Multiple Sclerosis in a Multi-Ethnic Population in Houston, Texas: A Retrospective Analysis
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Design and Setting
2.2. Cohort Identification and Selection
2.3. Outcome Measurements
2.4. Data Collection and Management
2.5. Statistical Analysis
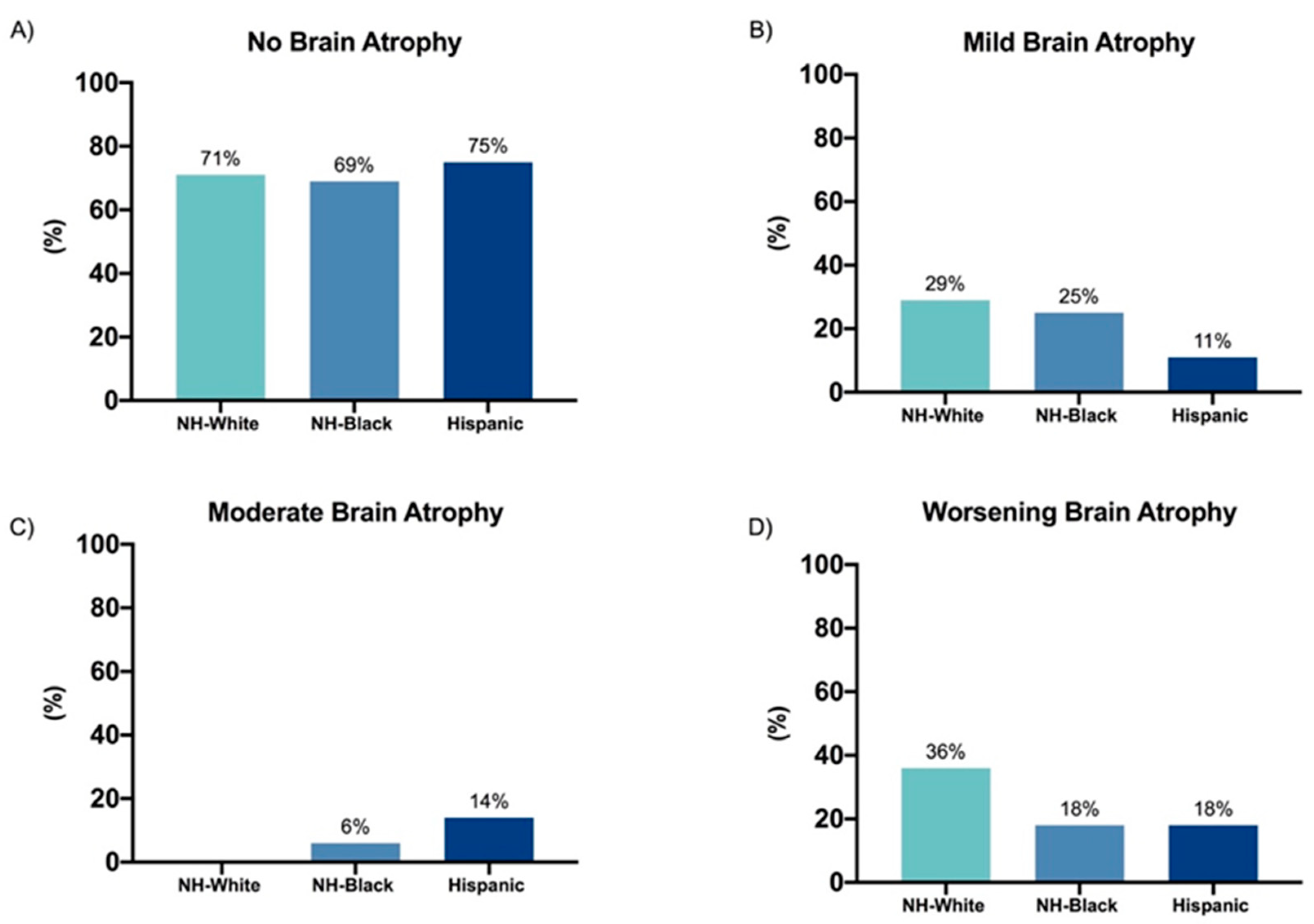
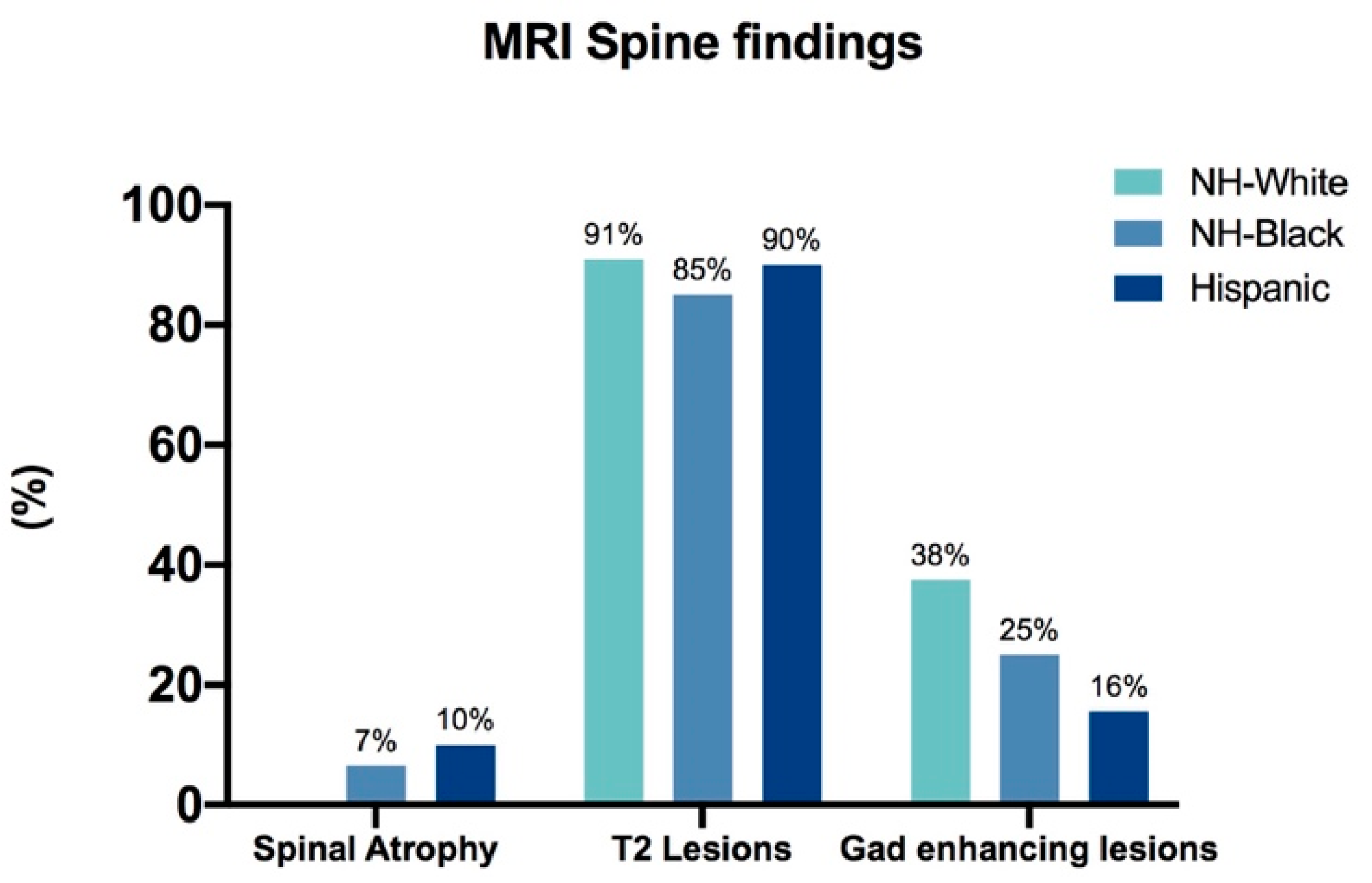
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Wallin, M.T.; Culpepper, W.J.; Campbell, J.D.; Nelson, L.M.; Langer-Gould, A.; Marrie, R.A.; Cutter, G.R.; Kaye, W.E.; Wagner, L.; Tremlett, H.; et al. The prevalence of MS in the United States: A population-based estimate using health claims data. Neurology 2019, 92, 1029–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallin, M.T.; Culpepper, W.J.; Nichols, E.; Bhutta, Z.A.; Gebrehiwot, T.T.; Hay, S.I.; Khalil, I.A.; Krohn, K.J.; Liang, X.; Naghavi, M.; et al. Global, regional, and national burden of multiple sclerosis 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 269–285. [Google Scholar] [CrossRef] [Green Version]
- Freedman, M.S. Present and emerging therapies for multiple sclerosis. Contin. Lifelong Learn. Neurol. 2013, 19, 968–991. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Culla, M.; Irizar, H.; Otaegui, D. The genetics of multiple sclerosis: Review of current and emerging candidates. Appl. Clin. Genet. 2013, 6, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Amezcua, L.; McCauley, J.L. Race and ethnicity on MS presentation and disease course. Mult. Scler. J. 2020, 26, 561–567. [Google Scholar] [CrossRef]
- Wallin, M.T.; Culpepper, W.J.; Coffman, P.; Pulaski, S.; Maloni, H.; Mahan, C.M.; Haselkorn, J.K.; Kurtzke, J.F.; Veterans Affairs Multiple Sclerosis Centres of Excellence Epidemiology Group. The Gulf War era multiple sclerosis cohort: Age and incidence rates by race, sex and service. Brain 2012, 135, 1778–1785. [Google Scholar] [CrossRef] [Green Version]
- Amezcua, L.; Rivas, E.; Joseph, S.; Zhang, J.; Liu, L. Multiple Sclerosis Mortality by Race/Ethnicity, Age, Sex, and Time Period in the United States, 1999–2015. Neuroepidemiology 2018, 50, 35–40. [Google Scholar] [CrossRef]
- Rivas-Rodríguez, E.; Amezcua, L. Ethnic Considerations and Multiple Sclerosis Disease Variability in the United States. Neurol. Clin. 2018, 36, 151–162. [Google Scholar] [CrossRef]
- U.S. Census Bureau QuickFacts: United States. Available online: https://www.census.gov/quickfacts/fact/table/US/PST045219#qf-headnote-a (accessed on 4 September 2020).
- Khan, O.; Williams, M.J.; Amezcua, L.; Javed, A.; Larsen, K.E.; Smrtka, J.M. Multiple Sclerosis in US Minority Populations Clinical Practice Insights. Neurol Clin Pr. 2015, 5, 132–142. [Google Scholar] [CrossRef] [Green Version]
- Diaz, V. Encouraging participation of minorities in research studies. Ann. Fam. Med. 2012, 10, 372–373. [Google Scholar] [CrossRef] [Green Version]
- Rosati, G. The prevalence of multiple sclerosis in the world: An update. Neurol. Sci. 2001, 22, 117–139. [Google Scholar] [CrossRef] [PubMed]
- Langer-Gould, A.; Brara, S.M.; Beaber, B.E.; Zhang, J.L. Incidence of multiple sclerosis in multiple racial and ethnic groups. Neurology 2013, 80, 1734–1739. [Google Scholar] [CrossRef] [PubMed]
- Kister, I.; Chamot, E.; Bacon, J.H.; Niewczyk, P.M.; De Guzman, R.A.; Apatoff, B.; Coyle, P.; Goodman, A.D.; Gottesman, M.; Granger, C.; et al. Rapid disease course in African Americans with multiple sclerosis. Neurology 2010, 75, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Marrie, R.A.; Cutter, G.; Tyry, T.; Vollmer, T.; Campagnolo, D. Does multiple sclerosis-associated disability differ between races? Neurology 2006, 66, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Weinstock-Guttman, B.; Jacobs, L.D.; Brownscheidle, C.M.; Baier, M.; Rea, D.F.; Apatoff, B.R.; Blitz, K.M.; Coyle, P.K.; Frontera, A.T.; Goodman, A.D.; et al. Multiple sclerosis characteristics in African American patients in the New York State Multiple Sclerosis Consortium. Mult. Scler. 2003, 9, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Cree, B.A.C.; Khan, O.; Bourdette, D.; Goodin, D.S.; Cohen, J.A.; Marrie, R.A.; Glidden, D.; Weinstock-Guttman, B.; Reich, D.; Patterson, N.; et al. Clinical Characteristics of African Americans vs Caucasian Americans with Multiple Sclerosis. Neurology 2004, 63, 2039–2045. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, R.J.; Wang, S.; Huang, C.; Graber, D. Profiles of nursing home residents with multiple sclerosis using the minimum data set. Mult. Scler. 2001, 7, 189–200. [Google Scholar] [CrossRef]
- Howard, J.; Battaglini, M.; Babb, J.S.; Arienzo, D.; Holst, B. MRI Correlates of Disability in African-Americans with Multiple Sclerosis. PLoS ONE 2012, 7, e43061. [Google Scholar] [CrossRef]
- Amezcua, L.; Lund, B.T.; Weiner, L.P.; Islam, T. Multiple sclerosis in Hispanics: A study of clinical disease expression. Mult. Scler. 2011, 17, 1010–1016. [Google Scholar] [CrossRef]
- Hadjixenofontos, A.; Beecham, A.H.; Manrique, C.P.; Pericak-Vance, M.A.; Tornes, L.; Ortega, M.; Rammohan, K.W.; McCauley, J.L.; Delgado, S.R. Clinical expression of multiple sclerosis in Hispanic whites of primarily Caribbean ancestry. Neuroepidemiology 2015, 44, 62–268. [Google Scholar] [CrossRef]
- Ventura, R.E.; Antezana, A.O.; Bacon, T.; Kister, I. Hispanic Americans and African Americans with multiple sclerosis have more severe disease course than Caucasian Americans. Mult. Scler. 2017, 23, 1554–1557. [Google Scholar] [CrossRef] [PubMed]
- Saadi, A.; Himmelstein, D.U.; Woolhandler, S.; Mejia, N.I. Racial disparities in neurologic health care access and utilization in the United States. Neurology 2017, 88, 2268–2275. [Google Scholar] [CrossRef] [PubMed]
- Shabas, D.; Heffner, M. Multiple sclerosis management for low-income minorities. Mult. Scler. J. 2005, 11, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Langille, M.M.; Islam, T.; Burnett, M.; Amezcua, L. Clinical Characteristics of Pediatric-Onset and Adult-Onset Multiple Sclerosis in Hispanic Americans. J. Child Neurol. 2016, 31, 1068–1073. [Google Scholar] [CrossRef]
- Avasarala, J. FDA-approved drugs for multiple sclerosis have no efficacy or disability data in non-Caucasian patients. CNS Spectr. 2019, 24, 279–280. [Google Scholar] [CrossRef] [Green Version]
- Minden, S.L.; Hoaglin, D.C.; Hadden, L.; Frankel, D.; Robbins, T.; Perloff, J. Access to and utilization of neurologists by people with multiple sclerosis. Neurology 2008, 70, 1141–1149. [Google Scholar] [CrossRef]
- Degelman, M.L.; Herman, K.M. Smoking and multiple sclerosis: A systematic review and meta-analysis using the Bradford Hill criteria for causation. Mult. Scler. Relat. Disord. 2017, 17, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Dong, D.; Carlson, J.; Ruberwa, J.; Snihur, T.; Al-Obaidi, N.; Bustillo, J. Unmasking the Masquerader: A Delayed Diagnosis of MS and Its 4.5 Years of Implications in an Older African American Male. Case Rep. Med. 2019. [Google Scholar] [CrossRef]
- Naismith, R.T.; Trinkaus, K.; Cross, A.H. Phenotype and prognosis in African-Americans with multiple sclerosis: A retrospective chart review. Mult. Scler. 2006, 12, 775–781. [Google Scholar] [CrossRef]
- Cree, B.A.C.; Al-Sabbagh, A.; Bennett, R.; Goodin, D. Response to interferon beta-1a treatment in African American multiple sclerosis patients. Arch. Neurol. 2005, 62, 1681–1683. [Google Scholar] [CrossRef] [Green Version]
| Characteristics | NH-White (n = 11) | NH-Black (n = 61) | Hispanic (n = 40) | p Value |
|---|---|---|---|---|
| Multiple Sclerosis (MS) type at diagnosis | p = 0.1859 | |||
| Relapsing remitting MS | 82% | 95% | 95% | |
| Primary progressive MS | 18% | 5% | 2.5% | |
| Secondary progressive MS | 0% | 0% | 2.5% | |
| Mean Age at diagnosis (years) | 39.9 (11.3) | 36.7 (11.4) | 32.4 (11.5) | p = 0.9918 |
| Female/Male ratio | 1.70/1 | 2.33/1 | 1.22/1 | p = 0.3765 |
| Active smokers | 55% | 44% | 30% | p = 0.3079 |
| Mean time from symptom onset to diagnosis (months) | 30.8 (38.9) | 32.9 (32.1) | 13.7 (15.4) | p = 0.9934 |
| Medical Evaluation at symptom onset | 64% | 63% | 70% | p = 0.8597 |
| Neurological Evaluation at symptom onset | 45% | 28% | 53% | p = 0.1778 |
| Adherence (Adherence/Ever used) | p = 0.0252 | |||
| Escalation therapy | 63.2% | 73% | 61.8% | |
| High efficacy therapy | 100% | 84.2% | 50% | |
| Relapse (Relapse/Ever used) | p = 0.00015 | |||
| Escalation therapy | 26.3% | 31.1% | 36.4% | |
| High efficacy therapy | 0% | 10.5% | 0% |
| Clinical Characteristics | NH-White (n = 11) | NH-Black (n = 61) | Hispanic (n = 40) | p Value |
|---|---|---|---|---|
| EDSS scores | ||||
| Mean EDSS score at diagnosis (SD) | 2.6 (2.1) | 2.2 (1.1) | 3.8 (1.9) | p = 0.9328 |
| Mean EDSS score at last presentation (SD) | 2.9 (2.8) | 4.2 (2.9) | 3.8 (2.3) | p = 0.9950 |
| Severe disability at diagnosis (EDSS > 4.5) | 14.3% | 50% | 31.6% | p = < 0.001 |
| Severe disability at last presentation (EDSS > 4.5) | 32.5% | 45.5% | 41% | p = < 0.001 |
| Symptoms at Presentation | p = 0.1473 | |||
| Motor | 72.7% | 57.4% | 47.5% | |
| Brainstem | 27.3% | 24.9% | 25% | |
| Cerebellar | 27.3% | 37.7% | 37.5% | |
| Gait | 27.3% | 26.2% | 15% | |
| Sensory | 72.7% | 37.7% | 52.5% | |
| Visual | 9.1% | 27.9% | 30% | |
| Cognitive | 9.1% | 9.8% | 5% | |
| Other or unknown | 36.4% | 18.1% | 15% |
| High EDSS Score at Last Presentation | ||
|---|---|---|
| OR | p-Value | |
| Usage of escalation therapies a | ||
| No | reference | |
| Yes | 1.60 (0.45–6.14) | 0.48 |
| Usage of high efficacy therapies b | ||
| No | reference | |
| Yes | 2.64 (0.87–8.33) | 0.09 |
| Smoker c | ||
| No | reference | |
| Yes | 2.44 (1.36–6.12) | 0.01 |
| Medical evaluation by Neurologist c | ||
| No | reference | |
| Yes | 0.40 (0.16–0.90) | 0.04 |
| Adherence to DMT c | ||
| Yes | reference | |
| No | 0.73 (0.31–1.62) | 0.43 |
| Time to diagnosis c | ||
| <=12 months | reference | |
| >12 months | 1.73 (0.75–4.01) | 0.2 |
| High EDSS Score at Diagnosis | ||
|---|---|---|
| OR | p-Value | |
| Smoker c | ||
| No | reference | |
| Yes | 2.79 (1.10–7.10) | 0.01 |
| Time to diagnosis c | ||
| <=12 months | reference | |
| >12 months | 1.15 (0.46–2.83) | 0.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mercado, V.; Dongarwar, D.; Fisher, K.; Salihu, H.M.; Hutton, G.J.; Cuascut, F.X. Multiple Sclerosis in a Multi-Ethnic Population in Houston, Texas: A Retrospective Analysis. Biomedicines 2020, 8, 534. https://doi.org/10.3390/biomedicines8120534
Mercado V, Dongarwar D, Fisher K, Salihu HM, Hutton GJ, Cuascut FX. Multiple Sclerosis in a Multi-Ethnic Population in Houston, Texas: A Retrospective Analysis. Biomedicines. 2020; 8(12):534. https://doi.org/10.3390/biomedicines8120534
Chicago/Turabian StyleMercado, Vicki, Deepa Dongarwar, Kristen Fisher, Hamisu M. Salihu, George J. Hutton, and Fernando X. Cuascut. 2020. "Multiple Sclerosis in a Multi-Ethnic Population in Houston, Texas: A Retrospective Analysis" Biomedicines 8, no. 12: 534. https://doi.org/10.3390/biomedicines8120534
APA StyleMercado, V., Dongarwar, D., Fisher, K., Salihu, H. M., Hutton, G. J., & Cuascut, F. X. (2020). Multiple Sclerosis in a Multi-Ethnic Population in Houston, Texas: A Retrospective Analysis. Biomedicines, 8(12), 534. https://doi.org/10.3390/biomedicines8120534