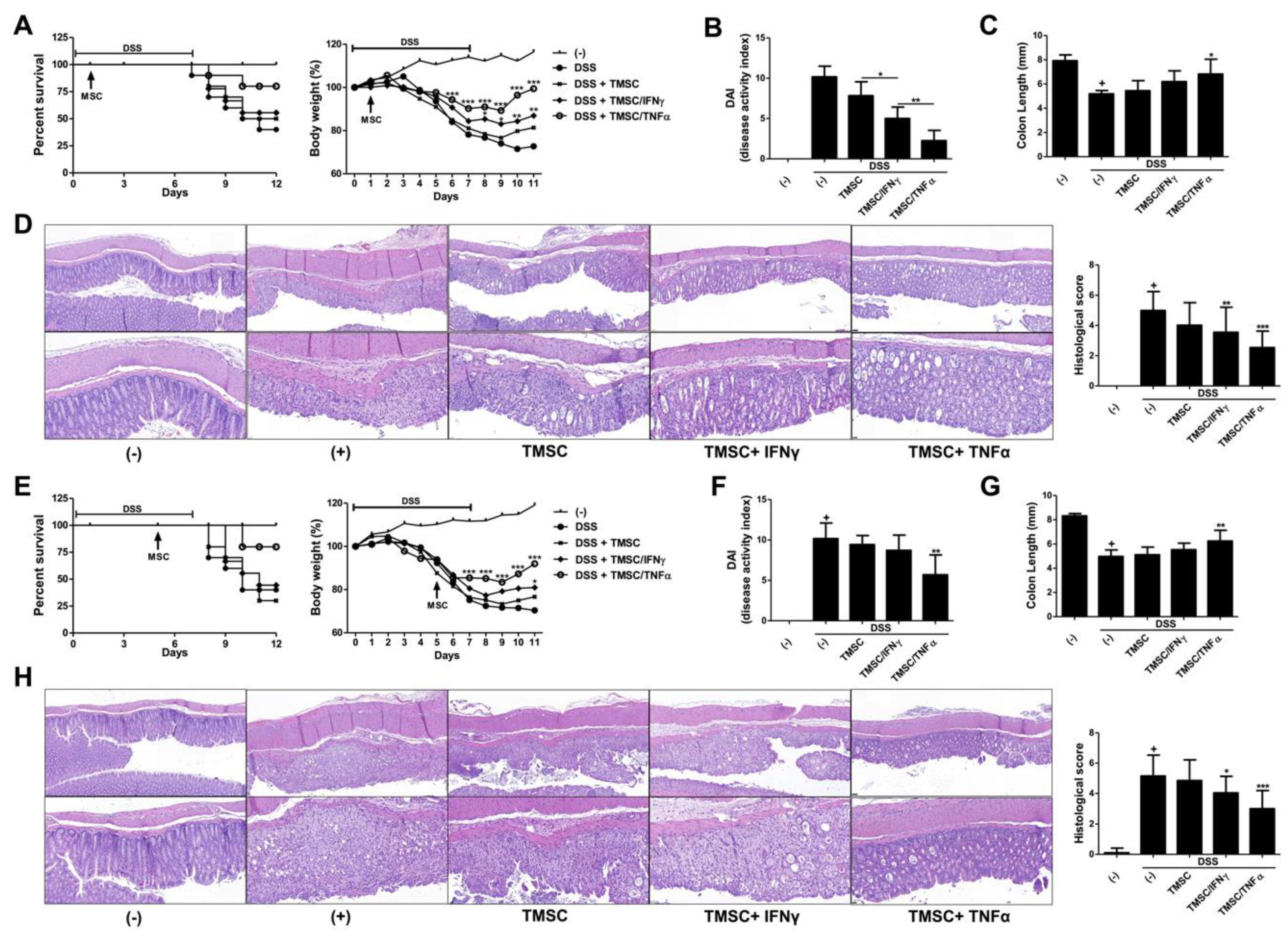
3.5. TNF-α-Primed TMSCs Exert both Prophylactic and Therapeutic Potency against DSS-Induced Murine Colitis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Saeedi, P.; Halabian, R.; Imani Fooladi, A.A. A revealing review of mesenchymal stem cells therapy, clinical perspectives and Modification strategies. Stem Cell Investig. 2019, 634. [Google Scholar] [CrossRef]
- Kolf, C.M.; Cho, E.; Tuan, R.S. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: Regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007, 9, 204. [Google Scholar] [CrossRef][Green Version]
- Shin, S.C.; Seo, Y.; Park, H.Y.; Jung, D.W.; Shin, T.H.; Son, H.; Kim, Y.K.; Lee, J.C.; Sung, E.S.; Jang, J.Y.; et al. Regenerative potential of tonsil mesenchymal stem cells on surgical cutaneous defect. Cell Death Dis. 2018, 9, 183. [Google Scholar] [CrossRef]
- Quarto, R.; Mastrogiacomo, M.; Cancedda, R.; Kutepov, S.M.; Mukhachev, V.; Lavroukov, A.; Kon, E.; Marcacci, M. Repair of large bone defects with the use of autologous bone marrow stromal cells. N. Engl. J. Med. 2001, 344, 385–386. [Google Scholar] [CrossRef]
- Lee, K.B.; Hui, J.H.; Song, I.C.; Ardany, L.; Lee, E.H. Injectable mesenchymal stem cell therapy for large cartilage defects--A porcine model. Stem Cells 2007, 25, 2964–2971. [Google Scholar] [CrossRef]
- Lee, M.; Jeong, S.Y.; Ha, J.; Kim, M.; Jin, H.J.; Kwon, S.J.; Chang, J.W.; Choi, S.J.; Oh, W.; Yang, Y.S.; et al. Low immunogenicity of allogeneic human umbilical cord blood-derived mesenchymal stem cells in vitro and in vivo. Biochem Biophys Res. Commun. 2014, 446, 983–989. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Y.; Yang, D.; Luo, F.; Liang, W.; Guo, S.; Xu, J. The immunomodulatory activity of human umbilical cord blood-derived mesenchymal stem cells in vitro. Immunology 2009, 126, 220–232. [Google Scholar] [CrossRef]
- Meisel, R.; Zibert, A.; Laryea, M.; Gobel, U.; Daubener, W.; Dilloo, D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood 2004, 103, 4619–4621. [Google Scholar] [CrossRef]
- Nasef, A.; Chapel, A.; Mazurier, C.; Bouchet, S.; Lopez, M.; Mathieu, N.; Sensebe, L.; Zhang, Y.; Gorin, N.C.; Thierry, D.; et al. Identification of IL-10 and TGF-beta transcripts involved in the inhibition of T-lymphocyte proliferation during cell contact with human mesenchymal Stem Cells. Gene Expr. 2007, 13, 217–226. [Google Scholar] [CrossRef]
- Ren, G.; Zhang, L.; Zhao, X.; Xu, G.; Zhang, Y.; Roberts, A.I.; Zhao, R.C.; Shi, Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2008, 2, 141–150. [Google Scholar] [CrossRef]
- Nemeth, K.; Leelahavanichkul, A.; Yuen, P.S.; Mayer, B.; Parmelee, A.; Doi, K.; Robey, P.G.; Leelahavanichkul, K.; Koller, B.H.; Brown, J.M.; et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2009, 15, 42–49. [Google Scholar] [CrossRef]
- Shin, T.H.; Kim, H.S.; Kang, T.W.; Lee, B.C.; Lee, H.Y.; Kim, Y.J.; Shin, J.H.; Seo, Y.; Won Choi, S.; Lee, S.; et al. Human umbilical cord blood-stem cells direct macrophage polarization and block inflammasome activation to alleviate rheumatoid arthritis. Cell Death Dis. 2016, 7, e2524. [Google Scholar] [CrossRef]
- Lee, R.H.; Seo, M.J.; Reger, R.L.; Spees, J.L.; Pulin, A.A.; Olson, S.D.; Prockop, D.J. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc. Natl. Acad. Sci. USA 2006, 103, 17438–17443. [Google Scholar] [CrossRef]
- Kim, H.S.; Shin, T.H.; Lee, B.C.; Yu, K.R.; Seo, Y.; Lee, S.; Seo, M.S.; Hong, I.S.; Choi, S.W.; Seo, K.W.; et al. Human umbilical cord blood mesenchymal stem cells reduce colitis in mice by activating NOD2 signaling to COX2. Gastroenterology 2013, 145, 1392–1403. [Google Scholar] [CrossRef]
- Goodwin, M.; Sueblinvong, V.; Eisenhauer, P.; Ziats, N.P.; LeClair, L.; Poynter, M.E.; Steele, C.; Rincon, M.; Weiss, D.J. Bone marrow-derived mesenchymal stromal cells inhibit Th2-mediated allergic airways inflammation in mice. Stem Cells 2011, 29, 1137–1148. [Google Scholar] [CrossRef]
- Nemeth, K.; Keane-Myers, A.; Brown, J.M.; Metcalfe, D.D.; Gorham, J.D.; Bundoc, V.G.; Hodges, M.G.; Jelinek, I.; Madala, S.; Karpati, S.; et al. Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc. Natl. Acad. Sci. USA 2010, 107, 5652–5657. [Google Scholar] [CrossRef]
- Kim, H.S.; Yun, J.W.; Shin, T.H.; Lee, S.H.; Lee, B.C.; Yu, K.R.; Seo, Y.; Lee, S.; Kang, T.W.; Choi, S.W.; et al. Human umbilical cord blood mesenchymal stem cell-derived PGE2 and TGF-beta1 alleviate atopic dermatitis by reducing mast cell degranulation. Stem Cells 2015, 33, 1254–1266. [Google Scholar] [CrossRef]
- Meng, F.; Xu, R.; Wang, S.; Xu, Z.; Zhang, C.; Li, Y.; Yang, T.; Shi, L.; Fu, J.; Jiang, T.; et al. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: A phase 1 clinical trial. Signal Transduct Target Ther. 2020, 5, 172. [Google Scholar] [CrossRef]
- Hass, R.; Kasper, C.; Bohm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 2011, 912. [Google Scholar] [CrossRef]
- Costa, L.A.; Eiro, N.; Fraile, M.; Gonzalez, L.O.; Saa, J.; Garcia-Portabella, P.; Vega, B.; Schneider, J.; Vizoso, F.J. Functional heterogeneity of mesenchymal stem cells from natural niches to culture conditions: Implications for further clinical uses. Cell Mol. Life Sci. 2020. [Google Scholar] [CrossRef]
- Seo, Y.; Shin, T.H.; Kim, H.S. Current Strategies to Enhance Adipose Stem Cell Function: An Update. Int. J. Mol. Sci. 2019, 20, 3827. [Google Scholar] [CrossRef]
- Janjanin, S.; Djouad, F.; Shanti, R.M.; Baksh, D.; Gollapudi, K.; Prgomet, D.; Rackwitz, L.; Joshi, A.S.; Tuan, R.S. Human palatine tonsil: A new potential tissue source of multipotent mesenchymal progenitor cells. Arthritis Res Ther. 2008, 10, R83. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.H.; Cho, K.A.; Park, H.S.; Kim, J.Y.; Woo, S.Y.; Jo, I.; Choi, Y.H.; Park, Y.M.; Jung, S.C.; Chung, S.M.; et al. Tonsil-derived mesenchymal stromal cells: Evaluation of biologic, immunologic and genetic factors for successful banking. Cytotherapy 2012, 14, 1193–1202. [Google Scholar] [CrossRef]
- Lee, B.J.; Kang, D.W.; Park, H.Y.; Song, J.S.; Kim, J.M.; Jang, J.Y.; Lee, J.C.; Wang, S.G.; Jung, J.S.; Shin, S.C. Isolation and Localization of Mesenchymal Stem Cells in Human Palatine Tonsil by W5C5 (SUSD2). Cell Physiol. Biochem. 2016, 38, 83–93. [Google Scholar] [CrossRef]
- Park, G.C.; Song, J.S.; Park, H.Y.; Shin, S.C.; Jang, J.Y.; Lee, J.C.; Wang, S.G.; Lee, B.J.; Jung, J.S. Role of Fibroblast Growth Factor-5 on the Proliferation of Human Tonsil-Derived Mesenchymal Stem Cells. Stem Cells Dev. 2016, 25, 1149–1160. [Google Scholar] [CrossRef]
- Seo, Y.; Shin, T.H.; Ahn, J.S.; Oh, S.J.; Shin, Y.Y.; Yang, J.W.; Park, H.Y.; Shin, S.C.; Kwon, H.K.; Kim, J.M.; et al. Human Tonsil-Derived Mesenchymal Stromal Cells Maintain Proliferating and ROS-Regulatory Properties via Stanniocalcin-1. Cells 2020, 9, 636. [Google Scholar] [CrossRef]
- Samivel, R.; Kim, E.H.; Chung, Y.J.; Mo, J.H. Immunomodulatory effect of tonsil-derived mesenchymal stem cells in a mouse model of allergic rhinitis. Am. J. Rhinol. Allergy. 2015, 29, 262–267. [Google Scholar] [CrossRef]
- Park, M.; Kim, Y.H.; Woo, S.Y.; Lee, H.J.; Yu, Y.; Kim, H.S.; Park, Y.S.; Jo, I.; Park, J.W.; Jung, S.C.; et al. Tonsil-derived mesenchymal stem cells ameliorate CCl4-induced liver fibrosis in mice via autophagy activation. Sci. Rep. 2015, 58616. [Google Scholar] [CrossRef]
- Jung, N.; Park, S.; Choi, Y.; Park, J.W.; Hong, Y.B.; Park, H.H.; Yu, Y.; Kwak, G.; Kim, H.S.; Ryu, K.H.; et al. Tonsil-Derived Mesenchymal Stem Cells Differentiate into a Schwann Cell Phenotype and Promote Peripheral Nerve Regeneration. Int. J. Mol. Sci. 2016, 17, 1867. [Google Scholar] [CrossRef]
- Nakanishi, M.; Rosenberg, D.W. Multifaceted roles of PGE2 in inflammation and cancer. Semin Immunopathol. 2013, 35, 123–137. [Google Scholar] [CrossRef]
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef]
- Crisostomo, P.R.; Wang, Y.; Markel, T.A.; Wang, M.; Lahm, T.; Meldrum, D.R. Human mesenchymal stem cells stimulated by TNF-alpha, LPS, or hypoxia produce growth factors by an NF kappa B- but not JNK-dependent mechanism. Am. J. Physiol. Cell Physiol. 2008, 294, C675–C682. [Google Scholar] [CrossRef]
- Ryan, J.M.; Barry, F.; Murphy, J.M.; Mahon, B.P. Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal Stem Cells. Clin. Exp. Immunol. 2007, 149, 353–363. [Google Scholar] [CrossRef]
- Krampera, M.; Cosmi, L.; Angeli, R.; Pasini, A.; Liotta, F.; Andreini, A.; Santarlasci, V.; Mazzinghi, B.; Pizzolo, G.; Vinante, F.; et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal Stem Cells. Stem Cells 2006, 24, 386–398. [Google Scholar] [CrossRef]
- Zheng, G.; Qiu, G.; Ge, M.; He, J.; Huang, L.; Chen, P.; Wang, W.; Xu, Q.; Hu, Y.; Shu, Q.; et al. Human adipose-derived mesenchymal stem cells alleviate obliterative bronchiolitis in a murine model via IDO. Respir Res. 2017, 18, 119. [Google Scholar] [CrossRef]
- Prasanna, S.J.; Gopalakrishnan, D.; Shankar, S.R. Vasandan, A.B. Pro-inflammatory cytokines, IFNgamma and TNFalpha, influence immune properties of human bone marrow and Wharton jelly mesenchymal stem cells differentially. PLoS ONE 2010, 5, e9016. [Google Scholar] [CrossRef]
- Cuerquis, J.; Romieu-Mourez, R.; Francois, M.; Routy, J.P.; Young, Y.K.; Zhao, J.; Eliopoulos, N. Human mesenchymal stromal cells transiently increase cytokine production by activated T cells before suppressing T-cell proliferation: Effect of interferon-gamma and tumor necrosis factor-alpha stimulation. Cytotherapy 2014, 16, 191–202. [Google Scholar] [CrossRef]
- Galipeau, J. Reply: “Function of Cryopreserved Mesenchymal Stromal Cells With and Without Interferon-gamma Prelicensing Is Context Dependent”. Stem Cells 2017, 35, 1440–1441. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, M.; Kim, Y.H.; Ryu, K.H.; Lee, K.H.; Cho, K.A.; Woo, S.Y. Tonsil-derived mesenchymal stem cells (T-MSCs) prevent Th17-mediated autoimmune response via regulation of the programmed death-1/programmed death ligand-1 (PD-1/PD-L1) pathway. J. Tissue Eng. Regen Med. 2018, 12, e1022–e1033. [Google Scholar] [CrossRef]
- Ryu, J.H.; Park, M.; Kim, B.K.; Kim, Y.H.; Woo, S.Y.; Ryu, K.H. Human tonsilderived mesenchymal stromal cells enhanced myelopoiesis in a mouse model of allogeneic bone marrow transplantation. Mol. Med. Rep. 2016, 14, 3045–3051. [Google Scholar] [CrossRef][Green Version]
- Yu, Y.; Song, E.M.; Lee, K.E.; Joo, Y.H.; Kim, S.E.; Moon, C.M.; Kim, H.Y.; Jung, S.A.; Jo, I. Therapeutic potential of tonsil-derived mesenchymal stem cells in dextran sulfate sodium-induced experimental murine colitis. PLoS ONE 2017, 12, e0183141. [Google Scholar] [CrossRef]
- Eichele, D.D.; Kharbanda, K.K. Dextran sodium sulfate colitis murine model: An indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J. Gastroenterol. 2017, 23, 6016–6029. [Google Scholar] [CrossRef]
- Qiu, Y.; Guo, J.; Mao, R.; Chao, K.; Chen, B.L.; He, Y.; Zeng, Z.R.; Zhang, S.H.; Chen, M.H. TLR3 preconditioning enhances the therapeutic efficacy of umbilical cord mesenchymal stem cells in TNBS-induced colitis via the TLR3-Jagged-1-Notch-1 pathway. Mucosal Immunol. 2017, 10, 727–742. [Google Scholar] [CrossRef]
- Mancheno-Corvo, P.; Menta, R.; del Rio, B.; Franquesa, M.; Ramirez, C.; Hoogduijn, M.J.; DelaRosa, O.; Dalemans, W.; Lombardo, E. T Lymphocyte Prestimulation Impairs in a Time-Dependent Manner the Capacity of Adipose Mesenchymal Stem Cells to Inhibit Proliferation: Role of Interferon gamma, Poly I:C, and Tryptophan Metabolism in Restoring Adipose Mesenchymal Stem Cell Inhibitory Effect. Stem Cells Dev. 2015, 24, 2158–2170. [Google Scholar] [CrossRef] [PubMed]
- Duijvestein, M.; Wildenberg, M.E.; Welling, M.M.; Hennink, S.; Molendijk, I.; van Zuylen, V.L.; Bosse, T.; Vos, A.C.; de Jonge-Muller, E.S.; Roelofs, H.; et al. Pretreatment with interferon-gamma enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cells 2011, 29, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Su, J.; Hu, Y.; Huang, Q.; Shi, H.; Wang, L.; Ren, J. Interleukin-25 primed mesenchymal stem cells achieve better therapeutic effects on dextran sulfate sodium-induced colitis via inhibiting Th17 immune response and inducing T regulatory cell phenotype. Am. J. Transl. Res. 2017, 9, 4149–4160. [Google Scholar]
- Taddio, A.; Tommasini, A.; Valencic, E.; Biagi, E.; Decorti, G.; De Iudicibus, S.; Cuzzoni, E.; Gaipa, G.; Badolato, R.; Prandini, A.; et al. Failure of interferon-gamma pre-treated mesenchymal stem cell treatment in a patient with Crohn’s disease. World J. Gastroenterol. 2015, 21, 4379–4384. [Google Scholar] [CrossRef]
- Song, W.J.; Li, Q.; Ryu, M.O.; Nam, A.; An, J.H.; Jung, Y.C.; Ahn, J.O.; Youn, H.Y. Canine adipose tissue-derived mesenchymal stem cells pre-treated with TNF-alpha enhance immunomodulatory effects in inflammatory bowel disease in mice. Res. Vet. Sci. 2019, 125176–125184. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, T.-H.; Ahn, J.-S.; Oh, S.-J.; Shin, Y.Y.; Yang, J.W.; Kang, M.-J.; Kim, J.M.; Lee, B.-J.; Seo, Y.; Kim, H.-S. TNF-α Priming Elicits Robust Immunomodulatory Potential of Human Tonsil-Derived Mesenchymal Stem Cells to Alleviate Murine Colitis. Biomedicines 2020, 8, 561. https://doi.org/10.3390/biomedicines8120561
Shin T-H, Ahn J-S, Oh S-J, Shin YY, Yang JW, Kang M-J, Kim JM, Lee B-J, Seo Y, Kim H-S. TNF-α Priming Elicits Robust Immunomodulatory Potential of Human Tonsil-Derived Mesenchymal Stem Cells to Alleviate Murine Colitis. Biomedicines. 2020; 8(12):561. https://doi.org/10.3390/biomedicines8120561
Chicago/Turabian StyleShin, Tae-Hoon, Ji-Su Ahn, Su-Jeong Oh, Ye Young Shin, Ji Won Yang, Min-Jung Kang, Ji Min Kim, Byung-Joo Lee, Yoojin Seo, and Hyung-Sik Kim. 2020. "TNF-α Priming Elicits Robust Immunomodulatory Potential of Human Tonsil-Derived Mesenchymal Stem Cells to Alleviate Murine Colitis" Biomedicines 8, no. 12: 561. https://doi.org/10.3390/biomedicines8120561
APA StyleShin, T.-H., Ahn, J.-S., Oh, S.-J., Shin, Y. Y., Yang, J. W., Kang, M.-J., Kim, J. M., Lee, B.-J., Seo, Y., & Kim, H.-S. (2020). TNF-α Priming Elicits Robust Immunomodulatory Potential of Human Tonsil-Derived Mesenchymal Stem Cells to Alleviate Murine Colitis. Biomedicines, 8(12), 561. https://doi.org/10.3390/biomedicines8120561





