Abstract
We aimed to investigate the role of positron emission computed tomography (PET/CT) with 18F-choline for predicting the outcome of metastatic castration-resistant prostate cancer (mCRPC) submitted to treatment with Radium-223 (223Ra-therapy). Clinical records of 20 mCRPC patients submitted to PET/CT with 18F-choline before 223Ra-therapy were retrospectively evaluated. The following PET-derived parameters were calculated: number of lesions, maximum and mean standardized uptake values (SUVmax, SUVmean), lean body mass corrected SUV peak (SULpeak), metabolic tumor volume (MATV), and total lesion activity (TLA). After 223Ra-therapy, all patients underwent regular follow-up until death. The predictive power of clinical and PET-derived parameters on overall survival (OS) was assessed by Kaplan–Meier analysis and the Cox proportional hazard method. All the patients showed 18F-choline-avid lesions at baseline PET/CT. Among the enrolled subjects, eleven (55%) completed all the six scheduled cycles of 223Ra-therapy; seven (35%) were responders according to imaging and biochemical parameters. Mean OS was 12.7 ± 1.4 months: by Kaplan–Meier analysis, number of lesions, PSA level and TLA were significantly correlated with OS. In multivariate Cox analysis, TLA remained the only significant predictor of survival (p = 0.003; hazard ratio = 7.6, 95% confidence interval = 1.9–29.5 months). 18F-choline PET may be useful for patients’ stratification before 223Ra-therapy. In particular, high metabolically active tumor burden (i.e., TLA) was predictive of poor outcome.
1. Introduction
Prostate cancer is a leading cause of death in developed countries. In particular, the condition of hormone-refractory metastatic disease, termed as “castration-resistant prostate cancer” (CRPC), is characterized by poor prognosis, with a reported median overall survival after CRPC onset ranging 9–30 months among patients without metastases and 9–13 months among patients with metastases (mCRPC) [1]. In 2004, the US Food and Drug Administration (FDA) approved docetaxel as a front line treatment for mCRPC [2]. Since 2010, carbazitaxel has been further introduced to manage mCRPC patients progressing during docetaxel [3]. Taxane-based chemotherapy has represented the treatment of choice in mCRPC for many years and proved useful for improving survival and achieving pain control [4].
The therapeutic landscape of mCRPC has been enormously changed by the recent introduction of novel therapeutic tools, such as new-generation antiandrogen drugs, (i.e., abiraterone acetate and enzalutamide) [5]. Recently, other treatments have been implemented such as poly(ADP-ribose) polymerase inhibitors (e.g., olaparib), which were demonstrated to provide survival benefit in mCRPC patients bearing defects in DNA repair genes, and immunotherapy with sipuleucel-T, consisting of autologous peripheral blood mononuclear cells, pulsed ex vivo and incubated with a fusion protein (prostatic acid phosphatase) [6,7]. Furthermore, prostate-specific membrane antigen (PSMA) has emerged as an effective molecular target for prostate cancer imaging and therapy, according to the so-called “theranostic” approach. In this regard, the radioligand PSMA-617 has been conjugated with the beta-emitting radioisotope lutetium-177 (177Lu) for the radionuclide therapy of mCRPC with favorable response rates in the first clinical trials [8].
Targeted alpha therapy (TAT) with the bone-seeking radiopharmaceutical 223radium-dichloride (223Ra-therapy) has been approved by the FDA and European Medical Agency (EMA) after the phase III clinical trial ALYSMPCA that demonstrated survival benefit and delayed the first symptomatic skeletal event in mCRPC patients with bone metastases submitted to 223Ra-therapy as compared to those undergoing standard of care treatment [9]. It has been reported that the outcome of 223Ra-therapy in clinical practice is strongly influenced by pre-treatment patients’ selection and prognostic stratification [10].
Several clinical parameters have been investigated as predictive biomarkers of outcome after 223Ra-therapy such as prostate specific antigen (PSA), hemoglobin level, Eastern Cooperative Oncology Group performance status (ECOG), neutrophil to lymphocyte ratio (NLR), alkaline phosphatase (ALP) and lactate dehydrogenase (LDH) concentration [11,12].
Positron emission computed tomography (PET/CT) with 18F-choline is a well-established imaging approach for the detection of prostate cancer recurrence after surgery/radiotherapy and has also been applied for the prognostication of mCRPC patients submitted to treatment with the novel antiandrogen drugs (i.e., enzalutamide and abiraterone acetate) [13]. Of note, PET-derived functional parameters, such as maximum and mean standardized uptake value (SUVmax and SUVmean) and lean body mass corrected SUV peak (SULpeak), have been investigated as potential biomarkers for patients’ prognostication before therapy. Furthermore, volumetric SUV-based parameters, namely Metabolically Active Tumor Volume (MATV) and Total Lesion Activity (TLA), both reflecting the burden of metabolically active disease, were found to have significant prognostic impact [13,14,15,16]. Nevertheless, the role of 18F-choline PET-derived biomarkers for patients’ selection before 223Ra-therapy has not been thoroughly investigated yet [14,15].
The aim of this retrospective, single-center, clinical study was to evaluate whether baseline PET/CT with 18F-choline, and in particular PET-derived parameters, may be applied to predict survival of mCRPC patients after 223Ra-therapy. Furthermore, as secondary endpoint of the study, we evaluated the role of 18F-choline PET/CT for the assessment of the response to treatment.
2. Experimental Section
2.1. Study Design
Clinical records of patients affected by mCRPC, treated with 223Ra-therapy between January 2017 and September 2019 in our Nuclear Medicine Department, and submitted to PET/CT with 18F-choline at baseline and within 1 month after the end of treatment, were retrospectively reviewed. According to our standard procedure, all the patients signed a written informed consent form, which encompassed the use of anonymized data for retrospective research purposes before each PET/CT scan and before the start of 223Ra-therapy.
All the subjects had to fulfill the following inclusion criteria: (a) age over 18 years, (b) an initial histopathology diagnosis of prostate cancer, (c) fulfillment of clinical criteria for CRPC according to the international guidelines [17], (d) at least 3 cycles of 223Ra-therapy administered, (e) at least six symptomatic bone metastases and no known visceral metastases, except for malignant lymphadenopathy with less than 3 cm in the short-axis diameter, (f) an Eastern Cooperative Oncology Group (ECOG) performance status (PS) score of 0–1 and adequate hematological, liver and renal function, (g) complete imaging and laboratory follow-up; in particular, clinical and biochemical parameters including PSA, ALP, NLR had to be available for patients’ inclusion.
All the subjects included in the analysis were submitted to therapy with 223Ra-dichloride (Xofigo®, Bayer AG, Germany), encompassing six intravenous injections of the radiopharmaceutical at a standard dose of 55 kBq/kg at four-week intervals until unacceptable toxicity or worsening of the overall performance status. The use of androgen deprivation therapy (ADT) continued during radionuclide treatment. Concomitant treatment with abiraterone and enzalutamide was not allowed. Patients also received the best standard of care, including glucocorticoids and analgesics.
2.2. PET/CT Imaging Procedure
All patients were submitted to PET/CT examination within 3 weeks before the start of 223Ra-therapy. All subjects fasted at least 4 h before tracer administration and underwent PET-CT scan 60 min after the intravenous administration of 3.7 KBq/kg of 18F-choline (18fluoromethylcholine/Cholscan®, Advanced Accelerator Applications, Venafro, Italy).
The PET/CT device consisted of a Discovery ST (GE, Milwaukee, WI, USA) with bismuth germanate crystal units arranged to form 24 rings combined with a 16-slice Light Speed Plus CT scanner. The average full width at half maximum (FWHM) axial resolution of PET (full width at half maximum) is 5.2 mm and system sensitivity 9.3 cps/KBq for 3D acquisition mode. Scanning was performed in 3D modality, with an acquisition time of 3 min per bed/position. Images were reconstructed using an ordered subset expectation maximization iterative algorithm (OSEM-SV, VUEPoint HD, GE, 2 iterations, 15 subsets). The CT was performed immediately before PET in the identical axial field of view using a standardized protocol consisting of automatic tube current modulation with auto mA, tube rotation time of 0.5 s/rotation, slice thickness of 3.75 mm. The CT data were resized from 512 × 512 to a 256 × 256 matrix to match the PET data. The data were transmitted to a nuclear medicine database, fused and displayed using dedicated software (PET VCAR, Advantage Workstation 4.7; GE Healthcare, Milwaukee, WI, USA).
2.3. PET-Derived Parameters Calculation
Images were interpreted by 2 experienced nuclear medicine physicians (L.F. and O.B.). Focal or diffuse 18F-choline uptake above the background, excluding normal physiological uptake was considered as a positive lesion. The following parameters were carried out from baseline PET/CT analysis: number of lesions detected on PET/CT scan, SUVmax, SUVmean, SULpeak, MATV and TLA. MATV was defined on the pre-treatment PET scan using a dedicated software (PET VCAR, Advantage Workstation 4.7; GE Healthcare, Milwaukee, WI, USA). Every lesion was segmented with a threshold of 42% of the maximum SUV value within the lesion’s bounding box. TLA was calculated as the product of MATV × SUVmean. All the lesions were considered and the TLA and MATV calculated were the sum of the TLA and MATV of all the metastatic lesions.
2.4. PET Response Assessment and Clinical Follow-Up
Metabolic response to 223Ra-therapy was assessed by 18F-choline PET/CT scan within 1 month after the end of treatment. Change in TLA (i.e., ΔTLA) between pre-treatment and post-treatment PET scans was calculated according to the following formula: [(pre-treatment TLA-post-treatment TLA)/pre-treatment TLA] × 100. The metabolic response was defined as a 50% or greater reduction in TLA between baseline and post-treatment PET scans. All patients showing a reduction in TLA inferior to the 50% threshold or an increased TLA were considered as non-responders.
All the subjects were subsequently submitted to monthly follow-up through clinical examination and laboratory tests and imaging follow-up by ceCT, bone scintigraphy and/or 18F-choline PET/CT examination every 6 months. All patients were monitored until death. A biochemical (ALP and PSA) response was defined as a reduction of ≥30% from the baseline values to the end of 223Ra-therapy [18].
2.5. Statistical Analysis
The study’s primary endpoint was to assess the role of 18F-choline PET-derived parameters to predict survival after 223Ra-therapy. Overall survival was calculated from the patients’ enrollment for 223Ra-therapy. Statistical analysis was performed using dedicated software (MedCalc 11.3.8.0; MedCalc Software, Mariakerke, Belgium). OS was calculated by the Kaplan–Meier method, measured from the date of the first administration of 223Ra-therapy to the death of the patients. Regression analysis and Pearson’s coefficient were used to calculate the correlation between clinical (i.e., PSA, NLR) and PET-derived parameters, Cox proportional hazard regression analysis was applied to identify prognostic factors on survival. Significance was established at p < 0.05.
3. Results
The interrogation of our database identified 20 mCRPC patients fulfilling inclusion criteria. All the included subjects were submitted to 223Ra-therapy between January 2017 and September 2019. Of the enrolled patients, five had been previously treated with chemoterapy, 12 had been previously submitted to anti-androgen therapy with abiraterone acetate and six had been treated with enzalutamide.
The median interval between PET/CT with 18F-choline and the start of 223Ra-therapy was 17 days.
Clinical-demographic characteristics of patients and the values of clinical and PET-derived quantitative variables are summarized in Table 1.

Table 1.
Patients’ clinical-demographic features and results of quantitative parameters.
Eleven patients (55%) completed all the six scheduled cycles of 223Ra-therapy (mean administered cycles 4.8 ± 1.3), the remaining nine discontinued radionuclide treatment due to evidence of progressive disease (n = 6, i.e., 30%) or hematological toxicity (n = 3, i.e., 15%). All the subjects underwent a follow-up PET/CT with 18F-choline for response assessment within 4 weeks after the end of 223Ra-therapy. Patients were subsequently submitted to monthly hematological/biochemical follow-up and monitoring by imaging every 6 months, or before in case of changes in patients’ symptoms or parameters, with 18F-choline PET/CT (n = 4), contrast enhanced computed tomography (n = 2) or bone scintigraphy (n= 5). In such cases, the choice of the most appropriate diagnostic approach was determined by the referring clinician on the basis of the specific clinical issue. During follow-up, all the patients deceased.
3.1. Baseline Clinical and PET-Derived Parameters
All the patients presented 18F-choline-avid lesions at baseline PET/CT scan. In particular, 18 patients (i.e., 90%) showed exclusive metastatic skeletal localizations, while two (i.e., 10%) had bone lesions and also presented mild tracer uptake in abdominal-pelvic lymph nodes with maximum diameter <3 cm.
As far as it concerns the number of lesions detected on baseline PET/CT scan, 15 subjects had less than 20 localizations, while five presented more than 20 metastases. As regards PET-derived parameters, median SUVmax resulted in 13.8, median SUVmean was 7, median SULpeak was 8.4, median MATV was 48.2 cc, and median TLA resulted in 351.5 g.
From a clinical/biochemical point of view, the median PSA resulted in 50.2 ng/mL and median NLR was 2.9. On regression analysis, a significant positive correlation (i.e., p < 0.05) was found between all the PET-derived parameters and PSA levels, with SUVmax being the variable with the strongest correlation (i.e., r = 0.63, p = 0.003), as shown in Table 2 and Figure 1. No significant correlations were found between PET-derived parameters and NLR.

Table 2.
PET-derived parameters and PSA levels at baseline: regression analysis.
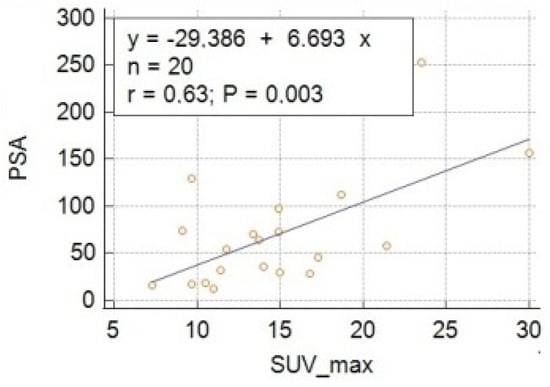
Figure 1.
Regression analysis showing the correlation between baseline levels of PSA and maximum standardized uptake value (SUVmax).
3.2. Predictive Value of Clinical and PET-Derived Parameters on Survival after 223Ra-Therapy
The mean OS of the entire cohort of patients was 12.7 ± 1.4 months (median OS = 9 months, 95% CI 7–27). PSA, the number of lesions and TLA significantly correlated with OS (Figure 2). To perform Kaplan–Meier analysis, PET-derived parameters (i.e., SUVmax, SUVmean, SULpeak, MATV, TLA), PSA and NLR were dichotomized at their median value; the other analyzed parameters were dichotomized as shown in Table 3.
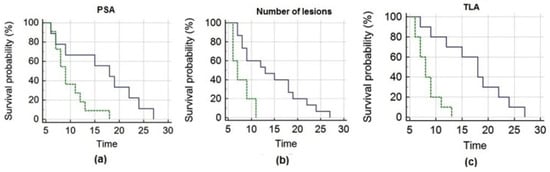
Figure 2.
Kaplan–Meier analysis depicts overall survival after 223Ra-therapy as a function of pre-treatment PSA (a), number of lesions (b) and TLA (c), respectively. Panel (a) shows that patients with PSA ≤ 50.2 ng/mL (blue line) had significantly longer survival than those with PSA > 50.2 ng/mL (green line). Panel (b) depicts that subjects with a number of lesions ≤ 20 (blue line) presented significantly longer survival than those presenting > 20 lesions (green line). Panel (c) demonstrates that subjects with TLA ≤ 351.5 g (blue line) survived significantly longer than those with TLA > 351.5 g (green line).

Table 3.
Results of Kaplan–Meier analysis of clinical and PET-derived parameters.
Parameters presenting p < 0.05 at Kaplan–Meier (i.e., number of lesions, PSA, TLA) were further submitted to multivariate analysis (Cox regression, stepwise): TLA remained the only significant predictor of survival (p = 0.003; hazard ratio = 7.6, 95% confidence interval = 1.9–29.5 months).
3.3. 18F-Choline PET/CT for Response Assessment to 223Ra-Therapy
A metabolic response (i.e., ΔTLA > −50%) was detected at post treatment 18F-choline PET/CT in seven patients (35%), while the remaining 13 (65%) were non-responders. In the sub-group of non-responders, 10 presented progression at skeletal level, while the remaining three had progressive disease both at the skeletal and visceral level (two with lymph node metastases and one with lymph node and hepatic metastases). In two out of the three patients with visceral involvement a correlative imaging with contrast enhanced CT was performed in order to better define the site and the number of pathological lymph nodes.
All the seven responders continued ADT until evidence of progressive disease, as a result of which they were submitted to chemotherapy (n = 6) or therapy with enzalutamide (n = 1), while among non-responders, nine were submitted to chemotherapy and the remaining four underwent palliative and supportive care.
All the patients showing metabolic response also presented biochemical response, while none of the non-metabolic responders presented a significant reduction in PSA/ALP value with respect to the baseline levels. In the responders’ cohort, the mean ΔTLA was −72% ± 14%, the mean ΔPSA was −76% ± 10%; on regression analysis, a moderately strong non-significant correlation (r = 0.53, p = 0.2) was registered between the two aforementioned parameters (i.e., ΔTLA and ΔPSA).
By Kaplan–Meier analysis, responders presented a significantly longer survival than non-responders (i.e., median OS = 19 months, 95% CI 15–27, vs. 8 months, 95% CI 7–13, p < 0.05), as shown by Figure 3. Emblematic examples of 18F-choline PET/CT findings in a patient with more favorable prognostic factors and clinical outcome are depicted in Figure 4, while Figure 5 describes a subject with poor prognostic factors and outcome.
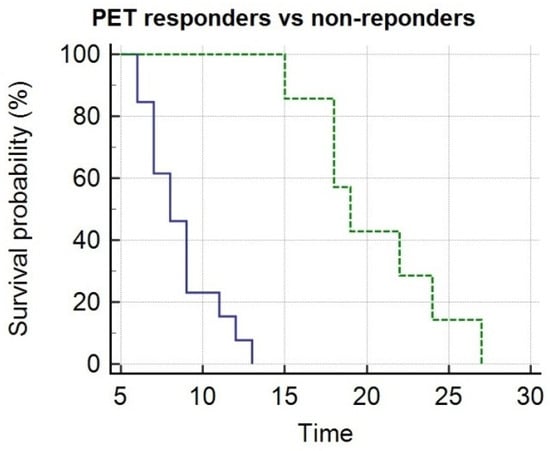
Figure 3.
Kaplan–Meier analysis depicts overall survival after 223Ra-therapy as function of metabolic response assessed at post treatment PET/CT with 18F-choline. PET responders (green line) presented significantly longer survival as compared with non-responders (blue line).
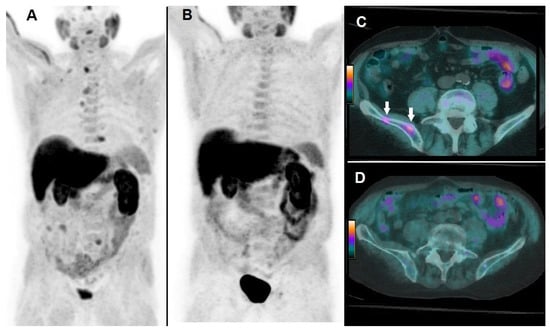
Figure 4.
A 74-year-old patient affected by mCRPC, previously treated with androgen deprivation therapy, subsequently submitted to 223Ra-therapy. At baseline, 18F-choline PET/CT demonstrated multiple sites of tracer uptake in bones, as evident in Whole Body (A) and the following parameters were registered: PSA = 17 ng/mL; number of lesions = 18; TLA = 235.3 g. 18F-choline PET/CT acquired after 6 cycles of 223Ra-therapy demonstrated complete metabolic response as depicted by Whole Body (B). 18F-choline PET/CT fused axial images of the pelvis acquired at baseline showed hypermetabolic lesions in the right iliac bone (C, white arrows), while post-treatment axial slices did not show any pathological sites of tracer uptake (D). Post-treatment PSA was 3 ng/mL, OS resulted in 27 months.
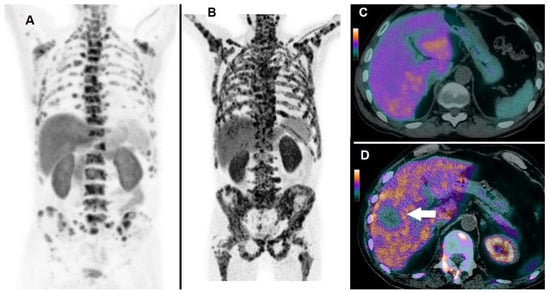
Figure 5.
A 66-year-old patient affected by mCRPC, previously treated with abiraterone acetate and docetaxel, then submitted to 223Ra-therapy. 18F-choline PET/CT acquired at baseline demonstrated multiple tracer uptake sites in bones, as evident in Whole Body (A); baseline pre-treatment parameters were as follows: PSA = 157 ng/mL; the number of lesions > 20; TLA = 3969.9 g. After 3 cycles, 223Ra-therapy was discontinued due to deterioration of patient’s performance status and increased PSA (i.e., 289 ng/mL). 18F-choline PET/CT acquired after 3 cycles of 223Ra-therapy demonstrated an impressive progression of skeletal disease, as shown by Whole Body (B). 18F-choline PET/CT fused images of the liver acquired at baseline did not show abnormal tracer uptake (C), while post-treatment axial slices demonstrated the appearance of a partially necrotic metastasis in the VIII/VII hepatic segment (D, white arrow). The patient’s OS resulted in 7 months.
3.4. Toxicities
Among the enrolled subjects, three cases (15%) of hematological toxicity were registered, one (5%) of whom was a grade 2 Hb toxicity not needing transfusion, since it spontaneously regressed after 223Ra-therapy discontinuation; the remaining two cases (10%) presented, respectively, grade 3 Hb toxicity and pancytopenia (grade 3 Hb and PLT toxicity and grade 2 lymphocyte toxicity) and were treated with transfusions.
4. Discussion
A growing amount of scientific data suggests that the clinical efficacy of 223Ra-therapy in real-life practice is strictly dependent on pre-treatment patients’ prognostic stratification [12,19]. This issue calls for an unmet need for clinical and imaging biomarkers suitable for identifying patients who can benefit from such a therapeutic regimen.
Although PET/CT scan with 18F-choline is routinely utilized for the detection of prostate cancer recurrence, its use for the prognostication of patients submitted to 223Ra-therapy is yet to be defined. To the best of our knowledge, this is the first study analyzing the prognostic role of 18F-choline PET-derived parameters on patients’ survival after 223Ra-therapy, showing that baseline overall burden of disease, as expressed by TLA, has an independent predictive impact on multivariate Cox analysis.
In a prospective study by Garcia et al. [20], 40 patients affected by mCRPC were submitted to bone scintigraphy and 18F-choline PET/CT prior to 223Ra-therapy. The following parameters were calculated on baseline PET/CT scan: number of lesions, SUVmax and average SUVmax. All the aforementioned quantitative data were significantly associated with survival after radionuclide therapy. Our results confirm disease extent, expressed by the number of lesions detected on PET/CT, as a relevant prognostic factor on Kaplan–Meier analysis; on the contrary, in our cohort SUVmax was not significantly correlated with OS. This discrepancy might be explained by the fact that SUVmax reflects only the lesion portion with the highest metabolic activity and does not provide information on volumetric tumor features. As a matter of fact, Garcia and collaborators in their analysis did not take into account MATV and TLA, which combine volumetric and functional information.
Caroli et al. retrospectively assessed the predictive value of 18F-choline PET-derived parameters in 94 mCRPC patients submitted to therapy with abiraterone acetate or enzalutamide: among the analyzed PET-derived parameters (SUVmax, SUVmean, TLA, MATV), the authors found that the sum of MATV (SMATV) and TLA (STLA) were correlated with survival at univariate analysis, but STLA (median value 495.070 g) remained the only predictor of survival on multivariate analysis [13].
Quaquarini and coworkers investigated the prognostic role of PET/CT with 18F-choline in 29 mCRPC patients submitted to chemotherapy with docetaxel, through the calculation of several PET-derived parameters: both SMATV (i.e., median 27.1 cc) and STLA (i.e., median 253.5 g) correlated with progression-free survival [16]. It has to be pointed out that, although the authors used a different terminology in the previously cited studies [13,16], both SMATV and STLA correspond to the parameters calculated in our study (i.e., MATV and TLA, respectively), reflecting the overall burden of metabolically active disease. Our results support the findings reported in the aforementioned papers, which indicate TLA as a relevant prognostic factor in mCRPC submitted to systemic therapy.
Further consideration should be made regarding the different approaches applied for the calculation of PET-derived volumetric parameters. Racaru et al. assessed the value of 18F-choline PET/CT for predicting toxicity associated with 223Ra-therapy [14]: in a group of 15 patients, MATV and TLA were shown to be capable of predicting Hb and PLT toxicity and were therefore suggested as potential tools for patients’ selection before treatment. In the previously cited paper, MATV was carried out through an open-source software, including voxels with an SUV of at least 3 and corresponding to a CT value more than – 1000 Hounsfield. In contrast to Racaru’s group, we used a segmentation method based on a fixed threshold of 42% of the SUVmax, substantially similar to that applied by Caroli et al. [13]. It is still debated which is the best approach for volumetric segmentation in order to obtain the most robust and reproducible results; further studies are needed to better define this technical issue.
Lastly, although the primary endpoint of our research was focused on survival, in our cohort of mCRPC patients, PET/CT with 18F-choline was shown to be a reliable approach for assessing response to 223Ra-therapy. In particular, as previously demonstrated by a preliminary report from our group [15], a reduction of at least 50% of the overall metabolically active tumor burden (i.e., TLA) after 223Ra-therapy correlated with a significantly longer survival. Of note, we found that all PET-responders presented a significant reduction in PSA/ ALP levels with respect to the baseline values, with a good correlation, although not statistically significant, between ΔTLA and ΔPSA. It has to be underlined that several reports indicate that PSA dosage might not represent a reliable approach for monitoring the response to treatment, since it may present a transitory increase after the first cycle of 223Ra-therapy, in relation to the so-called “PSA flare” due to the release of PSA from tumor cell lysis [21]. The correlation between ΔTLA and ΔPSA found in our cohort might be explained by the inclusion criteria we applied for the enrollment, since only patients who had completed three cycles of 223Ra-therapy were considered, thus minimizing the interference of “PSA flare” occurring in the first month of treatment.
In this regard, the bone-seeking radiopharmaceutical 18F-fluoride has been applied by Kairemo et al. [22] to assess tumor response in 10 subjects treated with 223Ra-therapy. The authors performed 18F-fluoride PET/CT at baseline, after the 1st and the 6th cycle and applied a modified version of PET response evaluation criteria (PERCIST). While PET/CT after the 1st cycle did not provide additional information, PET evaluation after the 6th cycle demonstrated metabolic response in all patients, with a correlation with PSA trend in 9/10 cases.
The main drawback of PET/CT with 18F-fluoride is represented by its capability to monitor only the metabolic response of bone metastases to 223Ra-therapy, while it is unable to detect eventual progression at the visceral level. In our cohort of patients, for example, 18F-choline PET/CT was shown to be useful in identifying the appearance of visceral metastases in 3 out of the 13 non-responder patients (i.e., 23%).
Our study presents some limitations, such as the small cohort of patients and its retrospective nature. Furthermore, clinical parameters other than those included in this retrospective analysis (such as LDH) might impact patients’ prognostic stratification. Further studies with larger cohorts are needed to better define the role of 18F-choline PET/CT for selection before 223Ra-therapy.
5. Conclusions
18F-choline PET/CT may represent a useful tool for patients’ prognostic stratification before 223Ra-therapy and for the assessment of response to treatment. In particular, PET-derived parameter TLA is an independent predictor of survival in multivariate analysis.
Author Contributions
Study conceptualization and design, L.F. and G.P.S.; methodology, L.F.; software, O.B.; validation, L.F., F.E. and O.B.; formal analysis, L.F.; investigation, G.P.S.; resources, G.P.S.; data curation, A.C.; writing—original draft preparation, L.F.; writing—review and editing, A.C., G.P.S, O.S.; visualization, A.C.; supervision, O.S. and F.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| mCRPC | Metastatic castration resistan prostate cancer |
| PET/CT | Positron emission computed tomography |
| TLA | Total lesion glycolysis |
| MATV | Metabolically active tumor volume |
References
- He, L.; Fang, H.; Chen, C.; Wu, Y.; Wang, Y.; Ge, H.; Wang, L.; Wan, Y.; He, H. Metastatic castration-resistant prostate cancer: Academic insights and perspectives through bibliometric analysis. Medicine (Baltimore) 2020, 99, e19760. [Google Scholar] [CrossRef] [PubMed]
- Corn, P.G.; Agarwal, N.; Araujo, J.C.; Sonpavde, G. Taxane-based Combination Therapies for Metastatic Prostate Cancer. Eur. Urol. Focus 2019, 5, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, M.; Dhingra, R.; Sharma, T.; Deep, A.; Narasimhan, B.; Phogat, P.; Sharma, P.C. Cabazitaxel: A novel drug for hormone-refractory prostate cancer. Mini-Rev. Med. Chem. 2013, 13, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Huebner, N.A.; Shariat, S.F.; Resch, I.; Gust, K.; Kramer, G. The role of taxane-based chemotherapy in the treatment of prostate cancer. Curr. Opin. Urol. 2020, 30, 527–533. [Google Scholar] [CrossRef]
- Swami, U.; McFarland, T.R.; Nussenzveig, R.; Agarwal, N. Advanced Prostate Cancer: Treatment Advances and Future Directions. Trends Cancer 2020, 6, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Sigorski, D.; Iżycka-Świeszewska, E.; Bodnar, L. Poly(ADP-Ribose) Polymerase Inhibitors in Prostate Cancer: Molecular Mechanisms, and Preclinical and Clinical Data. Target. Oncol. 2020. [Google Scholar] [CrossRef]
- Garcia, J.A. Sipuleucel-T in patients with metastatic castration-resistant prostate cancer: An insight for oncologists. Ther. Adv. Med. Oncol. 2011, 3, 101–108. [Google Scholar] [CrossRef]
- Rahbar, K.; Ahmadzadehfar, H.; Kratochwil, C.; Haberkorn, U.; Schäfers, M.; Essler, M.; Baum, R.P.; Kulkarni, H.R.; Schmidt, M.; Drzezga, A.; et al. German Multicenter Study Investigating 177Lu-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2017, 58, 85–90. [Google Scholar] [CrossRef]
- Sartor, O.; Coleman, R.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: Results from a phase 3, double-blind, randomised trial. Lancet Oncol. 2014, 15, 738–746. [Google Scholar] [CrossRef]
- Saad, F.; Carles, J.; Gillessen, S.; Heidenreich, A.; Heinrich, D.; Gratt, J.; Lévy, J.; Miller, K.; Nilsson, S.; Petrenciuc, O.; et al. Radium-223 and concomitant therapies in patients with metastatic castration-resistant prostate cancer: An international, early access, open-label, single-arm phase 3b trial. Lancet Oncol. 2016, 17, 1306–1316. [Google Scholar] [CrossRef]
- Frantellizzi, V.; Farcomeni, A.; Follacchio, G.A.; Pacilio, M.; Pellegrini, R.; Pani, R.; De Vincentis, G. A 3-variable prognostic score (3-PS) for overall survival prediction in metastatic castration-resistant prostate cancer treated with 223Radium-dichloride. Ann. Nucl. Med. 2018, 32, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O.; Coleman, R.E.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Vogelzang, N.J.; Bruland, Ø.; Kobina, S.; Wilhelm, S.; et al. An exploratory analysis of alkaline phosphatase, lactate dehydrogenase, and prostate-specific antigen dynamics in the phase 3 ALSYMPCA trial with radium-223. Ann. Oncol. 2017, 28, 1090–1097. [Google Scholar] [CrossRef] [PubMed]
- Caroli, P.; De Giorgi, U.; Scarpi, E.; Fantini, L.; Moretti, A.; Galassi, R.; Celli, M.; Conteduca, V.; Rossi, L.; Bianchi, E.; et al. Prognostic value of 18F-choline PET/CT metabolic parameters in patients with metastatic castration-resistant prostate cancer treated with abiraterone or enzalutamide. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 348–354. [Google Scholar] [CrossRef]
- Vija Racaru, L.; Sinigaglia, M.; Kanoun, S.; Ben Bouallègue, F.; Tal, I.; Brillouet, S.; Bauriaud-Mallet, M.; Zerdoud, S.; Dierickx, L.; Vallot, D.; et al. Fluorine-18-fluorocholine PET/CT parameters predictive for hematological toxicity to radium-223 therapy in castrate-resistant prostate cancer patients with bone metastases: A pilot study. Nucl. Med. Commun. 2018, 39, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Filippi, L.; Basile, P.; Schillaci, O.; Bagni, O. The Relationship Between Total Lesion Activity on 18F Choline Positron Emission Tomography-Computed Tomography and Clinical Outcome in Patients with Castration-Resistant Prostate Cancer Bone Metastases Treated with 223Radium. Cancer Biother. Radiopharm. 2020, 35, 398–403. [Google Scholar] [CrossRef]
- Quaquarini, E.; D’Ambrosio, D.; Sottotetti, F.; Gallivanone, F.; Hodolic, M.; Baiardi, P.; Palumbo, R.; Vellani, C.; Canevari, C.; Bernardo, A.; et al. Prognostic Value of 18 F-Fluorocholine PET Parameters in Metastatic Castrate-Resistant Prostate Cancer Patients Treated with Docetaxel. Contrast Media Mol. Imaging 2019, 2019, 1–7. [Google Scholar] [CrossRef]
- Cornford, P.; Bellmunt, J.; Bolla, M.; Briers, E.; De Santis, M.; Gross, T.; Henry, A.M.; Joniau, S.; Lam, T.B.; Mason, M.D.; et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur. Urol. 2017, 71, 630–642. [Google Scholar] [CrossRef]
- Prelaj, A.; Rebuzzi, S.; Buzzacchino, F.; Pozzi, C.; Ferrara, C.; Frantellizzi, V.; Follacchio, G.; Civitelli, L.; De Vincentis, G.; Tomao, S.; et al. Radium‑223 in patients with metastatic castration‑resistant prostate cancer: Efficacy and safety in clinical practice. Oncol. Lett. 2018. [Google Scholar] [CrossRef]
- Wong, W.W.; Anderson, E.M.; Mohammadi, H.; Daniels, T.B.; Schild, S.E.; Keole, S.R.; Choo, C.R.; Tzou, K.S.; Bryce, A.H.; Ho, T.H.; et al. Factors Associated with Survival Following Radium-223 Treatment for Metastatic Castration-resistant Prostate Cancer. Clin. Genitourin. Cancer 2017, 15, e969–e975. [Google Scholar] [CrossRef]
- García Vicente, A.M.; González García, B.; Amo-Salas, M.; García Carbonero, I.; Cassinello Espinosa, J.; Gómez-Aldaraví Gutierrez, J.L.; Suarez Hinojosa, L.; Soriano Castrejón, Á. Baseline 18F-Fluorocholine PET/CT and bone scan in the outcome prediction of patients treated with radium 223 dichloride. Clin. Transl. Oncol. 2019, 21, 289–297. [Google Scholar] [CrossRef]
- Rizzini, E.L.; Dionisi, V.; Ghedini, P.; Morganti, A.G.; Fanti, S.; Monari, F. Clinical aspects of mCRPC management in patients treated with radium-223. Sci. Rep. 2020, 10, 6681. [Google Scholar] [CrossRef] [PubMed]
- Kairemo, K.; Joensuu, T. Radium-223-Dichloride in Castration Resistant Metastatic Prostate Cancer—Preliminary Results of the Response Evaluation Using F-18-Fluoride PET/CT. Diagnostics 2015, 5, 413–427. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).