Radioprotection and Radiomitigation: From the Bench to Clinical Practice
Abstract
1. Introduction
2. Interaction of Ionizing Radiation with Living Matter
2.1. The Effects Are Determined by the Radiation Type and Its Penetration Capacity
2.2. Physical, Biological, and Chemical Regulatory Factors
2.3. Exposure to Ionizing Radiation: Adverse Effects
2.4. Acute and Chronic Radiation Syndromes
3. Radioprotectors, Radiomitigators, and Other Antiradiation Therapies
3.1. Radioprotectors
3.1.1. Thiol-Containing Molecules
3.1.2. Polyphenolic Phytochemicals
3.1.3. Nonpolyphenolic Phytochemicals
3.1.4. Vitamins
3.1.5. Oligoelements
3.1.6. Superoxide Dismutase
3.1.7. Ex-Rad
3.1.8. Nitroxides
3.1.9. Hormones and Hormone Analogues
3.1.10. Antibiotics
3.1.11. Adenosine Receptor Agonists
3.1.12. DNA-Binding Molecules
3.2. Radiomitigators
3.2.1. Glutamine
3.2.2. Probiotics
3.2.3. Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers
3.2.4. Statins
3.2.5. Somatostatin Analogues
3.2.6. Immunomodulators
3.2.7. Cytokines
3.2.8. Prostaglandins and Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
3.2.9. BIO 300
3.2.10. ABC294640
3.2.11. Cell Therapy
3.3. Radionuclides and Methods to Treat Contaminated Patients
3.3.1. Blockers
3.3.2. Dilution
3.3.3. Reducing Absorption
3.3.4. Displacement
3.3.5. Ion Exchange
3.3.6. Increased Turnover
3.3.7. Chelators and Functional Sorbents
3.3.8. Surgical Excision
3.3.9. Lung Lavage
4. Validated and Potential Biomarkers to Assess the Harmful Effects of Ionizing Radiation
4.1. Cytogenetics
4.2. Oxidative Stress-Induced Molecular Damage
4.3. Immune and Inflammation Mediators
4.4. Gene Expression
4.5. Gene Mutations
4.6. Epigenetics
4.7. Metabolomics, Proteomics, and Lipidomics
4.8. MicroRNAs (miRNAs)
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Zakariya, N.I.; Kahn, M.T.E. Benefits and Biological Effects of Ionizing Radiation. Sch. Acad. J. Biosci. SAJB 2014, 2, 583–591. [Google Scholar]
- Jeon, J. Review of Therapeutic Applications of Radiolabeled Functional Nanomaterials. Int. J. Mol. Sci. 2019, 20, 2323. [Google Scholar] [CrossRef] [PubMed]
- Nagataki, S.; Takamura, N. Radioactive Doses—Predicted and Actual—and Likely Health Effects. Clin. Oncol. R. Coll. Radiol. 2016, 28, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi-Gahrouei, D.; Gholami, M.; Setayandeh, S. A review on natural background radiation. Adv. Biomed. Res. 2013, 2, 65. [Google Scholar] [CrossRef] [PubMed]
- Martinez Marignac, V.L.; Mondragon, L.; Favant, J.L. Sources of ionizing radiation (IR) and their biological effects. An interdisciplinary view, from the physics to cell and molecular biology. Clin. Cancer Investig. J. 2019, 8, 129–138. [Google Scholar]
- Beaton, L.; Bandula, S.; Gaze, M.N.; Sharma, R.A. How rapid advances in imaging are defining the future of precision radiation oncology. Br. J. Cancer 2019, 120, 779–790. [Google Scholar] [CrossRef]
- Pacelli, R.; Caroprese, M.; Palma, G.; Oliviero, C.; Clemente, S.; Cella, L.; Conson, M. Technological evolution of radiation treatment: Implications for clinical applications. Semin. Oncol. 2019, 46, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Milano, M.T.; Mahesh, M.; Mettler, F.A.; Elee, J.; Vetter, R.J. Patient Radiation Exposure: Imaging During Radiation Oncology Procedures: Executive Summary of NCRP Report No. 184. J. Am. Coll. Radiol. JACR 2020, 17, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.A.; Symonds, P. Radiation Exposure and Health Effects—Is it Time to Reassess the Real Consequences? Clin. Oncol. R. Coll. Radiol. 2016, 28, 231–236. [Google Scholar] [CrossRef]
- Stewart, F.A.; Akleyev, A.V.; Hauer-Jensen, M.; Hendry, J.H.; Kleiman, N.J.; Macvittie, T.J.; Aleman, B.M.; Edgar, A.B.; Mabuchi, K.; Muirhead, C.R.; et al. ICRP publication 118: ICRP statement on tissue reactions and early and late effects of radiation in normal tissues and organs—Threshold doses for tissue reactions in a radiation protection context. Ann. ICRP 2012, 41, 1–322. [Google Scholar] [CrossRef] [PubMed]
- Bolus, N.E. Basic Review of Radiation Biology and Terminology. J. Nucl. Med. Technol. 2017, 45, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, P.G.S.; Narayanan, D.; Hallett, K.; Bernhard, E.J.; Ahmed, M.M.; Evans, G.; Vikram, B.; Weingarten, M.; Coleman, C.N. Radioprotectors and Radiomitigators for Improving Radiation Therapy: The Small Business Innovation Research (SBIR) Gateway for Accelerating Clinical Translation. Radiat. Res. 2015, 184, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, K.; Narayanan, D.; Vikram, B.; Evans, G.; Coleman, C.N.; Prasanna, P.G.S. Decreasing the Toxicity of Radiation Therapy: Radioprotectors and Radiomitigators Being Developed by the National Cancer Institute Through Small Business Innovation Research Contracts. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 188–196. [Google Scholar] [CrossRef]
- Citrin, D.; Cotrim, A.P.; Hyodo, F.; Baum, B.J.; Krishna, M.C.; Mitchell, J.B. Radioprotectors and mitigators of radiation-induced normal tissue injury. Oncologist 2010, 15, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, B.S.; Overgaard, J.; Bassler, N. In vitro RBE-LET dependence for multiple particle types. Acta Oncol. Stockh. Swed. 2011, 50, 757–762. [Google Scholar] [CrossRef]
- de Kruijff, R.M.; Wolterbeek, H.T.; Denkova, A.G. A critical review of alpha radionuclide therapy—How to deal with recoiling daughters? Pharmaceuticals 2015, 8, 321–336. [Google Scholar] [CrossRef]
- Kirwan, J.F.; Constable, P.H.; Murdoch, I.E.; Khaw, P.T. Beta irradiation: New uses for an old treatment: A review. Eye 2003, 17, 207–215. [Google Scholar] [CrossRef]
- Reisz, J.A.; Bansal, N.; Qian, J.; Zhao, W.; Furdui, C.M. Effects of ionizing radiation on biological molecules–mechanisms of damage and emerging methods of detection. Antioxid. Redox Signal. 2014, 21, 260–292. [Google Scholar] [CrossRef]
- Sasaki, M.S.; Endo, S.; Hoshi, M.; Nomura, T. Neutron relative biological effectiveness in Hiroshima and Nagasaki atomic bomb survivors: A critical review. J. Radiat. Res. (Tokyo) 2016, 57, 583–595. [Google Scholar] [CrossRef]
- Lühr, A.; von Neubeck, C.; Pawelke, J.; Seidlitz, A.; Peitzsch, C.; Bentzen, S.M.; Bortfeld, T.; Debus, J.; Deutsch, E.; Langendijk, J.A.; et al. “Radiobiology of Proton Therapy”: Results of an international expert workshop. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2018, 128, 56–67. [Google Scholar] [CrossRef]
- De Marzi, L.; Patriarca, A.; Scher, N.; Thariat, J.; Vidal, M. Exploiting the full potential of proton therapy: An update on the specifics and innovations towards spatial or temporal optimisation of dose delivery. Cancer Radiother. J. Soc. Francaise Radiother. Oncol. 2020, 24, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Malouff, T.D.; Mahajan, A.; Krishnan, S.; Beltran, C.; Seneviratne, D.S.; Trifiletti, D.M. Carbon Ion Therapy: A Modern Review of an Emerging Technology. Front. Oncol. 2020, 10, 82. [Google Scholar]
- Dumont, F.; Le Roux, A.; Bischoff, P. Radiation countermeasure agents: An update. Expert Opin. Ther. Pat. 2010, 20, 73–101. [Google Scholar] [CrossRef]
- Singh, V.K.; Seed, T.M. A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: Part I. Radiation sub-syndromes, animal models and FDA-approved countermeasures. Int. J. Radiat. Biol. 2017, 93, 851–869. [Google Scholar] [CrossRef] [PubMed]
- Rajaraman, P.; Hauptmann, M.; Bouffler, S.; Wojcik, A. Human individual radiation sensitivity and prospects for prediction. Ann. ICRP 2018, 47, 126–141. [Google Scholar] [CrossRef]
- NIRS. Available online: https://www.nirs.org/ (accessed on 25 April 2020).
- McBride, W.H.; Schaue, D. Radiation-induced tissue damage and response. J. Pathol. 2020, 250, 647–655. [Google Scholar] [CrossRef]
- Lorimore, S.A.; Coates, P.J.; Scobie, G.E.; Milne, G.; Wright, E.G. Inflammatory-type responses after exposure to ionizing radiation in vivo: A mechanism for radiation-induced bystander effects? Oncogene 2001, 20, 7085–7095. [Google Scholar] [CrossRef]
- Li, Y.-Q.; Chen, P.; Jain, V.; Reilly, R.M.; Wong, C.S. Early radiation-induced endothelial cell loss and blood-spinal cord barrier breakdown in the rat spinal cord. Radiat. Res. 2004, 161, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Di Maggio, F.M.; Minafra, L.; Forte, G.I.; Cammarata, F.P.; Lio, D.; Messa, C.; Gilardi, M.C.; Bravatà, V. Portrait of inflammatory response to ionizing radiation treatment. J. Inflamm. Lond. Engl. 2015, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Domina, E.A.; Philchenkov, A.; Dubrovska, A. Individual Response to Ionizing Radiation and Personalized Radiotherapy. Crit. Rev. Oncog. 2018, 23, 69–92. [Google Scholar] [CrossRef] [PubMed]
- Bentzen, S.M. Preventing or reducing late side effects of radiation therapy: Radiobiology meets molecular pathology. Nat. Rev. Cancer 2006, 6, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Straub, J.M.; New, J.; Hamilton, C.D.; Lominska, C.; Shnayder, Y.; Thomas, S.M. Radiation-induced fibrosis: Mechanisms and implications for therapy. J. Cancer Res. Clin. Oncol. 2015, 141, 1985–1994. [Google Scholar] [CrossRef]
- Kainthola, A.; Haritwal, T.; Tiwari, M.; Gupta, N.; Parvez, S.; Tiwari, M.; Prakash, H.; Agrawala, P.K. Immunological Aspect of Radiation-Induced Pneumonitis, Current Treatment Strategies, and Future Prospects. Front. Immunol. 2017, 8, 506. [Google Scholar] [CrossRef] [PubMed]
- Guipaud, O.; Jaillet, C.; Clément-Colmou, K.; François, A.; Supiot, S.; Milliat, F. The importance of the vascular endothelial barrier in the immune-inflammatory response induced by radiotherapy. Br. J. Radiol. 2018, 91, 20170762. [Google Scholar] [CrossRef]
- Vorobiev, A.I. Acute radiation disease and biological dosimetry in 1993. Stem Cells 1997, 15 (Suppl. 2), 269–274. [Google Scholar] [CrossRef] [PubMed]
- Stenke, L.; Lindberg, K.; Lagergren Lindberg, M.; Lewensohn, R.; Valentin, J.; Powles, R.; Dainiak, N. Coordination of management of the acute radiation syndrome. Radiat. Prot. Dosim. 2018, 182, 80–84. [Google Scholar] [CrossRef]
- Dörr, H.; Meineke, V. Acute radiation syndrome caused by accidental radiation exposure—Therapeutic principles. BMC Med. 2011, 9, 126. [Google Scholar] [CrossRef]
- Braga-Tanaka, I.; Tanaka, S.; Kohda, A.; Takai, D.; Nakamura, S.; Ono, T.; Tanaka, K.; Komura, J.-I. Experimental studies on the biological effects of chronic low dose-rate radiation exposure in mice: Overview of the studies at the Institute for Environmental Sciences. Int. J. Radiat. Biol. 2018, 94, 423–433. [Google Scholar] [CrossRef]
- Kwak, Y.-K.; Lee, S.-W.; Kay, C.S.; Park, H.H. Intensity-modulated radiotherapy reduces gastrointestinal toxicity in pelvic radiation therapy with moderate dose. PLoS ONE 2017, 12, e0183339. [Google Scholar] [CrossRef]
- Ojima, M.; Hirouchi, T.; Etani, R.; Ariyoshi, K.; Fujishima, Y.; Kai, M. Dose-Rate-Dependent PU.1 Inactivation to Develop Acute Myeloid Leukemia in Mice Through Persistent Stem Cell Proliferation After Acute or Chronic Gamma Irradiation. Radiat. Res. 2019, 192, 612–620. [Google Scholar] [CrossRef]
- Dale, W.M.; Davies, J.V.; Meredith, W.J. Further observations on the protection effect in radiation chemistry. Br. J. Cancer 1949, 3, 31–41. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Patt, H.M.; Tyree, E.B.; Straube, R.L.; Smith, D.E. Cysteine Protection against X Irradiation. Science 1949, 110, 213–214. [Google Scholar] [CrossRef]
- Denekamp, J.; Michael, B.D.; Rojas, A.; Stewart, F.A. Thiol radioprotection in vivo: The critical role of tissue oxygen concentration. Br. J. Radiol. 1981, 54, 1112–1114. [Google Scholar] [CrossRef] [PubMed]
- Koukourakis, M.I. Amifostine in clinical oncology: Current use and future applications. Anticancer Drugs 2002, 13, 181–209. [Google Scholar] [CrossRef] [PubMed]
- Koukourakis, M.I.; Giatromanolaki, A.; Zois, C.E.; Kalamida, D.; Pouliliou, S.; Karagounis, I.V.; Yeh, T.-L.; Abboud, M.I.; Claridge, T.D.W.; Schofield, C.J.; et al. Normal tissue radioprotection by amifostine via Warburg-type effects. Sci. Rep. 2016, 6, 30986. [Google Scholar] [CrossRef] [PubMed]
- Kouvaris, J.R.; Kouloulias, V.E.; Vlahos, L.J. Amifostine: The first selective-target and broad-spectrum radioprotector. Oncologist 2007, 12, 738–747. [Google Scholar] [CrossRef]
- Floersheim, G.L.; Bieri, A. Further studies on selective radioprotection by organic zinc salts and synergism of zinc aspartate with WR 2721. Br. J. Radiol. 1990, 63, 468–475. [Google Scholar] [CrossRef]
- Patchen, M.L.; MacVittie, T.J.; Solberg, B.D.; D’Alesandro, M.M.; Brook, I. Radioprotection by polysaccharides alone and in combination with aminothiols. Adv. Space Res. Off. J. Comm. Space Res. COSPAR 1992, 12, 233–248. [Google Scholar] [CrossRef]
- Prasanna, P.G.; Uma Devi, P. Modification of WR-2721 radiation protection from gastrointestinal injury and death in mice by 2-mercaptopropionylglycine. Radiat. Res. 1993, 133, 111–115. [Google Scholar] [CrossRef]
- Margulies, B.S.; Damron, T.A.; Allen, M.J. The differential effects of the radioprotectant drugs amifostine and sodium selenite treatment in combination with radiation therapy on constituent bone cells, Ewing’s sarcoma of bone tumor cells, and rhabdomyosarcoma tumor cells in vitro. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2008, 26, 1512–1519. [Google Scholar] [CrossRef]
- Miller, R.C.; Murley, J.S.; Grdina, D.J. Metformin exhibits radiation countermeasures efficacy when used alone or in combination with sulfhydryl containing drugs. Radiat. Res. 2014, 181, 464–470. [Google Scholar] [CrossRef]
- Liu, W.; Chen, Q.; Wu, S.; Xia, X.; Wu, A.; Cui, F.; Gu, Y.-P.; Zhang, X.; Cao, J. Radioprotector WR-2721 and mitigating peptidoglycan synergistically promote mouse survival through the amelioration of intestinal and bone marrow damage. J. Radiat. Res. (Tokyo) 2015, 56, 278–286. [Google Scholar] [CrossRef]
- Singh, V.K.; Fatanmi, O.O.; Wise, S.Y.; Newman, V.L.; Romaine, P.L.P.; Seed, T.M. The potentiation of the radioprotective efficacy of two medical countermeasures, gamma-tocotrienol and amifostine, by a combination prophylactic modality. Radiat. Prot. Dosim. 2016, 172, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.F.; Landauer, M.R. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology 2003, 189, 1–20. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Fischer, N.; Seo, E.-J.; Efferth, T. Prevention from radiation damage by natural products. Phytomed. Int. J. Phytother. Phytopharm. 2018, 47, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Landauer, M.R.; Srinivasan, V.; Seed, T.M. Genistein treatment protects mice from ionizing radiation injury. J. Appl. Toxicol. JAT 2003, 23, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, J.S.; Moon, C.; Kim, J.C.; Lee, Y.S.; Jang, J.S.; Jo, S.K.; Kim, S.H. Modification of gamma-radiation response in mice by green tea polyphenols. Phytother. Res. PTR 2008, 22, 1380–1383. [Google Scholar] [CrossRef]
- Koide, K.; Osman, S.; Garner, A.L.; Song, F.; Dixon, T.; Greenberger, J.S.; Epperly, M.W. The Use of 3,5,4′-Tri-O-acetylresveratrol as a Potential Prodrug for Resveratrol Protects Mice from γ-Irradiation-Induced Death. ACS Med. Chem. Lett. 2011, 2, 270. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Tiku, A.B. Biochemical and Molecular Mechanisms of Radioprotective Effects of Naringenin, a Phytochemical from Citrus Fruits. J. Agric. Food Chem. 2016, 64, 1676–1685. [Google Scholar] [CrossRef] [PubMed]
- Mun, G.-I.; Kim, S.; Choi, E.; Kim, C.S.; Lee, Y.-S. Pharmacology of natural radioprotectors. Arch. Pharm. Res. 2018, 41, 1033–1050. [Google Scholar] [CrossRef] [PubMed]
- Shoji, Y.; Nakashima, H. Nutraceutics and delivery systems. J. Drug Target. 2004, 12, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Estrela, J.M.; Mena, S.; Obrador, E.; Benlloch, M.; Castellano, G.; Salvador, R.; Dellinger, R.W. Polyphenolic Phytochemicals in Cancer Prevention and Therapy: Bioavailability versus Bioefficacy. J. Med. Chem. 2017, 60, 9413–9436. [Google Scholar] [CrossRef]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The Reciprocal Interactions between Polyphenols and Gut Microbiota and Effects on Bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef]
- Serreli, G.; Deiana, M. In vivo formed metabolites of polyphenols and their biological efficacy. Food Funct. 2019, 10, 6999–7021. [Google Scholar] [CrossRef]
- Sirerol, J.A.; Feddi, F.; Mena, S.; Rodriguez, M.L.; Sirera, P.; Aupí, M.; Pérez, S.; Asensi, M.; Ortega, A.; Estrela, J.M. Topical treatment with pterostilbene, a natural phytoalexin, effectively protects hairless mice against UVB radiation-induced skin damage and carcinogenesis. Free Radic. Biol. Med. 2015, 85, 1–11. [Google Scholar] [CrossRef]
- Estrela, J.M.; Ortega, A.; Mena, S.; Rodriguez, M.L.; Asensi, M. Pterostilbene: Biomedical applications. Crit. Rev. Clin. Lab. Sci. 2013, 50, 65–78. [Google Scholar] [CrossRef]
- Hall, S.; Desbrow, B.; Anoopkumar-Dukie, S.; Davey, A.K.; Arora, D.; McDermott, C.; Schubert, M.M.; Perkins, A.V.; Kiefel, M.J.; Grant, G.D. A review of the bioactivity of coffee, caffeine and key coffee constituents on inflammatory responses linked to depression. Food Res. Int. 2015, 76, 626–636. [Google Scholar] [CrossRef]
- Stelzer, K.J.; Koh, W.J.; Kurtz, H.; Greer, B.E.; Griffin, T.W. Caffeine consumption is associated with decreased severe late toxicity after radiation to the pelvis. Int. J. Radiat. Oncol. Biol. Phys. 1994, 30, 411–417. [Google Scholar] [CrossRef]
- George, K.C.; Hebbar, S.A.; Kale, S.P.; Kesavan, P.C. Caffeine protects mice against whole-body lethal dose of gamma-irradiation. J. Radiol. Prot. Off. J. Soc. Radiol. Prot. 1999, 19, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Kumar, A.; Adhikari, J.S.; Rizvi, M.A.; Chaudhury, N.K. Protective effect of sesamol against 60Co γ-ray-induced hematopoietic and gastrointestinal injury in C57BL/6 male mice. Free Radic. Res. 2015, 49, 1344–1361. [Google Scholar] [CrossRef]
- Fan, S.; Meng, Q.; Xu, J.; Jiao, Y.; Zhao, L.; Zhang, X.; Sarkar, F.H.; Brown, M.L.; Dritschilo, A.; Rosen, E.M. DIM (3,3′-diindolylmethane) confers protection against ionizing radiation by a unique mechanism. Proc. Natl. Acad. Sci. USA 2013, 110, 18650–18655. [Google Scholar] [CrossRef]
- Seifter, E.; Mendecki, J.; Holtzman, S.; Kanofsky, J.D.; Friedenthal, E.; Davis, L.; Weinzweig, J. Role of vitamin A and beta carotene in radiation protection: Relation to antioxidant properties. Pharmacol. Ther. 1988, 39, 357–365. [Google Scholar] [CrossRef]
- Jacobs, M.M. Vitamins and Minerals in the Prevention and Treatment of Cancer; CRC Press: Boca Raton, FL, USA, 2018; ISBN 978-1-351-09449-8. [Google Scholar]
- Yamamoto, T.; Kinoshita, M.; Shinomiya, N.; Hiroi, S.; Sugasawa, H.; Matsushita, Y.; Majima, T.; Saitoh, D.; Seki, S. Pretreatment with ascorbic acid prevents lethal gastrointestinal syndrome in mice receiving a massive amount of radiation. J. Radiat. Res. (Tokyo) 2010, 51, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Kinoshita, M.; Yamamoto, T.; Ito, M.; Nishida, T.; Takeuchi, M.; Saitoh, D.; Seki, S.; Mukai, Y. Treatment of irradiated mice with high-dose ascorbic acid reduced lethality. PLoS ONE 2015, 10, e0117020. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Weiss, J.F. Radioprotection by vitamin E: Injectable vitamin E administered alone or with WR-3689 enhances survival of irradiated mice. Int. J. Radiat. Oncol. Biol. Phys. 1992, 23, 841–845. [Google Scholar] [CrossRef]
- Singh, V.K.; Beattie, L.A.; Seed, T.M. Vitamin E: Tocopherols and tocotrienols as potential radiation countermeasures. J. Radiat. Res. (Tokyo) 2013, 54, 973–988. [Google Scholar] [CrossRef]
- Kumar, K.S.; Srinivasan, V.; Toles, R.; Jobe, L.; Seed, T.M. Nutritional approaches to radioprotection: Vitamin E. Mil. Med. 2002, 167, 57–59. [Google Scholar]
- Satyamitra, M.M.; Kulkarni, S.; Ghosh, S.P.; Mullaney, C.P.; Condliffe, D.; Srinivasan, V. Hematopoietic Recovery and Amelioration of Radiation-Induced Lethality by the Vitamin E Isoform δ-Tocotrienol. Radiat. Res. 2011, 175, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.P.; Perkins, M.W.; Hieber, K.; Kulkarni, S.; Kao, T.-C.; Reddy, E.P.; Reddy, M.V.R.; Maniar, M.; Seed, T.; Kumar, K.S. Radiation protection by a new chemical entity, Ex-Rad: Efficacy and mechanisms. Radiat. Res. 2009, 171, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Berbée, M.; Fu, Q.; Garg, S.; Kulkarni, S.; Kumar, K.S.; Hauer-Jensen, M. Pentoxifylline enhances the radioprotective properties of γ-tocotrienol: Differential effects on the hematopoietic, gastrointestinal and vascular systems. Radiat. Res. 2011, 175, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Nukala, U.; Thakkar, S.; Krager, K.J.; Breen, P.J.; Compadre, C.M.; Aykin-Burns, N. Antioxidant Tocols as Radiation Countermeasures (Challenges to be Addressed to Use Tocols as Radiation Countermeasures in Humans). Antioxidants 2018, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Kulkarni, S.; Fatanmi, O.O.; Wise, S.Y.; Newman, V.L.; Romaine, P.L.P.; Hendrickson, H.; Gulani, J.; Ghosh, S.P.; Kumar, K.S.; et al. Radioprotective Efficacy of Gamma-Tocotrienol in Nonhuman Primates. Radiat. Res. 2016, 185, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Haddad, P.; Kalaghchi, B.; Amouzegar-Hashemi, F. Pentoxifylline and vitamin E combination for superficial radiation-induced fibrosis: A phase II clinical trial. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2005, 77, 324–326. [Google Scholar] [CrossRef]
- Hosseinimehr, S.J. The protective effects of trace elements against side effects induced by ionizing radiation. Radiat. Oncol. J. 2015, 33, 66–74. [Google Scholar] [CrossRef]
- Weiss, J.F.; Srinivasan, V.; Kumar, K.S.; Landauer, M.R. Radioprotection by metals: selenium. Adv. Space Res. Off. J. Comm. Space Res. COSPAR 1992, 12, 223–231. [Google Scholar] [CrossRef]
- Verma, P.; Kunwar, A.; Indira Priyadarsini, K. Effect of Low-Dose Selenium Supplementation on the Genotoxicity, Tissue Injury and Survival of Mice Exposed to Acute Whole-Body Irradiation. Biol. Trace Elem. Res. 2017, 179, 130–139. [Google Scholar] [CrossRef]
- Kunwar, A.; Bansal, P.; Kumar, S.J.; Bag, P.P.; Paul, P.; Reddy, N.D.; Kumbhare, L.B.; Jain, V.K.; Chaubey, R.C.; Unnikrishnan, M.K.; et al. In vivo radioprotection studies of 3,3’-diselenodipropionic acid, a selenocystine derivative. Free Radic. Biol. Med. 2010, 48, 399–410. [Google Scholar] [CrossRef]
- Du, J.; Gu, Z.; Yan, L.; Yong, Y.; Yi, X.; Zhang, X.; Liu, J.; Wu, R.; Ge, C.; Chen, C.; et al. Poly(Vinylpyrollidone)- and Selenocysteine-Modified Bi2 Se3 Nanoparticles Enhance Radiotherapy Efficacy in Tumors and Promote Radioprotection in Normal Tissues. Adv. Mater. Deerfield Beach Fla 2017, 29, 1701268. [Google Scholar] [CrossRef] [PubMed]
- Ertekin, M.V.; Koç, M.; Karslioglu, I.; Sezen, O. Zinc sulfate in the prevention of radiation-induced oropharyngeal mucositis: A prospective, placebo-controlled, randomized study. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 167–174. [Google Scholar] [CrossRef]
- Watanabe, T.; Ishihara, M.; Matsuura, K.; Mizuta, K.; Itoh, Y. Polaprezinc prevents oral mucositis associated with radiochemotherapy in patients with head and neck cancer. Int. J. Cancer 2010, 127, 1984–1990. [Google Scholar] [CrossRef]
- Muecke, R.; Micke, O.; Schomburg, L.; Buentzel, J.; Kisters, K.; Adamietz, I.A. and on behalf of AKTE. Selenium in Radiation Oncology-15 Years of Experiences in Germany. Nutrients 2018, 10, 483. [Google Scholar] [CrossRef]
- Petkau, A.; Chelack, W.S.; Pleskach, S.D. Protection by superoxide dismutase of white blood cells in X-irradiated mice. Life Sci. 1978, 22, 867–882. [Google Scholar] [CrossRef]
- Eastgate, J.; Moreb, J.; Nick, H.S.; Suzuki, K.; Taniguchi, N.; Zucali, J.R. A role for manganese superoxide dismutase in radioprotection of hematopoietic stem cells by interleukin-1. Blood 1993, 81, 639–646. [Google Scholar] [CrossRef]
- Yan, S.; Brown, S.L.; Kolozsvary, A.; Freytag, S.O.; Lu, M.; Kim, J.H. Mitigation of radiation-induced skin injury by AAV2-mediated MnSOD gene therapy. J. Gene Med. 2008, 10, 1012–1018. [Google Scholar] [CrossRef]
- Patyar, R.R.; Patyar, S. Role of drugs in the prevention and amelioration of radiation induced toxic effects. Eur. J. Pharmacol. 2018, 819, 207–216. [Google Scholar] [CrossRef]
- Chen, H.-X.; Xiang, H.; Xu, W.-H.; Li, M.; Yuan, J.; Liu, J.; Sun, W.-J.; Zhang, R.; Li, J.; Ren, Z.-Q.; et al. Manganese Superoxide Dismutase Gene-Modified Mesenchymal Stem Cells Attenuate Acute Radiation-Induced Lung Injury. Hum. Gene Ther. 2017, 28, 523–532. [Google Scholar] [CrossRef]
- Batinic-Haberle, I.; Tovmasyan, A.; Spasojevic, I. Mn Porphyrin-Based Redox-Active Drugs: Differential Effects as Cancer Therapeutics and Protectors of Normal Tissue Against Oxidative Injury. Antioxid. Redox Signal. 2018, 29, 1691–1724. [Google Scholar] [CrossRef]
- Weitzel, D.H.; Tovmasyan, A.; Ashcraft, K.A.; Rajic, Z.; Weitner, T.; Liu, C.; Li, W.; Buckley, A.F.; Prasad, M.R.; Young, K.H.; et al. Radioprotection of the brain white matter by Mn(III) n-Butoxyethylpyridylporphyrin-based superoxide dismutase mimic MnTnBuOE-2-PyP5+. Mol. Cancer Ther. 2015, 14, 70–79. [Google Scholar] [CrossRef]
- Leu, D.; Spasojevic, I.; Nguyen, H.; Deng, B.; Tovmasyan, A.; Weitner, T.; Sampaio, R.S.; Batinic-Haberle, I.; Huang, T.-T. CNS bioavailability and radiation protection of normal hippocampal neurogenesis by a lipophilic Mn porphyrin-based superoxide dismutase mimic, MnTnBuOE-2-PyP5. Redox Biol. 2017, 12, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.P.; Kulkarni, S.; Hieber, K.; Toles, R.; Romanyukha, L.; Kao, T.-C.; Hauer-Jensen, M.; Kumar, K.S. Gamma-tocotrienol, a tocol antioxidant as a potent radioprotector. Int. J. Radiat. Biol. 2009, 85, 598–606. [Google Scholar] [CrossRef]
- Suman, S.; Datta, K.; Doiron, K.; Ren, C.; Kumar, R.; Taft, D.R.; Fornace, A.J.; Maniar, M. Radioprotective effects of ON 01210.Na upon oral administration. J. Radiat. Res. (Tokyo) 2012, 53, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Kang, A.D.; Cosenza, S.C.; Bonagura, M.; Manair, M.; Reddy, M.V.R.; Reddy, E.P. ON01210.Na (Ex-RAD®) mitigates radiation damage through activation of the AKT pathway. PLoS ONE 2013, 8, e58355. [Google Scholar] [CrossRef] [PubMed]
- Dent, P.; Yacoub, A.; Contessa, J.; Caron, R.; Amorino, G.; Valerie, K.; Hagan, M.P.; Grant, S.; Schmidt-Ullrich, R. Stress and radiation-induced activation of multiple intracellular signaling pathways. Radiat. Res. 2003, 159, 283–300. [Google Scholar] [CrossRef]
- Feng, T.; Wang, L.; Zhou, N.; Liu, C.; Cui, J.; Wu, R.; Jing, J.; Zhang, S.; Chen, H.; Wang, S. Salidroside, a scavenger of ROS, enhances the radioprotective effect of Ex-RAD® via a p53-dependent apoptotic pathway. Oncol. Rep. 2017, 38, 3094–3102. [Google Scholar] [CrossRef]
- Soule, B.P.; Hyodo, F.; Matsumoto, K.-I.; Simone, N.L.; Cook, J.A.; Krishna, M.C.; Mitchell, J.B. Therapeutic and clinical applications of nitroxide compounds. Antioxid. Redox Signal. 2007, 9, 1731–1743. [Google Scholar] [CrossRef]
- Hahn, S.M.; Tochner, Z.; Krishna, C.M.; Glass, J.; Wilson, L.; Samuni, A.; Sprague, M.; Venzon, D.; Glatstein, E.; Mitchell, J.B. Tempol, a stable free radical, is a novel murine radiation protector. Cancer Res. 1992, 52, 1750–1753. [Google Scholar]
- Ramachandran, L.; Nair, C.K.K. Prevention of γ-radiation induced cellular genotoxicity by tempol: Protection of hematopoietic system. Environ. Toxicol. Pharmacol. 2012, 34, 253–262. [Google Scholar] [CrossRef]
- Hu, L.; Wang, Y.; Cotrim, A.P.; Zhu, Z.; Gao, R.; Zheng, C.; Goldsmith, C.M.; Jin, L.; Zhang, C.; Mitchell, J.B.; et al. Effect of Tempol on the prevention of irradiation-induced mucositis in miniature pigs. Oral Dis. 2017, 23, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.S. Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol. Ther. 2010, 126, 119–145. [Google Scholar] [CrossRef]
- Zetner, D.; Andersen, L.P.H.; Rosenberg, J. Melatonin as Protection Against Radiation Injury: A Systematic Review. Drug Res. 2016, 66, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Vijayalaxmi; Reiter, R.J.; Herman, T.S.; Meltz, M.L. Melatonin and radioprotection from genetic damage: In vivo/in vitro studies with human volunteers. Mutat. Res. 1996, 371, 221–228. [Google Scholar] [CrossRef]
- Vijayalaxmi; Meltz, M.L.; Reiter, R.J.; Herman, T.S.; Kumar, K.S. Melatonin and protection from whole-body irradiation: Survival studies in mice. Mutat. Res. 1999, 425, 21–27. [Google Scholar] [CrossRef]
- Elsabagh, H.H.; Moussa, E.; Mahmoud, S.A.; Elsaka, R.O.; Abdelrahman, H. Efficacy of Melatonin in prevention of radiation-induced oral mucositis: A randomized clinical trial. Oral Dis. 2020, 26, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Vasin, M.V.; Chernov, G.A.; Koroleva, L.V.; L’vova, T.S.; Abramov, M.M.; Antipov, V.V.; Suvorov, N.N. Mechanism of the radiation-protective effect of indralin. Radiats Biol. Radioecol. 1996, 36, 36–46. [Google Scholar]
- Pulatova, M.K.; Sharygin, V.L.; Todorov, I.N. The activation of ribonucleotide reductase in animal organs as the cellular response against the treatment with DNA-damaging factors and the influence of radioprotectors on this effect. Biochim. Biophys. Acta 1999, 1453, 321–329. [Google Scholar] [CrossRef][Green Version]
- Vasin, M.V.; Chernov, G.A.; Antipov, V.V. Width of radiation protective effects of indralin in comparative studies using different animal species. Radiats Biol. Radioecol. 1997, 37, 896–904. [Google Scholar]
- Vasin, M.V.; Semenov, L.F.; Suvorov, N.N.; Antipov, V.V.; Ushakov, I.B.; Ilyin, L.A.; Lapin, B.A. Protective effect and the therapeutic index of indralin in juvenile rhesus monkeys. J. Radiat. Res. (Tokyo) 2014, 55, 1048–1055. [Google Scholar] [CrossRef]
- Vasin, M.V.; Antipov, V.V.; Komarova, S.N.; Semenova, L.A.; Galkin, A.A. Radioprotective properties of indralin combined with cystamine and mexamine. Radiats. Biol. Radioecol. 2011, 51, 243–246. [Google Scholar] [CrossRef]
- Byron, J.W.; Haigh, M.V.; Lajtha, L.G. Effect of an antibiotic regime on monkeys exposed to total-body irradiation. Nature 1964, 202, 977–979. [Google Scholar] [CrossRef] [PubMed]
- Mastromarino, A.; Wilson, R. Antibiotic radioprotection of mice exposed to supralethal whole-body irradiation independent of antibacterial activity. Radiat. Res. 1976, 68, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Pollard, J.M.; Norris, A.J.; McDonald, J.T.; Sun, Y.; Micewicz, E.; Pettijohn, K.; Damoiseaux, R.; Iwamoto, K.S.; Sayre, J.W.; et al. High Throughput Screening Identifies Two Classes of Antibiotics as Radioprotectors: Tetracyclines and Fluoroquinolones. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 7238. [Google Scholar] [CrossRef] [PubMed]
- Alok, A.; Chaudhury, N.K. Tetracycline hydrochloride: A potential clinical drug for radioprotection. Chem. Biol. Interact. 2016, 245, 90–99. [Google Scholar] [CrossRef]
- Hosek, B.; Bohácek, J.; Sikulová, J.; Pospísil, M.; Vacek, A. Protection of early cellular damage in 1 Gy-irradiated mice by the elevation of extracellular adenosine. Radiat. Environ. Biophys. 1992, 31, 289–297. [Google Scholar] [CrossRef]
- Pospísil, M.; Hofer, M.; Netíková, J.; Pipalová, I.; Vacek, A.; Bartonícková, A.; Volenec, K. Elevation of extracellular adenosine induces radioprotective effects in mice. Radiat. Res. 1993, 134, 323–330. [Google Scholar] [CrossRef]
- Pospísil, M.; Hofer, M.; Znojil, V.; Vácha, J.; Netíková, J.; Holá, J. Radioprotection of mouse hemopoiesis by dipyridamole and adenosine monophosphate in fractionated treatment. Radiat. Res. 1995, 142, 16–22. [Google Scholar] [CrossRef]
- Hofer, M.; Pospísil, M.; Znojil, V.; Holá, J.; Vacek, A.; Streitová, D. Adenosine A(3) receptor agonist acts as a homeostatic regulator of bone marrow hematopoiesis. Biomed. Pharmacother. Biomed. Pharmacother. 2007, 61, 356–359. [Google Scholar] [CrossRef]
- Hofer, M.; Pospísil, M.; Sefc, L.; Dusek, L.; Vacek, A.; Holá, J.; Hoferová, Z.; Streitová, D. Activation of adenosine A(3) receptors supports hematopoiesis-stimulating effects of granulocyte colony-stimulating factor in sublethally irradiated mice. Int. J. Radiat. Biol. 2010, 86, 649–656. [Google Scholar] [CrossRef]
- Hofer, M.; Pospíšil, M.; Dušek, L.; Hoferová, Z.; Weiterová, L. Inhibition of cyclooxygenase-2 promotes the stimulatory action of adenosine A3 receptor agonist on hematopoiesis in sublethally γ-irradiated mice. Biomed. Pharmacother. Biomed. Pharmacother. 2011, 65, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Hofer, M.; Pospíšil, M.; Dušek, L.; Hoferová, Z.; Komůrková, D. Agonist of the adenosine A3 receptor, IB-MECA, and inhibitor of cyclooxygenase-2, meloxicam, given alone or in a combination early after total body irradiation enhance survival of γ-irradiated mice. Radiat. Environ. Biophys. 2014, 53, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Yi, J.; Wang, R.; Cheng, L.; Wang, Z.; Lu, W. Protection of Spleen Tissue of γ-ray Irradiated Mice against Immunosuppressive and Oxidative Effects of Radiation by Adenosine 5′-Monophosphate. Int. J. Mol. Sci. 2018, 19, 1273. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Tosh, D.K.; Jain, S.; Gao, Z.-G. Historical and Current Adenosine Receptor Agonists in Preclinical and Clinical Development. Front. Cell. Neurosci. 2019, 13, 124. [Google Scholar] [CrossRef]
- Bischoff, G.; Hoffmann, S. DNA-binding of drugs used in medicinal therapies. Curr. Med. Chem. 2002, 9, 312–348. [Google Scholar] [CrossRef]
- Mishra, K.; Alsbeih, G. Appraisal of biochemical classes of radioprotectors: Evidence, current status and guidelines for future development. 3 Biotech 2017, 7, 292. [Google Scholar] [CrossRef]
- Martin, R.F.; Broadhurst, S.; D’Abrew, S.; Budd, R.; Sephton, R.; Reum, M.; Kelly, D.P. Radioprotection by DNA ligands. Br. J. Cancer. Suppl. 1996, 27, S99–S101. [Google Scholar] [PubMed]
- Lyubimova, N.V.; Coultas, P.G.; Yuen, K.; Martin, R.F. In vivo radioprotection of mouse brain endothelial cells by Hoechst 33342. Br. J. Radiol. 2001, 74, 77–82. [Google Scholar] [CrossRef]
- Martin, R.F.; Broadhurst, S.; Reum, M.E.; Squire, C.J.; Clark, G.R.; Lobachevsky, P.N.; White, J.M.; Clark, C.; Sy, D.; Spotheim-Maurizot, M.; et al. In vitro studies with methylproamine: A potent new radioprotector. Cancer Res. 2004, 64, 1067–1070. [Google Scholar] [CrossRef]
- Mishra, K.; Bhardwaj, R.; Chaudhury, N.K. Netropsin, a minor groove binding ligand: A potential radioprotective agent. Radiat. Res. 2009, 172, 698–705. [Google Scholar] [CrossRef]
- Turner, P.R.; Denny, W.A. The mutagenic properties of DNA minor-groove binding ligands. Mutat. Res. 1996, 355, 141–169. [Google Scholar] [CrossRef]
- Kobayashi, J.; Kato, A.; Ota, Y.; Ohba, R.; Komatsu, K. Bisbenzamidine derivative, pentamidine represses DNA damage response through inhibition of histone H2A acetylation. Mol. Cancer 2010, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, J.T. Interorgan amino acid transport and its regulation. J. Nutr. 2003, 133, 2068S–2072S. [Google Scholar] [CrossRef] [PubMed]
- Klimberg, V.S.; Souba, W.W.; Dolson, D.J.; Salloum, R.M.; Hautamaki, R.D.; Plumley, D.A.; Mendenhall, W.M.; Bova, F.J.; Khan, S.R.; Hackett, R.L.; et al. Prophylactic glutamine protects the intestinal mucosa from radiation injury. Cancer 1990, 66, 62–68. [Google Scholar] [CrossRef]
- Savarese, D.M.F.; Savy, G.; Vahdat, L.; Wischmeyer, P.E.; Corey, B. Prevention of chemotherapy and radiation toxicity with glutamine. Cancer Treat. Rev. 2003, 29, 501–513. [Google Scholar] [CrossRef]
- Cao, D.-D.; Xu, H.-L.; Xu, M.; Qian, X.-Y.; Yin, Z.-C.; Ge, W. Therapeutic role of glutamine in management of radiation enteritis: A meta-analysis of 13 randomized controlled trials. Oncotarget 2017, 8, 30595–30605. [Google Scholar] [CrossRef]
- Ersin, S.; Tuncyurek, P.; Esassolak, M.; Alkanat, M.; Buke, C.; Yilmaz, M.; Telefoncu, A.; Kose, T. The prophylactic and therapeutic effects of glutamine- and arginine-enriched diets on radiation-induced enteritis in rats. J. Surg. Res. 2000, 89, 121–125. [Google Scholar] [CrossRef]
- Yavas, C.; Yavas, G.; Celik, E.; Buyukyoruk, A.; Buyukyoruk, C.; Yuce, D.; Ata, O. Beta-Hydroxy-Beta-Methyl-Butyrate, L-glutamine, and L-arginine Supplementation Improves Radiation-Induce Acute Intestinal Toxicity. J. Diet. Suppl. 2019, 16, 576–591. [Google Scholar] [CrossRef]
- Pathak, S.; Soni, T.P.; Sharma, L.M.; Patni, N.; Gupta, A.K. A Randomized Controlled Trial to Evaluate the Role and Efficacy of Oral Glutamine in the Treatment of Chemo-radiotherapy-induced Oral Mucositis and Dysphagia in Patients with Oropharynx and Larynx Carcinoma. Cureus 2019, 11, e4855. [Google Scholar] [CrossRef]
- Kligler, B.; Cohrssen, A. Probiotics. Am. Fam. Physician 2008, 78, 1073–1078. [Google Scholar]
- Demirer, S.; Aydintug, S.; Aslim, B.; Kepenekci, I.; Sengül, N.; Evirgen, O.; Gerceker, D.; Andrieu, M.N.; Ulusoy, C.; Karahüseyinoglu, S. Effects of probiotics on radiation-induced intestinal injury in rats. Nutr. Burbank Los Angel. Cty. Calif 2006, 22, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Ciorba, M.A.; Stenson, W.F. Probiotic therapy in radiation-induced intestinal injury and repair. Ann. N. Y. Acad. Sci. 2009, 1165, 190–194. [Google Scholar] [CrossRef]
- Stacey, R.; Green, J.T. Radiation-induced small bowel disease: Latest developments and clinical guidance. Ther. Adv. Chronic Dis. 2014, 5, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, N.K.; Suppiah, S.; Veettil, S.K.; Ching, S.M.; Lee, K.W.; Menon, R.K.; Soo, M.J.; Deuraseh, I.; Hoo, F.K.; Sivaratnam, D. The Effects of Probiotic Supplementation on the Incidence of Diarrhea in Cancer Patients Receiving Radiation Therapy: A Systematic Review with Meta-Analysis and Trial Sequential Analysis of Randomized Controlled Trials. Nutrients 2019, 11, 2886. [Google Scholar] [CrossRef]
- Zha, J.-M.; Li, H.-S.; Lin, Q.; Kuo, W.-T.; Jiang, Z.-H.; Tsai, P.-Y.; Ding, N.; Wu, J.; Xu, S.-F.; Wang, Y.-T.; et al. Interleukin 22 Expands Transit-Amplifying Cells While Depleting Lgr5+ Stem Cells via Inhibition of Wnt and Notch Signaling. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 255–274. [Google Scholar] [CrossRef]
- Zhang, X.; Fisher, R.; Hou, W.; Shields, D.; Epperly, M.W.; Wang, H.; Wei, L.; Leibowitz, B.J.; Yu, J.; Alexander, L.M.; et al. Second-generation Probiotics Producing IL-22 Increase Survival of Mice After Total Body Irradiation. Vivo Athens Greece 2020, 34, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Charrier, S.; Michaud, A.; Badaoui, S.; Giroux, S.; Ezan, E.; Sainteny, F.; Corvol, P.; Vainchenker, W. Inhibition of angiotensin I-converting enzyme induces radioprotection by preserving murine hematopoietic short-term reconstituting cells. Blood 2004, 104, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.A.; Landauer, M.R.; Mog, S.R.; Barshishat-Kupper, M.; Zins, S.R.; Amare, M.F.; Day, R.M. Timing of captopril administration determines radiation protection or radiation sensitization in a murine model of total body irradiation. Exp. Hematol. 2010, 38, 270–281. [Google Scholar] [CrossRef]
- McCart, E.A.; Lee, Y.H.; Jha, J.; Mungunsukh, O.; Rittase, W.B.; Summers, T.A.; Muir, J.; Day, R.M. Delayed Captopril Administration Mitigates Hematopoietic Injury in a Murine Model of Total Body Irradiation. Sci. Rep. 2019, 9, 2198. [Google Scholar] [CrossRef]
- Rosen, E.M.; Day, R.; Singh, V.K. New approaches to radiation protection. Front. Oncol. 2014, 4, 381. [Google Scholar] [CrossRef]
- Kalman, N.S.; Zhao, S.S.; Anscher, M.S.; Urdaneta, A.I. Current Status of Targeted Radioprotection and Radiation Injury Mitigation and Treatment Agents: A Critical Review of the Literature. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 662–682. [Google Scholar] [CrossRef]
- Wang, H.; Sethi, G.; Loke, W.-K.; Sim, M.-K. Des-Aspartate-Angiotensin I Attenuates Mortality of Mice Exposed to Gamma Radiation via a Novel Mechanism of Action. PLoS ONE 2015, 10, e0138009. [Google Scholar] [CrossRef]
- McLaughlin, M.F.; Donoviel, D.B.; Jones, J.A. Novel Indications for Commonly Used Medications as Radiation Protectants in Spaceflight. Aerosp. Med. Hum. Perform. 2017, 88, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Pedro-Botet, J.; Pintó, X. LDL-cholesterol: The lower the better. Clin. E Investig. En Arterioscler. Publ. Soc. Espanola Arterioscler. 2019, 31 (Suppl. 2), 16–27. [Google Scholar] [CrossRef]
- Gaugler, M.-H.; Vereycken-Holler, V.; Squiban, C.; Vandamme, M.; Vozenin-Brotons, M.-C.; Benderitter, M. Pravastatin limits endothelial activation after irradiation and decreases the resulting inflammatory and thrombotic responses. Radiat. Res. 2005, 163, 479–487. [Google Scholar] [CrossRef]
- Fritz, G.; Henninger, C.; Huelsenbeck, J. Potential use of HMG-CoA reductase inhibitors (statins) as radioprotective agents. Br. Med. Bull. 2011, 97, 17–26. [Google Scholar] [CrossRef]
- Ma, S.; Ma, C.C.-H. Recent development in pleiotropic effects of statins on cardiovascular disease through regulation of transforming growth factor-beta superfamily. Cytokine Growth Factor Rev. 2011, 22, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.J.; Park, K.R.; Lee, J.H.; Kim, T.G.; Kim, Y.-H. Simvastatin Reduces Capsular Fibrosis around Silicone Implants. J. Korean Med. Sci. 2016, 31, 1273–1278. [Google Scholar] [CrossRef]
- Henninger, C.; Fritz, G. Statins in anthracycline-induced cardiotoxicity: Rac and Rho, and the heartbreakers. Cell Death Dis. 2017, 8, e2564. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Si-Tu, J.; Qiu, J.; Lu, L.; Mao, Y.; Zeng, H.; Chen, M.; Lai, C.; Chang, H.-J.; Wang, D. Statin and metformin therapy in prostate cancer patients with hyperlipidemia who underwent radiotherapy: A population-based cohort study. Cancer Manag. Res. 2019, 11, 1189–1197. [Google Scholar] [CrossRef]
- Ong, Z.Y.; Gibson, R.J.; Bowen, J.M.; Stringer, A.M.; Darby, J.M.; Logan, R.M.; Yeoh, A.S.; Keefe, D.M. Pro-inflammatory cytokines play a key role in the development of radiotherapy-induced gastrointestinal mucositis. Radiat. Oncol. Lond. Engl. 2010, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, L.; Hiatt, N. Injurious effect of pancreatic secretions on postradiation enteropathy. Gastroenterology 1967, 53, 923–929. [Google Scholar] [CrossRef]
- Heintges, T.; Lüthen, R.; Niederau, C. Inhibition of exocrine pancreatic secretion by somatostatin and its analogues. Digestion 1994, 55 (Suppl. 1), 1–9. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, H.; Sung, C.C.; Hauer-Jensen, M. The synthetic somatostatin analogue, octreotide, ameliorates acute and delayed intestinal radiation injury. Int. J. Radiat. Oncol. Biol. Phys. 1999, 45, 1289–1296. [Google Scholar] [CrossRef]
- Yavuz, M.N.; Yavuz, A.A.; Aydin, F.; Can, G.; Kavgaci, H. The efficacy of octreotide in the therapy of acute radiation-induced diarrhea: A randomized controlled study. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 195–202. [Google Scholar] [CrossRef]
- Grabenbauer, G.G.; Holger, G. Management of radiation and chemotherapy related acute toxicity in gastrointestinal cancer. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 655–664. [Google Scholar] [CrossRef]
- Wadler, S.; Haynes, H.; Wiernik, P.H. Phase I trial of the somatostatin analog octreotide acetate in the treatment of fluoropyrimidine-induced diarrhea. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1995, 13, 222–226. [Google Scholar] [CrossRef]
- Fu, Q.; Berbée, M.; Boerma, M.; Wang, J.; Schmid, H.A.; Hauer-Jensen, M. The somatostatin analog SOM230 (pasireotide) ameliorates injury of the intestinal mucosa and increases survival after total-body irradiation by inhibiting exocrine pancreatic secretion. Radiat. Res. 2009, 171, 698–707. [Google Scholar] [CrossRef]
- Fu, Q.; Berbée, M.; Wang, W.; Boerma, M.; Wang, J.; Schmid, H.A.; Hauer-Jensen, M. Preclinical evaluation of Som230 as a radiation mitigator in a mouse model: Postexposure time window and mechanisms of action. Radiat. Res. 2011, 175, 728–735. [Google Scholar] [CrossRef]
- Shuryak, I. Review of microbial resistance to chronic ionizing radiation exposure under environmental conditions. J. Environ. Radioact. 2019, 196, 50–63. [Google Scholar] [CrossRef]
- Hofer, M.; Pospísil, M. Glucan as stimulator of hematopoiesis in normal and gamma-irradiated mice. A survey of the authors’ results. Int. J. Immunopharmacol. 1997, 19, 607–609. [Google Scholar] [CrossRef]
- Patchen, M.L.; D’Alesandro, M.M.; Chirigos, M.A.; Weiss, J.F. Radioprotection by biological response modifiers alone and in combination with WR-2721. Pharmacol. Ther. 1988, 39, 247–254. [Google Scholar] [CrossRef]
- Pospísil, M.; Netíková, J.; Pipalová, I.; Jarý, J. Combined radioprotection by preirradiation peroral cystamine and postirradiation glucan administration. Folia Biol. (Praha) 1991, 37, 117–124. [Google Scholar]
- Patchen, M.L.; MacVittie, T.J.; Weiss, J.F. Combined modality radioprotection: The use of glucan and selenium with WR-2721. Int. J. Radiat. Oncol. Biol. Phys. 1990, 18, 1069–1075. [Google Scholar] [CrossRef]
- Hofer, M.; Pospísil, M.; Viklická, S.; Vacek, A.; Pipalová, I.; Bartonícková, A. Hematopoietic recovery in repeatedly irradiated mice can be enhanced by a repeatedly administered combination of diclofenac and glucan. J. Leukoc. Biol. 1993, 53, 185–189. [Google Scholar] [CrossRef]
- Patchen, M.L.; MacVittie, T.J.; Solberg, B.D.; Souza, L.M. Survival enhancement and hemopoietic regeneration following radiation exposure: Therapeutic approach using glucan and granulocyte colony-stimulating factor. Exp. Hematol. 1990, 18, 1042–1048. [Google Scholar] [PubMed]
- Pillai, T.G.; Uma Devi, P. Mushroom beta glucan: Potential candidate for post irradiation protection. Mutat. Res. 2013, 751, 109–115. [Google Scholar] [CrossRef]
- Rondanelli, M.; Opizzi, A.; Monteferrario, F. The biological activity of beta-glucans. Minerva Med. 2009, 100, 237–245. [Google Scholar] [PubMed]
- Novak, M.; Vetvicka, V. Glucans as biological response modifiers. Endocr. Metab. Immune Disord. Drug Targets 2009, 9, 67–75. [Google Scholar] [CrossRef]
- Whitnall, M.H.; Elliott, T.B.; Harding, R.A.; Inal, C.E.; Landauer, M.R.; Wilhelmsen, C.L.; McKinney, L.; Miner, V.L.; Jackson WE3rd, J.; Loria, R.M.; et al. Androstenediol stimulates myelopoiesis and enhances resistance to infection in gamma-irradiated mice. Int. J. Immunopharmacol. 2000, 22, 1–14. [Google Scholar] [CrossRef]
- Stickney, D.R.; Dowding, C.; Garsd, A.; Ahlem, C.; Whitnall, M.; McKeon, M.; Reading, C.; Frincke, J. 5-androstenediol stimulates multilineage hematopoiesis in rhesus monkeys with radiation-induced myelosuppression. Int. Immunopharmacol. 2006, 6, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Shafran, R.L.; Inal, C.E.; Jackson, W.E.; Whitnall, M.H. Effects of whole-body gamma irradiation and 5-androstenediol administration on serum G-CSF. Immunopharmacol. Immunotoxicol. 2005, 27, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Hofer, M.; Hoferová, Z.; Falk, M. Pharmacological Modulation of Radiation Damage. Does It Exist a Chance for Other Substances than Hematopoietic Growth Factors and Cytokines? Int. J. Mol. Sci. 2017, 18, 1385. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Liu, W.; Fan, T.; Zhong, H.; Zhou, H.; Guo, W.; Zhu, X. 5-Androstenediol prevents radiation injury in mice by promoting NF-κB signaling and inhibiting AIM2 inflammasome activation. Biomed. Pharmacother. Biomed. Pharmacother. 2020, 121, 109597. [Google Scholar] [CrossRef]
- Singh, V.K.; Romaine, P.L.P.; Seed, T.M. Medical Countermeasures for Radiation Exposure and Related Injuries: Characterization of Medicines, FDA-Approval Status and Inclusion into the Strategic National Stockpile. Health Phys. 2015, 108, 607–630. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Ducey, E.J.; Fatanmi, O.O.; Singh, P.K.; Brown, D.S.; Purmal, A.; Shakhova, V.V.; Gudkov, A.V.; Feinstein, E.; Shakhov, A. CBLB613: A TLR 2/6 agonist, natural lipopeptide of Mycoplasma arginini, as a novel radiation countermeasure. Radiat. Res. 2012, 177, 628–642. [Google Scholar] [CrossRef]
- Madonna, G.S.; Ledney, G.D.; Moore, M.M.; Elliott, T.B.; Brook, I. Treatment of mice with sepsis following irradiation and trauma with antibiotics and synthetic trehalose dicorynomycolate (S-TDCM). J. Trauma 1991, 31, 316–325. [Google Scholar] [CrossRef]
- Bascones-Martinez, A.; Mattila, R.; Gomez-Font, R.; Meurman, J.H. Immunomodulatory drugs: Oral and systemic adverse effects. Med. Oral Patol. Oral Cirugia Bucal 2014, 19, e24–e31. [Google Scholar] [CrossRef]
- Neta, R.; Douches, S.; Oppenheim, J.J. Interleukin 1 is a radioprotector. J. Immunol. Baltim. Md 1950 1986, 136, 2483–2485. [Google Scholar]
- Neta, R.; Oppenheim, J.J.; Schreiber, R.D.; Chizzonite, R.; Ledney, G.D.; MacVittie, T.J. Role of cytokines (interleukin 1, tumor necrosis factor, and transforming growth factor beta) in natural and lipopolysaccharide-enhanced radioresistance. J. Exp. Med. 1991, 173, 1177–1182. [Google Scholar] [CrossRef]
- Neta, R. Modulation of radiation damage by cytokines. Stem Cells Dayt. Ohio 1997, 15 (Suppl. 2), 87–94. [Google Scholar] [CrossRef]
- Xu, L.; Xiong, S.; Guo, R.; Yang, Z.; Wang, Q.; Xiao, F.; Wang, H.; Pan, X.; Zhu, M. Transforming growth factor β3 attenuates the development of radiation-induced pulmonary fibrosis in mice by decreasing fibrocyte recruitment and regulating IFN-γ/IL-4 balance. Immunol. Lett. 2014, 162, 27–33. [Google Scholar] [CrossRef]
- Finch, P.W.; Mark Cross, L.J.; McAuley, D.F.; Farrell, C.L. Palifermin for the protection and regeneration of epithelial tissues following injury: New findings in basic research and pre-clinical models. J. Cell. Mol. Med. 2013, 17, 1065–1087. [Google Scholar] [CrossRef] [PubMed]
- Vadhan-Raj, S.; Goldberg, J.D.; Perales, M.-A.; Berger, D.P.; van den Brink, M.R.M. Clinical applications of palifermin: Amelioration of oral mucositis and other potential indications. J. Cell. Mol. Med. 2013, 17, 1371–1384. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.-J.; Geng, Z.H.; Spence, J.R.; Geng, J.-G. Induction of intestinal stem cells by R-spondin 1 and Slit2 augments chemoradioprotection. Nature 2013, 501, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Shi, S.; Yuan, Z.; Chang, J.Y. Bevacizumab treatment for radiation brain necrosis: Mechanism, efficacy and issues. Mol. Cancer 2019, 18, 21. [Google Scholar] [CrossRef]
- Matuschek, C.; Bölke, E.; Nawatny, J.; Hoffmann, T.K.; Peiper, M.; Orth, K.; Gerber, P.A.; Rusnak, E.; Lammering, G.; Budach, W. Bevacizumab as a treatment option for radiation-induced cerebral necrosis. Strahlenther. Onkol. Organ Dtsch. Rontgengesellschaft Al 2011, 187, 135–139. [Google Scholar] [CrossRef]
- Levin, V.A.; Bidaut, L.; Hou, P.; Kumar, A.J.; Wefel, J.S.; Bekele, B.N.; Grewal, J.; Prabhu, S.; Loghin, M.; Gilbert, M.R.; et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 1487–1495. [Google Scholar] [CrossRef]
- Alessandretti, M.; Buzaid, A.C.; Brandão, R.; Brandão, E.P. Low-dose bevacizumab is effective in radiation-induced necrosis. Case Rep. Oncol. 2013, 6, 598–601. [Google Scholar] [CrossRef]
- Hanson, W.R. Radiation protection of murine intestine by WR-2721, 16,16-dimethyl prostaglandin E2, and the combination of both agents. Radiat. Res. 1987, 111, 361–373. [Google Scholar] [CrossRef]
- Hanson, W.R.; Houseman, K.A.; Nelson, A.K.; Collins, P.W. Radiation protection of the murine intestine by misoprostol, a prostaglandin E1 analogue, given alone or with WR-2721, is stereospecific. Prostaglandins Leukot. Essent. Fatty Acids 1988, 32, 101–105. [Google Scholar] [PubMed]
- Gentile, P.; Byer, D.; Pelus, L.M. In vivo modulation of murine myelopoiesis following intravenous administration of prostaglandin E2. Blood 1983, 62, 1100–1107. [Google Scholar] [CrossRef]
- Lee, T.K.; Stupans, I. Radioprotection: The non-steroidal anti-inflammatory drugs (NSAIDs) and prostaglandins. J. Pharm. Pharmacol. 2002, 54, 1435–1445. [Google Scholar] [CrossRef]
- Hofer, M.; Pospíšil, M.; Hoferová, Z.; Weiterová, L.; Komůrková, D. Stimulatory action of cyclooxygenase inhibitors on hematopoiesis: A review. Molecules 2012, 17, 5615–5625. [Google Scholar] [CrossRef]
- Hofer, M.; Pospísil, M.; Tkadlecek, L.; Viklická, S.; Pipalová, I.; Holá, J. Low survival of mice following lethal gamma-irradiation after administration of inhibitors of prostaglandin synthesis. Physiol. Res. 1992, 41, 157–161. [Google Scholar] [PubMed]
- Hofer, M.; Pospíšil, M.; Dušek, L.; Hoferová, Z.; Weiterová, L. A single dose of an inhibitor of cyclooxygenase 2, meloxicam, administered shortly after irradiation increases survival of lethally irradiated mice. Radiat. Res. 2011, 176, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Hoggatt, J.; Singh, P.; Stilger, K.N.; Plett, P.A.; Sampson, C.H.; Chua, H.L.; Orschell, C.M.; Pelus, L.M. Recovery from hematopoietic injury by modulating prostaglandin E(2) signaling post-irradiation. Blood Cells. Mol. Dis. 2013, 50, 147–153. [Google Scholar] [CrossRef]
- Verheij, M.; Stewart, F.A.; Oussoren, Y.; Weening, J.J.; Dewit, L. Amelioration of radiation nephropathy by acetylsalicylic acid. Int. J. Radiat. Biol. 1995, 67, 587–596. [Google Scholar] [CrossRef]
- Demirel, C.; Kilciksiz, S.C.; Gurgul, S.; Erdal, N.; Yigit, S.; Tamer, L.; Ayaz, L. Inhibition of Radiation-Induced Oxidative Damage in the Lung Tissue: May Acetylsalicylic Acid Have a Positive Role? Inflammation 2016, 39, 158–165. [Google Scholar] [CrossRef]
- Mathkour, M.A.; Suhaibani, E.A. Protective Effect of Aspirin on γ Radiation Induced Chromosomal Aberrations in Swiss Albino Male Mice. Res. J. Radiol. 2014, 1, 1–6. [Google Scholar] [CrossRef]
- Day, R.M.; Barshishat-Kupper, M.; Mog, S.R.; McCart, E.A.; Prasanna, P.G.S.; Davis, T.A.; Landauer, M.R. Genistein protects against biomarkers of delayed lung sequelae in mice surviving high-dose total body irradiation. J. Radiat. Res. (Tokyo) 2008, 49, 361–372. [Google Scholar] [CrossRef]
- Calveley, V.L.; Jelveh, S.; Langan, A.; Mahmood, J.; Yeung, I.W.T.; Van Dyk, J.; Hill, R.P. Genistein can mitigate the effect of radiation on rat lung tissue. Radiat. Res. 2010, 173, 602–611. [Google Scholar] [CrossRef]
- Jackson, I.L.; Zodda, A.; Gurung, G.; Pavlovic, R.; Kaytor, M.D.; Kuskowski, M.A.; Vujaskovic, Z. BIO 300, a nanosuspension of genistein, mitigates pneumonitis/fibrosis following high-dose radiation exposure in the C57L/J murine model. Br. J. Pharmacol. 2017, 174, 4738–4750. [Google Scholar] [CrossRef] [PubMed]
- Jackson, I.L.; Pavlovic, R.; Alexander, A.A.; Connors, C.Q.; Newman, D.; Mahmood, J.; Eley, J.; Harvey, A.J.; Kaytor, M.D.; Vujaskovic, Z. BIO 300, a Nanosuspension of Genistein, Mitigates Radiation-Induced Erectile Dysfunction and Sensitizes Human Prostate Cancer Xenografts to Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Seed, T.M. BIO 300: A promising radiation countermeasure under advanced development for acute radiation syndrome and the delayed effects of acute radiation exposure. Expert Opin. Investig. Drugs 2020, 29, 429–441. [Google Scholar] [CrossRef] [PubMed]
- French, K.J.; Zhuang, Y.; Maines, L.W.; Gao, P.; Wang, W.; Beljanski, V.; Upson, J.J.; Green, C.L.; Keller, S.N.; Smith, C.D. Pharmacology and antitumor activity of ABC294640, a selective inhibitor of sphingosine kinase-2. J. Pharmacol. Exp. Ther. 2010, 333, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Britten, C.D.; Garrett-Mayer, E.; Chin, S.H.; Shirai, K.; Ogretmen, B.; Bentz, T.A.; Brisendine, A.; Anderton, K.; Cusack, S.L.; Maines, L.W.; et al. A Phase I Study of ABC294640, a First-in-Class Sphingosine Kinase-2 Inhibitor, in Patients with Advanced Solid Tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 4642–4650. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.; Maines, L.; Schrecengost, R.; Zhuang, Y.; Keller, S.; Smith, R.; Green, C. Antitumor and anti-inflammatory effects of the sphingosine kinase-2 inhibitor ABC294640 in combination with radiation. Eur. J. Cancer 2016, 69, S61. [Google Scholar] [CrossRef]
- Nicolay, N.H.; Lopez Perez, R.; Debus, J.; Huber, P.E. Mesenchymal stem cells—A new hope for radiotherapy-induced tissue damage? Cancer Lett. 2015, 366, 133–140. [Google Scholar] [CrossRef]
- Francois, S.; Mouiseddine, M.; Allenet-Lepage, B.; Voswinkel, J.; Douay, L.; Benderitter, M.; Chapel, A. Human mesenchymal stem cells provide protection against radiation-induced liver injury by antioxidative process, vasculature protection, hepatocyte differentiation, and trophic effects. BioMed Res. Int. 2013, 2013, 151679. [Google Scholar] [CrossRef]
- Hao, L.; Wang, J.; Zou, Z.; Yan, G.; Dong, S.; Deng, J.; Ran, X.; Feng, Y.; Luo, C.; Wang, Y.; et al. Transplantation of BMSCs expressing hPDGF-A/hBD2 promotes wound healing in rats with combined radiation-wound injury. Gene Ther. 2009, 16, 34–42. [Google Scholar] [CrossRef]
- Kiang, J.G.; Gorbunov, N.V. Bone Marrow Mesenchymal Stem Cells Increase Survival after Ionizing Irradiation Combined with Wound Trauma: Characterization and Therapy. J. Cell Sci. Ther. 2014, 5, 1–8. [Google Scholar] [CrossRef]
- Hu, K.X.; Sun, Q.Y.; Guo, M.; Ai, H.S. The radiation protection and therapy effects of mesenchymal stem cells in mice with acute radiation injury. Br. J. Radiol. 2010, 83, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Wang, W.; Prabath, B.G.; Boerma, M.; Wang, J.; Zhou, D.; Hauer-Jensen, M. Bone marrow transplantation helps restore the intestinal mucosal barrier after total body irradiation in mice. Radiat. Res. 2014, 181, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Kiang, J.G. Adult Mesenchymal Stem Cells and Radiation Injury. Health Phys. 2016, 111, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Schoefinius, J.-S.; Brunswig-Spickenheier, B.; Speiseder, T.; Krebs, S.; Just, U.; Lange, C. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Provide Long-Term Survival After Total Body Irradiation Without Additional Hematopoietic Stem Cell Support. Stem Cells Dayt. Ohio 2017, 35, 2379–2389. [Google Scholar] [CrossRef]
- Chang, P.-Y.; Zhang, B.-Y.; Cui, S.; Qu, C.; Shao, L.-H.; Xu, T.-K.; Qu, Y.-Q.; Dong, L.-H.; Wang, J. MSC-derived cytokines repair radiation-induced intra-villi microvascular injury. Oncotarget 2017, 8, 87821–87836. [Google Scholar] [CrossRef][Green Version]
- Li, B.; Li, C.; Zhu, M.; Zhang, Y.; Du, J.; Xu, Y.; Liu, B.; Gao, F.; Liu, H.; Cai, J.; et al. Hypoxia-Induced Mesenchymal Stromal Cells Exhibit an Enhanced Therapeutic Effect on Radiation-Induced Lung Injury in Mice due to an Increased Proliferation Potential and Enhanced Antioxidant Ability. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 44, 1295–1310. [Google Scholar] [CrossRef]
- Shen, Y.; Jiang, X.; Meng, L.; Xia, C.; Zhang, L.; Xin, Y. Transplantation of Bone Marrow Mesenchymal Stem Cells Prevents Radiation-Induced Artery Injury by Suppressing Oxidative Stress and Inflammation. Oxid. Med. Cell. Longev. 2018, 2018, 5942916. [Google Scholar] [CrossRef]
- Liao, H.; Wang, H.; Rong, X.; Li, E.; Xu, R.-H.; Peng, Y. Mesenchymal Stem Cells Attenuate Radiation-Induced Brain Injury by Inhibiting Microglia Pyroptosis. BioMed Res. Int. 2017, 2017, 1948985. [Google Scholar] [CrossRef]
- Soria, B.; Martin-Montalvo, A.; Aguilera, Y.; Mellado-Damas, N.; López-Beas, J.; Herrera-Herrera, I.; López, E.; Barcia, J.A.; Alvarez-Dolado, M.; Hmadcha, A.; et al. Human Mesenchymal Stem Cells Prevent Neurological Complications of Radiotherapy. Front. Cell. Neurosci. 2019, 13, 204. [Google Scholar] [CrossRef]
- Wang, G.; Ren, X.; Yan, H.; Gui, Y.; Guo, Z.; Song, J.; Zhang, P. Neuroprotective Effects of Umbilical Cord-Derived Mesenchymal Stem Cells on Radiation-Induced Brain Injury in Mice. Ann. Clin. Lab. Sci. 2020, 50, 57–64. [Google Scholar]
- Kink, J.A.; Forsberg, M.H.; Reshetylo, S.; Besharat, S.; Childs, C.J.; Pederson, J.D.; Gendron-Fitzpatrick, A.; Graham, M.; Bates, P.D.; Schmuck, E.G.; et al. Macrophages Educated with Exosomes from Primed Mesenchymal Stem Cells Treat Acute Radiation Syndrome by Promoting Hematopoietic Recovery. Biol. Blood Marrow Transplant. 2019, 25, 2124–2133. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Bhanja, P.; Kabarriti, R.; Liu, L.; Alfieri, A.A.; Guha, C. Bone marrow stromal cell transplantation mitigates radiation-induced gastrointestinal syndrome in mice. PLoS ONE 2011, 6, e24072. [Google Scholar] [CrossRef] [PubMed]
- Na Nakorn, T.; Traver, D.; Weissman, I.L.; Akashi, K. Myeloerythroid-restricted progenitors are sufficient to confer radioprotection and provide the majority of day 8 CFU-S. J. Clin. Investig. 2002, 109, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Ducey, E.J.; Brown, D.S.; Whitnall, M.H. A review of radiation countermeasure work ongoing at the Armed Forces Radiobiology Research Institute. Int. J. Radiat. Biol. 2012, 88, 296–310. [Google Scholar] [CrossRef]
- Milano, F.; Merriam, F.; Nicoud, I.; Li, J.; Gooley, T.A.; Heimfeld, S.; Imren, S.; Delaney, C. Notch-Expanded Murine Hematopoietic Stem and Progenitor Cells Mitigate Death from Lethal Radiation and Convey Immune Tolerance in Mismatched Recipients. Stem Cells Transl. Med. 2017, 6, 566–575. [Google Scholar] [CrossRef]
- Payne, H.; Adamson, A.; Bahl, A.; Borwell, J.; Dodds, D.; Heath, C.; Huddart, R.; McMenemin, R.; Patel, P.; Peters, J.L.; et al. Chemical- and radiation-induced haemorrhagic cystitis: Current treatments and challenges. BJU Int. 2013, 112, 885–897. [Google Scholar] [CrossRef]
- Flemming, K. Model studies on chemical radiation protection. Part I. Inhibition of the basic processes of biological radiation effect by cysteine. Strahlentherapie 1960, 111, 339–358. [Google Scholar]
- Mansour, H.H.; Hafez, H.F.; Fahmy, N.M.; Hanafi, N. Protective effect of N-acetylcysteine against radiation induced DNA damage and hepatic toxicity in rats. Biochem. Pharmacol. 2008, 75, 773–780. [Google Scholar] [CrossRef]
- Langendorff, H.; Catsch, A. Studies on a biological radiation protection. XVI. Protective activity of cysteamine in fractionated total body irradiation of mice. Strahlentherapie 1956, 101, 536–541. [Google Scholar]
- Brucer, M.; Mewissen, D.J. Late effects of gamma radiation on mice protected with cysteamine or cystamine. Nature 1957, 179, 201–202. [Google Scholar]
- Dacquisto, M.P.; Rothe, W.E.; Blackburn, E.W. Mechanism of the protective action of 2-mercaptoethylguanidine (MEG) against whole-body radiation in mice. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1961, 4, 33–42. [Google Scholar] [CrossRef]
- Peebles, D.D.; Soref, C.M.; Copp, R.R.; Thunberg, A.L.; Fahl, W.E. ROS-scavenger and radioprotective efficacy of the new PrC-210 aminothiol. Radiat. Res. 2012, 178, 57–68. [Google Scholar] [CrossRef]
- Traversi, G.; Fiore, M.; Leone, S.; Basso, E.; Di Muzio, E.; Polticelli, F.; Degrassi, F.; Cozzi, R. Resveratrol and its methoxy-derivatives as modulators of DNA damage induced by ionising radiation. Mutagenesis 2016, 31, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Amini, P.; Saffar, H.; Nourani, M.R.; Motevaseli, E.; Najafi, M.; Ali Taheri, R.; Qazvini, A. Curcumin Mitigates Radiation-induced Lung Pneumonitis and Fibrosis in Rats. Int. J. Mol. Cell. Med. 2018, 7, 212–219. [Google Scholar]
- Tiwari, M.; Dixit, B.; Parvez, S.; Agrawala, P.K. EGCG, a tea polyphenol, as a potential mitigator of hematopoietic radiation injury in mice. Biomed. Pharmacother. Biomed. Pharmacother. 2017, 88, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Sinha, M.; Das, D.K.; Manna, K.; Datta, S.; Ray, T.; Sil, A.K.; Dey, S. Epicatechin ameliorates ionising radiation-induced oxidative stress in mouse liver. Free Radic. Res. 2012, 46, 842–849. [Google Scholar] [CrossRef]
- Rithidech, K.N.; Tungjai, M.; Reungpatthanaphong, P.; Honikel, L.; Simon, S.R. Attenuation of oxidative damage and inflammatory responses by apigenin given to mice after irradiation. Mutat. Res. 2012, 749, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Thresiamma, K.C.; George, J.; Kuttan, R. Protective effect of curcumin, ellagic acid and bixin on radiation induced genotoxicity. J. Exp. Clin. Cancer Res. CR 1998, 17, 431–434. [Google Scholar]
- Vasudeva, V.; Tenkanidiyoor, Y.S.; Radhakrishna, V.; Shivappa, P.; Lakshman, S.P.; Fernandes, R.; Patali, K.A. Palliative effects of lutein intervention in gamma-radiation-induced cellular damages in Swiss albino mice. Indian J. Pharmacol. 2017, 49, 26–33. [Google Scholar]
- Friedenthal, E.; Mendecki, J.; Davis, L.; Steifter, E. The role of vitamin A and analogues in prevention of radiation toxicity during radiotherapy of cancer. In Vitamins and Minerals in the Prevention and Treatment of Cancer; CRC Press: Boca Raton, FL, USA, 1991; pp. 275–278. [Google Scholar]
- Manda, K.; Ueno, M.; Moritake, T.; Anzai, K. alpha-Lipoic acid attenuates x-irradiation-induced oxidative stress in mice. Cell Biol. Toxicol. 2007, 23, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Ogata, H.; Izumo, Y. Mortality reduction in mice administered a single abundant dose of zinc, manganese or magnesium after irradiation by gamma-rays at sublethal doses. Radioisotopes 1990, 39, 573–576. [Google Scholar] [CrossRef][Green Version]
- Mollà, M.; Gironella, M.; Salas, A.; Closa, D.; Biete, A.; Gimeno, M.; Coronel, P.; Piqué, J.M.; Panés, J. Protective effect of superoxide dismutase in radiation-induced intestinal inflammation. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 1159–1166. [Google Scholar] [CrossRef]
- Zhang, X.; Epperly, M.W.; Kay, M.A.; Chen, Z.-Y.; Dixon, T.; Franicola, D.; Greenberger, B.A.; Komanduri, P.; Greenberger, J.S. Radioprotection in vitro and in vivo by minicircle plasmid carrying the human manganese superoxide dismutase transgene. Hum. Gene Ther. 2008, 19, 820–826. [Google Scholar] [CrossRef]
- Gauter-Fleckenstein, B.; Fleckenstein, K.; Owzar, K.; Jiang, C.; Batinic-Haberle, I.; Vujaskovic, Z. Comparison of two Mn porphyrin-based mimics of superoxide dismutase in pulmonary radioprotection. Free Radic. Biol. Med. 2008, 44, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Colon, J.; Hsieh, N.; Ferguson, A.; Kupelian, P.; Seal, S.; Jenkins, D.W.; Baker, C.H. Cerium oxide nanoparticles protect gastrointestinal epithelium from radiation-induced damage by reduction of reactive oxygen species and upregulation of superoxide dismutase 2. Nanomedicine Nanotechnol. Biol. Med. 2010, 6, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, Z.N.; Batinic-Haberle, I.; Anscher, M.S.; Huang, J.; Day, B.J.; Alexander, E.; Dewhirst, M.W.; Vujaskovic, Z. Long-term administration of a small molecular weight catalytic metalloporphyrin antioxidant, AEOL 10150, protects lungs from radiation-induced injury. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 573–580. [Google Scholar] [CrossRef]
- Zanoni, M.; Cortesi, M.; Zamagni, A.; Tesei, A. The Role of Mesenchymal Stem Cells in Radiation-Induced Lung Fibrosis. Int. J. Mol. Sci. 2019, 20, 3876. [Google Scholar] [CrossRef]
- Rosenthal, R.A.; Fish, B.; Hill, R.P.; Huffman, K.D.; Lazarova, Z.; Mahmood, J.; Medhora, M.; Molthen, R.; Moulder, J.E.; Sonis, S.T.; et al. Salen Mn complexes mitigate radiation injury in normal tissues. Anticancer Agents Med. Chem. 2011, 11, 359–372. [Google Scholar] [CrossRef]
- Wei, L.; Leibowitz, B.J.; Epperly, M.; Bi, C.; Li, A.; Steinman, J.; Wipf, P.; Li, S.; Zhang, L.; Greenberger, J.; et al. The GS-nitroxide JP4-039 improves intestinal barrier and stem cell recovery in irradiated mice. Sci. Rep. 2018, 8, 2072. [Google Scholar] [CrossRef]
- Loria, R.M.; Conrad, D.H.; Huff, T.; Carter, H.; Ben-Nathan, D. Androstenetriol and androstenediol. Protection against lethal radiation and restoration of immunity after radiation injury. Ann. N. Y. Acad. Sci. 2000, 917, 860–867. [Google Scholar] [CrossRef]
- Mehrotra, S.; Pecaut, M.J.; Gridley, D.S. Analysis of minocycline as a countermeasure against acute radiation syndrome. Vivo Athens Greece 2012, 26, 743–758. [Google Scholar]
- Hofer, M.; Pospíšil, M.; Komůrková, D.; Hoferová, Z. Granulocyte colony-stimulating factor in the treatment of acute radiation syndrome: A concise review. Molecules 2014, 19, 4770–4778. [Google Scholar] [CrossRef]
- Chainy, G.B.; Manna, S.K.; Chaturvedi, M.M.; Aggarwal, B.B. Anethole blocks both early and late cellular responses transduced by tumor necrosis factor: Effect on NF-kappaB, AP-1, JNK, MAPKK and apoptosis. Oncogene 2000, 19, 2943–2950. [Google Scholar] [CrossRef]
- Lazo, J.S.; Sharlow, E.R.; Epperly, M.W.; Lira, A.; Leimgruber, S.; Skoda, E.M.; Wipf, P.; Greenberger, J.S. Pharmacologic profiling of phosphoinositide 3-kinase inhibitors as mitigators of ionizing radiation-induced cell death. J. Pharmacol. Exp. Ther. 2013, 347, 669–680. [Google Scholar] [CrossRef]
- Thotala, D.K.; Geng, L.; Dickey, A.K.; Hallahan, D.E.; Yazlovitskaya, E.M. A new class of molecular targeted radioprotectors: GSK-3beta inhibitors. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 557–565. [Google Scholar] [CrossRef]
- Cai, X.; Hao, J.; Zhang, X.; Yu, B.; Ren, J.; Luo, C.; Li, Q.; Huang, Q.; Shi, X.; Li, W.; et al. The polyhydroxylated fullerene derivative C60(OH)24 protects mice from ionizing-radiation-induced immune and mitochondrial dysfunction. Toxicol. Appl. Pharmacol. 2010, 243, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Panganiban, R.A.M.; Day, R.M. Inhibition of IGF-1R prevents ionizing radiation-induced primary endothelial cell senescence. PLoS ONE 2013, 8, e78589. [Google Scholar] [CrossRef]
- Deng, W.; Kimura, Y.; Gududuru, V.; Wu, W.; Balogh, A.; Szabo, E.; Thompson, K.E.; Yates, C.R.; Balazs, L.; Johnson, L.R.; et al. Mitigation of the hematopoietic and gastrointestinal acute radiation syndrome by octadecenyl thiophosphate, a small molecule mimic of lysophosphatidic acid. Radiat. Res. 2015, 183, 465–475. [Google Scholar] [CrossRef]
- Shaw, J.; Zhang, J.; Zhang, M.; Valeriote, F.; Chen, B.; Shaw, J. Pretreatment with A Small-Molecule Tumor Necrosis Factor-Alpha (TNF-α) Inhibitor, UTL-5g, Reduced Radiation-Induced Acute Liver Toxicity in Mice. Am. J. Biomed. Sci 2012, 4, 123–131. [Google Scholar]
- Basile, L.A.; Ellefson, D.; Gluzman-Poltorak, Z.; Junes-Gill, K.; Mar, V.; Mendonca, S.; Miller, J.D.; Tom, J.; Trinh, A.; Gallaher, T.K. HemaMaxTM, a recombinant human interleukin-12, is a potent mitigator of acute radiation injury in mice and non-human primates. PLoS ONE 2012, 7, e30434. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Song, Y.; Han, Q.; Liu, W.; Xu, J.; Yu, Z.; Zhang, R.; Li, N. Intestinal epithelial cell-specific IGF1 promotes the expansion of intestinal stem cells during epithelial regeneration and functions on the intestinal immune homeostasis. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E638–E649. [Google Scholar] [CrossRef] [PubMed]
- Satyamitra, M.; Cary, L.; Dunn, D.; Holmes-Hampton, G.P.; Thomas, L.J.; Ghosh, S.P. CDX-301: A novel medical countermeasure for hematopoietic acute radiation syndrome in mice. Sci. Rep. 2020, 10, 1757. [Google Scholar] [CrossRef]
- Zhang, K.; Tian, Y.; Yin, L.; Zhang, M.; Beck, L.A.; Zhang, B.; Okunieff, P.; Zhang, L.; Vidyasagar, S. Fibroblast growth factor-peptide improves barrier function and proliferation in human keratinocytes after radiation. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 248–254. [Google Scholar] [CrossRef][Green Version]
- Singh, V.K.; Christensen, J.; Fatanmi, O.O.; Gille, D.; Ducey, E.J.; Wise, S.Y.; Karsunky, H.; Sedello, A.K. Myeloid progenitors: A radiation countermeasure that is effective when initiated days after irradiation. Radiat. Res. 2012, 177, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Sher, N.; Ofir, R. Placenta-Derived Adherent Stromal Cell Therapy for Hematopoietic Disorders: A Case Study of PLX-R18. Cell Transplant. 2018, 27, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Kiang, J.G.; Zhai, M.; Liao, P.-J.; Ho, C.; Gorbunov, N.V.; Elliott, T.B. Thrombopoietin Receptor Agonist Mitigates Hematopoietic Radiation Syndrome and Improves Survival after Whole-Body Ionizing Irradiation Followed by Wound Trauma. Mediators Inflamm. 2017, 2017, 7582079. [Google Scholar] [CrossRef]
- Rotolo, J.; Stancevic, B.; Zhang, J.; Hua, G.; Fuller, J.; Yin, X.; Haimovitz-Friedman, A.; Kim, K.; Qian, M.; Cardó-Vila, M.; et al. Anti-ceramide antibody prevents the radiation gastrointestinal syndrome in mice. J. Clin. Investig. 2012, 122, 1786–1790. [Google Scholar] [CrossRef]
- Chung, E.J.; Sowers, A.; Thetford, A.; McKay-Corkum, G.; Chung, S.I.; Mitchell, J.B.; Citrin, D.E. Mammalian Target of Rapamycin Inhibition With Rapamycin Mitigates Radiation-Induced Pulmonary Fibrosis in a Murine Model. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 857–866. [Google Scholar] [CrossRef]
- Kantara, C.; Moya, S.M.; Houchen, C.W.; Umar, S.; Ullrich, R.L.; Singh, P.; Carney, D.H. Novel regenerative peptide TP508 mitigates radiation-induced gastrointestinal damage by activating stem cells and preserving crypt integrity. Lab. Investig. J. Tech. Methods Pathol. 2015, 95, 1222–1233. [Google Scholar] [CrossRef][Green Version]
- Smith, H.S.; Cox, L.R.; Smith, E.J. 5-HT3 receptor antagonists for the treatment of nausea/vomiting. Ann. Palliat. Med. 2012, 1, 115–120. [Google Scholar] [PubMed]
- Mortezaee, K.; Shabeeb, D.; Musa, A.E.; Najafi, M.; Farhood, B. Metformin as a Radiation Modifier; Implications to Normal Tissue Protection and Tumor Sensitization. Curr. Clin. Pharmacol. 2019, 14, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Aaseth, J.; Nurchi, V.M.; Andersen, O. Medical Therapy of Patients Contaminated with Radioactive Cesium or Iodine. Biomolecules 2019, 9, 856. [Google Scholar] [CrossRef]
- Brigant, L.; Rozen, R.; Apfelbaum, M. Tritium dilution space measurement is not modified by a doubling in fluid intake. J. Appl. Physiol. 1993, 75, 412–415. [Google Scholar] [CrossRef]
- Dubrovina, Z.V. The absorption of several calcium compounds from the gastro-intestinal tract and their effect on radioactive strontium uptake in rats. Radiobiologiia 1966, 6, 78–80. [Google Scholar] [PubMed]
- Ogawa, K.; Fukuda, T.; Han, J.; Kitamura, Y.; Shiba, K.; Odani, A. Evaluation of Chlorella as a Decorporation Agent to Enhance the Elimination of Radioactive Strontium from Body. PLoS ONE 2016, 11, e0148080. [Google Scholar] [CrossRef]
- Sonawane, V.R.; Jagtap, V.S.; Pahuja, D.N.; Rajan, M.G.R.; Samuel, A.M. Difficulty in dislodging in vivo fixed radiostrontium. Health Phys. 2004, 87, 46–50. [Google Scholar] [CrossRef]
- Adams, T.G.; Casagrande, R. Modeling the Optimum Prussian Blue Treatment for Acute Radiation Syndrome Following 137Cs Ingestion. Health Phys. 2019, 116, 88–95. [Google Scholar] [CrossRef]
- Charles, M.L.; Laszlo, D. The effectiveness of oral ammonium chloride on increased excretion of radio-strontium in man. Int. J. Appl. Radiat. Isot. 1959, 5, 253–264. [Google Scholar] [CrossRef]
- Ohmachi, Y.; Imamura, T.; Ikeda, M.; Shishikura, E.; Kim, E.; Kurihara, O.; Sakai, K. Sodium bicarbonate protects uranium-induced acute nephrotoxicity through uranium-decorporation by urinary alkalinization in rats. J. Toxicol. Pathol. 2015, 28, 65–71. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, G.; Hnatowich, D.J. A Brief Review of Chelators for Radiolabeling Oligomers. Materials 2010, 3, 3204–3217. [Google Scholar] [CrossRef]
- Yantasee, W.; Sangvanich, T.; Creim, J.A.; Pattamakomsan, K.; Wiacek, R.J.; Fryxell, G.E.; Addleman, R.S.; Timchalk, C. Functional sorbents for selective capture of plutonium, americium, uranium, and thorium in blood. Health Phys. 2010, 99, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Ménétrier, F.; Grappin, L.; Raynaud, P.; Courtay, C.; Wood, R.; Joussineau, S.; List, V.; Stradling, G.N.; Taylor, D.M.; Bérard, P.; et al. Treatment of accidental intakes of plutonium and americium: Guidance notes. Appl. Radiat. Isot. Data Instrum. Methods Use Agric. Ind. Med. 2005, 62, 829–846. [Google Scholar] [CrossRef] [PubMed]
- Ansoborlo, E.; Amekraz, B.; Moulin, C.; Moulin, V.; Taran, F.; Bailly, T.; Burgada, R.; Henge-Napoli, M.H.; Jeanson, A.; Den Auwer, C.; et al. Review of actinide decorporation with chelating agents. Comptes Rendus Chim. 2007. [Google Scholar] [CrossRef]
- Gusev, I.; Guskova, A.; Mettler, F.A. Medical Management of Radiation Accidents; CRC Press: Boca Raton, FL, USA, 2001; ISBN 978-1-4200-3719-7. [Google Scholar]
- Morgan, C.; Bingham, D.; Holt, D.C.B.; Jones, D.M.; Lewis, N.J. Therapeutic whole lung lavage for inhaled plutonium oxide revisited. J. Radiol. Prot. Off. J. Soc. Radiol. Prot. 2010, 30, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, M.A. Biomarkers for human radiation exposure. J. Biomed. Sci. 2008, 15, 557–563. [Google Scholar] [CrossRef]
- Battershill, J.M.; Burnett, K.; Bull, S. Factors affecting the incidence of genotoxicity biomarkers in peripheral blood lymphocytes: Impact on design of biomonitoring studies. Mutagenesis 2008, 23, 423–437. [Google Scholar] [CrossRef]
- Samarth, R.M.; Samarth, M.; Matsumoto, Y. Utilization of cytogenetic biomarkers as a tool for assessment of radiation injury and evaluation of radiomodulatory effects of various medicinal plants—A review. Drug Des. Devel. Ther. 2015, 9, 5355–5372. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.M. Cytogenetic Biomarkers of Radiation Exposure. Clin. Oncol. R. Coll. Radiol. G. B. 2019, 31, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Obe, G.; Pfeiffer, P.; Savage, J.R.K.; Johannes, C.; Goedecke, W.; Jeppesen, P.; Natarajan, A.T.; Martínez-López, W.; Folle, G.A.; Drets, M.E. Chromosomal aberrations: Formation, identification and distribution. Mutat. Res. 2002, 504, 17–36. [Google Scholar] [CrossRef]
- Bender, M.A.; Gooch, P.C. Persistent chromosome aberrations in irradiated human subjects. Radiat. Res. 1962, 16, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Blakely, W.F.; Romanyukha, A.; Hayes, S.M.; Reyes, R.A.; Stewart, H.M.; Hoefer, M.H.; Williams, A.; Sharp, T.; Huff, L.A. U.S. Department of Defense Multiple-Parameter Biodosimetry Network. Radiat. Prot. Dosim. 2016, 172, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Sproull, M.; Camphausen, K. State-of-the-Art Advances in Radiation Biodosimetry for Mass Casualty Events Involving Radiation Exposure. Radiat. Res. 2016, 186, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Newman, V.L.; Romaine, P.L.; Hauer-Jensen, M.; Pollard, H.B. Use of biomarkers for assessing radiation injury and efficacy of countermeasures. Expert Rev. Mol. Diagn. 2016, 16, 65–81. [Google Scholar] [CrossRef]
- Pernot, E.; Hall, J.; Baatout, S.; Benotmane, M.A.; Blanchardon, E.; Bouffler, S.; El Saghire, H.; Gomolka, M.; Guertler, A.; Harms-Ringdahl, M.; et al. Ionizing radiation biomarkers for potential use in epidemiological studies. Mutat. Res. 2012, 751, 258–286. [Google Scholar] [CrossRef]
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxid. Med. Cell. Longev. 2017, 2017, 6501046. [Google Scholar] [CrossRef]
- Barshishat-Kupper, M.; Tipton, A.J.; McCart, E.A.; McCue, J.; Mueller, G.P.; Day, R.M. Effect of ionizing radiation on liver protein oxidation and metabolic function in C57BL/6J mice. Int. J. Radiat. Biol. 2014, 90, 1169–1178. [Google Scholar] [CrossRef]
- Huang, S.; Koutrakis, P.; Grady, S.T.; Vieira, C.L.Z.; Schwartz, J.D.; Coull, B.A.; Hart, J.E.; Laden, F.; Zhang, J.J.; Garshick, E. Effects of particulate matter gamma radiation on oxidative stress biomarkers in COPD patients. J. Expo. Sci. Environ. Epidemiol. 2020. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2’ -deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta Int. J. Clin. Chem. 2003, 329, 23–38. [Google Scholar] [CrossRef]
- Halliwell, B.; Lee, C.Y.J. Using isoprostanes as biomarkers of oxidative stress: Some rarely considered issues. Antioxid. Redox Signal. 2010, 13, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Goldstein, M.; Christmann, M.; Becker, H.; Heylmann, D.; Kaina, B. Human monocytes are severely impaired in base and DNA double-strand break repair that renders them vulnerable to oxidative stress. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 21105–21110. [Google Scholar] [CrossRef] [PubMed]
- Manda, K.; Glasow, A.; Paape, D.; Hildebrandt, G. Effects of ionizing radiation on the immune system with special emphasis on the interaction of dendritic and T cells. Front. Oncol. 2012, 2, 102. [Google Scholar] [CrossRef]
- Wunderlich, R.; Ernst, A.; Rödel, F.; Fietkau, R.; Ott, O.; Lauber, K.; Frey, B.; Gaipl, U.S. Low and moderate doses of ionizing radiation up to 2 Gy modulate transmigration and chemotaxis of activated macrophages, provoke an anti-inflammatory cytokine milieu, but do not impact upon viability and phagocytic function. Clin. Exp. Immunol. 2015, 179, 50–61. [Google Scholar] [CrossRef] [PubMed]
- de Andrade Carvalho, H.; Villar, R.C. Radiotherapy and immune response: The systemic effects of a local treatment. Clin. Sao Paulo Braz. 2018, 73, e557s. [Google Scholar]
- Schaue, D.; Kachikwu, E.L.; McBride, W.H. Cytokines in radiobiological responses: A review. Radiat. Res. 2012, 178, 505–523. [Google Scholar] [CrossRef] [PubMed]
- Ao, X.; Zhao, L.; Davis, M.A.; Lubman, D.M.; Lawrence, T.S.; Kong, F.-M. Radiation produces differential changes in cytokine profiles in radiation lung fibrosis sensitive and resistant mice. J. Hematol. Oncol. 2009, 2, 6. [Google Scholar] [CrossRef]
- Petit-Frère, C.; Capulas, E.; Lyon, D.A.; Norbury, C.J.; Lowe, J.E.; Clingen, P.H.; Riballo, E.; Green, M.H.; Arlett, C.F. Apoptosis and cytokine release induced by ionizing or ultraviolet B radiation in primary and immortalized human keratinocytes. Carcinogenesis 2000, 21, 1087–1095. [Google Scholar] [CrossRef]
- Albanese, J.; Martens, K.; Karanitsa, L.V.; Karkanitsa, L.V.; Schreyer, S.K.; Dainiak, N. Multivariate analysis of low-dose radiation-associated changes in cytokine gene expression profiles using microarray technology. Exp. Hematol. 2007, 35, 47–54. [Google Scholar] [CrossRef]
- Babini, G.; Bellinzona, V.E.; Morini, J.; Baiocco, G.; Mariotti, L.; Unger, K.; Ottolenghi, A. Mechanisms of the induction of apoptosis mediated by radiation-induced cytokine release. Radiat. Prot. Dosim. 2015, 166, 165–169. [Google Scholar] [CrossRef]
- Bravatà, V.; Minafra, L.; Forte, G.I.; Cammarata, F.P.; Russo, G.; Di Maggio, F.M.; Augello, G.; Lio, D.; Gilardi, M.C. Cytokine profile of breast cell lines after different radiation doses. Int. J. Radiat. Biol. 2017, 93, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Centurione, L.; Aiello, F.B. DNA Repair and Cytokines: TGF-β, IL-6, and Thrombopoietin as Different Biomarkers of Radioresistance. Front. Oncol. 2016, 6, 175. [Google Scholar] [CrossRef] [PubMed]
- Amundson, S.A.; Fornace, A.J. Gene expression profiles for monitoring radiation exposure. Radiat. Prot. Dosim. 2001, 97, 11–16. [Google Scholar] [CrossRef]
- Amundson, S.A.; Bittner, M.; Fornace, A.J. Functional genomics as a window on radiation stress signaling. Oncogene 2003, 22, 5828–5833. [Google Scholar] [CrossRef]
- Snyder, A.R.; Morgan, W.F. Gene expression profiling after irradiation: Clues to understanding acute and persistent responses? Cancer Metastasis Rev. 2004, 23, 259–268. [Google Scholar] [CrossRef]
- Rashi-Elkeles, S.; Elkon, R.; Shavit, S.; Lerenthal, Y.; Linhart, C.; Kupershtein, A.; Amariglio, N.; Rechavi, G.; Shamir, R.; Shiloh, Y. Transcriptional modulation induced by ionizing radiation: p53 remains a central player. Mol. Oncol. 2011, 5, 336–348. [Google Scholar] [CrossRef]
- Willers, H.; Wang, M.; Benes, C.H. TP53 mutation status: Emerging biomarker for precision radiation medicine? Oncoscience 2018, 5, 258–259. [Google Scholar] [CrossRef] [PubMed]
- Detours, V.; Versteyhe, S.; Dumont, J.E.; Maenhaut, C. Gene expression profiles of post-Chernobyl thyroid cancers. Curr. Opin. Endocrinol. Diabetes Obes. 2008, 15, 440–445. [Google Scholar] [CrossRef]
- Lu, T.-P.; Hsu, Y.-Y.; Lai, L.-C.; Tsai, M.-H.; Chuang, E.Y. Identification of gene expression biomarkers for predicting radiation exposure. Sci. Rep. 2014, 4, 6293. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, M.; Neumann, R. Global Gene Expression Alterations as a Crucial Constituent of Human Cell Response to Low Doses of Ionizing Radiation Exposure. Int. J. Mol. Sci. 2016, 17, 55. [Google Scholar] [CrossRef]
- Li, S.; Lu, X.; Feng, J.B.; Tian, M.; Liu, Q.J. Identification and Validation of Candidate Radiation-responsive Genes for Human Biodosimetr. Biomed. Environ. Sci. BES 2017, 30, 834–840. [Google Scholar]
- Unverricht-Yeboah, M.; Giesen, U.; Kriehuber, R. Comparative gene expression analysis after exposure to 123I-iododeoxyuridine, γ- and α-radiation-potential biomarkers for the discrimination of radiation qualities. J. Radiat. Res. (Tokyo) 2018, 59, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Nishida, T.; Sato, Y.; Nakai, Y.; Kashiwakura, I. Identification of Radiation-Dose-Dependent Expressive Genes in Individuals Exposed to External Ionizing Radiation. Radiat. Res. 2020, 193, 274–285. [Google Scholar] [CrossRef]
- O’Brien, G.; Cruz-Garcia, L.; Majewski, M.; Grepl, J.; Abend, M.; Port, M.; Tichý, A.; Sirak, I.; Malkova, A.; Donovan, E.; et al. FDXR is a biomarker of radiation exposure in vivo. Sci. Rep. 2018, 8, 684. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente van Bentem, S.; Mentzen, W.I.; de la Fuente, A.; Hirt, H. Towards functional phosphoproteomics by mapping differential phosphorylation events in signaling networks. Proteomics 2008, 8, 4453–4465. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Oh, T.J.; Yoon, B.S. Evaluation of hprt mutant assay as a biomarker monitoring the specific environmental mutagen. Biotechnol. Lett. 2000, 22, 1401–1406. [Google Scholar] [CrossRef]
- Ma, S.; Liu, X.; Jiao, B.; Yang, Y.; Liu, X. Low-dose radiation-induced responses: Focusing on epigenetic regulation. Int. J. Radiat. Biol. 2010, 86, 517–528. [Google Scholar] [CrossRef]
- Weigel, C.; Schmezer, P.; Plass, C.; Popanda, O. Epigenetics in radiation-induced fibrosis. Oncogene 2015, 34, 2145–2155. [Google Scholar] [CrossRef]
- Lowe, D.J.; Herzog, M.; Mosler, T.; Cohen, H.; Felton, S.; Beli, P.; Raj, K.; Galanty, Y.; Jackson, S.P. Chronic irradiation of human cells reduces histone levels and deregulates gene expression. Sci. Rep. 2020, 10, 2200. [Google Scholar] [CrossRef]
- Miousse, I.R.; Kutanzi, K.R.; Koturbash, I. Effects of ionizing radiation on DNA methylation: From experimental biology to clinical applications. Int. J. Radiat. Biol. 2017, 93, 457–469. [Google Scholar] [CrossRef]
- Mahmood, N.; Rabbani, S.A. Targeting DNA Hypomethylation in Malignancy by Epigenetic Therapies. Adv. Exp. Med. Biol. 2019, 1164, 179–196. [Google Scholar] [PubMed]
- Jones, P.A. Functions of DNA methylation: Islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 2012, 13, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, V.; Dantzer, F.; Ame, J.-C.; de Murcia, G. Poly(ADP-ribose): Novel functions for an old molecule. Nat. Rev. Mol. Cell Biol. 2006, 7, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Magné, N.; Toillon, R.-A.; Bottero, V.; Didelot, C.; Houtte, P.V.; Gérard, J.-P.; Peyron, J.-F. NF-kappaB modulation and ionizing radiation: Mechanisms and future directions for cancer treatment. Cancer Lett. 2006, 231, 158–168. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, X.; Wang, G.; Wen, X.; Zhang, X.; Hoffman, A.R.; Li, W.; Hu, J.-F.; Cui, J. Loss of insulin-like growth factor II imprinting is a hallmark associated with enhanced chemo/radiotherapy resistance in cancer stem cells. Oncotarget 2016, 7, 51349–51364. [Google Scholar] [CrossRef]
- Ilnytskyy, Y.; Koturbash, I.; Kovalchuk, O. Radiation-induced bystander effects in vivo are epigenetically regulated in a tissue-specific manner. Environ. Mol. Mutagen. 2009, 50, 105–113. [Google Scholar] [CrossRef]
- Koturbash, I. 2017 Michael Fry Award Lecture When DNA is Actually Not a Target: Radiation Epigenetics as a Tool to Understand and Control Cellular Response to Ionizing Radiation. Radiat. Res. 2018, 190, 5. [Google Scholar] [CrossRef]
- Li, L.; Kim, J.-H.; Park, H.-T.; Lee, J.-H.; Park, M.-K.; Lee, J.-W.; Lee, J.-C.; Lee, M.-J. Effect of ionizing radiation at low dose on transgenerational carcinogenesis by epigenetic regulation. Lab. Anim. Res. 2017, 33, 92–97. [Google Scholar] [CrossRef]
- Ivashkevich, A.; Redon, C.E.; Nakamura, A.J.; Martin, R.F.; Martin, O.A. Use of the γ-H2AX assay to monitor DNA damage and repair in translational cancer research. Cancer Lett. 2012, 327, 123–133. [Google Scholar] [CrossRef]
- Scherthan, H.; Hieber, L.; Braselmann, H.; Meineke, V.; Zitzelsberger, H. Accumulation of DSBs in gamma-H2AX domains fuel chromosomal aberrations. Biochem. Biophys. Res. Commun. 2008, 371, 694–697. [Google Scholar] [CrossRef]
- Johansson, P.; Fasth, A.; Ek, T.; Hammarsten, O. Validation of a flow cytometry-based detection of γ-H2AX, to measure DNA damage for clinical applications. Cytometry B Clin. Cytom. 2017, 92, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.S.; Uppal, M.; Randhawa, S.; Cheema, M.S.; Aghdam, N.; Usala, R.L.; Ghosh, S.P.; Cheema, A.K.; Dritschilo, A. Radiation Metabolomics: Current Status and Future Directions. Front. Oncol. 2016, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Lindon, J.C.; Holmes, E.; Nicholson, J.K. Metabonomics techniques and applications to pharmaceutical research & development. Pharm. Res. 2006, 23, 1075–1088. [Google Scholar] [PubMed]
- Coy, S.L.; Cheema, A.K.; Tyburski, J.B.; Laiakis, E.C.; Collins, S.P.; Fornace, A. Radiation metabolomics and its potential in biodosimetry. Int. J. Radiat. Biol. 2011, 87, 802–823. [Google Scholar] [CrossRef]
- Jones, J.W.; Scott, A.J.; Tudor, G.; Xu, P.-T.; Jackson, I.L.; Vujaskovic, Z.; Booth, C.; MacVittie, T.J.; Ernst, R.K.; Kane, M.A. Identification and quantitation of biomarkers for radiation-induced injury via mass spectrometry. Health Phys. 2014, 106, 106–119. [Google Scholar] [CrossRef]
- Pannkuk, E.L.; Fornace, A.J.; Laiakis, E.C. Metabolomic applications in radiation biodosimetry: Exploring radiation effects through small molecules. Int. J. Radiat. Biol. 2017, 93, 1151–1176. [Google Scholar] [CrossRef]
- Tyburski, J.B.; Patterson, A.D.; Krausz, K.W.; Slavík, J.; Fornace, A.J.; Gonzalez, F.J.; Idle, J.R. Radiation metabolomics. 1. Identification of minimally invasive urine biomarkers for gamma-radiation exposure in mice. Radiat. Res. 2008, 170, 1–14. [Google Scholar] [CrossRef]
- Wang, C.; Yang, J.; Nie, J. Plasma phospholipid metabolic profiling and biomarkers of rats following radiation exposure based on liquid chromatography-mass spectrometry technique. Biomed. Chromatogr. BMC 2009, 23, 1079–1085. [Google Scholar] [CrossRef]
- Tyburski, J.B.; Patterson, A.D.; Krausz, K.W.; Slavík, J.; Fornace, A.J.; Gonzalez, F.J.; Idle, J.R. Radiation metabolomics. 2. Dose- and time-dependent urinary excretion of deaminated purines and pyrimidines after sublethal gamma-radiation exposure in mice. Radiat. Res. 2009, 172, 42–57. [Google Scholar] [CrossRef]
- Lanz, C.; Patterson, A.D.; Slavík, J.; Krausz, K.W.; Ledermann, M.; Gonzalez, F.J.; Idle, J.R. Radiation metabolomics. 3. Biomarker discovery in the urine of gamma-irradiated rats using a simplified metabolomics protocol of gas chromatography-mass spectrometry combined with random forests machine learning algorithm. Radiat. Res. 2009, 172, 198–212. [Google Scholar] [CrossRef]
- Johnson, C.H.; Patterson, A.D.; Krausz, K.W.; Lanz, C.; Kang, D.W.; Luecke, H.; Gonzalez, F.J.; Idle, J.R. Radiation metabolomics. 4. UPLC-ESI-QTOFMS-Based metabolomics for urinary biomarker discovery in gamma-irradiated rats. Radiat. Res. 2011, 175, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Brenner, D.J.; Brown, T.R. Identification of urinary biomarkers from X-irradiated mice using NMR spectroscopy. Radiat. Res. 2011, 175, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.H.; Patterson, A.D.; Krausz, K.W.; Kalinich, J.F.; Tyburski, J.B.; Kang, D.W.; Luecke, H.; Gonzalez, F.J.; Blakely, W.F.; Idle, J.R. Radiation metabolomics. 5. Identification of urinary biomarkers of ionizing radiation exposure in nonhuman primates by mass spectrometry-based metabolomics. Radiat. Res. 2012, 178, 328–340. [Google Scholar] [CrossRef]
- Pannkuk, E.L.; Laiakis, E.C.; Authier, S.; Wong, K.; Fornace, A.J. Global Metabolomic Identification of Long-Term Dose-Dependent Urinary Biomarkers in Nonhuman Primates Exposed to Ionizing Radiation. Radiat. Res. 2015, 184, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, Z.; Zhang, X.; Qiao, Y.; Wu, S.; Dong, F.; Chen, Y. Selection of candidate radiation biomarkers in the serum of rats exposed to gamma-rays by GC/TOFMS-based metabolomics. Radiat. Prot. Dosim. 2013, 154, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Goudarzi, M.; Weber, W.; Mak, T.D.; Chung, J.; Doyle-Eisele, M.; Melo, D.; Brenner, D.J.; Guilmette, R.A.; Fornace, A.J. Development of urinary biomarkers for internal exposure by cesium-137 using a metabolomics approach in mice. Radiat. Res. 2014, 181, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Goudarzi, M.; Weber, W.M.; Mak, T.D.; Chung, J.; Doyle-Eisele, M.; Melo, D.R.; Brenner, D.J.; Guilmette, R.A.; Fornace, A.J. Metabolomic and lipidomic analysis of serum from mice exposed to an internal emitter, cesium-137, using a shotgun LC-MS(E) approach. J. Proteome Res. 2015, 14, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Laiakis, E.C.; Mak, T.D.; Anizan, S.; Amundson, S.A.; Barker, C.A.; Wolden, S.L.; Brenner, D.J.; Fornace, A.J. Development of a metabolomic radiation signature in urine from patients undergoing total body irradiation. Radiat. Res. 2014, 181, 350–361. [Google Scholar] [CrossRef]
- Pannkuk, E.L.; Laiakis, E.C.; Mak, T.D.; Astarita, G.; Authier, S.; Wong, K.; Fornace, A.J. A Lipidomic and Metabolomic Serum Signature from Nonhuman Primates Exposed to Ionizing Radiation. Metabolomics Off. J. Metabolomic Soc. 2016, 12. [Google Scholar] [CrossRef]
- Golla, S.; Golla, J.P.; Krausz, K.W.; Manna, S.K.; Simillion, C.; Beyoğlu, D.; Idle, J.R.; Gonzalez, F.J. Metabolomic Analysis of Mice Exposed to Gamma Radiation Reveals a Systemic Understanding of Total-Body Exposure. Radiat. Res. 2017, 187, 612–629. [Google Scholar] [CrossRef]
- Cheema, A.K.; Hinzman, C.P.; Mehta, K.Y.; Hanlon, B.K.; Garcia, M.; Fatanmi, O.O.; Singh, V.K. Plasma Derived Exosomal Biomarkers of Exposure to Ionizing Radiation in Nonhuman Primates. Int. J. Mol. Sci. 2018, 19, 3427. [Google Scholar] [CrossRef] [PubMed]
- Cheema, A.K.; Mehta, K.Y.; Rajagopal, M.U.; Wise, S.Y.; Fatanmi, O.O.; Singh, V.K. Metabolomic Studies of Tissue Injury in Nonhuman Primates Exposed to Gamma-Radiation. Int. J. Mol. Sci. 2019, 20, 3360. [Google Scholar] [CrossRef] [PubMed]
- Kultova, G.; Tichy, A.; Rehulkova, H.; Myslivcova-Fucikova, A. The hunt for radiation biomarkers: Current situation. Int. J. Radiat. Biol. 2020, 96, 370–382. [Google Scholar] [CrossRef]
- Sun, J.-L.; Li, S.; Lu, X.; Feng, J.-B.; Cai, T.-J.; Tian, M.; Liu, Q.-J. Identification of the differentially expressed protein biomarkers in rat blood plasma in response to gamma irradiation. Int. J. Radiat. Biol. 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, Q.; Hu, Y.; Qi, Z.; Lin, Z.; Ying, W.; Zhou, M. Proteomic Profiling for Serum Biomarkers in Mice Exposed to Ionizing Radiation. Dose-Response Publ. Int. Hormesis Soc. 2019, 17, 1559325819894794. [Google Scholar] [CrossRef]
- Blakely, W.F.; Bolduc, D.L.; Debad, J.; Sigal, G.; Port, M.; Abend, M.; Valente, M.; Drouet, M.; Hérodin, F. Use of Proteomic and Hematology Biomarkers for Prediction of Hematopoietic Acute Radiation Syndrome Severity in Baboon Radiation Models. Health Phys. 2018, 115, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Jacob, N.K.; Cooley, J.V.; Yee, T.N.; Jacob, J.; Alder, H.; Wickramasinghe, P.; Maclean, K.H.; Chakravarti, A. Identification of sensitive serum microRNA biomarkers for radiation biodosimetry. PLoS ONE 2013, 8, e57603. [Google Scholar] [CrossRef]
- Yu, J.; Qiu, X.; Shen, X.; Shi, W.; Wu, X.; Gu, G.; Zhu, B.; Ju, S. miR-202 expression concentration and its clinical significance in the serum of multiple myeloma patients. Ann. Clin. Biochem. 2014, 51, 543–549. [Google Scholar] [CrossRef]
- Acharya, S.S.; Fendler, W.; Watson, J.; Hamilton, A.; Pan, Y.; Gaudiano, E.; Moskwa, P.; Bhanja, P.; Saha, S.; Guha, C.; et al. Serum microRNAs are early indicators of survival after radiation-induced hematopoietic injury. Sci. Transl. Med. 2015, 7, 287ra69. [Google Scholar] [CrossRef]
- Małachowska, B.; Tomasik, B.; Stawiski, K.; Kulkarni, S.; Guha, C.; Chowdhury, D.; Fendler, W. Circulating microRNAs as Biomarkers of Radiation Exposure: A Systematic Review and Meta-Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 390–402. [Google Scholar] [CrossRef]
- Port, M.; Herodin, F.; Valente, M.; Drouet, M.; Ullmann, R.; Doucha-Senf, S.; Lamkowski, A.; Majewski, M.; Abend, M. MicroRNA Expression for Early Prediction of Late Occurring Hematologic Acute Radiation Syndrome in Baboons. PLoS ONE 2016, 11, e0165307. [Google Scholar] [CrossRef]
- Gao, F.; Liu, P.; Narayanan, J.; Yang, M.; Fish, B.L.; Liu, Y.; Liang, M.; Jacobs, E.R.; Medhora, M. Changes in miRNA in the lung and whole blood after whole thorax irradiation in rats. Sci. Rep. 2017, 7, 44132. [Google Scholar] [CrossRef] [PubMed]
- Tucker, J.D.; Divine, G.W.; Grever, W.E.; Thomas, R.A.; Joiner, M.C.; Smolinski, J.M.; Auner, G.W. Gene expression-based dosimetry by dose and time in mice following acute radiation exposure. PLoS ONE 2013, 8, e83390. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shingara, J.; Keiger, K.; Shelton, J.; Laosinchai-Wolf, W.; Powers, P.; Conrad, R.; Brown, D.; Labourier, E. An optimized isolation and labeling platform for accurate microRNA expression profiling. RNA 2005, 11, 1461–1470. [Google Scholar] [CrossRef] [PubMed]
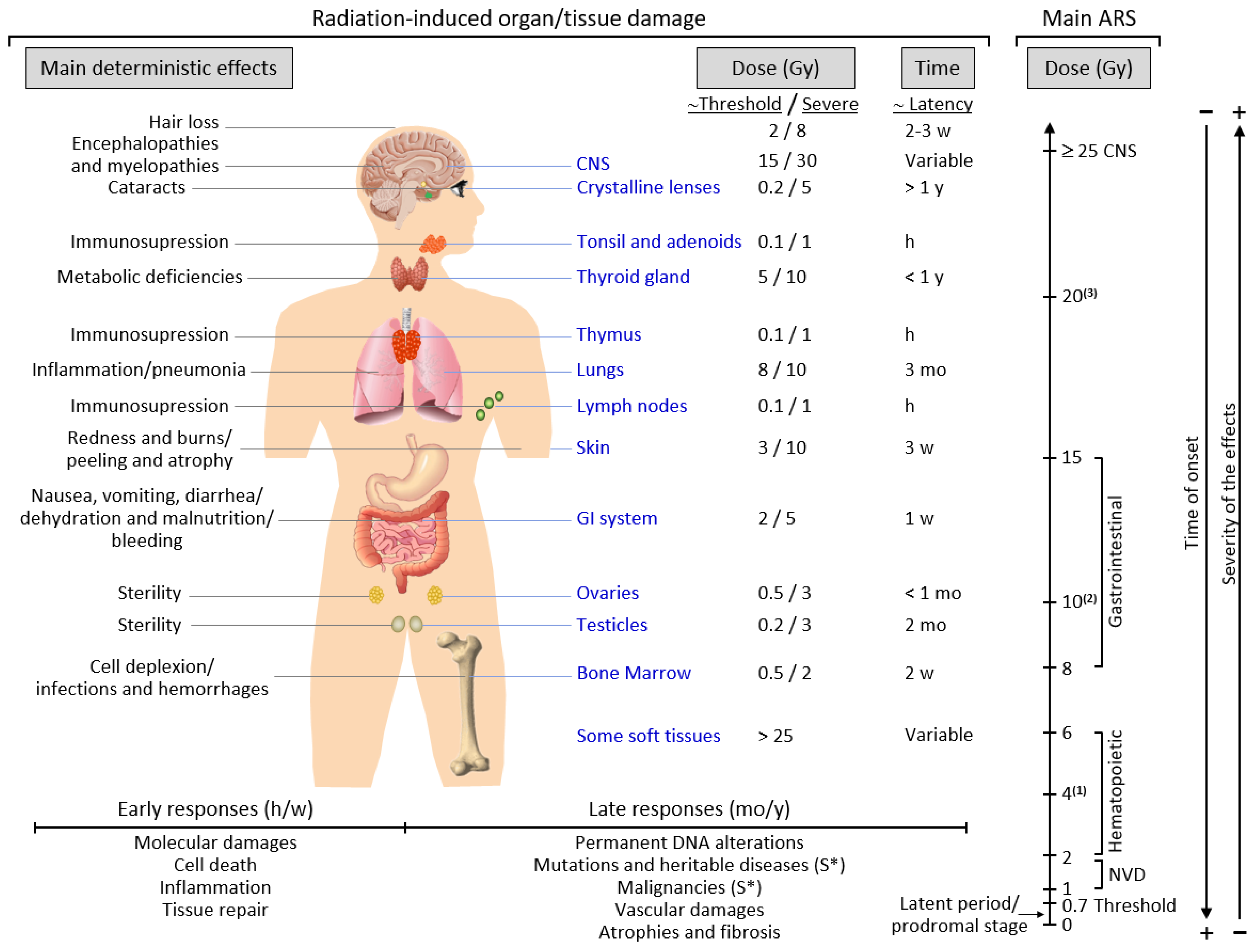
| Compound | Main Mechanism(s) of Action | Approved | Ref. |
|---|---|---|---|
| Radioprotectors | |||
| Thiol-containing molecules | Scavenging of free radicals, hydrogen donation, repair of damaged DNA, and Warburg-type- and HIF1α-dependent effects | ||
| Amifostine (WR-2721) | RP | [47] | |
| 2-mercaptoethane sodium sulphonate | RP | [250] | |
| Cysteine | + | [251] | |
| N-Acetyl cysteine | + | [252] | |
| Cysteamine | + | [253] | |
| Cystamine | [254] | ||
| 2-Mercaptoethylguanidine | [255] | ||
| Aminothiol PrC-210 | [256] | ||
| Polyphenolic phytochemicals | Antioxidant and free-radical-scavenging activities, upregulation of physiological antioxidant defenses, anti-inflammatory properties | ||
| Genistein | + | [60] | |
| Resveratrol | + | [62] | |
| Pterostilbene | + | [257] | |
| Naringenin | + | [63] | |
| Curcumin | + | [258] | |
| Epigallocatechin-3-gallate | + | [259] | |
| Epicatechin | + | [260] | |
| Apigenin | + | [261] | |
| Ellagic acid | + | [262] | |
| Lutein | + | [263] | |
| Nonpolyphenolic phytochemicals | |||
| Caffeine | Antioxidant and anti-inflammatory properties | + | [73] |
| 3,4-Methylenedioxyphenol | Protection of the hematopoietic and gastrointestinal systems | [74] | |
| 3,3′-Diindolylmethane | Stimulation of an ataxia-telangiectasia mutated-driven DNA damage response like response and NF-κB survival signaling | + | [75] |
| Vitamins | |||
| Tocopherol and derivatives | Antioxidants and free radical scavengers, immunomodulators | + | [81] |
| δ- and ɣ-Tocotrienol | Antioxidants and free radical scavengers, immunomodulators, G-CSF increase, bone marrow and gastrointestinal protection | + | [83] |
| Ascorbic acid | Antioxidant and free-radical scavenger, gastrointestinal protection | + | [79] |
| β-Carotene | Antimutagenic and antioxidant activity | + | [264] |
| Retinol | Anti-inflammatory, MMP inhibitor, free-radical scavenger | + | [76] |
| α-Lipoic acid | Antioxidant and free-radical scavenger | + | [265] |
| Oligoelements | |||
| Zinc | Development and functions of different immune cells, inhibition of NF-κB signaling, promotes DNA repair, stabilizes sulfhydryls, SOD1 activity | + | [266] |
| Copper | + | [267] | |
| Manganese | SOD2 activity | + | [268] |
| Mn-porphyrin derivatives | Free-radical scavengers | + | [269] |
| Selenium and sodium selenite | Increases the activity DNA glycosylases, GSH peroxidase activity | + | [91] |
| Selenomethionine | Upregulates p53-mediated base excision repair pathways | + | [90] |
| Selenopropionic acid | Inhibition of DNA strand break formation | [92] | |
| Selenocysteine | Forms part of the catalytic center of GSH peroxidases | [93] | |
| Cerium oxide nanoparticles | Free radical scavenging activity, SOD2 regulation | [270] | |
| Superoxide dismutase (SOD) | Antioxidant | ||
| SOD2 | + | [97] | |
| AEOL 10150 (Mn porphyrin SOD mimic) | [271] | ||
| AAV2-Mn-SOD-hrGFP | [100] | ||
| SOD2 gene-modified mesenchymal stem cells | [272] | ||
| BMX-001 (a SOD2 mimic) | + | [103] | |
| EUK-134, EUK-189, EUK-207 (synthetic MN complexes SOD/catalase mimetics) | + | [273] | |
| Ex-Rad (recilisib sodium) | Upregulation of PI3-kinase/AKT pathways, hematopoietic and gastrointestinal protection | [107] | |
| Nitrosides | |||
| Tempol | Antioxidant activity, prevention of chromosomal aberrations | + | [113] |
| JP4-039 (mitochondria-targeted GS-nitroxide) | Enhances intestinal barrier and stem-cell recovery | [274] | |
| Hormones and hormone analogues | |||
| Melatonin | Antioxidant and anti-inflammatory activity | + | [115] |
| Indraline (alpha-adrenomimetic) | Increase in DNA and protein biosynthesis and ribonucleotide reductase | [122] | |
| Androstenetriol | Stimulate the innate immune system | [275] | |
| Antibiotics | |||
| Streptomycin, kanamycin, neomycin, gentamicin | Protect hematopoietic system | + | [125] |
| Tetracyclines | Protect hematopoietic stem/progenitor cell population, free-radical-scavenging activity, protect DNA | + | [1] |
| Fluoroquinolones, minocyclin | Antioxidant and free-radical-scavenging activity, protect hematopoietic system | + | [276] |
| Adenosine receptor agonists | |||
| AMP and dipyridamole | Systemic vasodilation, hypotension, hypoxia and elevation of cAMPin sensitive cells), hematopoiesis stimulation, favors DNA stability | + | [130] |
| IB-MECA | Hematopoiesis stimulation | + | [131] |
| G-CSF | Hematopoiesis stimulation | + | [277] |
| DNA-binding molecules | Favor stability and prevent damage of DNA | ||
| Hoechst | [140] | ||
| Methylproamine | [141] | ||
| Netropsin | [142] | ||
| Pentamidine | + | [144] | |
| Other | |||
| Anethole and anethole ditholethione (unsaturated ether related to lignols) | Anti-inflammatory (inhibit TNF-induced NF-κB activation), increase GSH | [278] | |
| LY294002 and PX-867 | PI3K inhibitors that decrease radiation-induced DNA damage | [279] | |
| SB-415286 (a specific inhibitor of GSK-3β) | Protection for radiation-induced necrosis in brain and the intestine | [280] | |
| C60(OH) (a polyhydroxylated fullerene derivative) | Protects from radiation-induced immune and mitochondrial dysfunction | [281] | |
| Radiomitigators | |||
| l-Glutamine | Reducing the incidence of gastrointestinal, neurological, and cardiac complications, accelerates healing of the irradiated bowel | + | [147] |
| Probiotics | Mitigate the radiation-induced gastrointestinal damage, reduce diarrheal symptoms | [155] | |
| 2nd-generation producing IL22 | Prevents loss of intestinal crypt cells (principally Leu-rich repeat-containing G-protein-coupled receptor 5 stem cells) | [158] | |
| ACE inhibitors and angiotensin II receptor blockers | |||
| Perindopril | Increases bone marrow cellularity and the hematopoietic progenitors CFU-GM, BFU-E, and CFU-Meg, mitigates the hematopoietic toxicity | + | [159] |
| Captopril | Upregulates the hematopoietic progenitor cell cycle | [160] | |
| Angiotensin receptor blockers | Interact with the angiotensin AT1 receptor to specifically release PGE2 | + | [164] |
| Statins | Reduce the mRNA expression of pro-inflammatory and pro-fibrotic cytokines, accelerate the repair of DNA double-strand breaks and mitigate DNA damage | [168] | |
| Simvastatin | Reduces cardiac dysfunction and capsular fibrosis | + | [171] |
| Somatostatin analogues | |||
| Octreotide | Ameliorates acute and delayed intestinal radiation injury | RM | [177] |
| SOM230 (pasireotide) | Ameliorates injury of the intestinal mucosa | + | [180] |
| Immunomodulators | |||
| β-Glucan | Stimulates hematopoiesis | + | [189] |
| 5-Androstenediol | Stimulates hematopoiesis and the innate immune system, inhibits the inflammasome-mediated pyroptosis | RM | [196] |
| Peptidoglycan | Ameliorates intestinal and bone marrow damages | [53] | |
| CBLB502 | Activates TLR5 and triggers NF-κB signaling, mobilizing an innate immune response | + | [197] |
| CBLB613 | Protects against the hematopoietic syndrome | [248] | |
| Antibiotics and synthetic trehalose dicornomycolate | Prevent sepsis following irradiation | + | [199] |
| Cytokines | |||
| G-CSF and filgrastim, GM-CSF, and sargramostim | Upregulate the production of neutrophils within the bone marrow and minimizes the radiation-induced hematopoietic syndrome | RM | [197] |
| TGF-β | Decreases pulmonary fibrosis | + | [204] |
| Palifermin (recombinant N-terminal truncated KGF) | Stimulates epithelial cell proliferation, differentiation, and upregulates cytoprotective mechanisms | + | [206] |
| RSPO1 | Efficacy against radiation-induced mucositis, promotes gastrointestinal epithelial cell proliferation | RM | [207] |
| AG1024 (an IGF-1R inhibitor) | Prevents radiation-induced endothelial cell senescence | + | [282] |
| Bevacizumab (anti-VEGF antibody) | Ameliorates the edema associated with radiation necrosis | + | [211] |
| Octadecenyl thiophosphate (a synthetic mimic of the GF-like mediator lysophosphatidic acid) | Mitigates the hematopoietic and gastrointestinal ARS | RM | [283] |
| UTL-5g (TNF-α inhibitor) | Protects in the acute phase of radiation-induced liver injury | + | [284] |
| IL-1 | Protects the hematopoietic stem cells | + | [203] |
| IL-12 and rhIL12 (HemaMax) | Promote hematopoiesis and recovery of immune functions | RM | [285] |
| IGF-1 | Protection of intestinal stem cells | + | [286] |
| CDX-301 (recombinant human protein form of the Fms-related tyrosine kinase 3 ligand) | Stimulates expansion and differentiation of hematopoieticprogenitor and stem cells | + | [287] |
| FGF-peptide | Improves barrier function and proliferation in human keratinocytes, promotes stem cell self-renewal | + | [288] |
| NSAIDs | |||
| Meloxicam (selective COX2 inhibitor) | Stimulates hematopoiesis | + | [218] |
| Acetylsalicylic acid | Ameliorates radiation-induced kidney and lung damage, and reduces chromosomal aberrations | + | [221] |
| Bio 300 (genistein nanoparticles) | Reduces the formation of collagen-rich lesions in irradiated lungs | RM | [223] |
| ABC294640 (inhibitor of sphingosine kinase-2) | Reduces radiation-induced gastrointestinal inflammation | + | [230] |
| Cell therapy | |||
| MSCs | Ameliorate radiation-induced injury in liver and brain, promote healing of the irradiated skin, and mitigate radiation-induced gastrointestinal and hematopoietic syndromes | RM | [245] |
| Bone marrow stromal cell transplantation | Restitutes irradiated intestinal stem cells niche and mitigate radiation-induced gastrointestinal syndrome | + | [246] |
| Transplantation of myeloid progenitors and megakaryocyte/erythrocyte-restricted progenitors | Protection against the radiation-induced hematopoietic syndrome | + | [289] |
| PLX-R18 (allogenic placenta-derived stromal cells) | Maintenance and renewal of hematopoietic progenitor cells | RM | [290] |
| CLT-008 (human myeloid progenitor cells) | Promote neutrophil and platelet recovery, and human myeloidprogenitor cell persistence | RM | [289] |
| Others | |||
| ALXN4100TPO (thrombopoietin receptor agonist) | Stimulates platelet production | RM | [291] |
| 2A2 (an anti-ceramide antibody) | Prevents ceramide-induced endothelial cell apoptosis within the mucosal microvascular network | [292] | |
| Rapamycin (a specific inhibitor of mTOR) | Mitigates radiation-induced pulmonary fibrosis, and protects hematopoietic cells and the liver | RM | [293] |
| TP508 (rusalatide acetate) | Mitigates effects of radiation by restoring endothelial function, andrecovery of bone marrow stem cells and stem cells in intestinal and colonic crypts | RM | [294] |
| Granistron, ondansetron (serotonin 5-HT3 receptor antagonists) | Mitigate radiation-induced nausea and vomiting | RM | [295] |
| Poly N-(acetyl, arginyl) glucosamine | Mitigates the radiation-induced proctitis, reduces inflammation andlesions in radiation-induced oral mucositis | + | [13] |
| Metformin | Targets endogenous ROS production within cells, and enhancesDNA repair capacity | RM | [296] |
| Biomarkers | Assay | Methodology | Dose Range | Time Window after Exposure | TBI | PBI | Applicable for ARS Scoring | Automation | Recommended for Networks |
|---|---|---|---|---|---|---|---|---|---|
| Cytogenetics | DCA | Fluorescence + Giemsa staining | 0.1–5 Gy | Before renewal of PBL | yes | yes | yes | yes | yes |
| CBMN | Fluorescence + DAPI | 0.2–4 Gy but with limited sensitivity at dose <1 Gy | In lymphocytes: before renewal of PBL In reticulocytes: not yet established | yes | yes | TBD | yes | yes | |
| PCC | PEG-induced fusion of G0 lymphocytes with mitotic CHO cells | PCC fragments: 0.2–20 Gy PCC rings: 1–20 Gy | PCC fragments: ideally immediately after exposure PCC rings: before renewal of PBL | yes | limited | no | no | yes | |
| FISH | Cell cycle control for FISH painting using the FpG method | 0.25–4 Gy | Years | yes | no | TBD | no | yes | |
| Oxidative stress | 8-hydroxy-2′-deoxyguanosine, isoprostanes and protein carbonyls | UPLC–MS/MS, ELISA | >0.1 Gy | 1–2 h | yes | yes | no | no | yes |
| Immune and inflammatory mediators | IL-1, IL-1ra, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-11, IL-12, IL-12β, IL-13, IL-18, TNF-α, TNF-a/b, IFN-γ, TGF-β, GM-CSF, M-CSF | ELISA | >1.2 mGy | Within 24 h | yes | no | yes | yes | yes |
| Gene expression and mutations | Gene expression (ATM/P53 pathway, MAPK cascades, NF-κB activation, GADD45, CDKN1A, genes associated with the nucleotide excision repair pathway, TP53, PPP1R14C, TNFAIP8L1, DNAJC1, PRTFDC1, KLF10, TNFAIP8L1, Slfn4, Itgb5, Smim3, Tmem40, Litaf, Gp1bb, Cxx1c, FDXR) | RT-PCR | 0.1–10 Gy | Hours–days | yes | yes | yes | yes | yes |
| gpa | ELISA | >1 Gy | Years | ||||||
| hprt | Direct DNA sequencing or immunofluorescence | >90 mGy | Months | ||||||
| Epigenetics | Gene methylation and repetitive elements | HPLC-UV, LC–MS/MS, ELISA, qPCR or PCR and sequencing | 0.1–10 Gy | Hours–months | yes | TBD | TBD | yes | yes |
| γ-H2AX | Immunofluorescence staining, flow cytometry, electro-chemiluminescence | 0.01–8 Gy | Minutes–days | yes | yes | TBD | yes | yes | |
| Omics | Metabolomics: urine metabolites (N-hexanoylglycine and beta-thymidine, glyoxylate, threonate, thymine, uracil, p-cresol, citrate, 2-oxoglutarate, adipate, pimelate, suberate, azelaate, thymidine, 2′-deoxyuridine, 2′-deoxyxanthosine, N(1)-acetylspermidine, N-acetylglucosamine/galactosamine-6-sulfate, N-acetyltaurine, N-hexanoylglycine, taurine, creatine, succinate, methylamine, citrate, 2-oxoglutarate, N-methyl-nicotinamide, hippurate, choline, xanthine, hypoxanthine, uric acid, creatine, creatinine), blood serum metabolites (inositol, serine, lysine, glycine, threonine, glycerol, isocitrate, gluconic acid, stearic acid, methylglutarylcarnitine) | LC or GC coupled with MS and/or NMR spectroscopy | 0.5–10 Gy | Hours–months | yes | no | yes | yes | yes |
| Proteomics: plasma (ferredoxin reductase, alpha-2-macroglobulin, chromogranin-A, glutathione peroxidase 3) | HPLC–LC–MS/MS, SELDI-TOF-MS, | 1–14 Gy | 10 min–40 weeks post-irradiation | yes | no | TBD | yes | yes | |
| DIGE, MALDI-TOF/TOF, Western blot, tissue arrays | |||||||||
| Lipidomics: blood serum (as linoleic acid, palmitic acid, phosphatidylcholines, glycerolipids, glycerophospholipids and esterified sterols) | LC or GC coupled with MS and/or NMR spectroscopy | 6–10 Gy | 2–3 days post-irradiation | yes | no | TBD | yes | yes | |
| microRNAs (miRNAs) | Blood serum (miR-150, miR-30a, miR-30c, miR-34a, miR-200b, miR-29a, miR-29b, miR-144-5p, miR-144-3p, miR-142-5p, miR-19a-3p) | Microarrays, RT-PCR, nanostring nCounter technology, NGS | 0.5–12 Gy | Hours–months | yes | no | no | yes | yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obrador, E.; Salvador, R.; Villaescusa, J.I.; Soriano, J.M.; Estrela, J.M.; Montoro, A. Radioprotection and Radiomitigation: From the Bench to Clinical Practice. Biomedicines 2020, 8, 461. https://doi.org/10.3390/biomedicines8110461
Obrador E, Salvador R, Villaescusa JI, Soriano JM, Estrela JM, Montoro A. Radioprotection and Radiomitigation: From the Bench to Clinical Practice. Biomedicines. 2020; 8(11):461. https://doi.org/10.3390/biomedicines8110461
Chicago/Turabian StyleObrador, Elena, Rosario Salvador, Juan I. Villaescusa, José M. Soriano, José M. Estrela, and Alegría Montoro. 2020. "Radioprotection and Radiomitigation: From the Bench to Clinical Practice" Biomedicines 8, no. 11: 461. https://doi.org/10.3390/biomedicines8110461
APA StyleObrador, E., Salvador, R., Villaescusa, J. I., Soriano, J. M., Estrela, J. M., & Montoro, A. (2020). Radioprotection and Radiomitigation: From the Bench to Clinical Practice. Biomedicines, 8(11), 461. https://doi.org/10.3390/biomedicines8110461








