Engineering and Preclinical Evaluation of Western Reserve Oncolytic Vaccinia Virus Expressing A167Y Mutant Herpes Simplex Virus Thymidine Kinase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Virus
2.2. WOTS-418 Engineering and Characterization
2.3. Virus Titration by Plaque Assay
2.4. Virus Purification
2.5. Firefly Luciferase Assay
2.6. PCR
2.7. Western Blotting
2.8. Restriction Enzyme Digest Analysis
2.9. Gene Sequencing
2.10. Cytotoxicity Assay (In Vitro)
2.11. qPCR
2.12. Animal Models
2.13. Statistical Analysis
3. Results
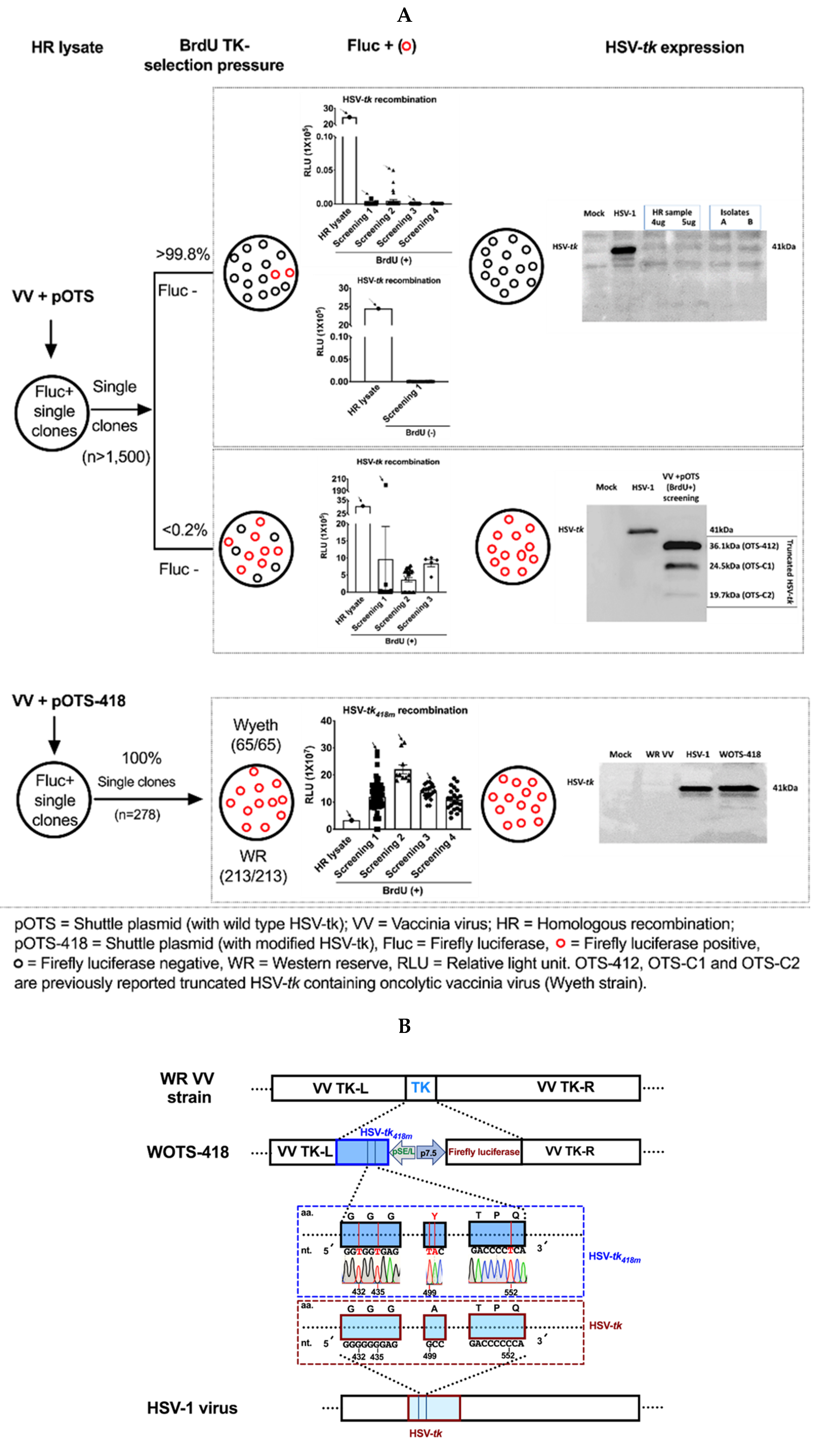
3.1. Engineering and Characterization of WOTS-418
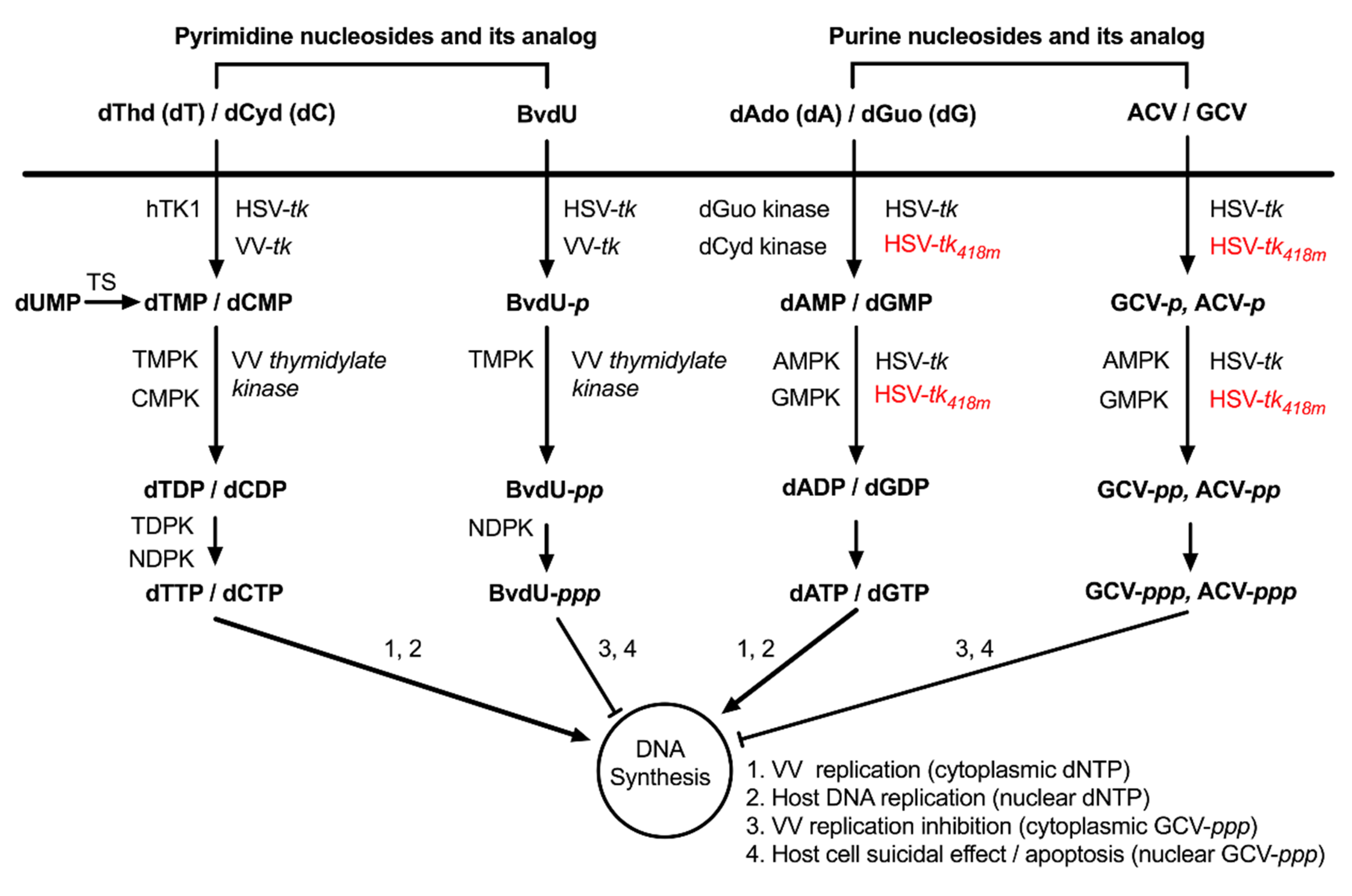
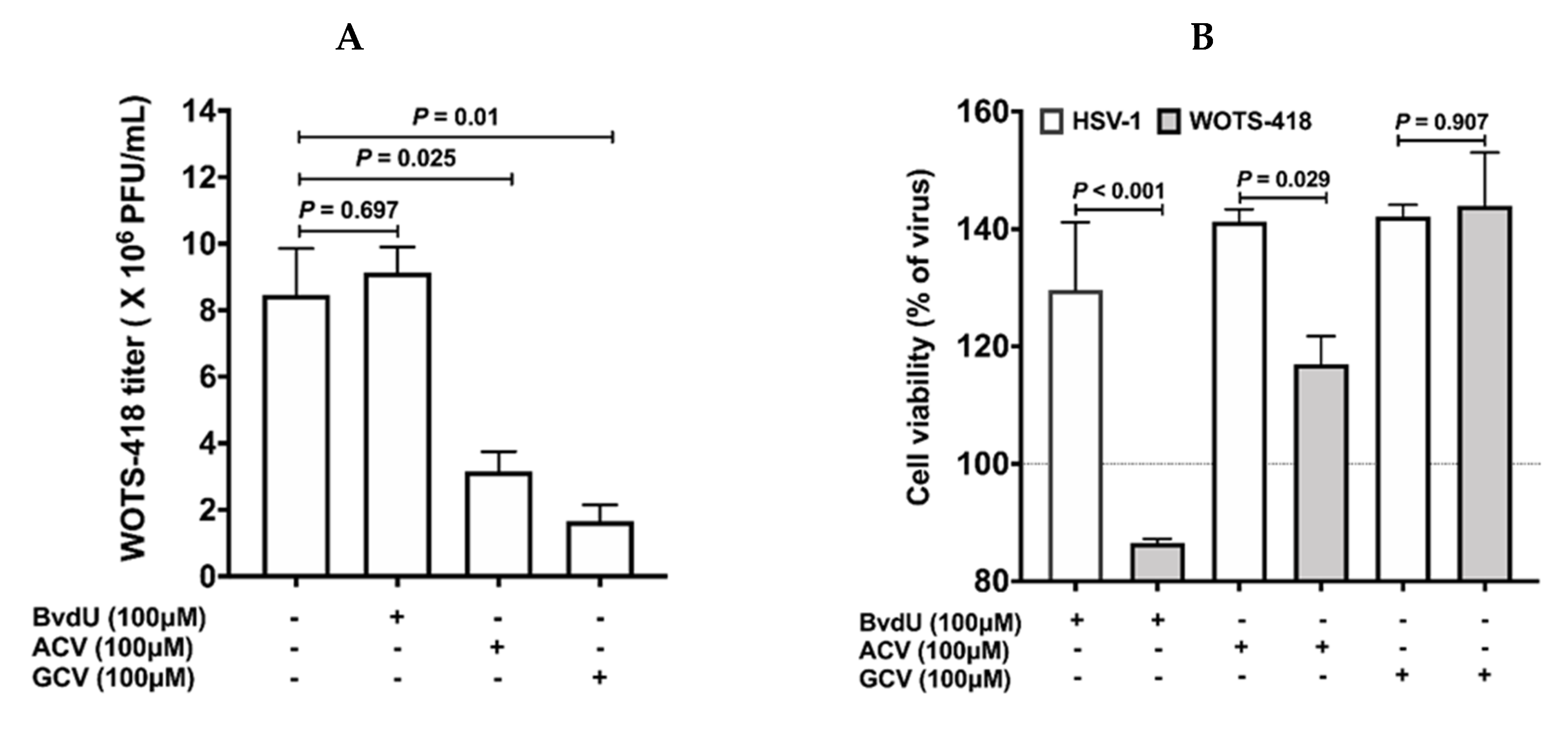
3.2. WOTS-418 Showed Enhanced Antiviral Potency to Purine Analogs (ACV and GCV) but No Antiviral Potency to Pyrimidine Analog
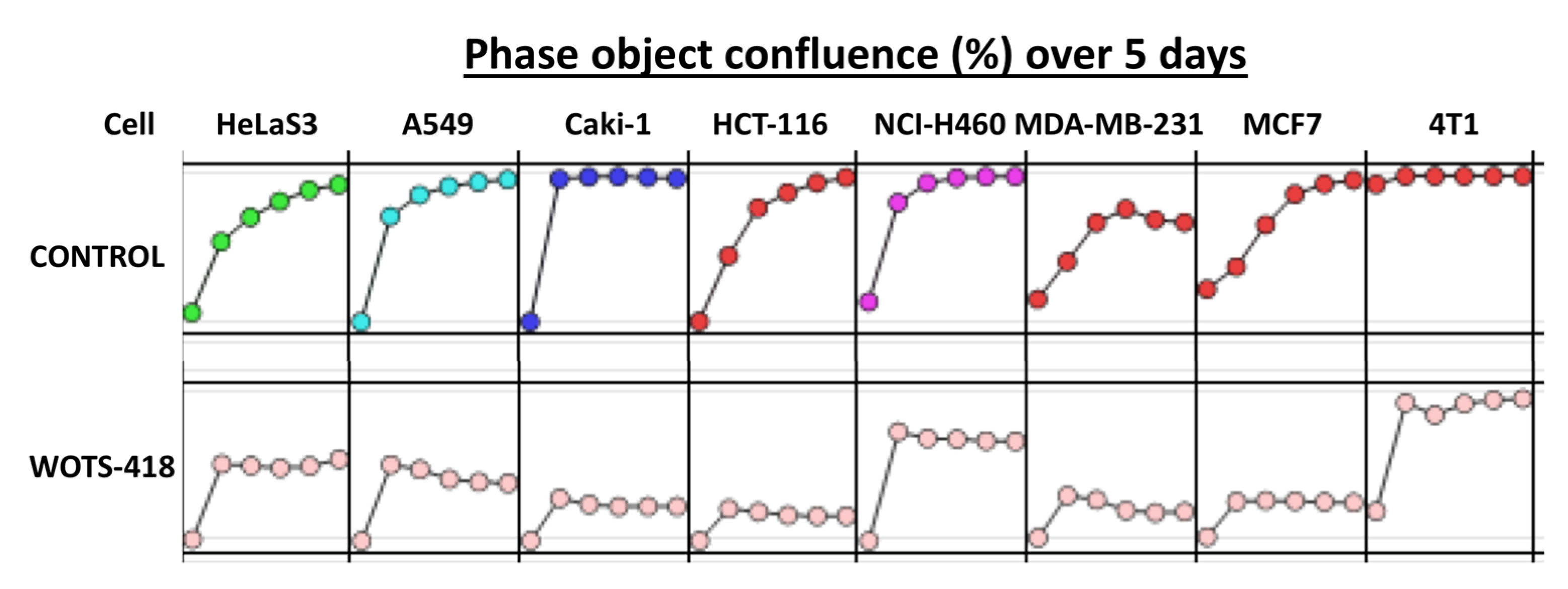
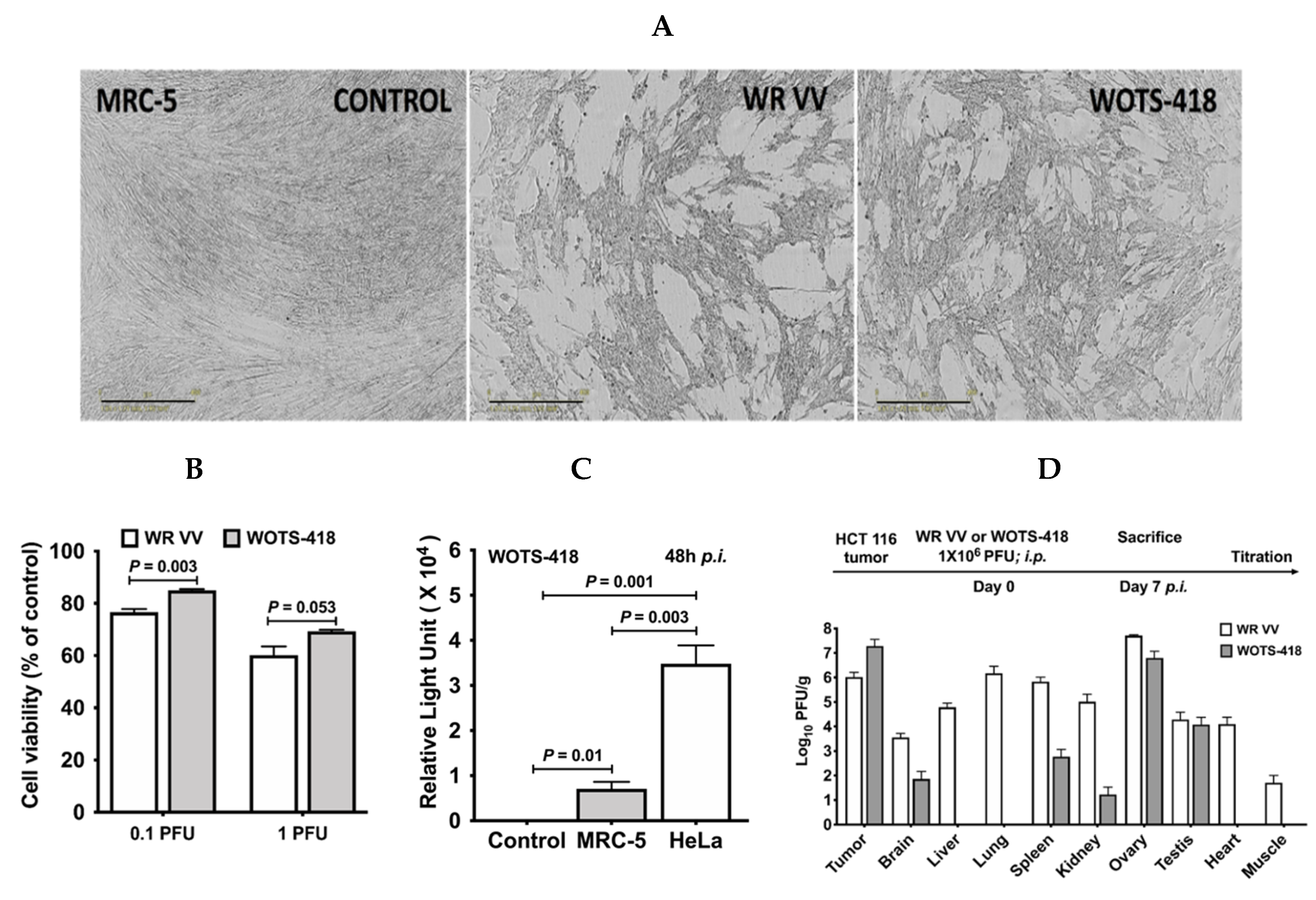
3.3. WOTS-418 Replication and Cytolysis Were Attenuated in Normal Human Cell Line but Not in Cancer Cells
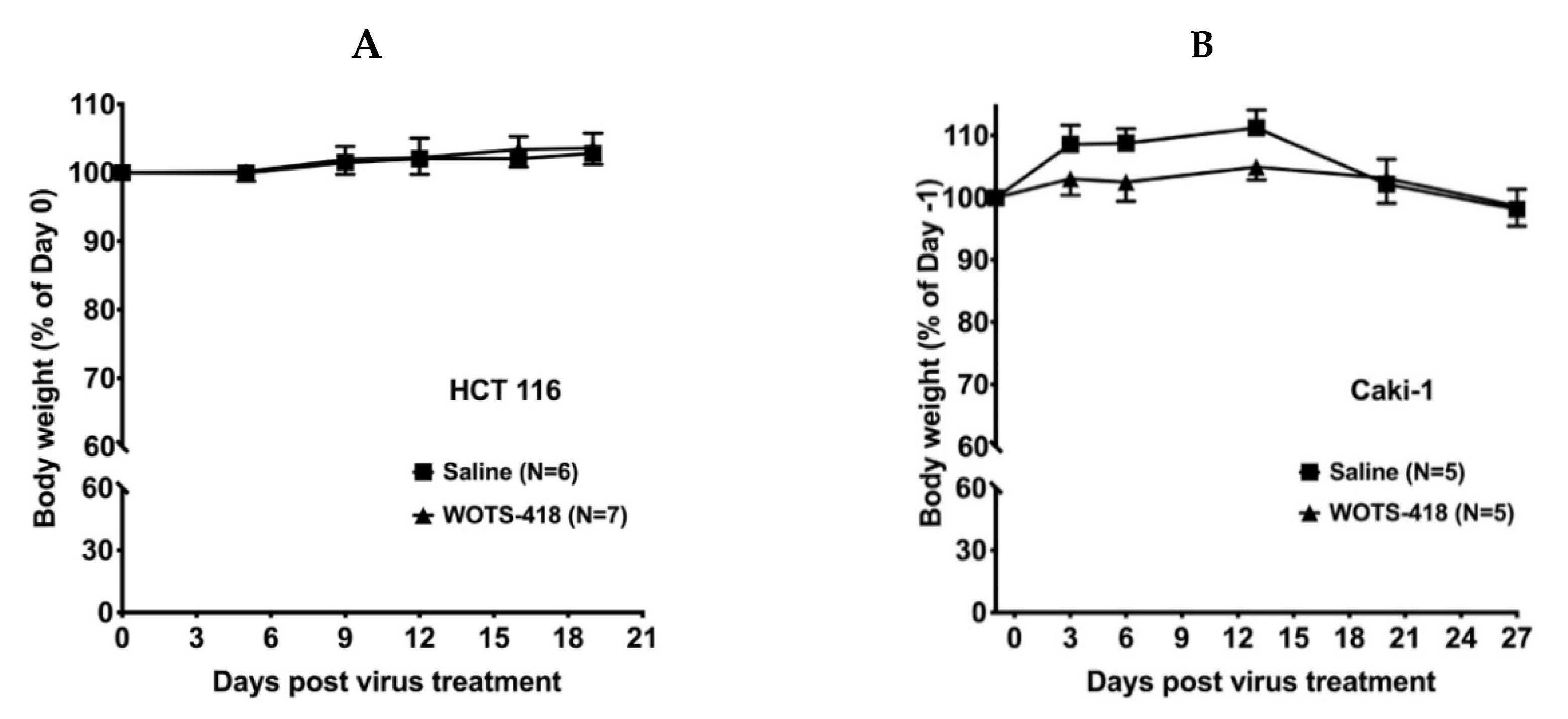
3.4. WOTS-418 Showed Significant Antitumor Response in Human Solid Tumor Models
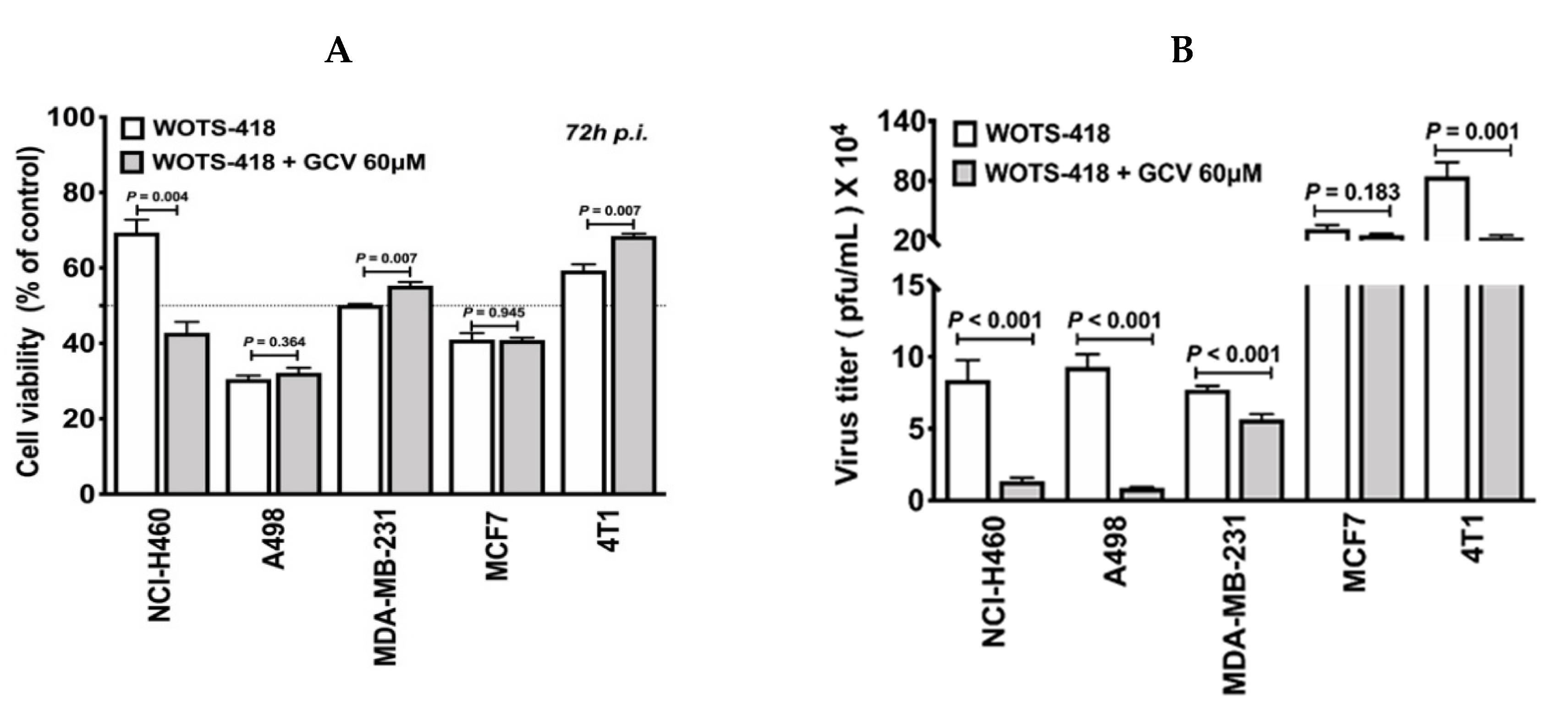
3.5. WOTS-418/GCV Combination Showed No Significant Suicidal Effect In Vitro Except in NCI-H460 Cancer Cells
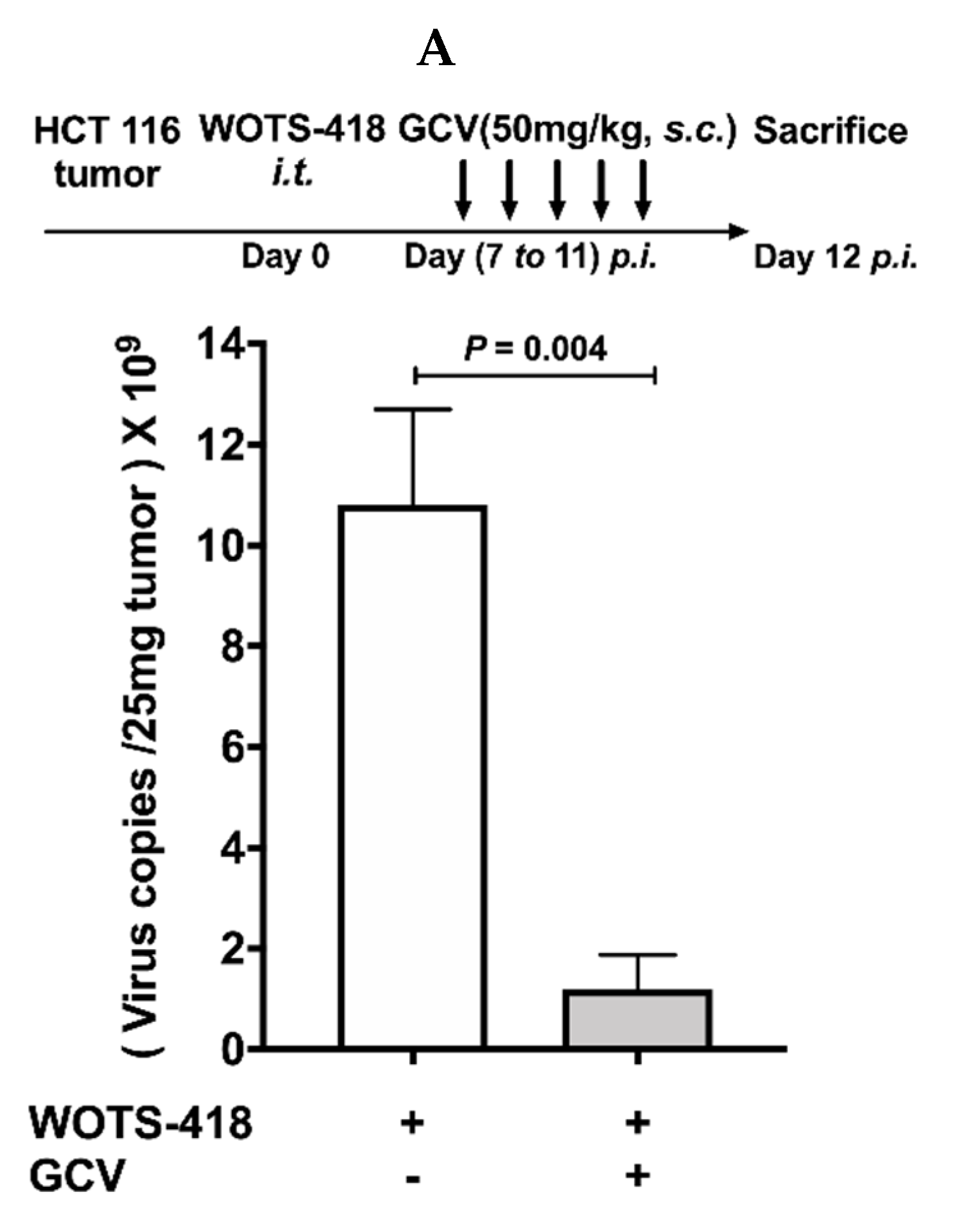
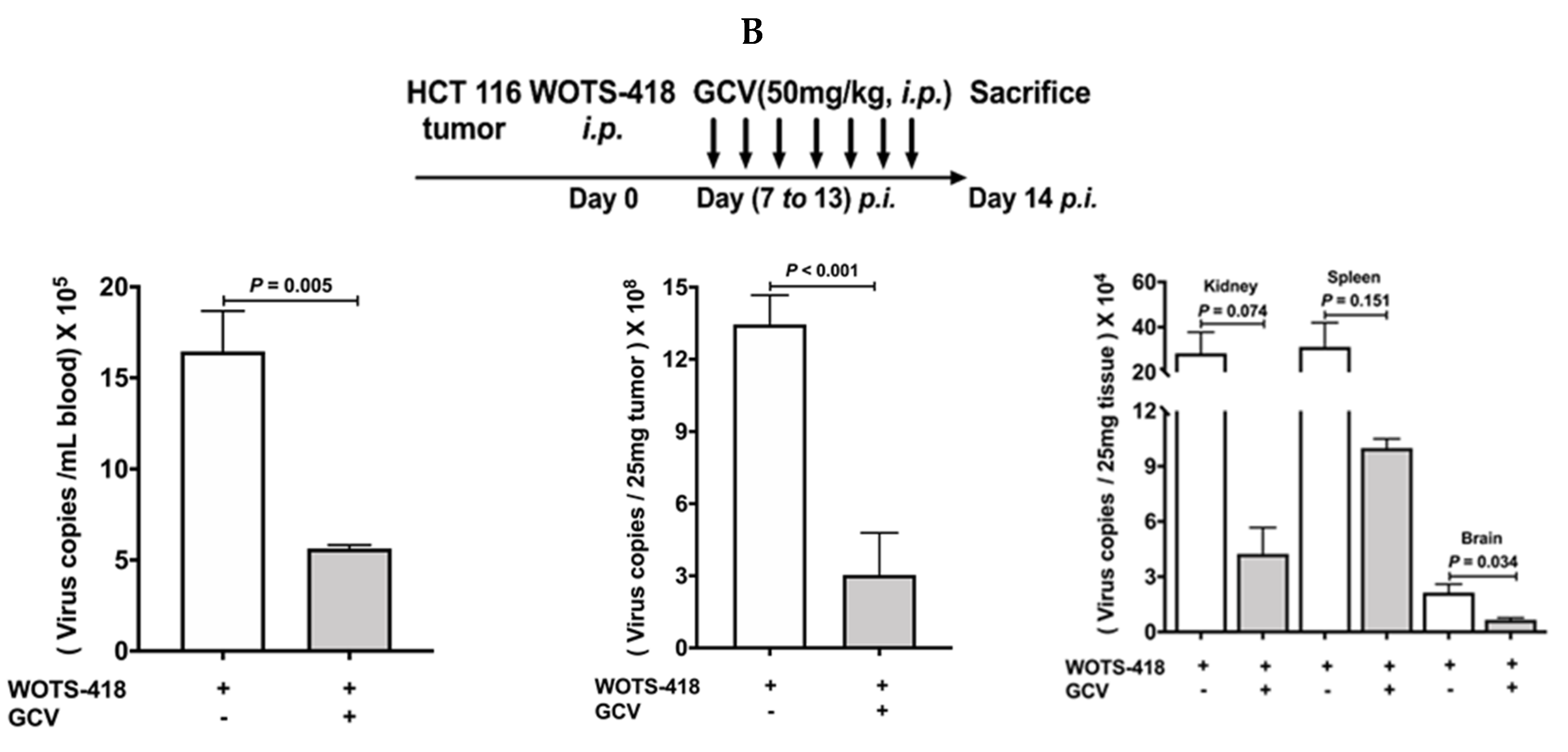
3.6. WOTS-418/GCV Combination Significantly Inhibit Virus Replication Not Only in Tumors but Also in Normal Tissues
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability Statement
Appendix A

References
- Park, B.-H.; Hwang, T.; Liu, T.-C.; Sze, D.Y.; Kim, J.-S.; Kwon, H.-C.; Oh, S.Y.; Han, S.-Y.; Yoon, J.-H.; Hong, S.-H.; et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: A phase I trial. Lancet Oncol. 2008, 9, 533–542. [Google Scholar] [CrossRef]
- Hwang, T.-H.; Moon, A.; Burke, J.; Ribas, A.; Stephenson, J.; Breitbach, C.J.; Daneshmand, M.; De Silva, N.; Parato, K.; Diallo, J.-S.; et al. A mechanistic proof-of-concept clinical trial with JX-594, a targeted multi-mechanistic oncolytic poxvirus, in patients with metastatic melanoma. Mol. Ther. J. Am. Soc. Gene Ther. 2011, 19, 1913–1922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Downs-Canner, S.; Guo, Z.S.; Ravindranathan, R.; Breitbach, C.J.; O’Malley, M.E.; Jones, H.L.; Moon, A.; McCart, J.A.; Shuai, Y.; Zeh, H.J.; et al. Phase 1 Study of Intravenous Oncolytic Poxvirus (vvDD) in Patients with Advanced Solid Cancers. Mol. Ther. 2016, 24, 1492–1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.G.; Hwang, T.H. 2597 Phase 2 trial of Pexa-Vec (pexastimogene devacirepvec; JX-594), an oncolytic and immunotherapeutic vaccinia virus, in patients with metastatic, refractory renal cell carcinoma (RCC). Eur. J. Cancer 2015, 51, S510. [Google Scholar] [CrossRef]
- Park, S.H.; Breitbach, C.J.; Lee, J.; Park, J.O.; Lim, H.Y.; Kang, W.K.; Moon, A.; Mun, J.-H.; Sommermann, E.M.; Maruri Avidal, L.; et al. Phase 1b Trial of Biweekly Intravenous Pexa-Vec (JX-594), an Oncolytic and Immunotherapeutic Vaccinia Virus in Colorectal Cancer. Mol. Ther. 2015, 23, 1532–1540. [Google Scholar] [CrossRef]
- Wang, J.; Neuhard, J.; Eriksson, S. Expression of human cytosolic thymidine kinase in Escherichia coli. A bacterial whole cell system for screening of potential antiproliferative nucleoside analogs. Adv. Exp. Med. Biol. 1998, 431, 673–676. [Google Scholar]
- Eriksson, S.; Kierdaszuk, B.; Munch-Petersen, B.; Oberg, B.; Johansson, N.G. Comparison of the substrate specificities of human thymidine kinase 1 and 2 and deoxycytidine kinase toward antiviral and cytostatic nucleoside analogs. Biochem. Biophys. Res. Commun. 1991, 176, 586–592. [Google Scholar] [CrossRef]
- El Omari, K.; Solaroli, N.; Karlsson, A.; Balzarini, J.; Stammers, D.K. Structure of vaccinia virus thymidine kinase in complex with dTTP: Insights for drug design. BMC Struct. Biol. 2006, 6, 22. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.S.; Summers, W.P.; Walker, J.; Summers, W.C.; Prusoff, W.H. Characterization of pyrimidine deoxyribonucleoside kinase (thymidine kinase) and thymidylate kinase as a multifunctional enzyme in cells transformed by herpes simplex virus type 1 and in cells infected with mutant strains of herpes simplex virus. J. Virol. 1979, 30, 942–945. [Google Scholar] [CrossRef] [Green Version]
- Jordan, A.; Reichard, P. Ribonucleotide reductases. Annu. Rev. Biochem. 1998, 67, 71–98. [Google Scholar] [CrossRef]
- Dijkwel, P.A.; Hamlin, J.L. Initiation of DNA replication in the dihydrofolate reductase locus is confined to the early S period in CHO cells synchronized with the plant amino acid mimosine. Mol. Cell. Biol. 1992, 12, 3715–3722. [Google Scholar] [CrossRef] [Green Version]
- Parker, W.B. Enzymology of purine and pyrimidine antimetabolites used in the treatment of cancer. Chem. Rev. 2009, 109, 2880–2893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galmarini, C.M.; Mackey, J.R.; Dumontet, C. Nucleoside analogues and nucleobases in cancer treatment. Lancet Oncol. 2002, 3, 415–424. [Google Scholar] [CrossRef]
- Galmarini, C.M.; Jordheim, L.; Dumontet, C. Pyrimidine nucleoside analogs in cancer treatment. Expert Rev. Anticancer Ther. 2003, 3, 717–728. [Google Scholar] [CrossRef]
- Robak, T.; Lech-Maranda, E.; Korycka, A.; Robak, E. Purine nucleoside analogs as immunosuppressive and antineoplastic agents: Mechanism of action and clinical activity. Curr. Med. Chem. 2006, 13, 3165–3189. [Google Scholar] [CrossRef]
- Slavik, M. Nucleoside analogs in the treatment of neoplastic and nonneoplastic diseases. Ann. N. Y. Acad. Sci. 1975, 255, 266–268. [Google Scholar] [CrossRef] [PubMed]
- Balzarini, J.; Liekens, S.; Esnouf, R.; De Clercq, E. The A167Y Mutation Converts the Herpes Simplex Virus Type 1 Thymidine Kinase into a Guanosine Analogue Kinase. Biochemistry 2002, 41, 6517–6524. [Google Scholar] [CrossRef] [PubMed]
- Balzarini, J.; Liekens, S.; Solaroli, N.; El Omari, K.; Stammers, D.K.; Karlsson, A. Engineering of a single conserved amino acid residue of herpes simplex virus type 1 thymidine kinase allows a predominant shift from pyrimidine to purine nucleoside phosphorylation. J. Biol. Chem. 2006, 281, 19273–19279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degrève, B.; Esnouf, R.; De Clercq, E.; Balzarini, J. Selective abolishment of pyrimidine nucleoside kinase activity of herpes simplex virus type 1 thymidine kinase by mutation of alanine-167 to tyrosine. Mol. Pharmacol. 2000, 58, 1326–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, S.M.B.U.; Lee, B.; Jiang, F.; Kim, E.-K.; Ahn, S.C.; Hwang, T.-H. Engineering and Characterization of Oncolytic Vaccinia Virus Expressing Truncated Herpes Simplex Virus Thymidine Kinase. Cancers 2020, 12, 228. [Google Scholar] [CrossRef] [Green Version]
- Thorne, S.H.; Hwang, T.-H.H.; O’Gorman, W.E.; Bartlett, D.L.; Sei, S.; Kanji, F.; Brown, C.; Werier, J.; Cho, J.-H.; Lee, D.-E.; et al. Rational strain selection and engineering creates a broad-spectrum, systemically effective oncolytic poxvirus, JX-963. J. Clin. Investig. 2007, 117, 3350–3358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeh, H.J.; Downs-Canner, S.; McCart, J.A.; Guo, Z.S.; Rao, U.N.M.; Ramalingam, L.; Thorne, S.H.; Jones, H.L.; Kalinski, P.; Wieckowski, E.; et al. First-in-man study of western reserve strain oncolytic vaccinia virus: Safety, systemic spread, and antitumor activity. Mol. Ther. J. Am. Soc. Gene Ther. 2015, 23, 202–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiewrodt, R.; Amin, K.; Kiefer, M.; Jovanovic, V.P.; Kapoor, V.; Force, S.; Chang, M.; Lanuti, M.; Black, M.E.; Kaiser, L.R.; et al. Adenovirus-mediated gene transfer of enhanced Herpes simplex virus thymidine kinase mutants improves prodrug-mediated tumor cell killing. Cancer Gene Ther. 2003, 10, 353–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wildner, O.; Blaese, R.M.; Morris, J.C. Therapy of Colon Cancer with Oncolytic Adenovirus Is Enhanced by the Addition of Herpes Simplex Virus-thymidine kinase. Cancer Res. 1999, 59, 410–413. [Google Scholar]



| Primer List | Sequence | Purpose |
|---|---|---|
| HSV1-TK SF | 5′-CCT CGT CGC AAT ATC GCA TTT T-3′ | PCR |
| HSV1-TK SR | 5’-CTC CAG CGG TTC CAT CTT C-3′ | PCR |
| SEQ 1 | 5′-AGT TAG CCT CCC CCA TCT CC-3′ | Sequencing |
| SEQ 2 | 5′-CGA CAG ATC TAG GCC TGG TA-3′ | Sequencing |
| SEQ 3 | 5′-CCC TGC TGC AAC TTA CCT CC-3′ | Sequencing |
| SEQ 4 | 5′-CTC CAG CGG TTC CAT CTT C-3′ | Sequencing |
| E9L Forward | 5′-CAA CTC TTA GCC GAA GCG TAT GAG-3′ | qPCR |
| E9L Reverse | 5′-GAA CAT TTT TGG CAG AGA GAG CC-3′ | qPCR |
| Probe | 5′-6-FAM -CAG GCT ACC AGT TCA A-MGB/NFQ-3′ | qPCR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, S.M.B.U.; Hong, Y.M.; Ornella, M.s.C.; Ngabire, D.; Jang, H.; Cho, E.; Kim, E.-K.; Hale, J.J.J.; Kim, C.H.; Ahn, S.C.; et al. Engineering and Preclinical Evaluation of Western Reserve Oncolytic Vaccinia Virus Expressing A167Y Mutant Herpes Simplex Virus Thymidine Kinase. Biomedicines 2020, 8, 426. https://doi.org/10.3390/biomedicines8100426
Islam SMBU, Hong YM, Ornella MsC, Ngabire D, Jang H, Cho E, Kim E-K, Hale JJJ, Kim CH, Ahn SC, et al. Engineering and Preclinical Evaluation of Western Reserve Oncolytic Vaccinia Virus Expressing A167Y Mutant Herpes Simplex Virus Thymidine Kinase. Biomedicines. 2020; 8(10):426. https://doi.org/10.3390/biomedicines8100426
Chicago/Turabian StyleIslam, S. M. Bakhtiar UL, Young Mi Hong, Mefotse saha Cyrelle Ornella, Daniel Ngabire, Hyunjung Jang, Euna Cho, Eung-Kyun Kim, Jessye Jin Joo Hale, Cy Hyun Kim, Soon Cheol Ahn, and et al. 2020. "Engineering and Preclinical Evaluation of Western Reserve Oncolytic Vaccinia Virus Expressing A167Y Mutant Herpes Simplex Virus Thymidine Kinase" Biomedicines 8, no. 10: 426. https://doi.org/10.3390/biomedicines8100426
APA StyleIslam, S. M. B. U., Hong, Y. M., Ornella, M. s. C., Ngabire, D., Jang, H., Cho, E., Kim, E.-K., Hale, J. J. J., Kim, C. H., Ahn, S. C., Cho, M., & Hwang, T.-H. (2020). Engineering and Preclinical Evaluation of Western Reserve Oncolytic Vaccinia Virus Expressing A167Y Mutant Herpes Simplex Virus Thymidine Kinase. Biomedicines, 8(10), 426. https://doi.org/10.3390/biomedicines8100426