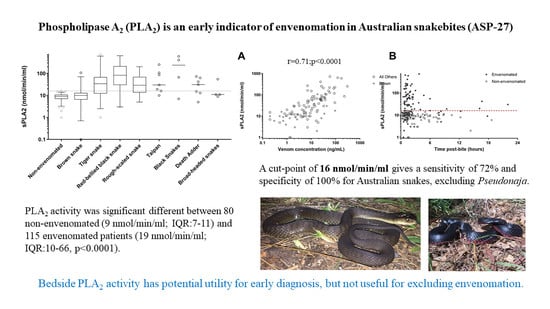
Phospholipase A2 (PLA2) as an Early Indicator of Envenomation in Australian Elapid Snakebites (ASP-27)
Abstract
1. Introduction
2. Experimental Section
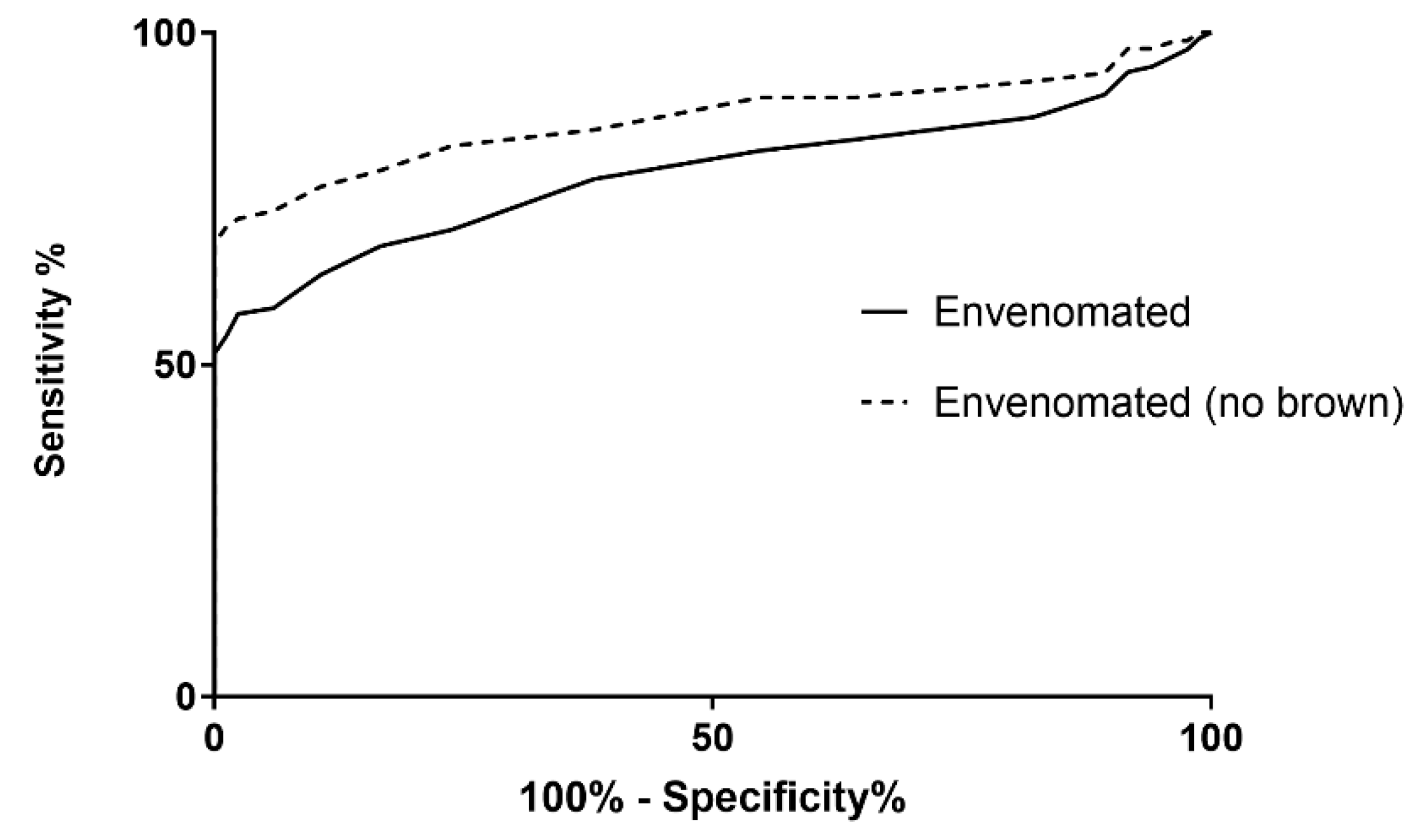
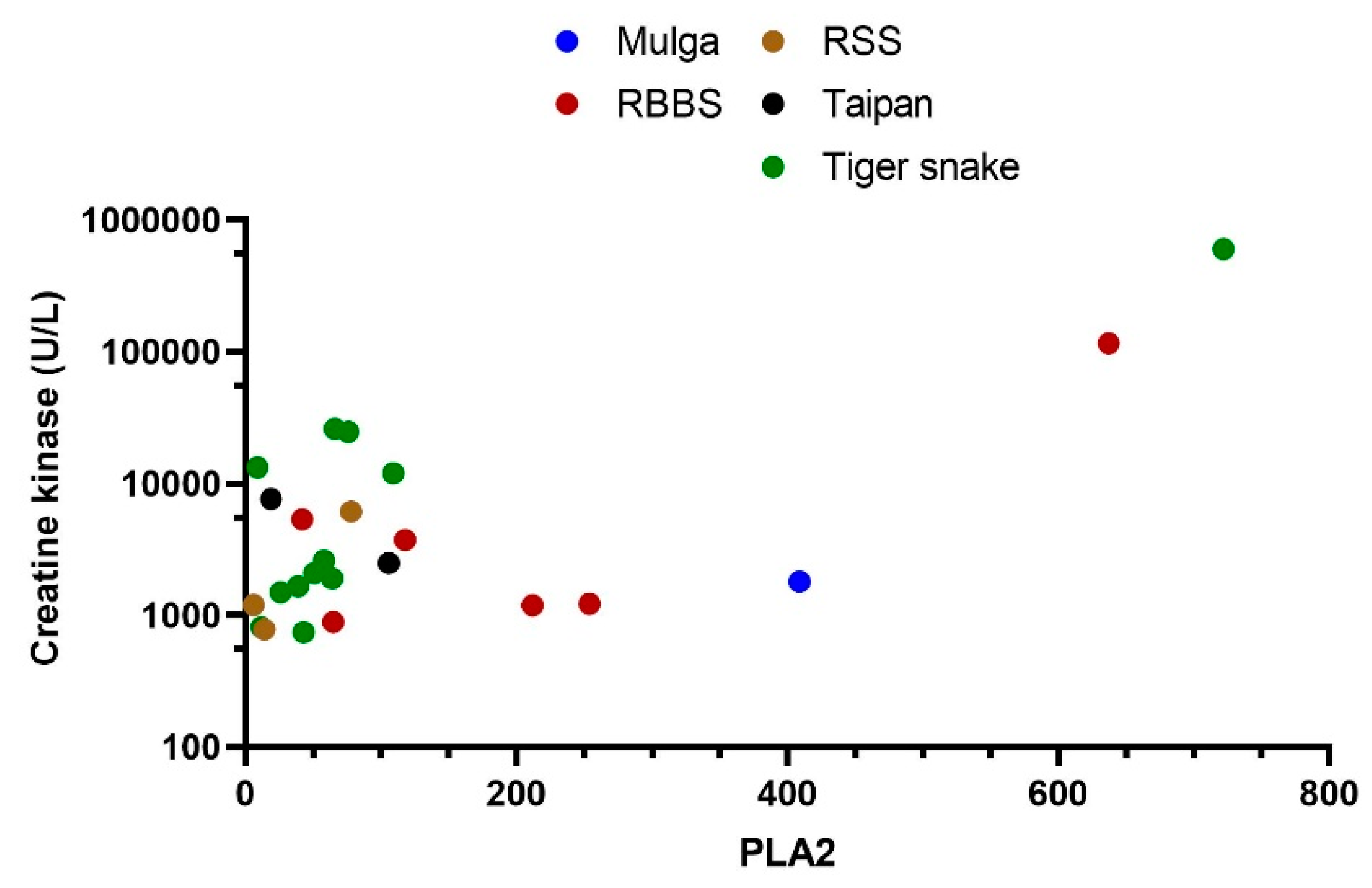
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Longbottom, J.; Shearer, F.M.; Devine, M.; Alcoba, G.; Chappuis, F.; Weiss, D.J.; Ray, S.E.; Ray, N.; Warrell, D.A.; de Castañeda, R.R.; et al. Vulnerability to snakebite envenoming: A global mapping of hotspots. Lancet 2018, 392, 673–684. [Google Scholar] [CrossRef]
- Kasturiratne, A.; Wickremasinghe, A.R.; de Silva, N.; Gunawardena, N.K.; Pathmeswaran, A.; Premaratna, R.; Savioli, L.; Lalloo, D.G.; de Silva, H.J. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008, 5, e218. [Google Scholar] [CrossRef] [PubMed]
- Isbister, G.K. Antivenom efficacy or effectiveness: The Australian experience. Toxicology 2010, 268, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.I.; Ryan, N.M.; O’Leary, M.A.; Brown, S.G.; Isbister, G.K. Australian taipan (Oxyuranus spp.) envenoming: Clinical effects and potential benefits of early antivenom therapy—Australian Snakebite Project (ASP-25). Clin. Toxicol. 2017, 55, 115–122. [Google Scholar] [CrossRef]
- Churchman, A.; O’Leary, M.A.; Buckley, N.A.; Page, C.B.; Tankel, A.; Gavaghan, C.; Holdgate, A.; Brown, S.G.; Isbister, G.K. Clinical effects of red-bellied black snake (Pseudechis porphyriacus) envenoming and correlation with venom concentrations: Australian Snakebite Project (ASP-11). Med. J. Aust. 2010, 193, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Lalloo, D.G.; Trevett, A.J.; Korinhona, A.; Nwokolo, N.; Laurenson, I.F.; Paul, M.; Black, J.; Naraqi, S.; Mavo, B.; Saweri, A.; et al. Snake bites by the Papuan taipan (Oxyuranus scutellatus canni): Paralysis, hemostatic and electrocardiographic abnormalities, and effects of antivenom. Am. J. Trop. Med. Hyg. 1995, 52, 525–531. [Google Scholar] [CrossRef]
- Silva, A.; Maduwage, K.; Sedgwick, M.; Pilapitiya, S.; Weerawansa, P.; Dahanayaka, N.J.; Buckley, N.A.; Johnston, C.; Siribaddana, S.; Isbister, G.K. Neuromuscular Effects of Common Krait (Bungarus caeruleus) Envenoming in Sri Lanka. PLoS Negl. Trop. Dis. 2016, 10, e0004368. [Google Scholar] [CrossRef]
- Johnston, C.I.; Brown, S.G.; O’Leary, M.A.; Currie, B.J.; Greenberg, R.; Taylor, M.; Barnes, C.; White, J.; Isbister, G.K.; ASP investigators. Mulga snake (Pseudechis australis) envenoming: A spectrum of myotoxicity, anticoagulant coagulopathy, haemolysis and the role of early antivenom therapy—Australian Snakebite Project (ASP-19). Clin. Toxicol. 2013, 51, 417–424. [Google Scholar] [CrossRef]
- Kularatne, S.A.; Silva, A.; Weerakoon, K.; Maduwage, K.; Walathara, C.; Paranagama, R.; Mendis, S. Revisiting Russell’s viper (Daboia russelii) bite in Sri Lanka: Is abdominal pain an early feature of systemic envenoming? PLoS ONE 2014, 9, e90198. [Google Scholar] [CrossRef]
- Isbister, G.K.; Scorgie, F.E.; O’leary, M.A.; Seldon, M.; Brown, S.G.; Lincz, L.F.; ASP Investigators. Factor deficiencies in venom-induced consumption coagulopathy resulting from Australian elapid envenomation: Australian Snakebite Project (ASP-10). J. Thromb. Haemost. 2010, 8, 2504–2513. [Google Scholar] [CrossRef]
- Isbister, G.K.; Maduwage, K.; Shahmy, S.; Mohamed, F.; Abeysinghe, C.; Karunathilake, H.; Ariaratnam, C.A.; Buckley, N.A. Diagnostic 20-min whole blood clotting test in Russell’s viper envenoming delays antivenom administration. QJM 2013, 106, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, I.; Shihana, F.; Dissanayake, D.M.; Buckley, N.A.; Maduwage, K.; Isbister, G.K. Performance of the 20-minute whole blood clotting test in detecting venom induced consumption coagulopathy from Russell’s viper (Daboia russelii) bites. Thromb. Haemost. 2017, 117, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.; Isbister, G.K. Australian Snakebite Myotoxicity (ASP-23). Clin. Toxicol. 2020, 28, 5. [Google Scholar]
- Tasoulis, T.; Isbister, G.K. A Review and Database of Snake Venom Proteomes. Toxins 2017, 9, 290. [Google Scholar] [CrossRef]
- Maduwage, K.; O’Leary, M.A.; Isbister, G.K. Diagnosis of snake envenomation using a simple phospholipase A2 assay. Sci. Rep. 2014, 4, 4827. [Google Scholar] [CrossRef]
- Johnston, C.I.; Ryan, N.M.; Page, C.B.; Buckley, N.A.; Brown, S.G.; O’Leary, M.A.; Isbister, G.K. The Australian Snakebite Project, 2005–2015 (ASP-20). Med. J. Aust. 2017, 207, 119–125. [Google Scholar] [CrossRef]
- Ireland, G.; Brown, S.G.; Buckley, N.A.; Stormer, J.; Currie, B.J.; White, J.; Spain, D.; Isbister, G.K. Changes in serial laboratory test results in snakebite patients: When can we safely exclude envenoming? Med. J. Aust. 2010, 193, 285–290. [Google Scholar] [CrossRef]
- Kulawickrama, S.; O’Leary, M.A.; Hodgson, W.C.; Brown, S.G.; Jacoby, T.; Davern, K.; Isbister, G.K. Development of a sensitive enzyme immunoassay for measuring taipan venom in serum. Toxicon 2010, 55, 1510–1518. [Google Scholar] [CrossRef]
- Hart, A.J.; Hodgson, W.C.; O’Leary, M.; Isbister, G.K. Pharmacokinetics and pharmacodynamics of the myotoxic venom of Pseudechis australis (mulga snake) in the anesthetised rat. Clin. Toxicol. 2014, 52, 604–610. [Google Scholar] [CrossRef]
- Chapman, R.; Lin, Y.; Burnapp, M.; Bentham, A.; Hillier, D.; Zabron, A.; Khan, S.; Tyreman, M.; Stevens, M.M. Multivalent nanoparticle networks enable point-of-care detection of human phospholipase-A2 in serum. ACS Nano 2015, 9, 2565–2573. [Google Scholar] [CrossRef]
- Aili, D.; Mager, M.; Roche, D.; Stevens, M.M. Hybrid nanoparticle-liposome detection of phospholipase activity. Nano Lett. 2011, 11, 1401–1405. [Google Scholar] [CrossRef]
- Tasoulis, T.; Lee, M.S.Y.; Ziajko, M.; Dunstan, N.; Sumner, J.; Isbister, G.K. Activity of two key toxin groups in Australian elapid venoms show a strong correlation to phylogeny but not to diet. BMC Evol. Biol. 2020, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Isbister, G.K.; Brown, S.G.; Page, C.B.; McCoubrie, D.L.; Greene, S.L.; Buckley, N.A. Snakebite in Australia: A practical approach to diagnosis and treatment. Med. J. Aust. 2013, 199, 763–768. [Google Scholar] [CrossRef] [PubMed]
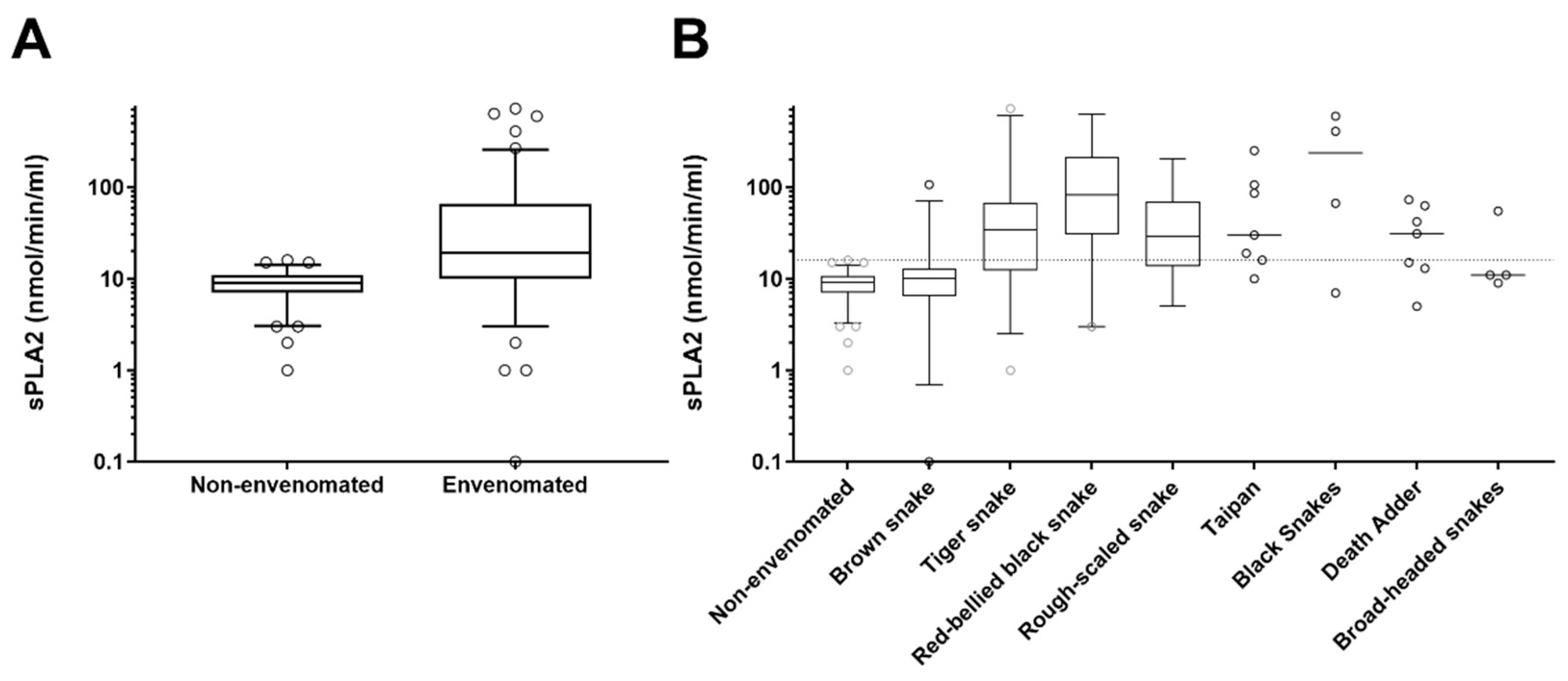
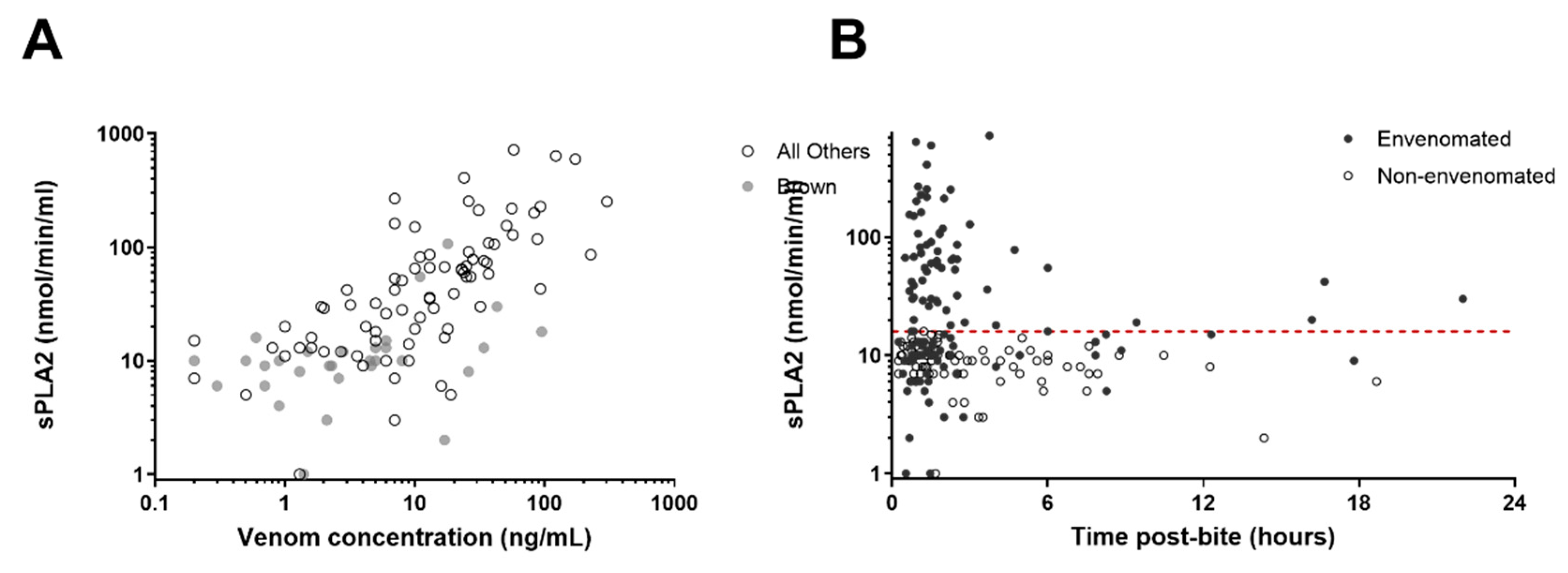
| Snake | No. | Phospholipase A2 (nmol/min/mL); Median, IQR and Range | Venom Concentration (ng/L); Median, IQR and Range |
|---|---|---|---|
| Non-envenomated | 80 | 9 (7 to 11; 1 to 16) | NA |
| Brown snake (Pseudonaja textilis) | 33 | 10 (6.5 to 13; 1 to 107) | 2.6 (0.9 to 8; 0.2 to 95) |
| Tiger snake (Notechis scutatus) | 24 | 34 (12 to 68; 1 to 68) | 7 (2.2 to 25; 0.2 to 93) |
| Red-bellied black snake (Pseudechis porphyriacus) | 19 | 82 (30 to 212; 3 to 637) | 11 (3 to 51; 0.2 to 122) |
| Rough-scale snake (Tropidechis carinatus) | 17 | 29 (14 to 69; 5 to 201) | 14 (5.8 to 27; 0.5 to 83) |
| Taipan (Oxyuranus scutellatus) | 7 | 30 (16 to 106; 10 to 252) | 32 (17 to 227; 9 to 303) |
| Death Adder (Acanthophis antarcticus) | 7 | 31 (13 to 63; 5 to 73) | 7 (3.2 to 23; 1.3 to 36) |
| Mulga snake (Pseudechis australis) | 3 | 7, 67, 409 | 7, 17, 24 |
| Collett’s Snake (Pseudechis colletti) | 1 | 597 | 173 |
| Stephen’s banded snake (Hoplocephalus stephensii) | 2 | 9, 55 | 25 |
| Broad-headed snake (Hoplocepalus bungaroides) | 2 | 11, 11 | 3.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isbister, G.K.; Mirajkar, N.; Fakes, K.; Brown, S.G.A.; Veerati, P.C. Phospholipase A2 (PLA2) as an Early Indicator of Envenomation in Australian Elapid Snakebites (ASP-27). Biomedicines 2020, 8, 459. https://doi.org/10.3390/biomedicines8110459
Isbister GK, Mirajkar N, Fakes K, Brown SGA, Veerati PC. Phospholipase A2 (PLA2) as an Early Indicator of Envenomation in Australian Elapid Snakebites (ASP-27). Biomedicines. 2020; 8(11):459. https://doi.org/10.3390/biomedicines8110459
Chicago/Turabian StyleIsbister, Geoffrey K., Nandita Mirajkar, Kellie Fakes, Simon G. A. Brown, and Punnam Chander Veerati. 2020. "Phospholipase A2 (PLA2) as an Early Indicator of Envenomation in Australian Elapid Snakebites (ASP-27)" Biomedicines 8, no. 11: 459. https://doi.org/10.3390/biomedicines8110459
APA StyleIsbister, G. K., Mirajkar, N., Fakes, K., Brown, S. G. A., & Veerati, P. C. (2020). Phospholipase A2 (PLA2) as an Early Indicator of Envenomation in Australian Elapid Snakebites (ASP-27). Biomedicines, 8(11), 459. https://doi.org/10.3390/biomedicines8110459