Lectin-Like Transcript 1 (LLT1) Checkpoint: A Novel Independent Prognostic Factor in HPV-Negative Oropharyngeal Squamous Cell Carcinoma
Abstract
1. Introduction
2. Results
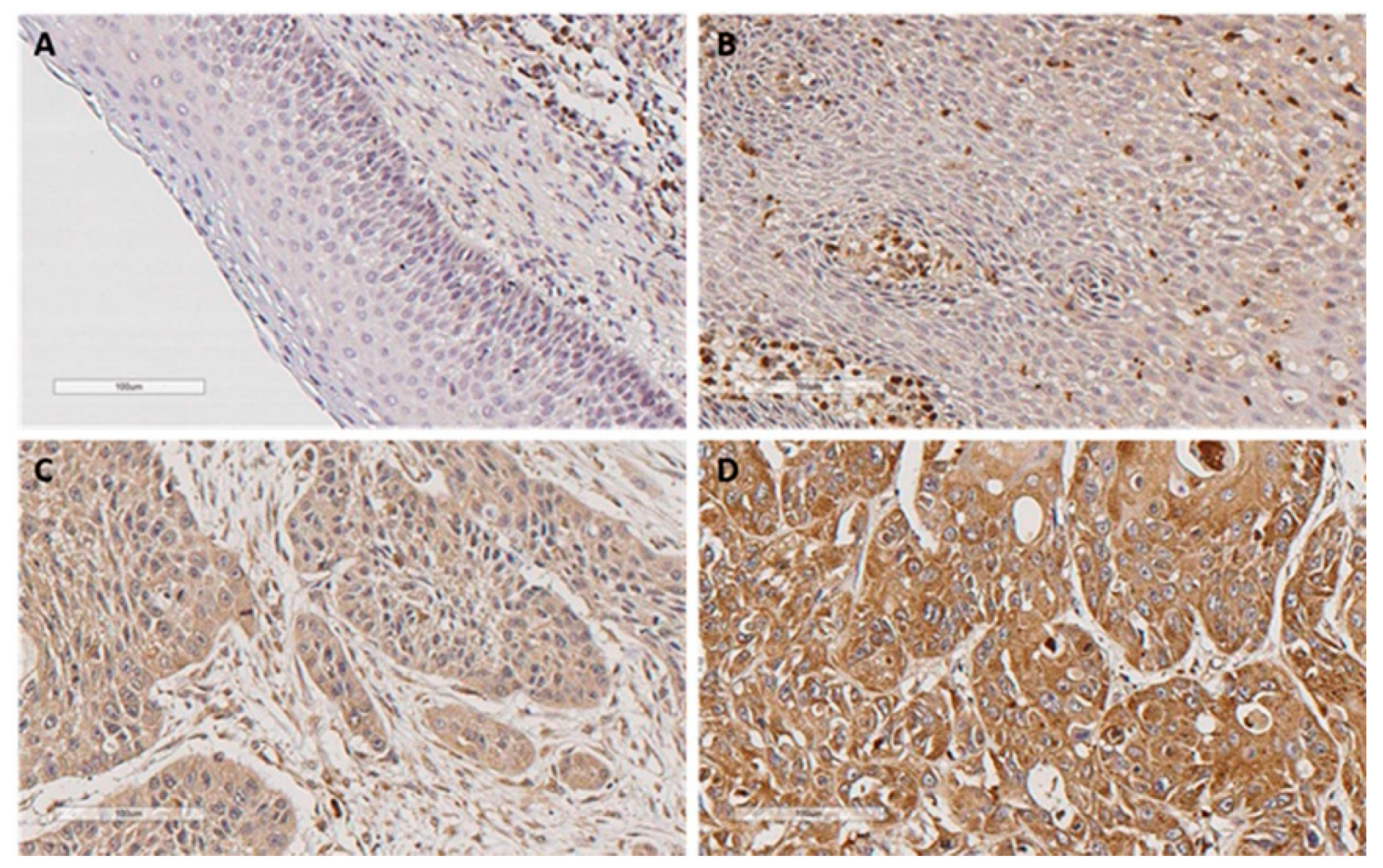
2.1. Immunohistochemical Analysis of LLT1 Expression in Oropharyngeal Squamous Cell Carcinomas (OPSCC) Tissue Specimens
2.2. Correlations of LLT1 Expression with Clinicopathological Parameters
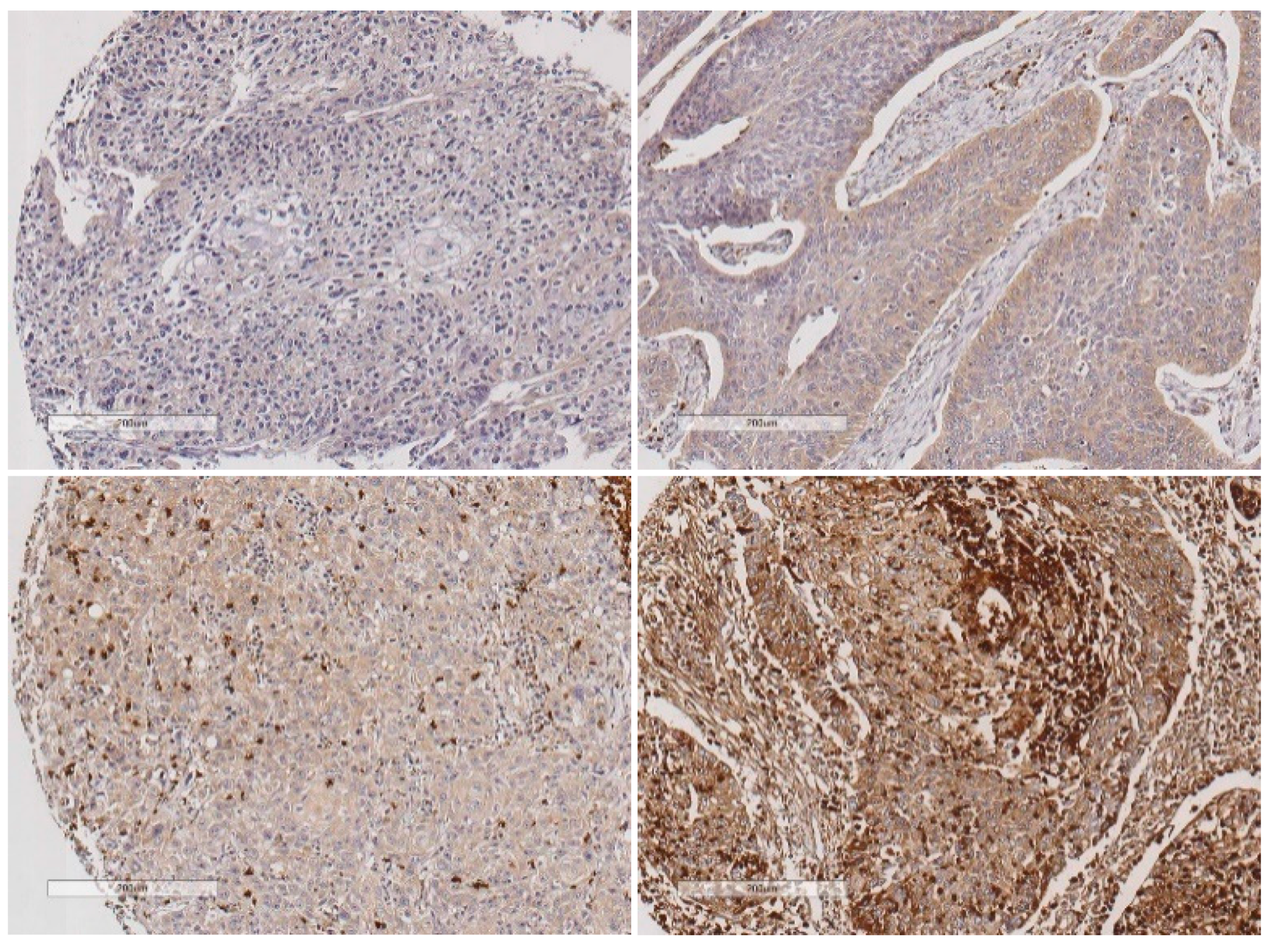
2.3. Evaluation of LLT1 Protein Expression in the Intratumor Immune Microenvironment
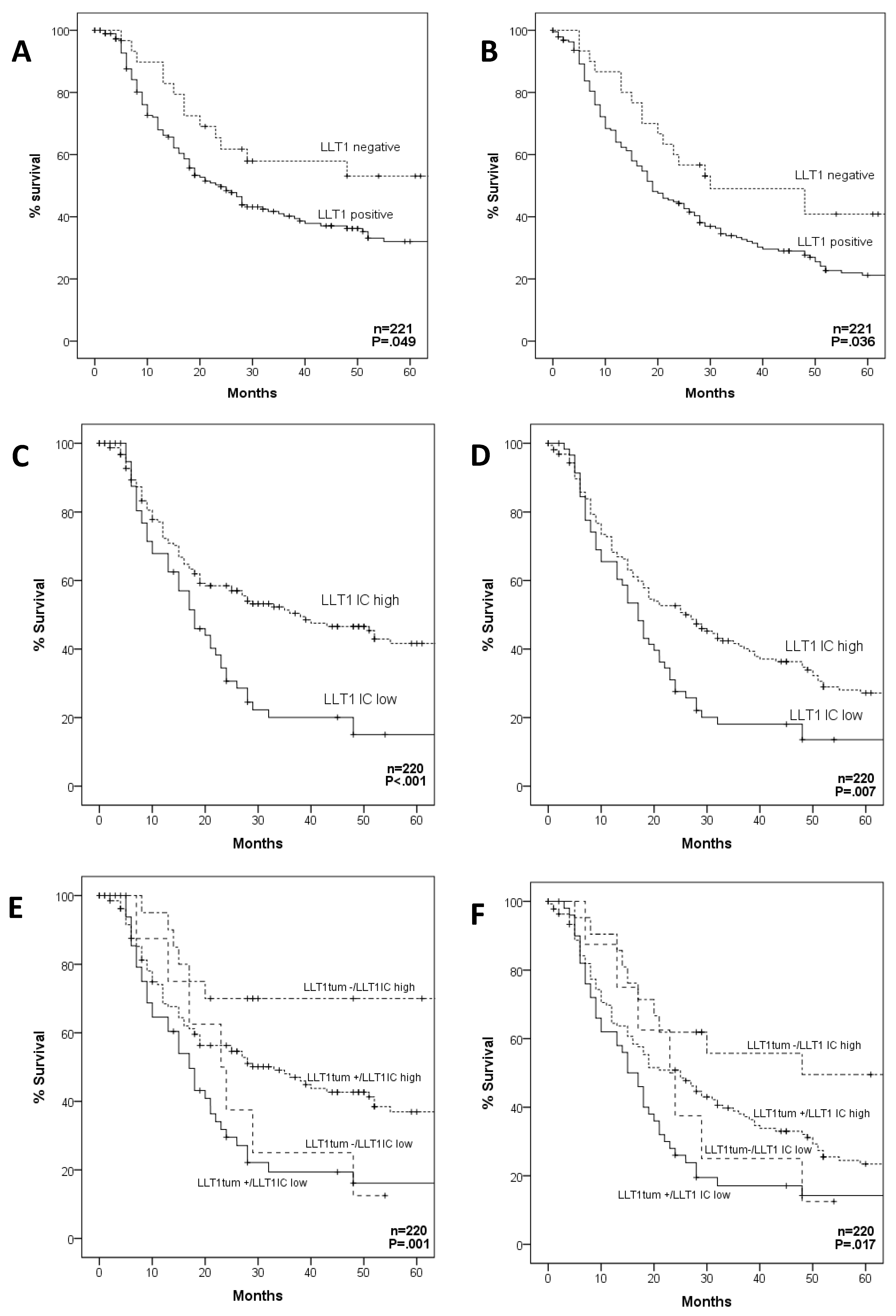
2.4. Impact of LLT1 Expression on OPSCC Survival
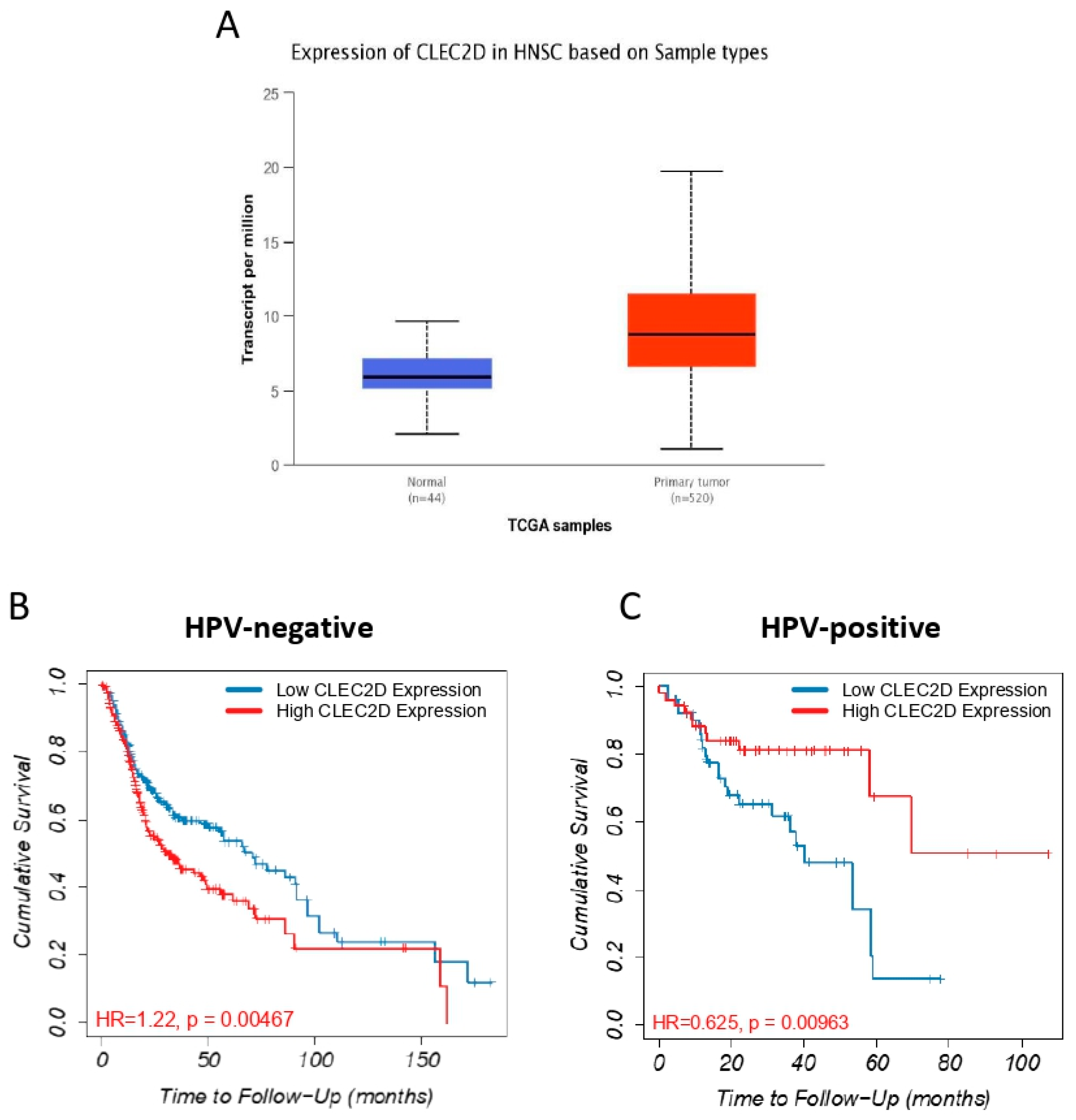
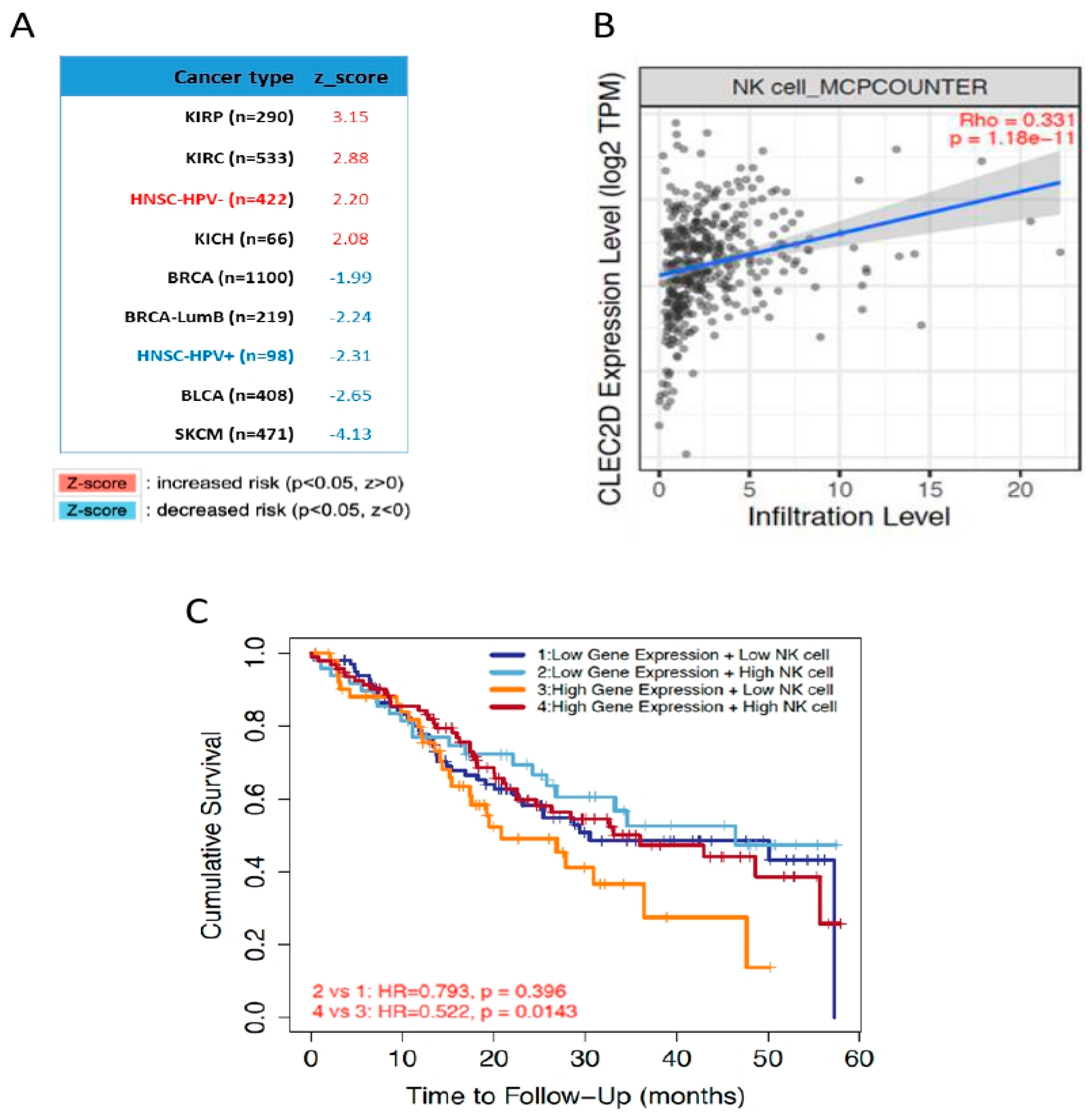
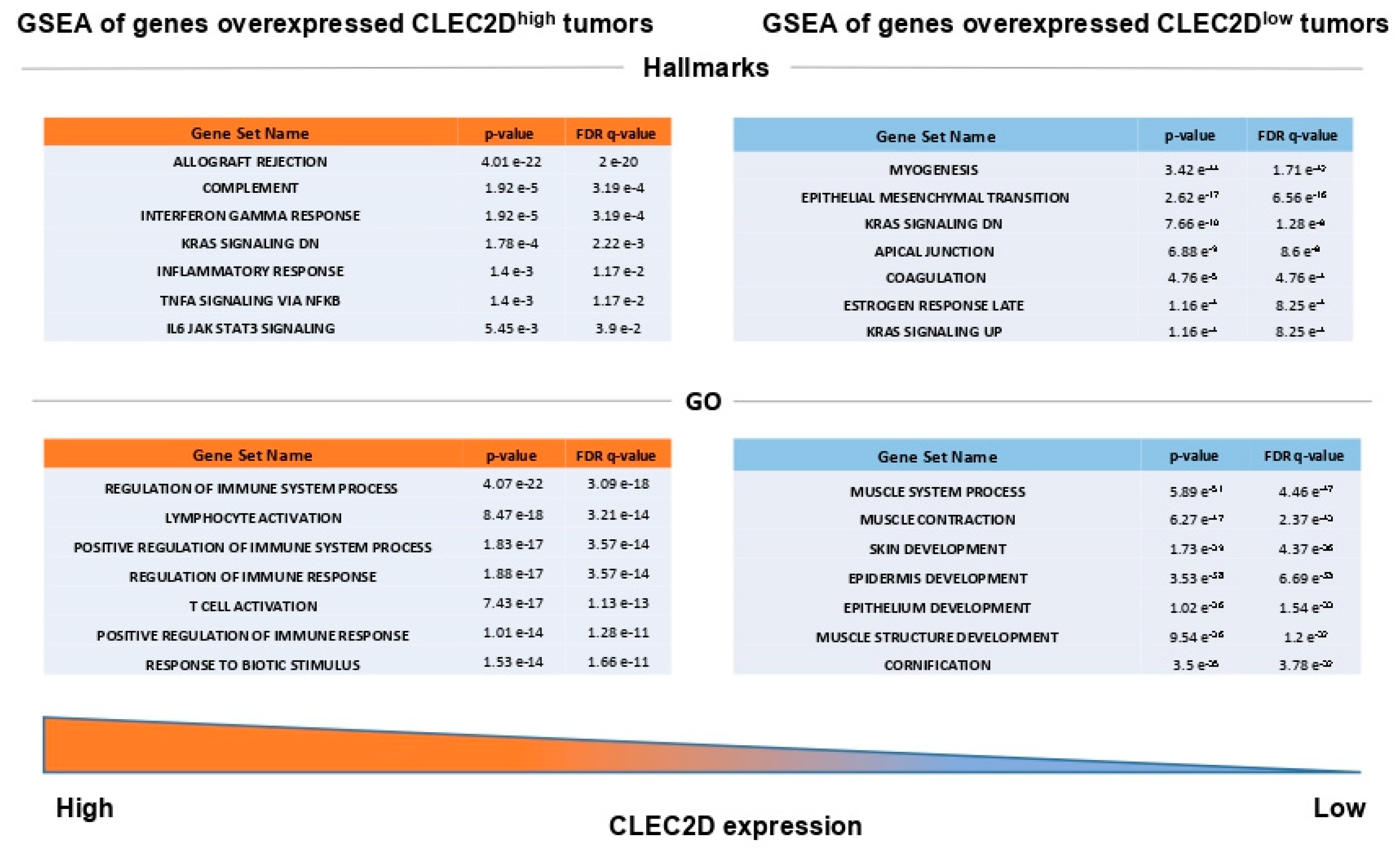
2.5. In Silico Analysis of mRNA Levels Using The Cancer Genome Atlas (TCGA) HNSCC Database
3. Discussion
4. Materials and Methods
4.1. Patients and Tissue Specimens
4.2. Tissue Microarray (TMA) Construction
4.3. Immunohistochemical Study
4.4. In Silico Analysis of CLEC2D mRNA Expression Using The Cancer Genome Atlas (TCGA) HNSCC Database
4.5. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer 2018, 18, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Wondergem, N.E.; Nauta, I.H.; Muijlwijk, T.; Leemans, C.R.; van de Ven, R. The Immune Microenvironment in Head and Neck Squamous Cell Carcinoma: On Subsets and Subsites. Curr. Oncol. Rep. 2020, 22, 81. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Llibre, A.; Klenerman, P.; Willberg, C.B. Multi-functional lectin-like transcript-1: A new player in human immune regulation. Immunol. Lett. 2016, 177, 62–69. [Google Scholar] [CrossRef]
- Bialoszewska, A.; Malejczyk, J. Biological and Clinical Significance of Human NKRP1A/LLT1 Receptor/Ligand Interactions. Crit. Rev. Immunol. 2018, 38, 479–489. [Google Scholar] [CrossRef]
- Suto, Y.; Yabe, T.; Maenaka, K.; Tokunaga, K.; Tadokoro, K.; Juji, T. The human natural killer gene complex (NKC) is located on chromosome 12p13.1-p13.2. Immunogenetics 1997, 46, 159–162. [Google Scholar] [CrossRef]
- Germain, C.; Bihl, F.; Zahn, S.; Poupon, G.; Dumaurier, M.J.; Rampanarivo, H.H.; Padkjær, S.B.; Spee, P.; Braud, V.M. Characterization of alternatively spliced transcript variants of CLEC2D gene. J. Biol. Chem. 2010, 285, 36207–36215. [Google Scholar] [CrossRef]
- Buller, C.W.; Mathew, P.A.; Mathew, S.O. Roles of NK cell receptors 2B4 (CD244), CS1 (CD319), and LLT1 (CLEC2D) in cancer. Cancers 2020, 12, 1755. [Google Scholar] [CrossRef]
- Kurioka, A.; Cosgrove, C.; Simoni, Y.; van Wilgenburg, B.; Geremia, A.; Björkander, S.; Sverremark-Ekström, E.; Thurnheer, C.; Günthard, H.F.; Khanna, N.; et al. CD161 Defines a Functionally Distinct Subset of Pro-Inflammatory Natural Killer Cells. Front. Immunol. 2018, 9, 486. [Google Scholar] [CrossRef]
- Rosen, D.B.; Cao, W.; Avery, D.T.; Tangye, S.G.; Liu, Y.J.; Houchins, J.P.; Lanier, L.L. Functional consequences of interactions between human NKR-P1A and its ligand LLT1 expressed on activated dendritic cells and B cells. J. Immunol. 2008, 180, 6508–6517. [Google Scholar] [CrossRef] [PubMed]
- Llibre, A.; Garner, L.; Partridge, A.; Freeman, G.J.; Klenerman, P.; Willberg, C.B. Expression of lectin-like transcript-1 in human tissues. F1000Research 2016, 5, 2929. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.O.; Chaudhary, P.; Powers, S.B.; Vishwanatha, J.K.; Mathew, P.A. Overexpression of LLT1 (OCIL, CLEC2D) on prostate cancer cells inhibits NK cell-mediated killing through LLT1-NKRP1A (CD161) interaction. Oncotarget 2016, 7, 68650–68661. [Google Scholar] [CrossRef]
- Liu, J.; Lichtenberg, T.; Hoadley, K.A.; Poisson, L.M.; Lazar, A.J.; Cherniack, A.D.; Kovatich, A.J.; Benz, C.C.; Levine, D.A.; Lee, A.V.; et al. An Integrated TCGA Pan-Cancer Clinical Data Resource to Drive High-Quality Survival Outcome Analytics. Cell 2018, 173, 400–416.e11. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Fu, J.; Zeng, Z.; Cohen, D.; Li, J.; Chen, Q.; Li, B.; Liu, X.S. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020, 48, W509–W514. [Google Scholar] [CrossRef] [PubMed]
- Becht, E.; Giraldo, N.A.; Lacroix, L.; Buttard, B.; Elarouci, N.; Petitprez, F.; Selves, J.; Laurent-Puig, P.; Sautès-Fridman, C.; Fridman, W.H.; et al. Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression. Genome Biol. 2016, 17, 218. [Google Scholar] [CrossRef] [PubMed]
- Mirza, A.H.; Thomas, G.; Ottensmeier, C.H.; King, E.V. Importance of the immune system in head and neck cancer. Head Neck. 2019, 41, 2789–2800. [Google Scholar] [CrossRef]
- Gentles, A.J.; Newman, A.M.; Liu, C.L.; Bratman, S.V.; Feng, W.; Kim, D.; Nair, V.S.; Xu, Y.; Khuong, A.; Hoang, C.D.; et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 2015, 21, 938–945. [Google Scholar] [CrossRef]
- Braud, V.M.; Biton, J.; Becht, E.; Knockaert, S.; Mansuet-Lupo, A.; Cosson, E.; Damotte, D.; Alifano, M.; Validire, P.; Anjuere, F.; et al. Expression of LLT1 and its receptor CD161 in lung cancer is associated with better clinical outcome. Oncoimmunology 2018, 7, e1423184. [Google Scholar] [CrossRef]
- Marrufo, A.M.; Mathew, S.O.; Chaudhary, P.; Malaer, J.D.; Vishwanatha, J.K.; Mathew, P.A. Blocking LLT1 (CLEC2D, OCIL)-NKRP1A (CD161) interaction enhances natural killer cell-mediated lysis of triple-negative breast cancer cells. Am. J. Cancer Res. 2018, 8, 1050–1063. [Google Scholar]
- Sun, Y.; Malaer, J.D.; Mathew, P.A. Lectin-like transcript 1 as a natural killer cell-mediated immunotherapeutic target for triple negative breast cancer and prostate cancer. J. Cancer Metastasis Treat 2019, 5, 80. [Google Scholar] [CrossRef][Green Version]
- Santos-Juanes, J.; Fernández-Vega, I.; Lorenzo-Herrero, S.; Sordo-Bahamonde, C.; Martínez-Camblor, P.; García-Pedrero, J.M.; Vivanco, B.; Galache-Osuna, C.; Vazquez-Lopez, F.; Gonzalez, S.; et al. Lectin-like transcript 1 (LLT1) expression is associated with nodal metastasis in patients with head and neck cutaneous squamous cell carcinoma. Arch. Dermatol. Res. 2019, 311, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, P.; Ganguly, N. Transcriptomic analyses of genes differentially expressed by high-risk and low-risk human papilloma virus E6 oncoproteins. Virusdisease 2015, 26, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lu, J.; Tian, H.; Du, W.; Zhao, L.; Feng, J.; Yuan, D.; Li, Z. Increased expression of PD L1 by the human papillomavirus 16 E7 oncoprotein inhibits anticancer immunity. Mol. Med. Rep. 2017, 15, 1063–1070. [Google Scholar] [CrossRef]
- Stanley, M.A. Immune responses to human papilloma viruses. Indian J. Med. Res. 2009, 130, 266–276. [Google Scholar]
- Lyford-Pike, S.; Peng, S.; Young, G.D.; Taube, J.M.; Westra, W.H.; Akpeng, B.; Bruno, T.C.; Richmon, J.D.; Wang, H.; Bishop, J.A.; et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013, 73, 1733–1741. [Google Scholar] [CrossRef]
- Rodrigo, J.P.; Heideman, D.A.; García-Pedrero, J.M.; Fresno, M.F.; Brakenhoff, R.H.; Díaz Molina, J.P.; Snijders, P.J.; Hermsen, M.A. Time trends in the prevalence of HPV in oropharyngeal squamous cell carcinomas in northern Spain (1990-2009). Int. J. Cancer 2014, 134, 487–492. [Google Scholar] [CrossRef]
- Balermpas, P.; Rödel, F.; Krause, M.; Linge, A.; Lohaus, F.; Baumann, M.; Tinhofer, I.; Budach, V.; Sak, A.; Stuschke, M.; et al. The PD-1/PD-L1 axis and human papilloma virus in patients with head and neck cancer after adjuvant chemoradiotherapy: A multicentre study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG). Int. J. Cancer 2017, 141, 594–603. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.V.; Varambally, S. UALCAN: A portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef]
| Characteristic | No. Cases (%) | LLT1-Positive Expression (%) | p |
|---|---|---|---|
| Age, mean (range) | 57.94 (30.4–85.4 years) | ||
| Gender | 0.59 | ||
| Male | 214 (97%) | 183 (85%) | |
| Female | 7 (3%) | 7 (100%) | |
| Tobacco | 0.53 | ||
| No | 4 (2%) | 4 (100%) | |
| <50 Pack-year | 114 (51%) | 96 (84%) | |
| >50 Pack-year | 99 (45%) | 87 (88%) | |
| Unknown | 4 (2%) | 3 (75%) | |
| Alcohol | 1 | ||
| No | 4 (2%) | 4 (100%) | |
| Yes | 213 (96%) | 183 (86%) | |
| Unknown | 4 (2%) | 3 (75%) | |
| pT classification | 0.43 | ||
| T1 | 12 (5%) | 11 (92%) | |
| T2 | 53 (24%) | 43 (81%) | |
| T3 | 77 (35%) | 64 (83%) | |
| T4 | 79 (36%) | 72 (91%) | |
| pN classification | 0.99 | ||
| N0 | 52 (23%) | 45 (86%) | |
| N1 | 28 (13%) | 24 (86%) | |
| N2 | 110 (50%) | 94 (85%) | |
| N3 | 31 (14%) | 27 (87%) | |
| Stage | 0.3 | ||
| I | 2 (1%) | 2 (100%) | |
| II | 18 (8%) | 13 (72%) | |
| III | 38 (17%) | 32 (84%) | |
| IV | 163 (74%) | 143 (88%) | |
| Degree of differentiation | 0.64 | ||
| Well-differentiated | 99 (45%) | 87 (88%) | |
| Moderately-differentiated | 83 (37%) | 69 (83%) | |
| Poorly-differentiated | 39 (18%) | 34 (87%) | |
| Tumor recurrence | 0.1 | ||
| No | 85 (38.5%) | 69 (81%) | |
| Yes | 136 (61.5%) | 121 (89%) | |
| Follow-up | 0.1 | ||
| Alive without disease | 48 (21%) | 36 (75%) | |
| Dead by the disease | 123 (56%) | 110 (89%) | |
| Dead by other causes | 50 (23%) | 44 (88%) | |
| Total | 221 | 190 (86%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez-Canteli, M.; Hermida-Prado, F.; Sordo-Bahamonde, C.; Montoro-Jiménez, I.; Pozo-Agundo, E.; Allonca, E.; Vallina-Álvarez, A.; Álvarez-Marcos, C.; Gonzalez, S.; García-Pedrero, J.M.; et al. Lectin-Like Transcript 1 (LLT1) Checkpoint: A Novel Independent Prognostic Factor in HPV-Negative Oropharyngeal Squamous Cell Carcinoma. Biomedicines 2020, 8, 535. https://doi.org/10.3390/biomedicines8120535
Sanchez-Canteli M, Hermida-Prado F, Sordo-Bahamonde C, Montoro-Jiménez I, Pozo-Agundo E, Allonca E, Vallina-Álvarez A, Álvarez-Marcos C, Gonzalez S, García-Pedrero JM, et al. Lectin-Like Transcript 1 (LLT1) Checkpoint: A Novel Independent Prognostic Factor in HPV-Negative Oropharyngeal Squamous Cell Carcinoma. Biomedicines. 2020; 8(12):535. https://doi.org/10.3390/biomedicines8120535
Chicago/Turabian StyleSanchez-Canteli, Mario, Francisco Hermida-Prado, Christian Sordo-Bahamonde, Irene Montoro-Jiménez, Esperanza Pozo-Agundo, Eva Allonca, Aitana Vallina-Álvarez, César Álvarez-Marcos, Segundo Gonzalez, Juana M. García-Pedrero, and et al. 2020. "Lectin-Like Transcript 1 (LLT1) Checkpoint: A Novel Independent Prognostic Factor in HPV-Negative Oropharyngeal Squamous Cell Carcinoma" Biomedicines 8, no. 12: 535. https://doi.org/10.3390/biomedicines8120535
APA StyleSanchez-Canteli, M., Hermida-Prado, F., Sordo-Bahamonde, C., Montoro-Jiménez, I., Pozo-Agundo, E., Allonca, E., Vallina-Álvarez, A., Álvarez-Marcos, C., Gonzalez, S., García-Pedrero, J. M., & Rodrigo, J. P. (2020). Lectin-Like Transcript 1 (LLT1) Checkpoint: A Novel Independent Prognostic Factor in HPV-Negative Oropharyngeal Squamous Cell Carcinoma. Biomedicines, 8(12), 535. https://doi.org/10.3390/biomedicines8120535






