Combined Treatment with Polynucleotides and Hyaluronic Acid Improves Tissue Repair in Experimental Colitis
Abstract
1. Introduction
2. Material and Methods
2.1. Animals and Drugs
2.2. DNBS Model
2.3. Evaluation of Body Weight and Food Intake
2.4. Macroscopic Damage Score
2.5. Microscopic Damage Score
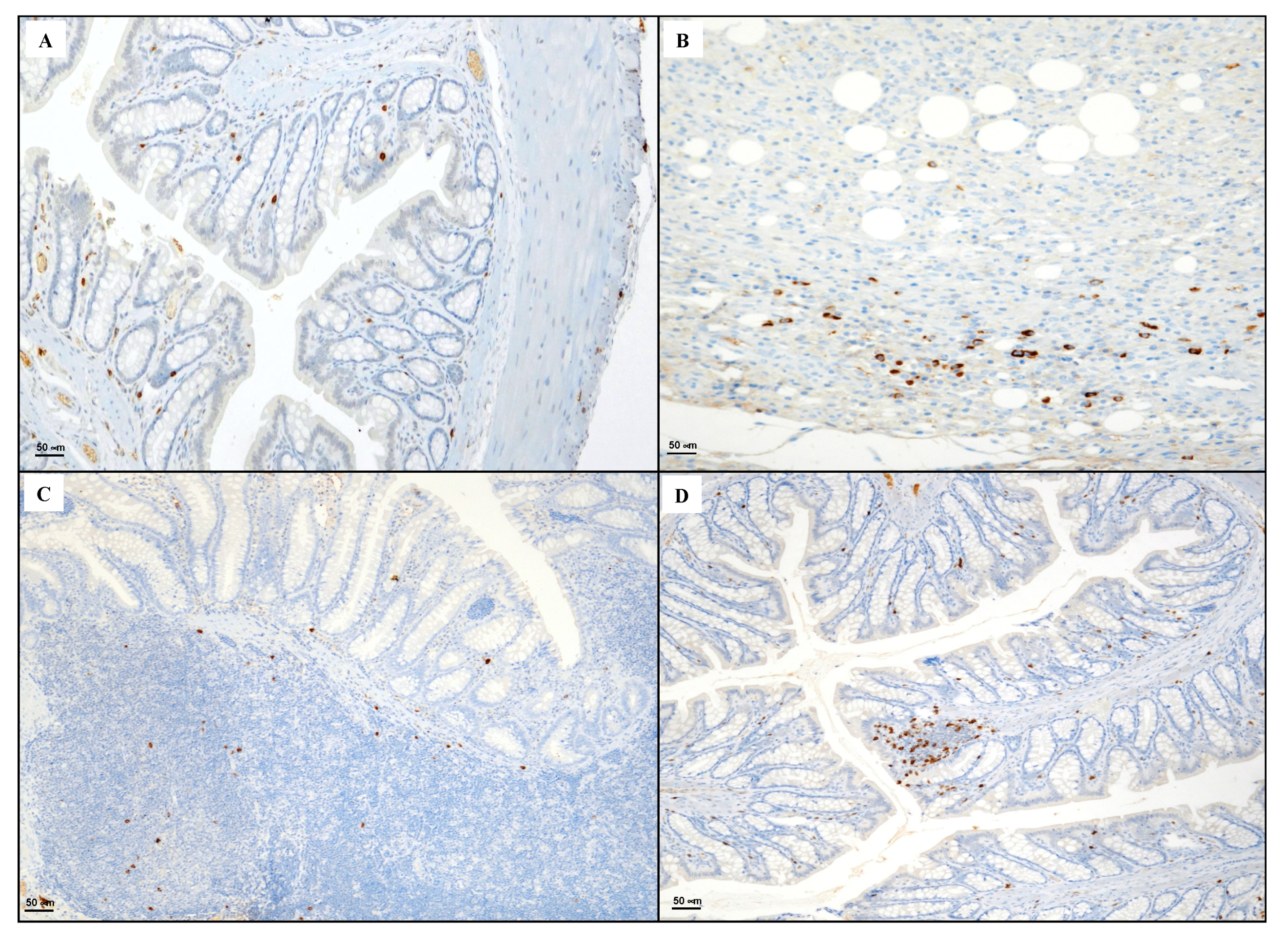
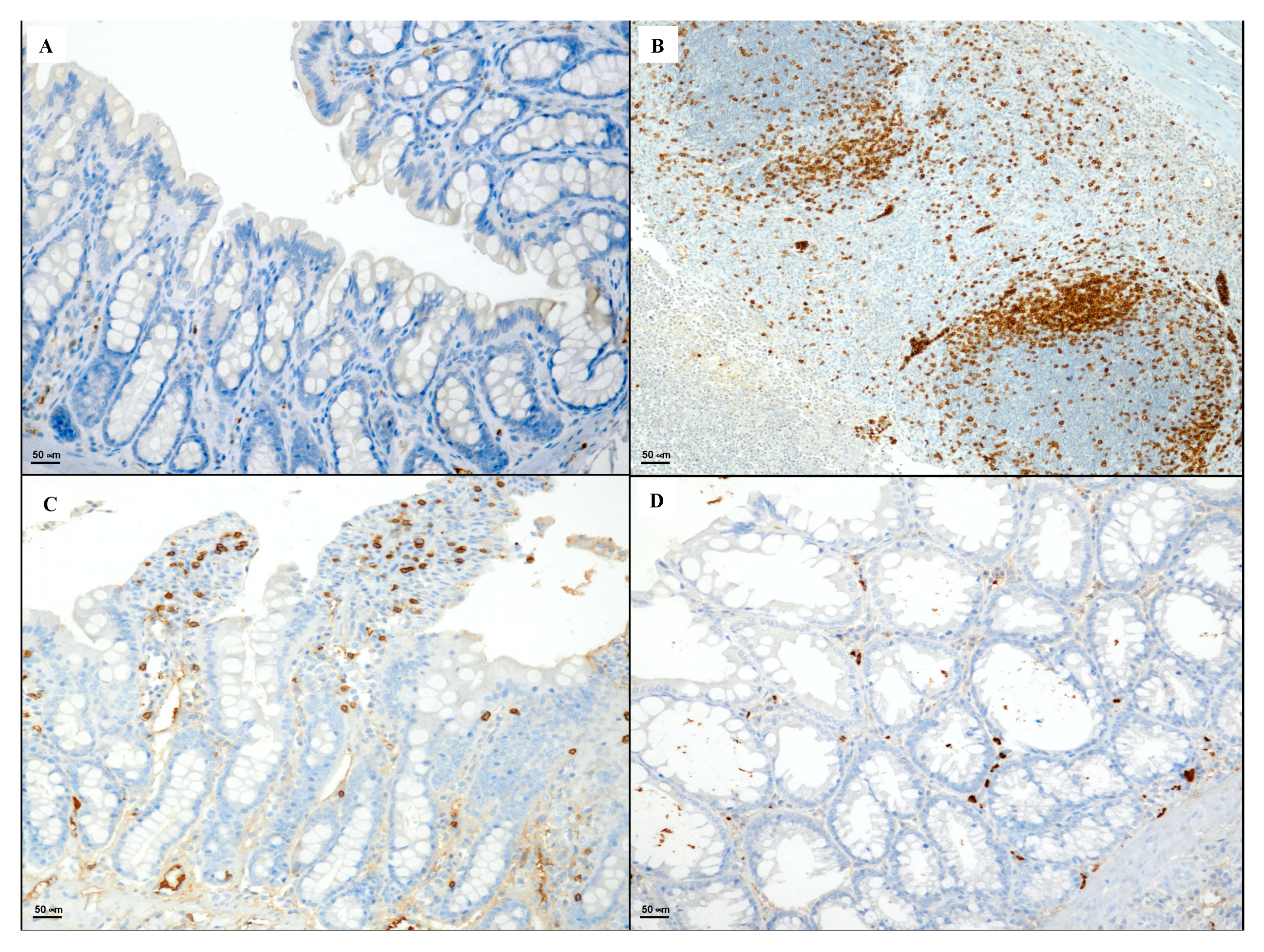
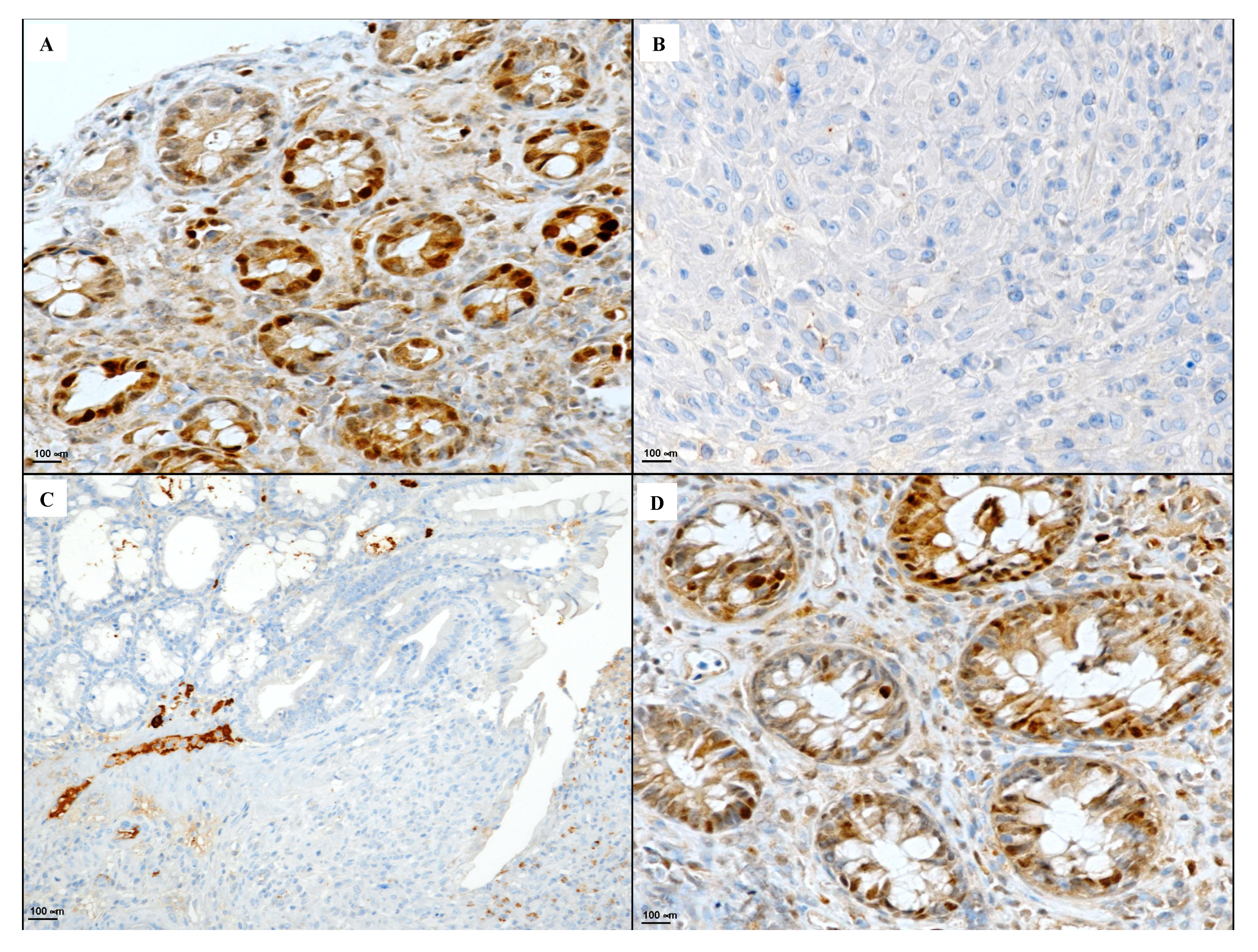
2.6. Immunohistochemical Evaluation of CD3, CD20, CD44 and Bcl-2
2.7. Measurement of Myeloperoxidase Activity
2.8. Malondialdehyde Measurement
2.9. Statistical Analysis
3. Results
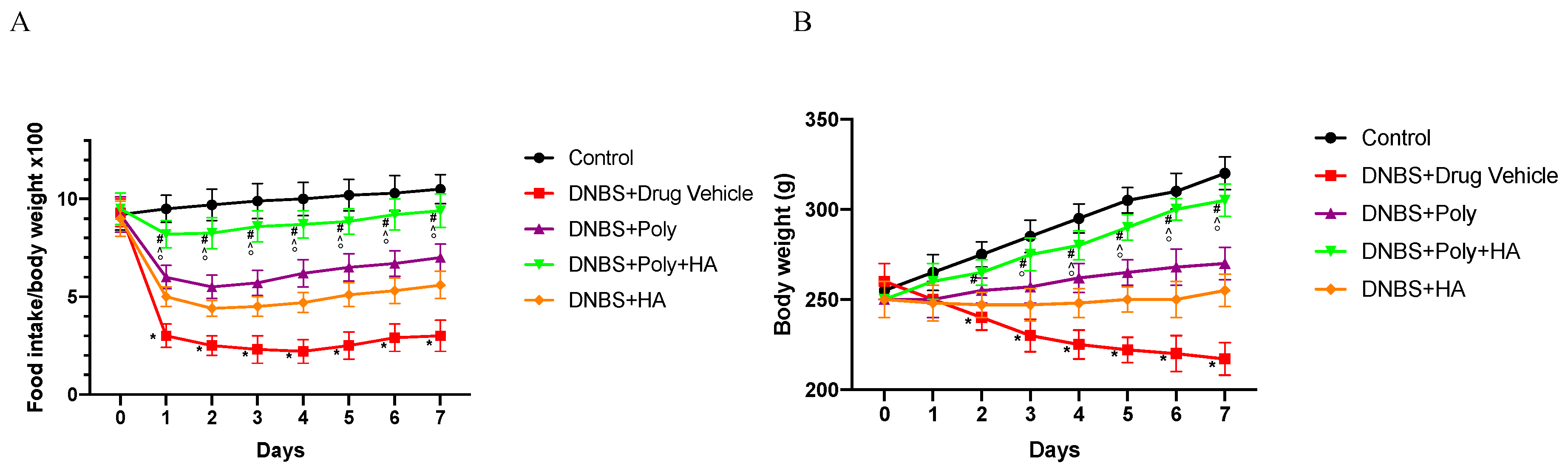
3.1. Effects of Polynucleotides and Hyaluronic Acid on Clinical Signs
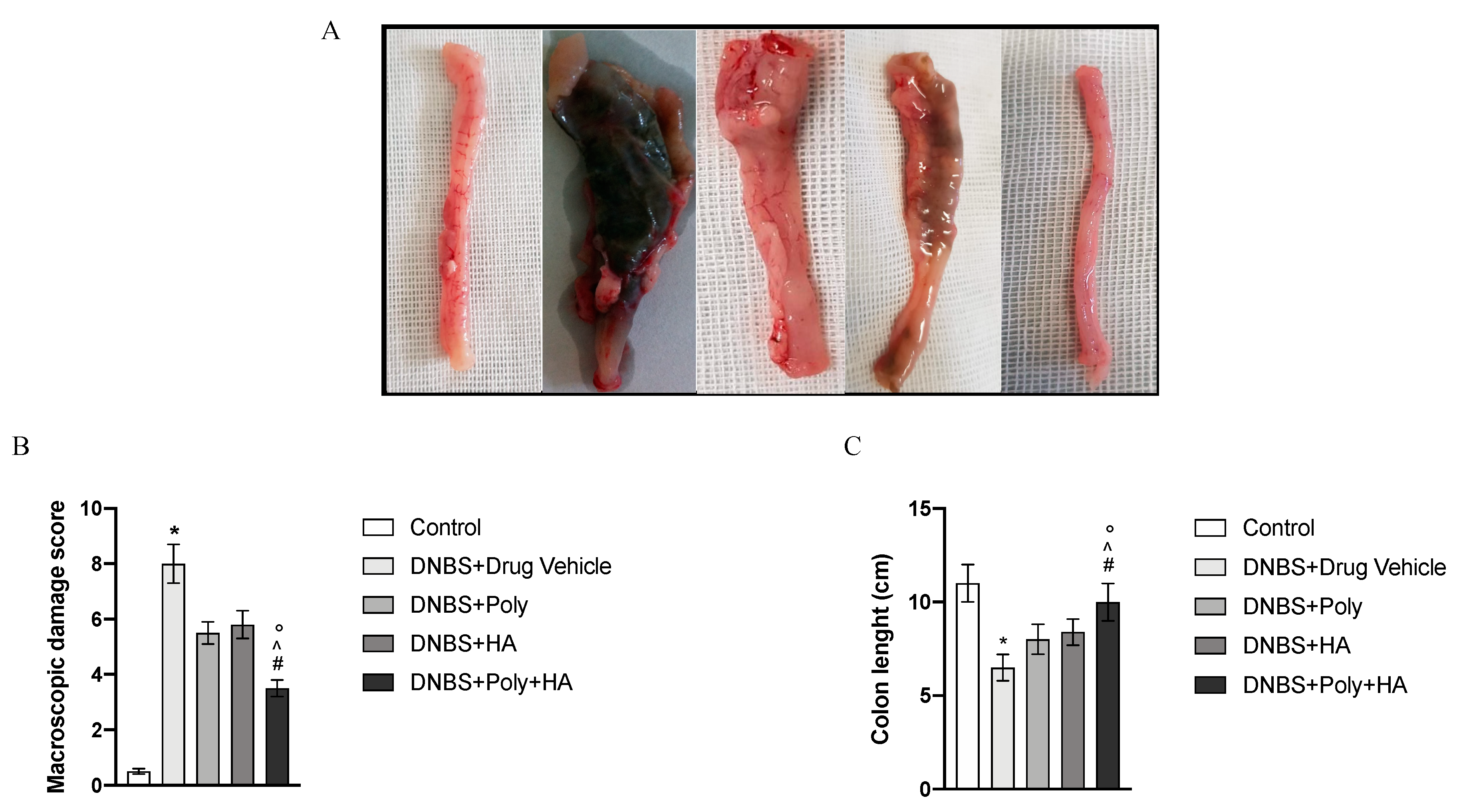
3.2. Effects of Polynucleotides and Hyaluronic Acid on Macroscopic Damage
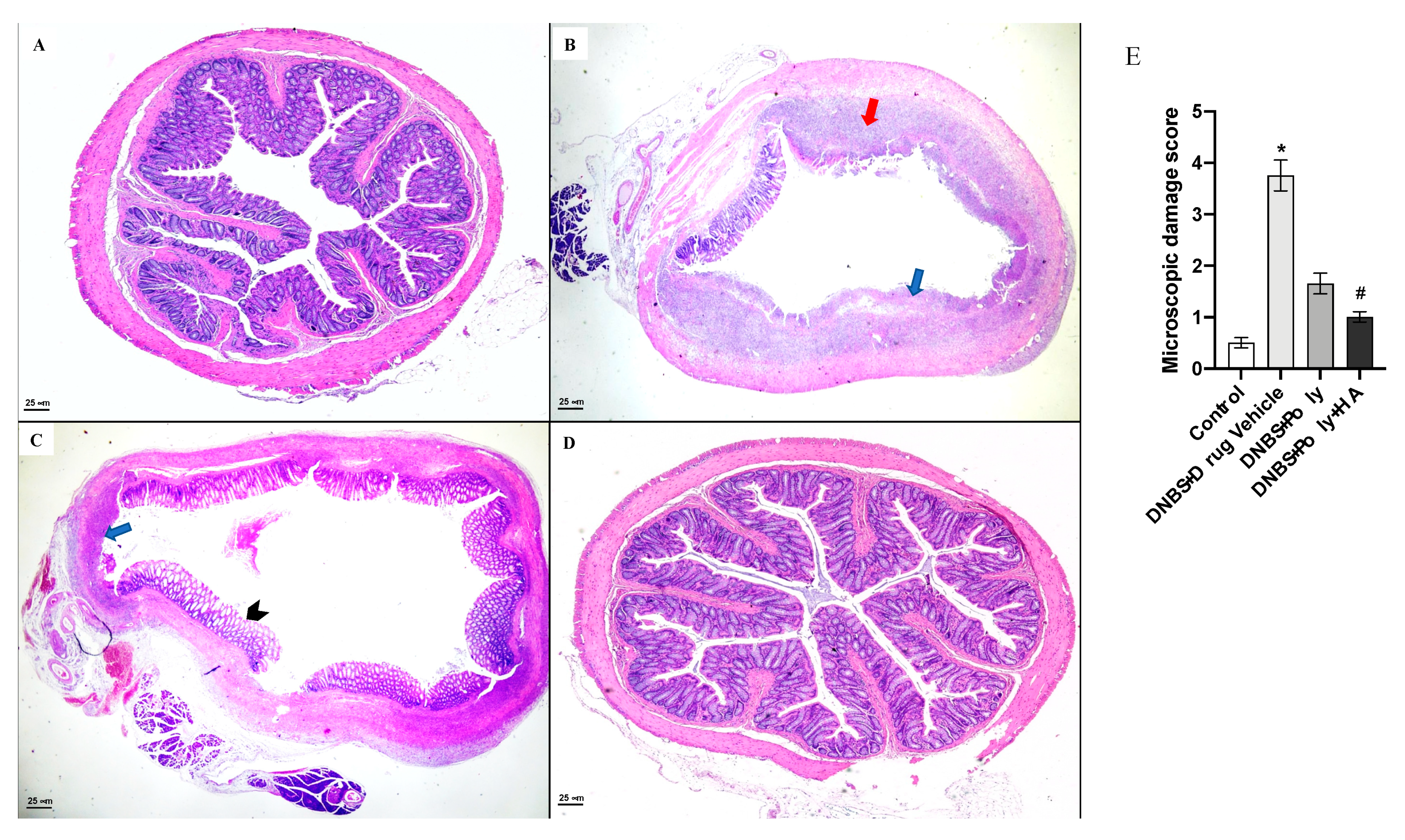
3.3. Polynucleotides and Hyaluronic Acid Reduces Histological Damage
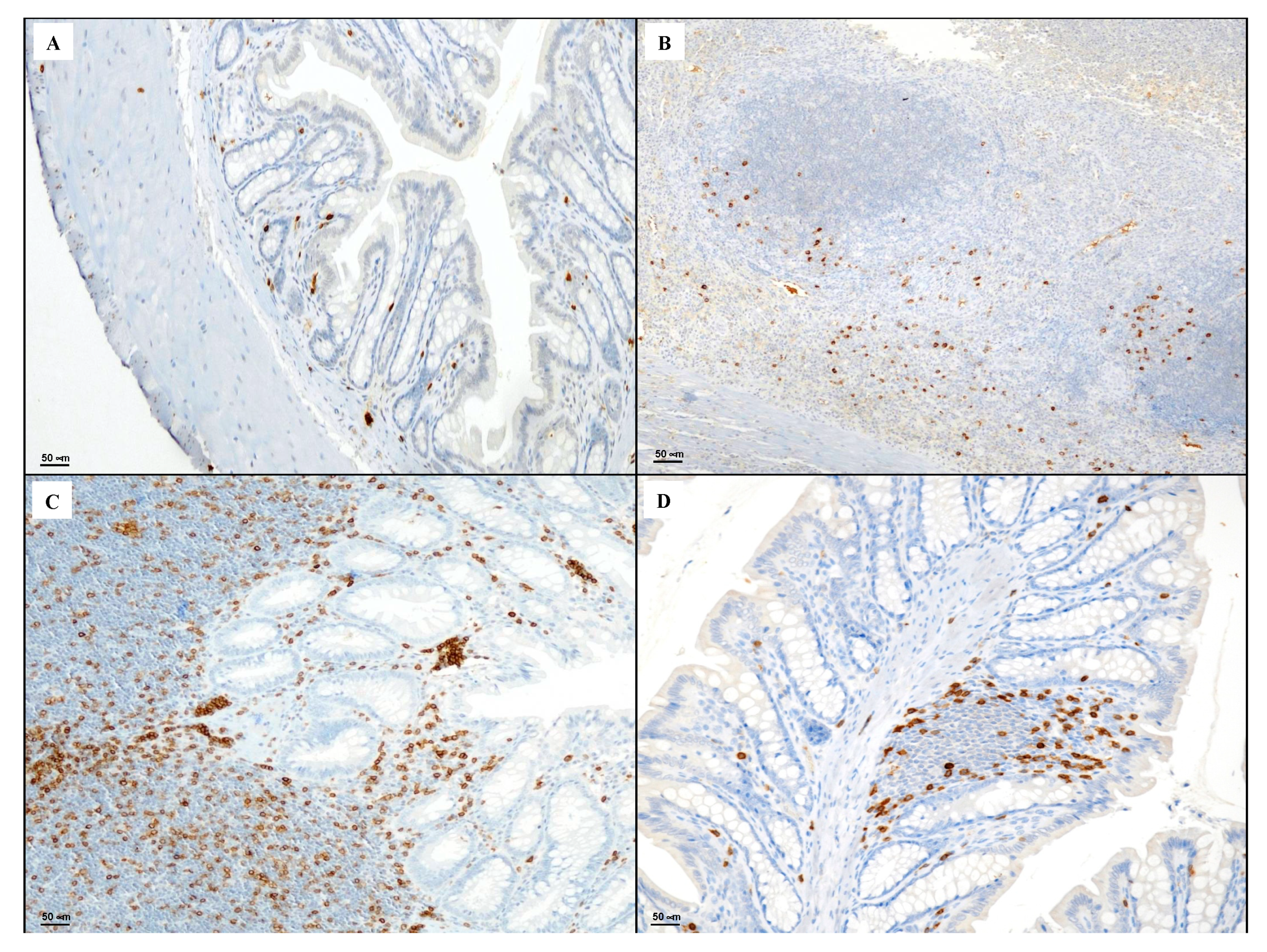
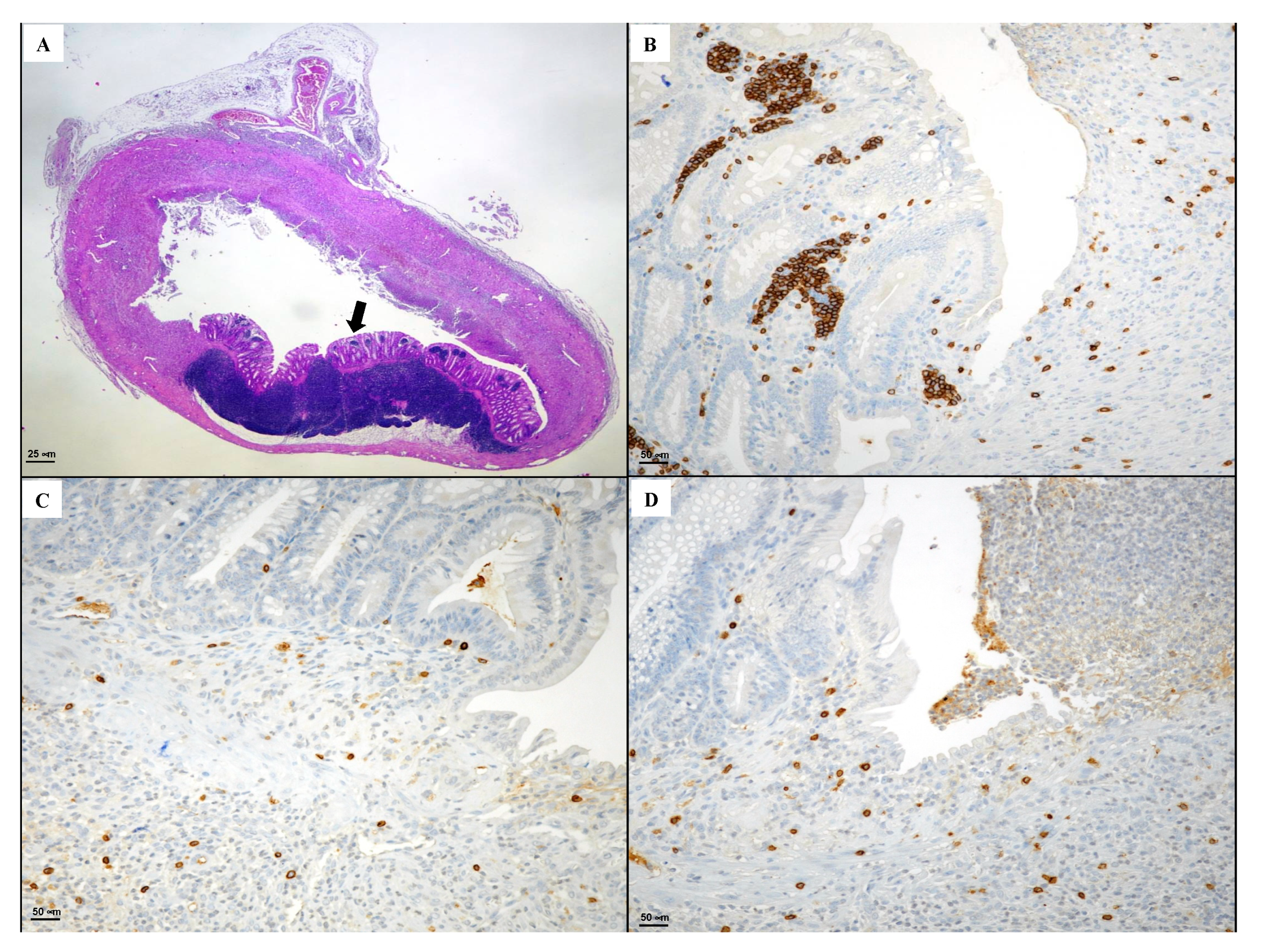
3.4. Immunohistochemical Evaluation of CD3, CD20, CD44 and Bcl-2
3.5. Effects of Polynucleotides and Hyaluronic Acid on Lipid Peroxidation and Neutrophil Infiltration
3.6. Effects of Hyaluronic Acid on Tissue Repair
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Podolsky, D.K. Inflammatory bowel disease. N. Engl. J. Med. 2002, 347, 417–429. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Sandborn, W.J. Inflammatory bowel disease: Clinical aspects and established and evolving therapies. Lancet 2007, 369, 1641–1657. [Google Scholar] [CrossRef]
- Pavlick, K.P.; Laroux, F.S.; Fuseler, J.; Wolf, R.E.; Gray, L.; Hoffman, J.; Grisham, M.B. Role of reactive metabolites of oxygen and nitrogen in inflammatory bowel disease. Free Radic. Biol. Med. 2002, 33, 311–322. [Google Scholar] [CrossRef]
- Rahimian, R.; Fakhfouri, G.; Daneshmand, A.; Mohammadi, H.; Bahremand, A.; Rasouli, M.R.; Mousavizadeh, K.; Dehpour, A.R. Adenosine A2A receptors and uric acid mediate protective effects of inosine against TNBS-induced colitis in rats. Eur. J. Pharmacol. 2010, 649, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Shaulian, E.; Karin, M. AP-1 as a regulator of cell life and death. Nat. Cell Biol. 2012, 4, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Burger, D.; Travis, S. Conventional medical management of inflammatory bowel disease. Gastroenterology 2011, 140, 1827–1837. [Google Scholar] [CrossRef]
- Odashima, M.; Bamias, G.; Rivera-Nieves, J.; Linden, J.; Nast, C.C.; Moskaluk, C.A.; Marini, M.; Sugawara, K.; Kozaiwa, K.; Otaka, M.; et al. Activation of A2A adenosine receptor attenuates intestinal inflammation in animal models of inflammatory bowel disease. Gastroenterology 2005, 129, 26–33. [Google Scholar] [CrossRef]
- Desrosiers, M.D.; Cembrola, K.M.; Fakir, M.J.; Stephens, L.A.; Jama, F.M.; Shameli, A.; Mehal, W.Z.; Santamaria, P.; Shy, Y. Adenosine deamination sustains dendritic cell activation in inflammation. J. Immunol. 2007, l179, 1884–1892. [Google Scholar] [CrossRef]
- Fortin, A.; Harbour, D.; Fernandes, M.; Borgeat, P.; Bourgoin, S. Differential expression of adenosine receptors in human neutrophils: Up-regulation by specific Th1 cytokines and lipopolysaccharide. J. Leukoc. Biol. 2006, 79, 574–585. [Google Scholar] [CrossRef]
- Haskó, G.; Linden, J.; Cronstein, B.; Pacher, P. Adenosine receptors: Therapeutic aspects for inflammatory and immune diseases. Nat. Rev. Drug Discov. 2008, 7, 759–770. [Google Scholar]
- Chiu, C.T.; Kuo, S.N.; Hung, S.W.; Yang, C.Y. Combined Treatment with Hyaluronic Acid and Mesalamine Protects Rats from Inflammatory Bowel Disease Induced by Intracolonic Administration of Trinitrobenzenesulfonic Acid. Molecules 2017, 22, 904. [Google Scholar] [CrossRef]
- Wittig, B.M.; Stallmach, A.; Zeitz, M.; Gunthert, U. Functional involvement of cd44 variant 7 in gut immune response. Pathobiology 2002, 70, 184–189. [Google Scholar] [CrossRef]
- Johnson, P.; Ruffell, B. Cd44 and its role in inflammation and inflammatory diseases. Inflamm. Allergy Drug Targets 2009, 8, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, L.Y.; Bikle, D. Selective Hyaluronan-CD44 Signaling Promotes miRNA-21 Expression and Interacts with Vitamin D Function during Cutaneous Squamous Cell Carcinomas Progression Following UV Irradiation. Front. Immunol. 2015, 6, 224. [Google Scholar] [CrossRef]
- Pallio, G.; Bitto, A.; Pizzino, G.; Galfo, F.; Irrera, N.; Squadrito, F.; Squadrito, G.; Pallio, S.; Anastasi, G.P.; Cutroneo, G.; et al. Adenosine Receptor Stimulation by Polydeoxyribonucleotide Improves Tissue Repair and Symptomology in Experimental Colitis. Front. Pharmacol. 2016, 7, 273. [Google Scholar] [CrossRef] [PubMed]
- Squadrito, F.; Micali, A.; Rinaldi, M.; Irrera, N.; Marini, H.; Puzzolo, D.; Pisani, A.; Lorenzini, C.; Valenti, A.; Laurà, R.; et al. Polydeoxyribonucleotide, an Adenosine-A2(A) Receptor Agonist, Preserves Blood Testis Barrier from Cadmium-Induced Injury. Front. Pharmacol. 2017, 7, 537. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Galfo, F.; Oteri, G.; Atteritano, M.; Pallio, G.; Mannino, F.; D’Amore, A.; Pellegrino, E.; Aliquò, F.; et al. Adenosine Receptor Stimulation Improves Glucocorticoid-Induced Osteoporosis in a Rat Model. Front. Pharmacol. 2017, 8, 558. [Google Scholar] [CrossRef]
- McGrath, J.; Drummond, G.; Kilkenny, C.; Wainwright, C. Guidelines for reporting experiments involving animals: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1573–1576. [Google Scholar] [CrossRef] [PubMed]
- Morampudi, V.; Bhinder, G.; Wu, X.; Dai, C.; Sham, H.P.; Vallance, B.A.; Jacobson, K. DNBS/TNBS colitis models: Providing insights into inflammatory bowel disease and effects of dietary fat. J. Vis. Exp. 2014, 27, e51297. [Google Scholar] [CrossRef]
- Kim, H.S.; Berstad, A. Experimental colitis in animal models. Scand. J. Gastroenterol. 1992, 27, 529–537. [Google Scholar] [CrossRef]
- Irrera, N.; Arcoraci, V.; Mannino, F.; Vermiglio, G.; Pallio, G.; Minutoli, L.; Bagnato, G.; Anastasi, G.P.; Mazzon, E.; Bramanti, P.; et al. Activation of A2A Receptor by PDRN Reduces Neuronal Damage and Stimulates WNT/β-CATENIN Driven Neurogenesis in Spinal Cord Injury. Front. Pharmacol. 2018, 9, 506. [Google Scholar] [CrossRef] [PubMed]
- Marini, H.R.; Puzzolo, D.; Micali, A.; Adamo, E.B.; Irrera, N.; Pisani, A.; Pallio, G.; Trichilo, V.; Malta, C.; Bitto, A.; et al. Neuroprotective Effects of Polydeoxyribonucleotide in a Murine Model of Cadmium Toxicity. Oxid. Med. Cell. Longev. 2018, 2018, 4285694, Erratum in: Oxid. Med. Cell. Longev. 2018, 13, 9176012. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Mahaseth, M.; Zhang, Y. Hyaluronic acid as a rescue therapy for trinitrobenzene sulfonic acid-induced colitis through Cox-2 and PGE2 in a Toll-like receptor 4-dependent way. J. Zhejiang Univ. Sci. B 2011, 12, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Pallio, G.; Bitto, A.; Pizzino, G.; Galfo, F.; Irrera, N.; Minutoli, L.; Arcoraci, V.; Squadrito, G.; Macrì, A.; Squadrito, F.; et al. Use of a balanced dual cyclooxygenase-1/2 and 5-lypoxygenase inhibitor in experimental colitis. Eur. J. Pharmacol. 2016, 789, 152–162. [Google Scholar] [CrossRef]
- Wallace, J.L.; Keenan, C.M.; Gale, D.; Shoupe, T.S. Exacerbation of experimental colitis by nonsteroidal anti-inflammatory drugs is not related to elevated leukotriene B4 synthesis. Gastroenterology 1992, 102, 18–27. [Google Scholar] [CrossRef]
- Pizzino, G.; Bitto, A.; Pallio, G.; Irrera, N.; Galfo, F.; Interdonato, M.; Mecchio, A.; De Luca, F.; Minutoli, L.; Squadrito, F.; et al. Blockade of the JNK signalling as a rational therapeutic approach to modulate the early and late steps of the inflammatory cascade in polymicrobial sepsis. Mediat. Inflamm. 2015, 2015, 591572. [Google Scholar] [CrossRef]
- Bitto, A.; Giuliani, D.; Pallio, G.; Irrera, N.; Vandini, E.; Canalini, F.; Zaffe, D.; Ottani, A.; Minutoli, L.; Rinaldi, M.; et al. Effects of COX1-2/5-LOX blockade in Alzheimer transgenic 3xTg-AD mice. Inflamm. Res. 2017, 66, 389–398. [Google Scholar] [CrossRef]
- Mullane, K.M.; Kraemer, R.; Smith, B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J. Pharmacol. Methods 1985, 14, 157–167. [Google Scholar] [CrossRef]
- Gugliandolo, E.; D’amico, R.; Cordaro, M.; Fusco, R.; Siracusa, R.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Effect of PEA-OXA on neuropathic pain and functional recovery after sciatic nerve crush. J. Neuroinflammation 2018, 15, 264. [Google Scholar] [CrossRef]
- Gugliandolo, E.; Fusco, R.; D’Amico, R.; Militi, A.; Oteri, G.; Wallace, J.L.; Di Paola, R.; Cuzzocrea, S. Anti-inflammatory effect of ATB-352, a H2S -releasing ketoprofen derivative, on lipopolysaccharide-induced periodontitis in rats. Pharmacol. Res. 2018, 132, 220–231. [Google Scholar] [CrossRef]
- Ferlito, M.; Romanenko, O.G.; Ashton, S.; Squadrito, F.; Halushka, P.V.; Cook, J.A. Effect of cross-tolerance between endotoxin and TNF-alpha or IL-1beta on cellular signaling and mediator production. J. Leukoc. Biol. 2001, 70, 821–829. [Google Scholar] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Bitto, A.; Pallio, G.; Mannino, F.; Arcoraci, V.; Aliquò, F.; Minutoli, L.; De Ponte, C.; D’andrea, P.; et al. Cadmium-Induced Oxidative Stress Impairs Glycemic Control in Adolescents. Oxid. Med. Cell. Longev. 2017, 2017, 6341671. [Google Scholar] [CrossRef] [PubMed]
- Interdonato, M.; Bitto, A.; Pizzino, G.; Irrera, N.; Pallio, G.; Mecchio, A.; Cuspilici, A.; Minutoli, L.; Altavilla, D.; Squadrito, F. Levels of heavy metals in adolescents living in the industrialised area of Milazzo-Valle del Mela (northern Sicily). J. Environ. Public Health 2014, 2014, 326845. [Google Scholar] [CrossRef]
- Minutoli, L.; Marini, H.; Rinaldi, M.; Bitto, A.; Irrera, N.; Pizzino, G.; Pallio, G.; Calò, M.; Adamo, E.B.; Trichilo, V.; et al. A dual inhibitor of cyclooxygenase and 5-lipoxygenase protects against kainic acid-induced brain injury. Neuromol. Med. 2015, 17, 192–201. [Google Scholar] [CrossRef]
- Antonioli, L.; Fornai, M.; Colucci, R.; Awwad, O.; Ghisu, N.; Tuccori, M.; Da Settimo, F.; La Motta, C.; Natale, G.; Duranti, E.; et al. The blockade of adenosine deaminase ameliorates chronic experimental colitis through the recruitment of adenosine A2A and A3 receptors. J. Pharmacol. Exp. Ther. 2010, 335, 434–442. [Google Scholar] [CrossRef]
- Siegmund, B.; Rieder, F.; Albrich, S.; Wolf, K.; Bidlingmaier, C.; Firestein, G.S.; Boyle, D.; Lehr, H.A.; Loher, F.; Hartmann, G.; et al. Adenosine kinase inhibitor GP515 improves experimental colitis in mice. J. Pharmacol. Exp. Ther. 2001, 296, 99–105. [Google Scholar]
- Naganuma, M.; Wiznerowicz, E.B.; Lappas, C.M.; Linden, J.; Worthington, M.T.; Ernst, P.B. Cutting edge: Critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis. J. Immunol. 2006, 177, 2765–2769. [Google Scholar] [CrossRef]
- Fiorino, G.; Gilardi, D.; Naccarato, P.; Sociale, O.R.; Danese, S. Safety and efficacy of sodium hyaluronate (IBD98E) in the induction of clinical and endoscopic remission in subjects with distal ulcerative colitis. Dig. Liver Dis. 2014, 46, 330–334. [Google Scholar] [CrossRef]
- Sammarco, G.; Shalaby, M.; Elangovan, S.; Petti, L.; Roda, G.; Restelli, S.; Arena, V.; Ungaro, F.; Fiorino, G.; Day, A.J.; et al. Hyaluronan Accelerates Intestinal Mucosal Healing through Interaction with TSG-6. Cells 2019, 8, 1074. [Google Scholar] [CrossRef]
- Haskò, G.; Kuhel, D.G.; Chen, J.F.; Schwarzschild, M.A.; Deitch, E.A.; Mabley, J.G.; Marton, A.; Szabò, C. Adenosine inhibits IL-12 and TNF-α production via adenosine A2a receptor-dependent and independent mechanisms. FASEB J. 2000, 14, 2065–2074. [Google Scholar] [PubMed]
- Kreckler, L.M.; Wan, T.C.; Ge, Z.D.; Auchampach, J.A. Adenosine inhibits tumor necrosis factor-α release from mouse peritoneal macrophages via A2A and A2B but not the A3 adenosine receptor. J. Pharmacol. Exp. Ther. 2006, 317, 172–180. [Google Scholar] [PubMed]
- Cronstein, B.N.; Kramer, S.B.; Weissmann, G.; Hirschhorn, R. Adenosine: A physiological modulator of superoxide anion generation by human neutrophils. J. Exp. Med. 1983, 158, 1160–1177. [Google Scholar] [PubMed]
- Cronstein, B.N.; Rosenstein, E.D.; Kramer, S.B.; Weissmann, G.; Hirschhorn, R. Adenosine; a physiologic modulator of superoxide anion generation by human neutrophils. Adenosine acts via an A2 receptor on human neutrophils. J. Immunol. 1985, 135, 1366–1371. [Google Scholar]
- Sullivan, G.W.; Lee, D.D.; Ross, W.G.; Di Vietro, J.A.; Lappas, C.M.; Lawrence, M.B.; Linden, J. Activation of A2A adenosine receptors inhibits expression of α4/β1 integrin (verylate antigen-4) on stimulated human neutrophils. J. Leukoc. Biol. 2004, 75, 127–134. [Google Scholar]
- Mayne, M.; Fotheringham, J.; Yan, H.J.; Power, C.; Del Bigio, M.R.; Peeling, J.; Geiger, J.D. Adenosine A2A receptor activation reduces proinflammatory events and decreases cell death following intracerebral hemorrhage. Ann. Neurol. 2001, 49, 727–735. [Google Scholar]
- Naor, D. Editorial: Interaction Between Hyaluronic Acid and Its Receptors (CD44, RHAMM) Regulates the Activity of Inflammation and Cancer. Front. Immunol. 2016, 8, 7–39. [Google Scholar]
- Cyphert, J.M.; Trempus, C.S.; Garantziotis, S. Size Matters: Molecular Weight Specificity of Hyaluronan Effects in Cell Biology. Int. J. Cell Biol. 2015, 2015, 563818. [Google Scholar]
- Ceuleers, H.; Van Spaendonk, H.; Hanning, N.; Heirbaut, J.; Lambeir, A.M.; Joossens, J.; Augustyns, K.; De Man, J.G.; De Meester, I.; De Winter, B.Y. Visceral hypersensitivity in inflammatory bowel diseases and irritable bowel syndrome: The role of proteases. World J. Gastroenterol. 2016, 22, 10275–10286. [Google Scholar]
- Srinath, A.; Young, E.; Szigethy, E. Pain management in patients with inflammatory bowel disease: Translational approaches from bench to bedside. Inflamm. Bowel Dis. 2014, 20, 2433–2449. [Google Scholar]
- Squadrito, F.; Bitto, A.; Altavilla, D.; Arcoraci, V.; De Caridi, G.; De Feo, M.E.; Corrao, S.; Pallio, G.; Sterrantino, C.; Minutoli, L.; et al. The effect of PDRN, an adenosine receptor A2A agonist, on the healing of chronic diabetic foot ulcers: Results of a clinical trial. J. Clin. Endocrinol. Metab. 2015, 99, E746–E753, Erratum in: J. Clin. Endocrinol. Metab. 2015, 100, 763. [Google Scholar]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 25, 701. [Google Scholar] [CrossRef]
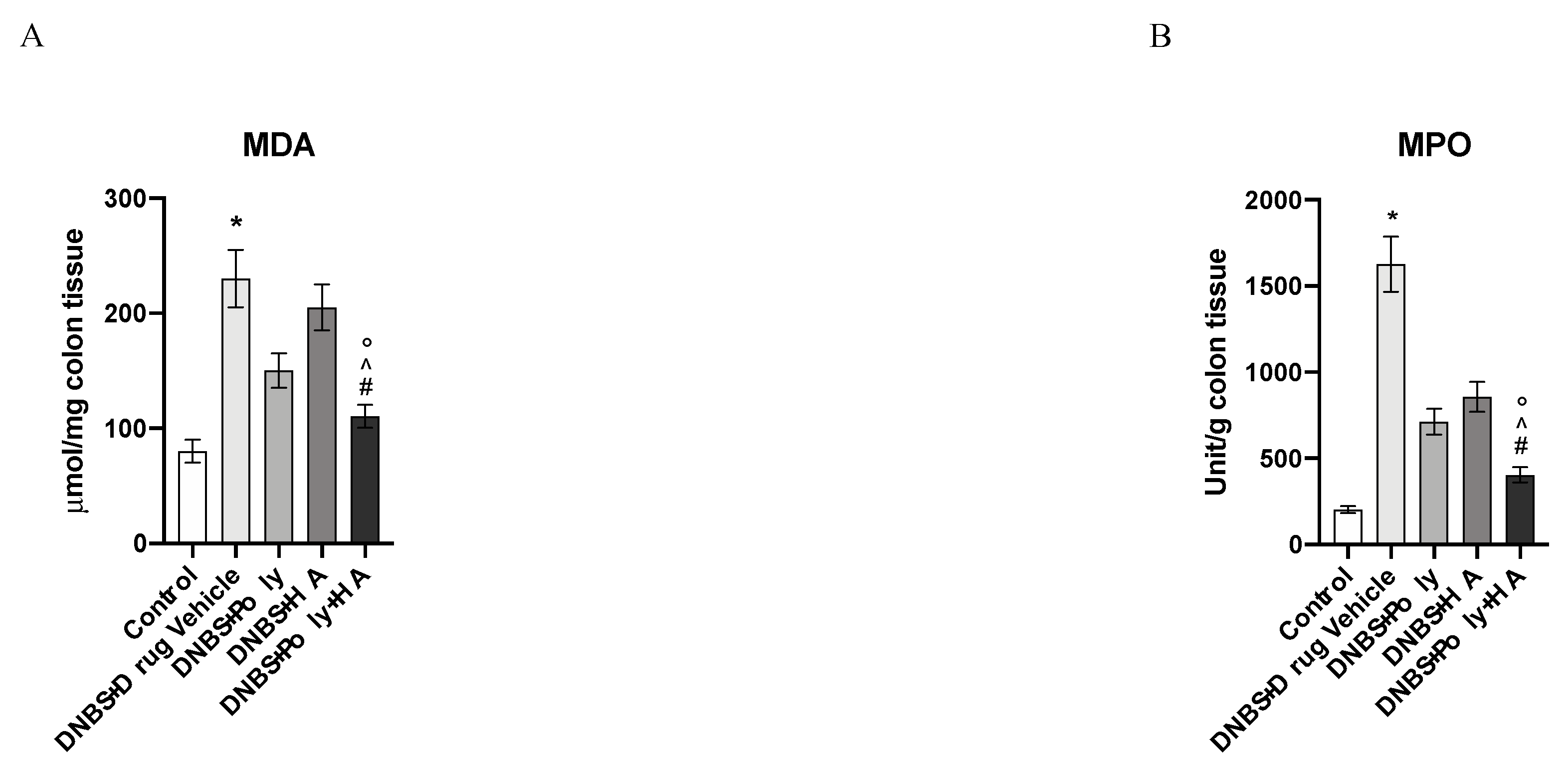
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pallio, G.; Bitto, A.; Ieni, A.; Irrera, N.; Mannino, F.; Pallio, S.; Altavilla, D.; Squadrito, F.; Scarpignato, C.; Minutoli, L. Combined Treatment with Polynucleotides and Hyaluronic Acid Improves Tissue Repair in Experimental Colitis. Biomedicines 2020, 8, 438. https://doi.org/10.3390/biomedicines8100438
Pallio G, Bitto A, Ieni A, Irrera N, Mannino F, Pallio S, Altavilla D, Squadrito F, Scarpignato C, Minutoli L. Combined Treatment with Polynucleotides and Hyaluronic Acid Improves Tissue Repair in Experimental Colitis. Biomedicines. 2020; 8(10):438. https://doi.org/10.3390/biomedicines8100438
Chicago/Turabian StylePallio, Giovanni, Alessandra Bitto, Antonio Ieni, Natasha Irrera, Federica Mannino, Socrate Pallio, Domenica Altavilla, Francesco Squadrito, Carmelo Scarpignato, and Letteria Minutoli. 2020. "Combined Treatment with Polynucleotides and Hyaluronic Acid Improves Tissue Repair in Experimental Colitis" Biomedicines 8, no. 10: 438. https://doi.org/10.3390/biomedicines8100438
APA StylePallio, G., Bitto, A., Ieni, A., Irrera, N., Mannino, F., Pallio, S., Altavilla, D., Squadrito, F., Scarpignato, C., & Minutoli, L. (2020). Combined Treatment with Polynucleotides and Hyaluronic Acid Improves Tissue Repair in Experimental Colitis. Biomedicines, 8(10), 438. https://doi.org/10.3390/biomedicines8100438







