Leptin, Leptin Receptor, KHDRBS1 (KH RNA Binding Domain Containing, Signal Transduction Associated 1), and Adiponectin in Bone Metastasis from Breast Carcinoma: An Immunohistochemical Study
Abstract
1. Introduction
2. Experimental Section
2.1. Tissue Collection and Preparation
2.2. Immunohistochemistry Analysis (IHC)
2.3. Statistical Analysis
3. Results
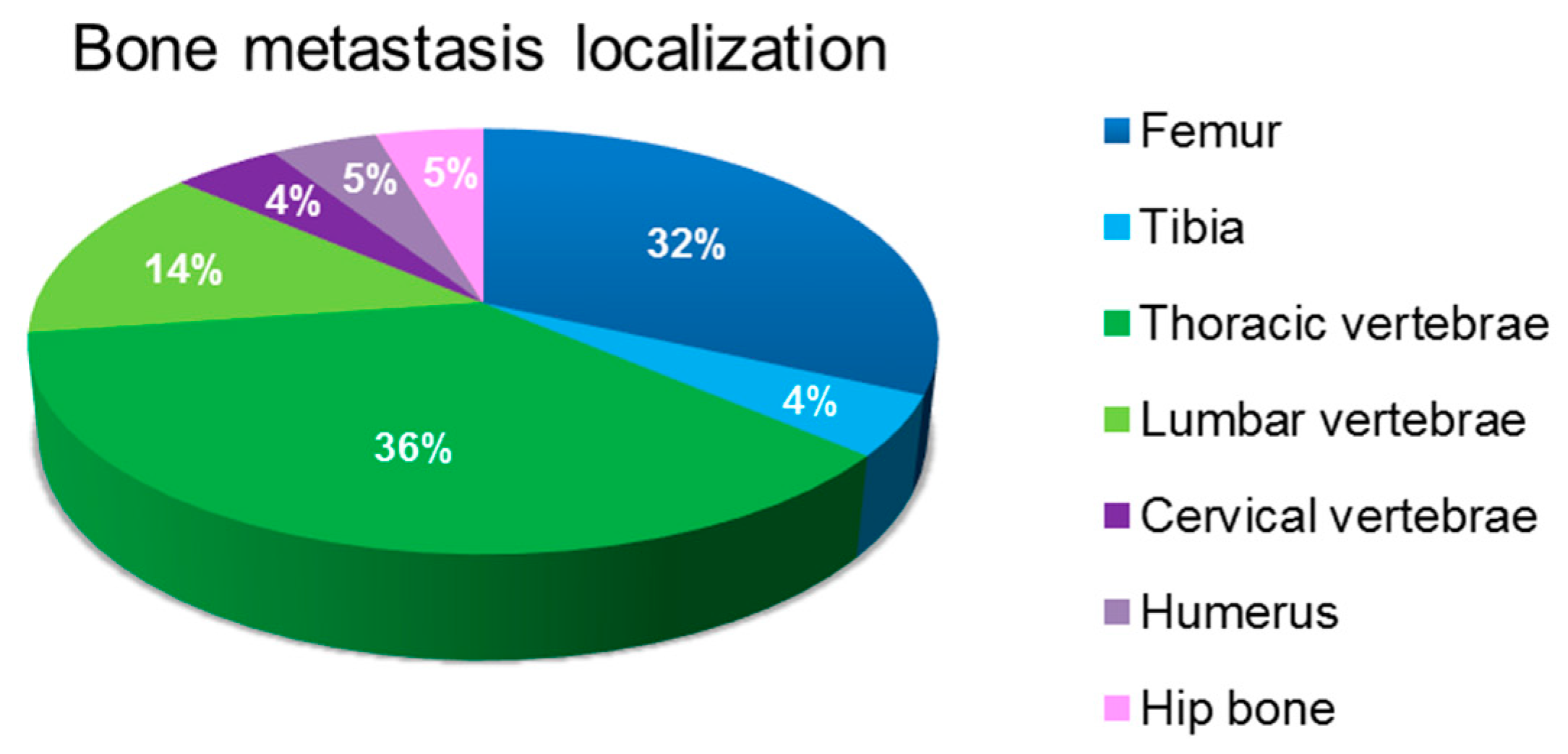
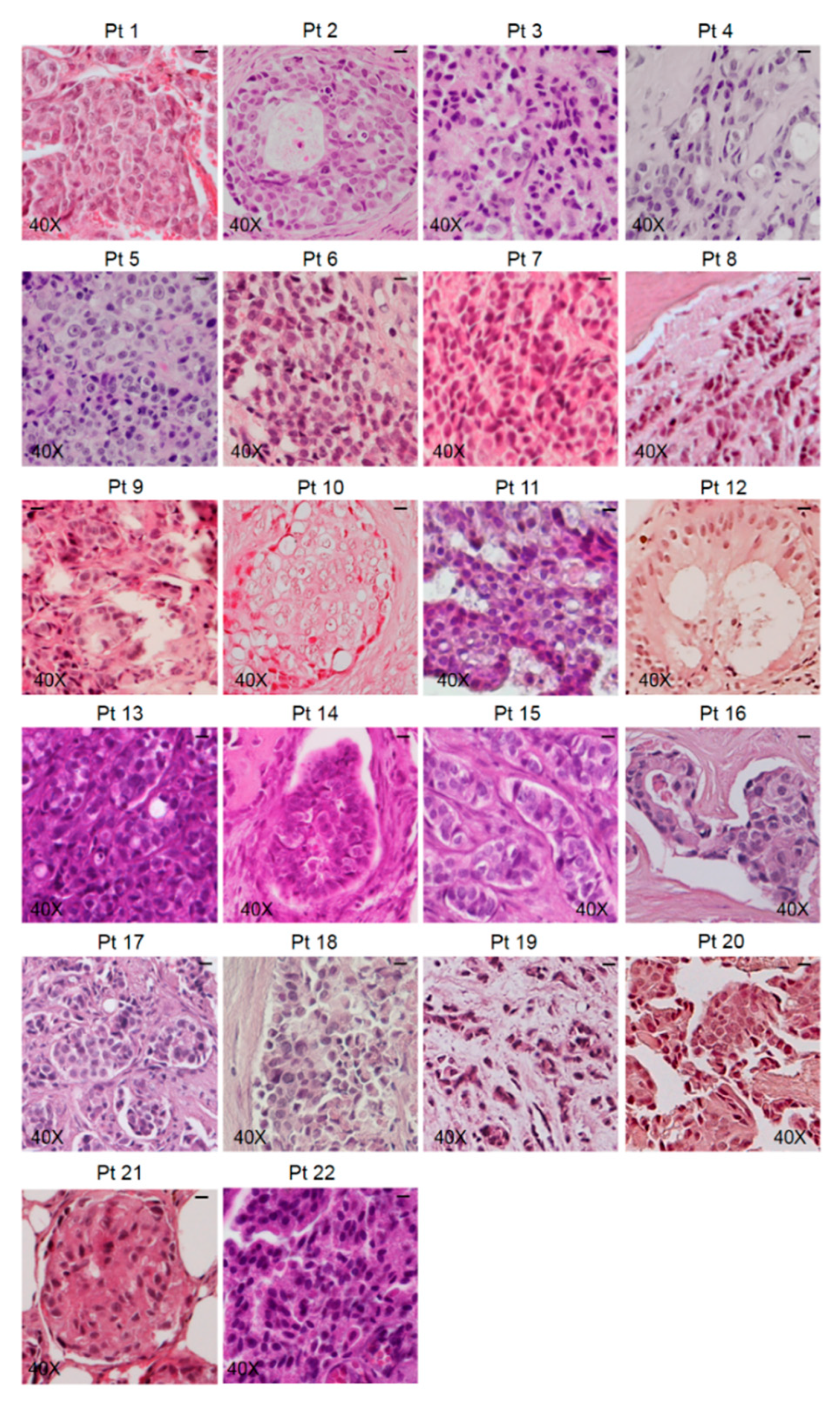
3.1. Characterization of Bone Metastasis Samples
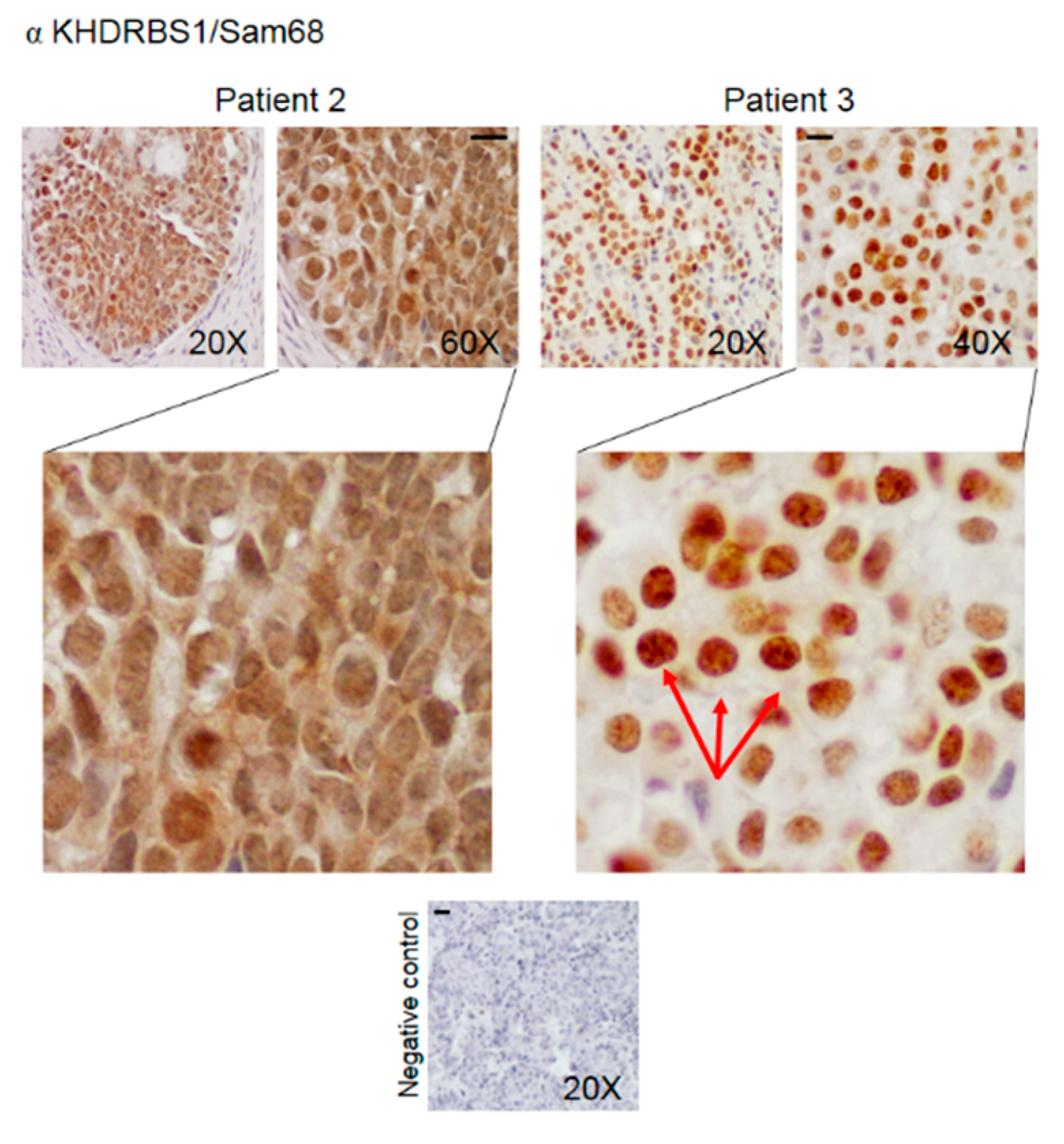
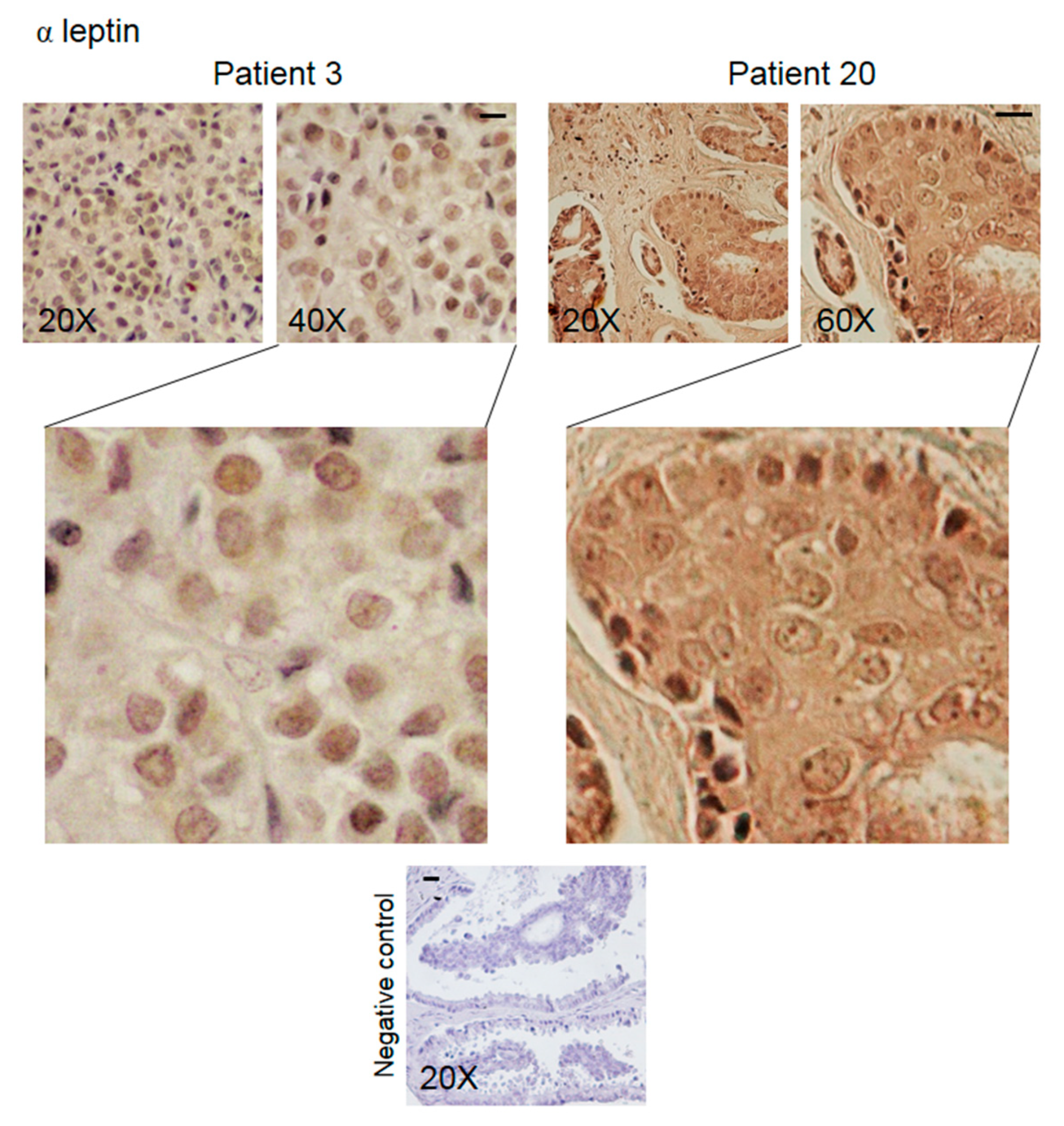
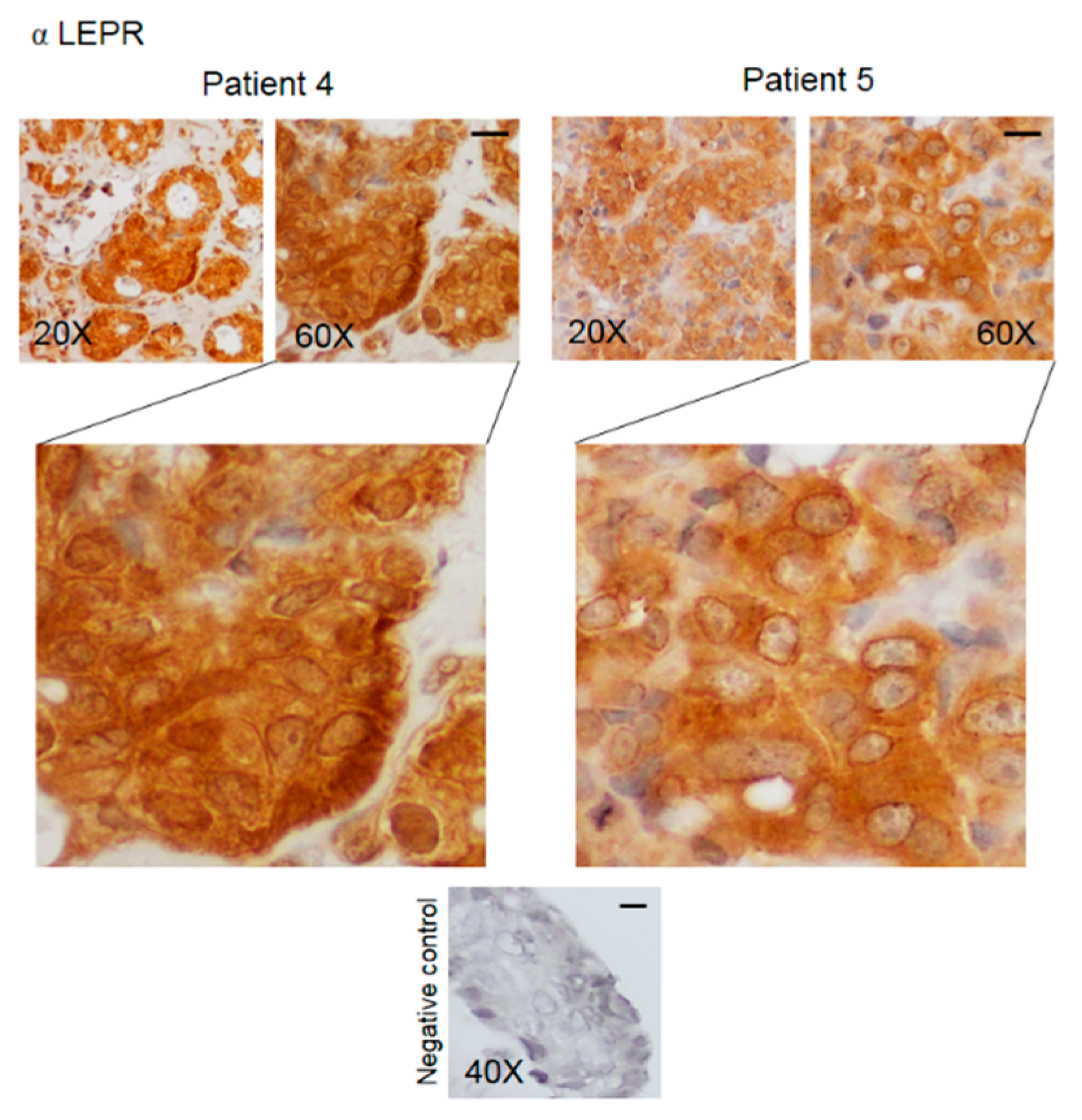
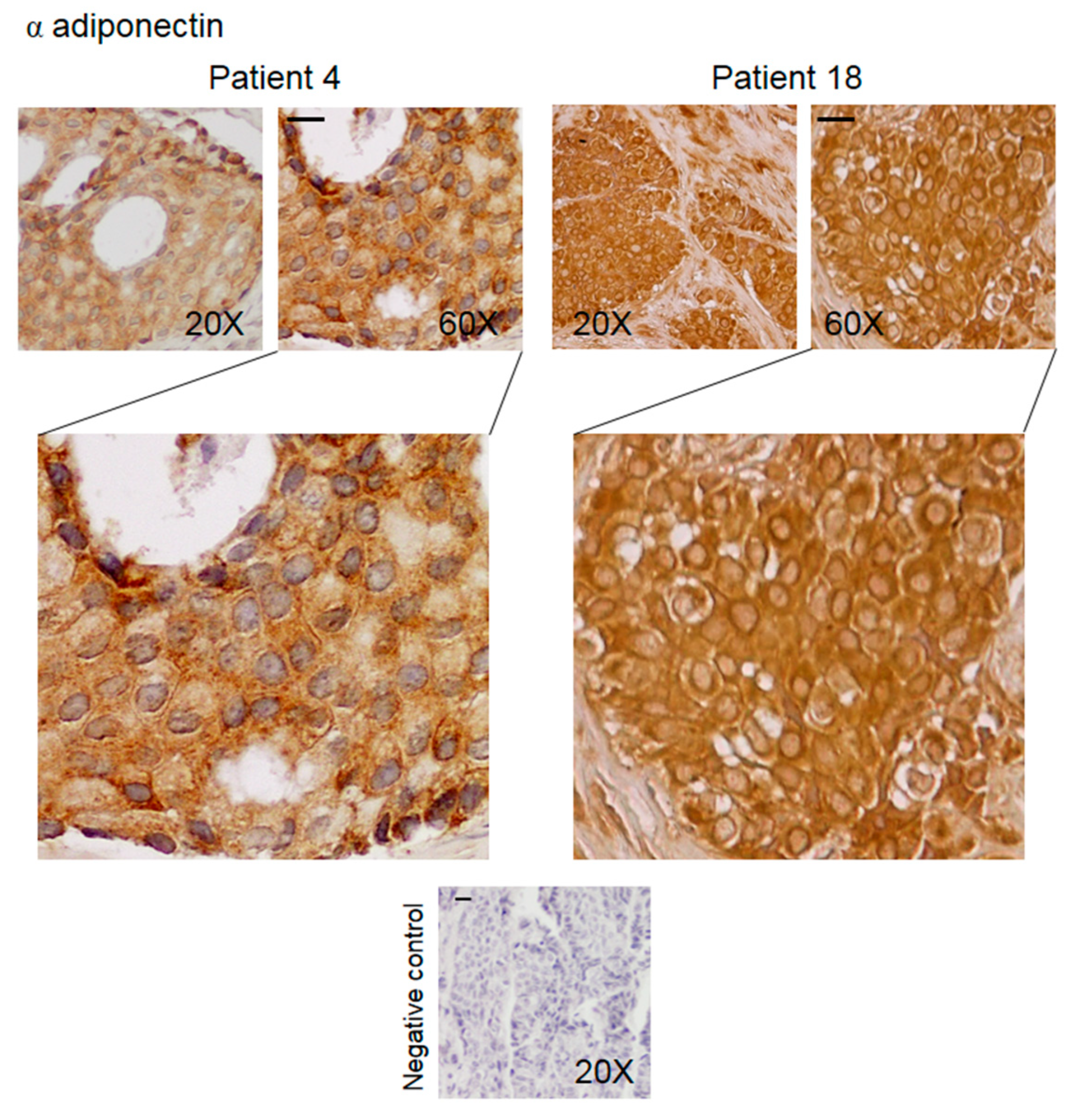
3.2. Pattern of Expression of KHDRBS1, Leptin, Leptin Receptor (LEPR), and Adiponectin in Human Bone Metastatic Tissue from Breast Cancer
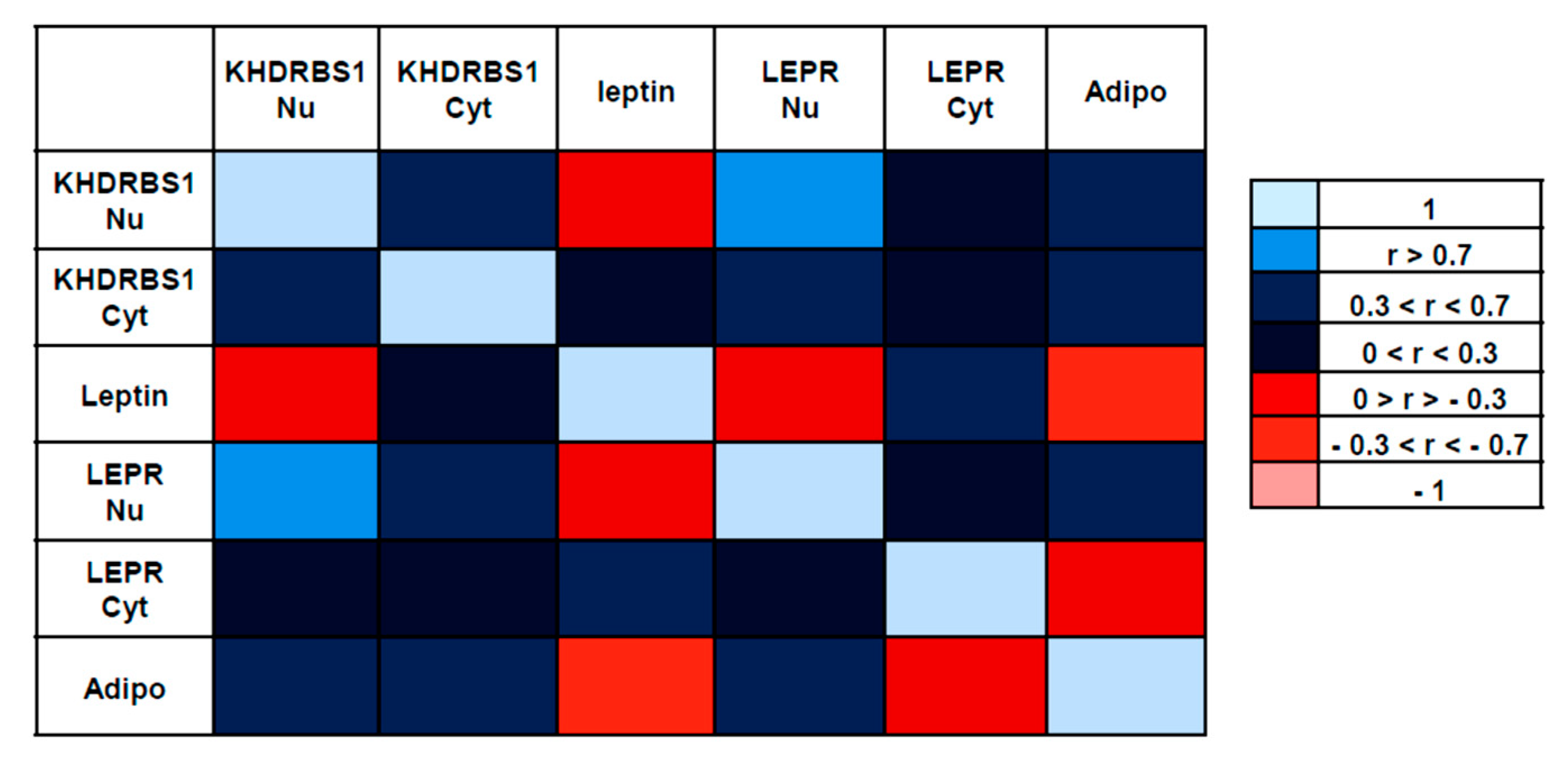
3.3. Correlations between the Examined Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Prim. 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Kan, C.; Vargas, G.; Pape, F.L.; Clézardin, P. Cancer Cell Colonisation in the Bone Microenvironment. Int. J. Mol. Sci. 2016, 17, 1674. [Google Scholar] [CrossRef] [PubMed]
- Turpin, A.; Duterque-Coquillaud, M.; Vieillard, M.H. Bone Metastasis: Current State of Play. Transl. Oncol. 2020, 13, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Vernet, C.; Artzt, K. STAR, a gene family involved in signal transduction and activation of RNA. Trends Genet. 1997, 13, 479–484. [Google Scholar] [CrossRef]
- Lukong, K.E.; Richard, S. Sam68, the KH domain-containing superSTAR. Biochim. Biophys. Acta Rev. Cancer 2003, 1653, 73–86. [Google Scholar] [CrossRef]
- Bielli, P.; Busà, R.; Paronetto, M.P.; Sette, C. The RNA-binding protein Sam68 is a multifunctional player in human cancer. Endocr. Relat. Cancer 2011, 18, R91–R102. [Google Scholar] [CrossRef]
- Song, L.; Wang, L.; Li, Y.; Xiong, H.; Wu, J.; Li, J.; Li, M. Sam68 up-regulation correlates with, and its down-regulation inhibits, proliferation and tumourigenicity of breast cancer cells. J. Pathol. 2010, 222, 227–237. [Google Scholar] [CrossRef]
- Ostrander, J.H.; Daniel, A.R.; Lange, C.A. Brk/PTK6 signalling in normal and cancer cell models. Curr. Opin. Pharmacol. 2010, 10, 662–669. [Google Scholar] [CrossRef]
- Crompton, M.R. BRK tyrosine kinase expression in a high proportion of human breast carcinomas. Oncogene 1997, 15, 799–805. [Google Scholar] [CrossRef]
- Frisone, P.; Pradella, D.; Di Matteo, A.; Belloni, E.; Ghigna, C.; Paronetto, M.P. SAM68: Signal Transduction and RNA Metabolism in Human Cancer. Biomed. Res. Int. 2015, 2015, 528954. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.T.; Liu, J.L.; Wang, Z.G.; Cui, Y.-M.; Shi, L.; Li, T.-T.; Zhao, X.-H.; Chen, X.-T.; Ding, Y.-Q.; Song, L.-B. High expression level and nuclear localization of Sam68 are associated with progression and poor prognosis in colorectal cancer. BMC Gastroenterol. 2013, 13, 126. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, A.; Sánchez-Jiménez, F.; Vilariño-García, T.; de la Cruz, L.; Virizuela, J.A.; Sánchez-Margalet, V. Sam68 Mediates the Activation of Insulin and Leptin Signalling in Breast Cancer Cells. PLoS ONE 2016, 11, e0158218. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maroni, P.; Citterio, L.; Piccoletti, R.; Bendinelli, P. Sam68 and ERKs regulate leptin-induced expression of OB-Rb mRNA in C2C12 myotubes. Mol. Cell. Endocrinol. 2009, 309, 26–31. [Google Scholar] [CrossRef][Green Version]
- Maroni, P. Leptin, Adiponectin, and Sam68 in Bone Metastasis from Breast Cancer. Int. J. Mol. Sci. 2020, 21, 1051. [Google Scholar] [CrossRef] [PubMed]
- Barone, I.; Giordano, C.; Bonofiglio, D.; Andò, S.; Catalano, S. Leptin, obesity and breast cancer: Progress to understanding the molecular connections. Curr. Opin. Pharmacol. 2016, 31, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, M.E.; Ray, A.; Nkhata, K.J.; Malakhov, D.A.; Rogozina, O.P.; Dogan, S.; Cleary, M.P. Obesity and breast cancer: Status of leptin and adiponectin in pathological processes. Cancer Metastasis Rev. 2010, 29, 641–653. [Google Scholar] [CrossRef]
- Maroni, P.; Bendinelli, P.; Morelli, D.; Drago, L.; Luzzati, A.; Perrucchini, G.; Bonini, C.; Matteucci, E.; Desiderio, M.A. High SPARC Expression Starting from Dysplasia, Associated with Breast Carcinoma, Is Predictive for Bone Metastasis without Enhancement of Plasma Levels. Int. J. Mol. Sci. 2015, 16, 28108–28122. [Google Scholar] [CrossRef]
- Rajan, P.; Dalgliesh, C.; Bourgeois, C.F.; Heiner, M.; Emami, K.; Clark, E.L.; Bindereif, A.; Stevenin, J.; Robson, C.N.; Leung, H.Y.; et al. Proteomic identification of heterogeneous nuclear ribonucleoprotein L as a novel component of SLM/Sam68 Nuclear Bodies. BMC Cell Biol. 2009, 10, 82. [Google Scholar] [CrossRef]
- Raut, P.K.; Park, P.H. Globular adiponectin antagonizes leptin-induced growth of cancer cells by modulating inflammasomes activation: Critical role of HO-1 signaling. Biochem. Pharmacol. 2020, 180, 114186. [Google Scholar] [CrossRef]
- Santoni, M.; Conti, A.; Procopio, G.; Porta, C.; Ibrahim, T.; Barni, S.; Guida, F.M.; Fontana, A.; Berruti, A.; Berardi, R.; et al. Bone metastases in patients with metastatic renal cell carcinoma: Are they always associated with poor prognosis? J. Exp. Clin. Cancer Res. 2015, 34, 10. [Google Scholar] [CrossRef] [PubMed]
- Silvestris, N.; Pantano, F.; Ibrahim, T.; Gamucci, T.; De Vita, F.; Di Palma, T.; Pedrazzoli, P.; Barni, S.; Bernardo, A.; Febbraro, A.; et al. Natural history of malignant bone disease in gastric cancer: Final results of a multicenter bone metastasis survey. PLoS ONE 2013, 8, e74402. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Kitayama, J.; Nagawa, H. Enhanced expression of leptin and leptin receptor (OB-R) in human breast cancer. Clin Cancer Res. 2004, 10, 4325–4331. [Google Scholar] [CrossRef] [PubMed]
- Jardé, T.; Caldefie-Chézet, F.; Damez, M.; Mishellany, F.; Penault-Llorca, F.; Guillot, J.; Vasson, M.P. Leptin and leptin receptor involvement in cancer development: A study on human primary breast carcinoma. Oncol. Rep. 2008, 19, 905–911. [Google Scholar] [CrossRef]
- Al-Shibli, S.M.; Amjad, N.M.; Al-Kubaisi, M.K.; Mizan, S. Subcellular localization of leptin and leptin receptor in breast cancer detected in an electron microscopic study. Biochem. Biophys. Res. Commun. 2017, 482, 1102–1106. [Google Scholar] [CrossRef]
- Jardé, T.; Perrier, S.; Vasson, M.P.; Caldefie-Chézet, F. Molecular mechanisms of leptin and adiponectin in breast cancer. Eur. J. Cancer 2011, 47, 33–43. [Google Scholar] [CrossRef]
- Llanos, A.A.M.; Lin, Y.; Chen, W.; Yao, S.; Norin, J.; Chekmareva, M.A.; Omene, C.; Cong, L.; Omilian, A.R.; Khoury, T.; et al. Immunohistochemical analysis of adipokine and adipokine receptor expression in the breast tumor microenvironment: Associations of lower leptin receptor expression with estrogen receptor-negative status and triple-negative subtype. Breast Cancer Res. 2020, 22, 18. [Google Scholar] [CrossRef]
- Elmquist, J.K.; Coppari, R.; Balthasar, N.; Ichinose, M.; Lowell, B.B. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J. Comp. Neurol. 2005, 493, 63–71. [Google Scholar] [CrossRef]
- Martin-Romero, C.; Sanchez-Margalet, V. Human leptin activates PI3K and MAPK pathways in human peripheral blood mononuclear cells: Possible role of Sam68. Cell. Immunol. 2001, 212, 83–91. [Google Scholar] [CrossRef]
- Locatelli, A.; Lange, C.A. Met receptors induce Sam68-dependent cell migration by activation of alternate extracellular signal-regulated kinase family members. J. Biol. Chem. 2011, 286, 21062–21072. [Google Scholar] [CrossRef]
- Li, Z.; Yu, C.P.; Zhong, Y.; Liu, T.J.; Huang, Q.D.; Zhao, X.H.; Huang, H.; Tu, H.; Jiang, S.; Zhang, Y.; et al. Sam68 expression and cytoplasmic localization is correlated with lymph node metastasis as well as prognosis in patients with early-stage cervical cancer. Ann. Oncol. 2012, 23, 638. [Google Scholar] [CrossRef] [PubMed]
- Huang, S. Review: Perinucleolar structures. J. Struct. Biol. 2000, 129, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Boisvert, F.-M.; Bazzet-Jones, D.P.; Richard, S. A role for the GSG domain in localizing Sam68 to novel nuclear structures in cancer cell lines. Mol. Biol. Cell 1999, 10, 3015–3033. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Resnick, R.J.; Rakowski, C.; Shalloway, D.; Taylor, S.J.; Blobel, G.A. Physical and functional interaction between the transcriptional cofactor CBP and the KH domain protein Sam68. Mol. Cancer Res. 2002, 1, 48–55. [Google Scholar] [PubMed]
- Valacca, C.; Bonomi, S.; Buratti, E.; Pedrotti, S.; Baralle, F.E.; Sette, C.; Ghigna, C.; Biamonti, G. Sam68 regulates EMT through alternative splicing-activated nonsense-mediated mRNA decay of the SF2/ASF proto-oncogene. J. Cell Biol. 2010, 191, 87–99. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, L.; Yuan, M.; Kuang, Z.; Zou, Y.; Tang, T.; Zhang, W.; Hu, X.; Xia, T.; Cao, T.; et al. Sam68 Promotes the Progression of Human Breast Cancer through inducing Activation of EphA3. Curr. Cancer Drug Targets 2020, 20, 76–83. [Google Scholar] [CrossRef]
- Genty, N.; Paly, J.; Edery, M.; Kelly, P.A.; Djiane, J.; Salesse, R. Endocytosis and degradation of prolactin and its receptor in Chinese hamster ovary cells stably transfected with prolactin receptor cDNA. Mol. Cell Endocrinol. 1994, 99, 221–228. [Google Scholar] [CrossRef]
- Levin, I.; Cohen, J.; Supino-Rosin, L.; Yoshimura, A.; Watowich, S.S.; Neumann, D. Identification of a cytoplasmic motif in the erythropoietin receptor required for receptor internalization. FEBS Lett. 1998, 427, 164–170. [Google Scholar] [CrossRef]
- Barr, V.A.; Lane, K.; Taylor, S.I. Subcellular localization and internalization of the four human leptin receptor isoforms. J. Biol. Chem. 1999, 274, 21416–21424. [Google Scholar] [CrossRef]
- Belouzard, S.; Delcroix, D.; Rouillé, Y. Low levels of expression of leptin receptor at the cell surface result from constitutive endocytosis and intracellular retention in the biosynthetic pathway. J. Biol. Chem. 2004, 279, 28499–28508. [Google Scholar] [CrossRef]
- Angelucci, A.; Clementi, L.; Alesse, E. Leptin in Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1259, 89–112. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Lee, Y.Y.; Yu, B.Y.; Yang, B.S.; Cho, K.H.; Yoon, D.K.; Roh, Y.K. Adiponectin induces growth arrest and apoptosis of MDA-MB-231 breast cancer cell. Arch. Pharm. Res. 2005, 28, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, M.E.; Nkhata, K.J.; Mizuno, N.K.; Ray, A.; Cleary, M.P. Effects of adiponectin on breast cancer cell growth and signaling. Br. J. Cancer 2008, 98, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lam, J.B.; Lam, K.S.; Liu, J.; Lam, M.C.; Hoo, R.L.; Wu, D.; Cooper, G.J.; Xu, A. Adiponectin modulates the glycogen synthase kinase-3 beta/beta-catenin signaling pathway and attenuates mammary tumorigenesis of MDA-MB-231 cells in nude mice. Cancer Res. 2006, 66, 11462–11470. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, E.; Benaitreau, D.; Dieudonne, M.N.; Leneveu, M.C.; Serazin, V.; Giudicelli, Y.; Pecquery, R. Adiponectin mediates an antiproliferative response in human MDA-MB 231 breast cancer cells. Oncol. Rep. 2008, 20, 971–977. [Google Scholar] [PubMed]
- Nakayama, S.; Miyoshi, Y.; Ishihara, H.; Noguchi, S. Growth-inhibitory effect of adiponectin via adiponectin receptor 1 on human breast cancer cells through inhibition of S-phase entry without inducing apoptosis. Breast Cancer Res. Treat. 2008, 112, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Mauro, L.; Pellegrino, M.; De Amicis, F.; Ricchio, E.; Giordano, F.; Rizza, P.; Catalano, S.; Bonofiglio, D.; Sisci, D.; Panno, M.L.; et al. Evidences that estrogen receptor α interferes with adiponectin effects on breast cancer cell growth. Cell Cycle 2014, 13, 553–564. [Google Scholar] [CrossRef]
- Mauro, L.; Pellegrino, M.; Giordano, F.; Ricchio, E.; Rizza, P.; De Amicis, F.; Catalano, S.; Bonofiglio, D.; Panno, M.L.; Andò, S. Estrogen receptor-α drives adiponectin effects on cyclin D1 expression in breast cancer cells. FASEB J. 2015, 29, 2150–2160. [Google Scholar] [CrossRef]
| Characteristic | No. | % |
|---|---|---|
| Age at surgery (years) | ||
| <50 | 4 | 18 |
| >50 <70 | 12 | 55 |
| >70 | 6 | 27 |
| Ki67 status | ||
| high (>15%) | 15 | 68 |
| low (<15%) | 3 | 14 |
| n.e. | 4 | 16 |
| ER status | ||
| ER+ | 21 | 95 |
| ER− | 1 | 5 |
| PR status | ||
| PR+ | 10 | 45 |
| PR− | 12 | 55 |
| HER2 status | ||
| HER2+ (>3+) | 3 | 14 |
| HER2− | 17 | 77 |
| n.e. | 2 | 9 |
| Staining Intensity | KHDRBS1 Nuclei | KHDRBS1 Cytosol | Leptin | LEPR Nuclei | LEPR Cytosol | Adiponectin |
|---|---|---|---|---|---|---|
| Negative | 4 | 6 | 3 | 1 | 1 | / |
| Low | 1 | 5 | 5 | 4 | 1 | 3 |
| Moderate | 2 | 10 | 11 | 10 | 5 | 11 |
| High | 15 (68%) | 1 | 3 | 7 | 15 | 8 |
| Mean score (n = 22) | 2.24 ± 0.29 ** | 1.09 ± 0.18 | 1.55 ± 0.19 | 1.84 ± 0.19 | 2.5 ± 0.19 § | 2.11 ± 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maroni, P.; Luzzati, A.; Perrucchini, G.; Cannavò, L.; Bendinelli, P. Leptin, Leptin Receptor, KHDRBS1 (KH RNA Binding Domain Containing, Signal Transduction Associated 1), and Adiponectin in Bone Metastasis from Breast Carcinoma: An Immunohistochemical Study. Biomedicines 2020, 8, 510. https://doi.org/10.3390/biomedicines8110510
Maroni P, Luzzati A, Perrucchini G, Cannavò L, Bendinelli P. Leptin, Leptin Receptor, KHDRBS1 (KH RNA Binding Domain Containing, Signal Transduction Associated 1), and Adiponectin in Bone Metastasis from Breast Carcinoma: An Immunohistochemical Study. Biomedicines. 2020; 8(11):510. https://doi.org/10.3390/biomedicines8110510
Chicago/Turabian StyleMaroni, Paola, Alessandro Luzzati, Giuseppe Perrucchini, Luca Cannavò, and Paola Bendinelli. 2020. "Leptin, Leptin Receptor, KHDRBS1 (KH RNA Binding Domain Containing, Signal Transduction Associated 1), and Adiponectin in Bone Metastasis from Breast Carcinoma: An Immunohistochemical Study" Biomedicines 8, no. 11: 510. https://doi.org/10.3390/biomedicines8110510
APA StyleMaroni, P., Luzzati, A., Perrucchini, G., Cannavò, L., & Bendinelli, P. (2020). Leptin, Leptin Receptor, KHDRBS1 (KH RNA Binding Domain Containing, Signal Transduction Associated 1), and Adiponectin in Bone Metastasis from Breast Carcinoma: An Immunohistochemical Study. Biomedicines, 8(11), 510. https://doi.org/10.3390/biomedicines8110510






