Distinct Features of Autoimmune Gastritis in Patients with Open-Type Chronic Gastritis in Japan
Abstract
1. Introduction
2. Materials and Methods
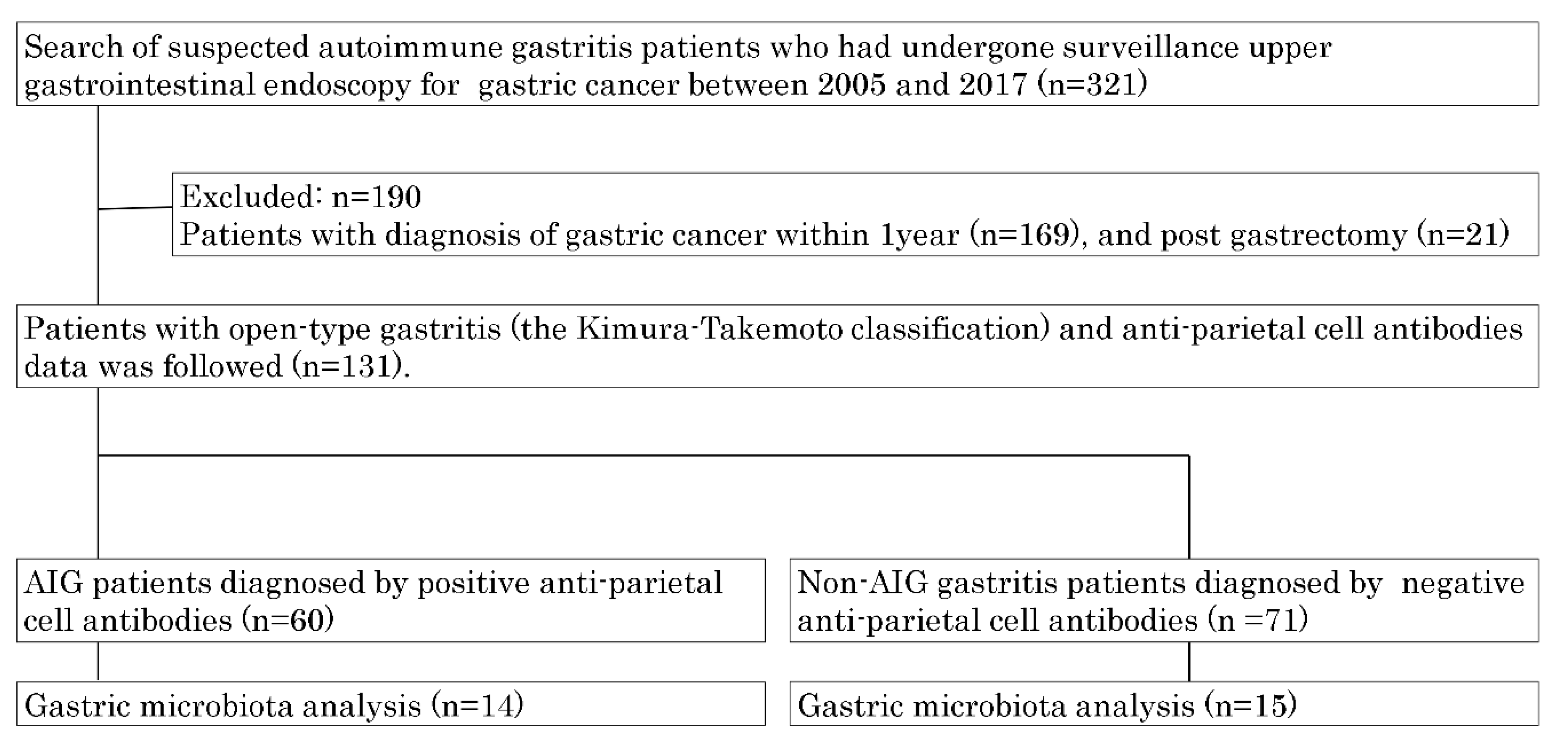
2.1. Study Design and Settings and Patients
2.2. Clinical Characteristics
2.3. Upper Gastrointestinal Endoscopy and Histological Examination
2.4. 16S rRNA Gene Sequencing and Analyses
2.5. Survival Analyses
2.6. Statistical Analyses
3. Results
3.1. Patients Characteristics
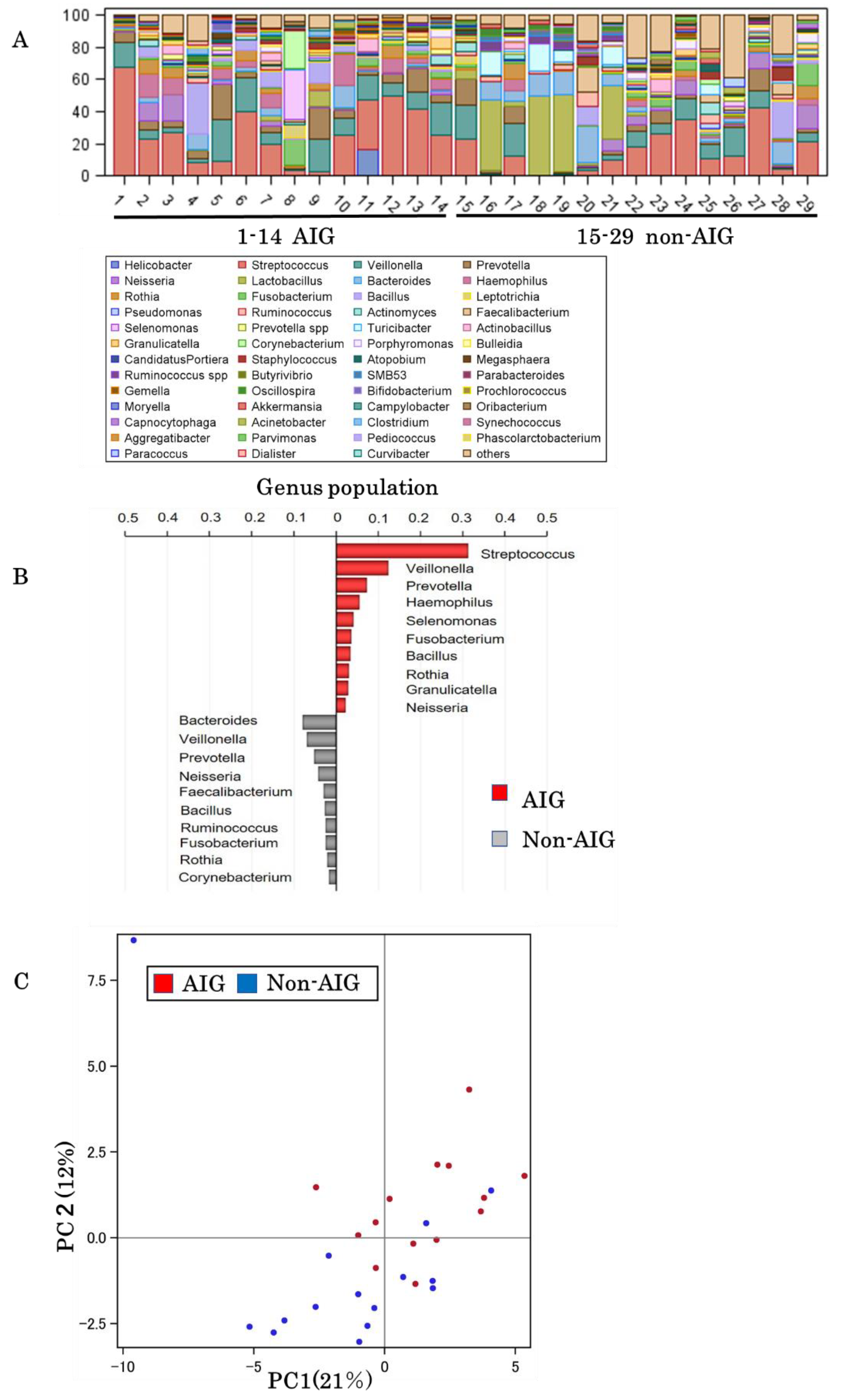
3.2. Gastric Microbiota
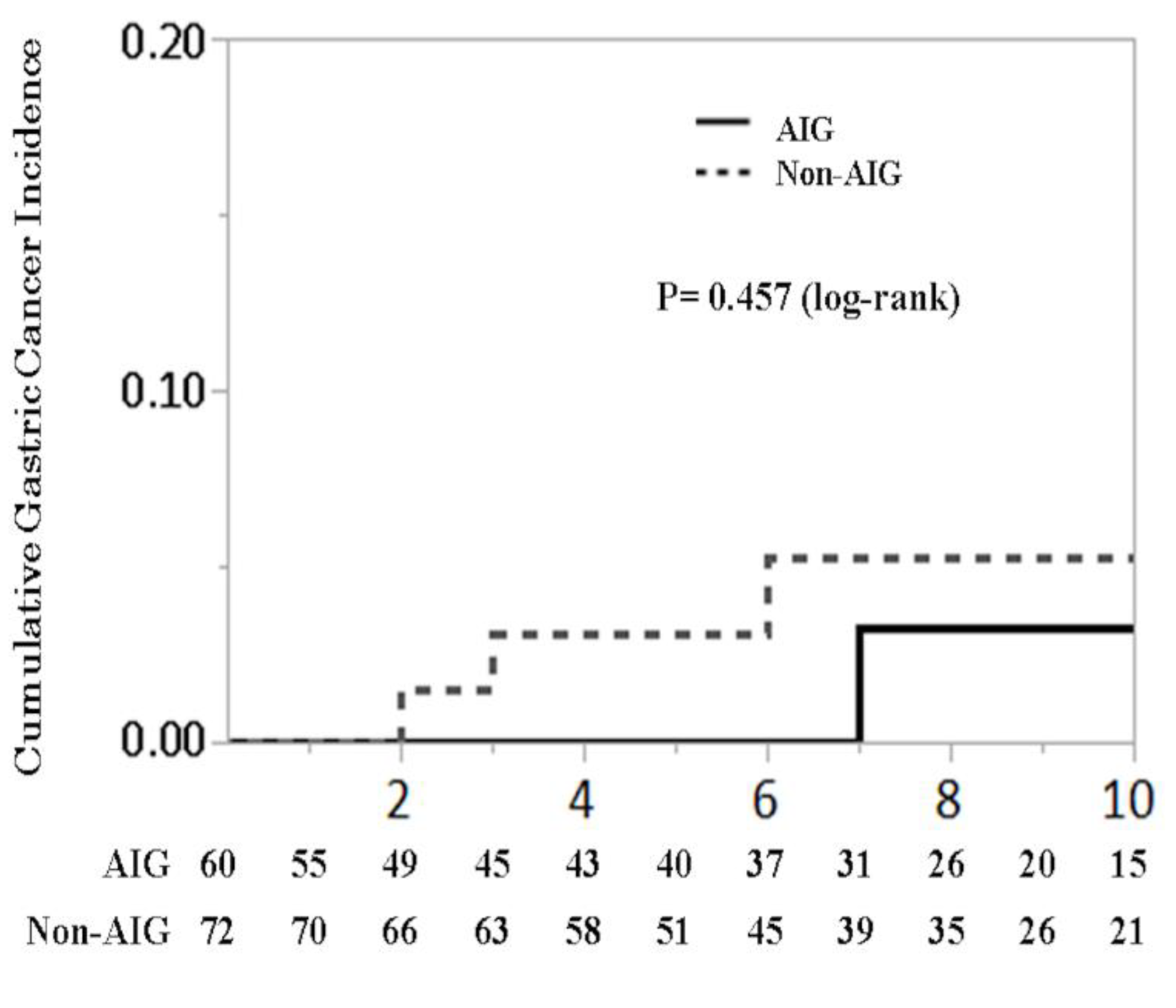
3.3. Cumulative Gastric Cancer Incidence
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Neumann, W.L.; Coss, E.; Rugge, M.; Genta, R.M. Autoimmune atrophic gastritis--pathogenesis, pathology and management. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, Y.; Sethi, N.; Sepulveda, A.R.; Bass, A.J.; Wang, T.C. Oesophageal adenocarcinoma and gastric cancer: Should we mind the gap? Nat. Rev. Cancer 2016, 16, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Shichijo, S.; Hirata, Y.; Niikura, R.; Hayakawa, Y.; Yamada, A.; Ushiku, T.; Fukayama, M.; Koike, K. Histologic intestinal metaplasia and endoscopic atrophy are predictors of gastric cancer development after Helicobacter pylori eradication. Gastrointest. Endosc. 2016, 84, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, Y.; Chang, W.; Jin, G.; Wang, T.C. Gastrin and upper GI cancers. Curr. Opin. Pharmacol. 2016, 31, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Furuta, T.; Baba, S.; Yamade, M.; Uotani, T.; Kagami, T.; Suzuki, T.; Tani, S.; Hamaya, Y.; Iwaizumi, M.; Osawa, S.; et al. High incidence of autoimmune gastritis in patients misdiagnosed with two or more failures of H. pylori eradication. Aliment. Pharmacol. Ther. 2018, 48, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Niikura, R.; Hayakawa, Y.; Hirata, Y.; Konishi, M.; Suzuki, N.; Ihara, S.; Yamada, A.; Ushiku, T.; Fujishiro, M.; Fukayama, M.; et al. Distinct Chemopreventive Effects of Aspirin in Diffuse and Intestinal-Type Gastric Cancer. Cancer Prev. Res. 2018, 11, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Kulnigg-Dabsch, S. Autoimmune gastritis. Wien. Med. Wochenschr. 2016, 166, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Shichijo, S.; Hirata, Y.; Sakitani, K.; Yamamoto, S.; Serizawa, T.; Niikura, R.; Watabe, H.; Yoshida, S.; Yamada, A.; Yamaji, Y.; et al. Distribution of intestinal metaplasia as a predictor of gastric cancer development. J. Gastroenterol. Hepatol. 2015, 30, 1260–1264. [Google Scholar] [CrossRef] [PubMed]
- Niikura, R.; Hayakawa, Y.; Hirata, Y.; Ogura, K.; Fujishiro, M.; Yamada, A.; Ushiku, T.; Konishi, M.; Fukayama, M.; Koike, K. The Reduction in Gastric Atrophy after Helicobacter pylori Eradication Is Reduced by Treatment with Inhibitors of Gastric Acid Secretion. Int. J. Mol. Sci. 2019, 20, 1913. [Google Scholar] [CrossRef] [PubMed]
- Eissele, R.; Patberg, H.; Koop, H.; Krack, W.; Lorenz, W.; McKnight, A.T.; Arnold, R. Effect of gastrin receptor blockade on endocrine cells in rats during achlorhydria. Gastroenterology 1992, 103, 1596–1601. [Google Scholar] [CrossRef]
- Parsons, B.N.; Ijaz, U.Z.; D’Amore, R.; Burkitt, M.D.; Eccles, R.; Lenzi, L.; Duckworth, C.A.; Moore, A.R.; Tiszlavicz, L.; Varro, A.; et al. Comparison of the human gastric microbiota in hypochlorhydric states arising as a result of Helicobacter pylori-induced atrophic gastritis, autoimmune atrophic gastritis and proton pump inhibitor use. PLoS Pathog. 2017, 13, e1006653. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.A.; Goodrich, J.K.; Maxan, M.E.; Freedberg, D.E.; Abrams, J.A.; Poole, A.C.; Sutter, J.L.; Welter, D.; Ley, R.E.; Bell, J.T.; et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut 2016, 65, 749–756. [Google Scholar] [CrossRef]
- Waldum, H.L.; Fossmark, R. Role of Autoimmune Gastritis in Gastric Cancer. Clin. Transl. Gastroenterol. 2019, 10, e00080. [Google Scholar] [CrossRef]

| AIG (n = 60) | Non-AIG (n = 71) | p Value | |
|---|---|---|---|
| Mean age | 75.80 ± 9.59 | 73.32 ± 9.58 | 0.143 |
| Sex, female (%) | 30 (50.00) | 26 (36.62) | 0.123 |
| H. pylori infectious states (%) | |||
| Positive | 17(28.33) | 20 (28.17) | 0.662 |
| Eradicated | 20 (33.33) | 29 (40.85) | |
| Negative | 18 (30.00) | 15 (21.13) | |
| Unknown | 5 (8.33) | 7 (9.86) | |
| Comorbidity (%) | |||
| Diabetes mellitus | 13 (21.67) | 18 (25.35) | 0.621 |
| Chronic heart disease | 11 (18.33) | 15 (21.13) | 0.690 |
| Ischemic heart disease | 8 (13.33) | 11 (15.49) | 0.726 |
| Thyroid diseases | 15 (25.00) | 6 (8.33) | 0.009 |
| Collagen diseases | 4 (6.67) | 1 (1.39) | 0.106 |
| Medication (%) | |||
| Low dose aspirin | 6 (10.00) | 8 (11.27) | 0.815 |
| Proton pump inhibitors | 20 (33.33) | 20 (28.17) | 0.523 |
| Histamine 2 receptor antagonist | 19 (31.67) | 16 (22.54) | 0.239 |
| Non-steroidal anti-inflammatory drugs | 12 (20.00) | 12 (16.90) | 0.648 |
| Steroids | 3 (5.00) | 9 (12.68) | 0.129 |
| Laboratory data * | |||
| Hemoglobin, g/dL | 10.45 ± 2.67 | 10.16 ± 2.71 | 0.530 |
| Mean corpuscular volume, fL | 88.99 ± 7.26 | 88.16 ± 6.46 | 0.489 |
| Gastrin, pg/mL | 1412 ± 2023 | 353 ± 471 | <0.001 |
| Vitamin B12, pg/mL | 273 ± 276 | 520 ± 473 | 0.074 |
| Folic acid, ng/mL | 11.33 ± 5.22 | 6.93 ± 2.80 | 0.016 |
| Iron, μg/dL | 61.98 ± 37.70 | 60.47 ± 39.00 | 0.848 |
| Pepsinogen I, ng/mL | 37.30 ± 41.25 | 63.82 ± 63.27 | 0.154 |
| Pepsinogen II, ng/mL | 17.92 ± 12.58 | 20.26 ± 6.21 | 0.695 |
| Pepsinogen I / II, ng/mL | 1.96 ± 1.99 | 3.36 ± 2.18 | 0.069 |
| Endoscopic findings | |||
| Atrophy, O-I/O-II/O-III (%) | 14/14/32 (23.33/23.33/53.33) | 23/21/27 (32.39/29.58/38.03) | 0.211 |
| Corpus dominant atrophy (%) | 19 (31.67) | 5 (7.04) | <0.001 |
| Histological findings †,* (%) | |||
| Atrophy, Group A/B/C | 4/4/18 (15.38/15.38/69.23) | 6/3/18 (22.22/11.11/66.67) | 0.769 |
| Neutrophils infiltration, Group A/B/C | 12/2/13 (44.44/7.41/48.15) | 12/3/16 (38.71/9.68/51.61) | 0.889 |
| Intestinal Metaplasia, Group A/B/C | 17/1/13 (54.84/3.23/41.94) | 20/6/9 (57.14/17.14/25.71) | 0.116 |
| ECL (%) | 8 (27.59) | 2 (6.67) | 0.032 |
| AIG (n = 14) | Non-AIG (n = 15) | p Value | |
|---|---|---|---|
| Mean age | 71.1 ± 10.17 | 69.4 ± 9.71 | 0.667 |
| Sex, female | 8 (57.14) | 6 (40.00) | 0.356 |
| H. Pylori infectious states (%) | |||
| Positive | 0 (0.00) | 0 (0.00) | 0.029 |
| Eradicated | 10 (71.43) | 15 (100.00) | |
| Negative | 4 (28.57) | 0 (0.00) | |
| Unknown | 0 (0.00) | 0 (0.00) | |
| Comorbidity (%) | |||
| Diabetes mellitus | 2 (14.29) | 1 (6.67) | 0.498 |
| Chronic heart disease | 1 (7.14) | 1 (6.67) | 0.960 |
| Ischemic heart disease | 2 (14.29) | 1 (6.67) | 0.501 |
| Thyroid diseases | 7 (50.00) | 0 (0.00) | 0.002 |
| Collagen diseases | 2 (14.29) | 0 (0.00) | 0.129 |
| Medication (%) | |||
| Low dose aspirin | 0 (0.00) | 0 (0.00) | 0 |
| Proton pump inhibitors | 5 (35.71) | 4 (26.67) | 0.599 |
| Histamine 2 receptor antagonist | 1 (7.14) | 3 (20.00) | 0.316 |
| Non-steroidal anti-inflammatory drugs | 3 (21.43) | 0 (0.00) | 0.002 |
| Steroids | 0 (0.00) | 2 (13.33) | 0.157 |
| Laboratory data * | |||
| Hemoglobin, g/dL | 12.09 ± 1.89 | 12.89 ± 1.75 | 0.246 |
| Mean corpuscular volume, fL | 89.73 ± 7.38 | 90.81 ± 5.97 | 0.666 |
| Gastrin, pg/mL | 3507 ± 2011 | 381 ± 385 | 0.020 |
| Vitamin B12, pg/mL | 150 ± 88 | 846 | <0.001 |
| Folic acid, ng/mL | 10.82 ± 3.84 | 3.7 | 0.103 |
| Iron, μg/dL | 74.45 ± 39.77 | 53.20 ± 22.59 | 0.289 |
| Pepsinogen I, ng/mL | 23.2 ± 35.47 | 100.8 ± 79.27 | 0.015 |
| Pepsinogen II, ng/mL | 13.25 ± 6.93 | 32.86 ± 28.76 | 0.044 |
| Pepsinogen I/ II, ng/mL | 1.64 ± 2.50 | 3.12 ± 1.73 | 0.255 |
| Endoscopic findings | |||
| Atrophy, O-I/O-II/O-III (%) | 1/0/13 (7.14/0/92.86) | 5/4/6 (33.33/26.67/40.00) | 0.01 |
| Corpus dominant atrophy (%) | 10 (71.43) | 1 (6.67) | <0.001 |
| Histological findings †,* (%) | |||
| Atrophy Group A/B/C | 1/0/12 (7.69/0.00/92.31) | 3/2/10 (20.00/13.33/66.67) | 0.217 |
| Neutrophils infiltration Group A/B/C | 4/2/7 (30.77/15.38/53.85) | 6/2/7 (40.00/13.33/46.67) | 0.879 |
| Intestinal Metaplasia Group A/B/C | 6/0/8 (42.86/0.00/57.14) | 7/5/3 (46.67/33.33/20.00) | 0.026 |
| ECL (%) | 7 (58.85) | 0 (0.00) | 0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsuboi, M.; Niikura, R.; Hayakawa, Y.; Hirata, Y.; Ushiku, T.; Koike, K. Distinct Features of Autoimmune Gastritis in Patients with Open-Type Chronic Gastritis in Japan. Biomedicines 2020, 8, 419. https://doi.org/10.3390/biomedicines8100419
Tsuboi M, Niikura R, Hayakawa Y, Hirata Y, Ushiku T, Koike K. Distinct Features of Autoimmune Gastritis in Patients with Open-Type Chronic Gastritis in Japan. Biomedicines. 2020; 8(10):419. https://doi.org/10.3390/biomedicines8100419
Chicago/Turabian StyleTsuboi, Mayo, Ryota Niikura, Yoku Hayakawa, Yoshihiro Hirata, Tetsuo Ushiku, and Kazuhiko Koike. 2020. "Distinct Features of Autoimmune Gastritis in Patients with Open-Type Chronic Gastritis in Japan" Biomedicines 8, no. 10: 419. https://doi.org/10.3390/biomedicines8100419
APA StyleTsuboi, M., Niikura, R., Hayakawa, Y., Hirata, Y., Ushiku, T., & Koike, K. (2020). Distinct Features of Autoimmune Gastritis in Patients with Open-Type Chronic Gastritis in Japan. Biomedicines, 8(10), 419. https://doi.org/10.3390/biomedicines8100419





