Comprehensive Analysis of Neurotoxin-Induced Ablation of Dopaminergic Neurons in Zebrafish Larvae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Zebrafish Care and Husbandry
2.2. Environmental Toxin Preparation and Exposure
2.3. Live Confocal Imaging
2.4. RNA Isolation and qRT-PCR
2.5. Swimming Activity
2.6. Statistical Analysis
3. Results
3.1. Effective LC50 Dose for PD Phenotypic Induction (Survival Curve/Median Lethal Concentration)
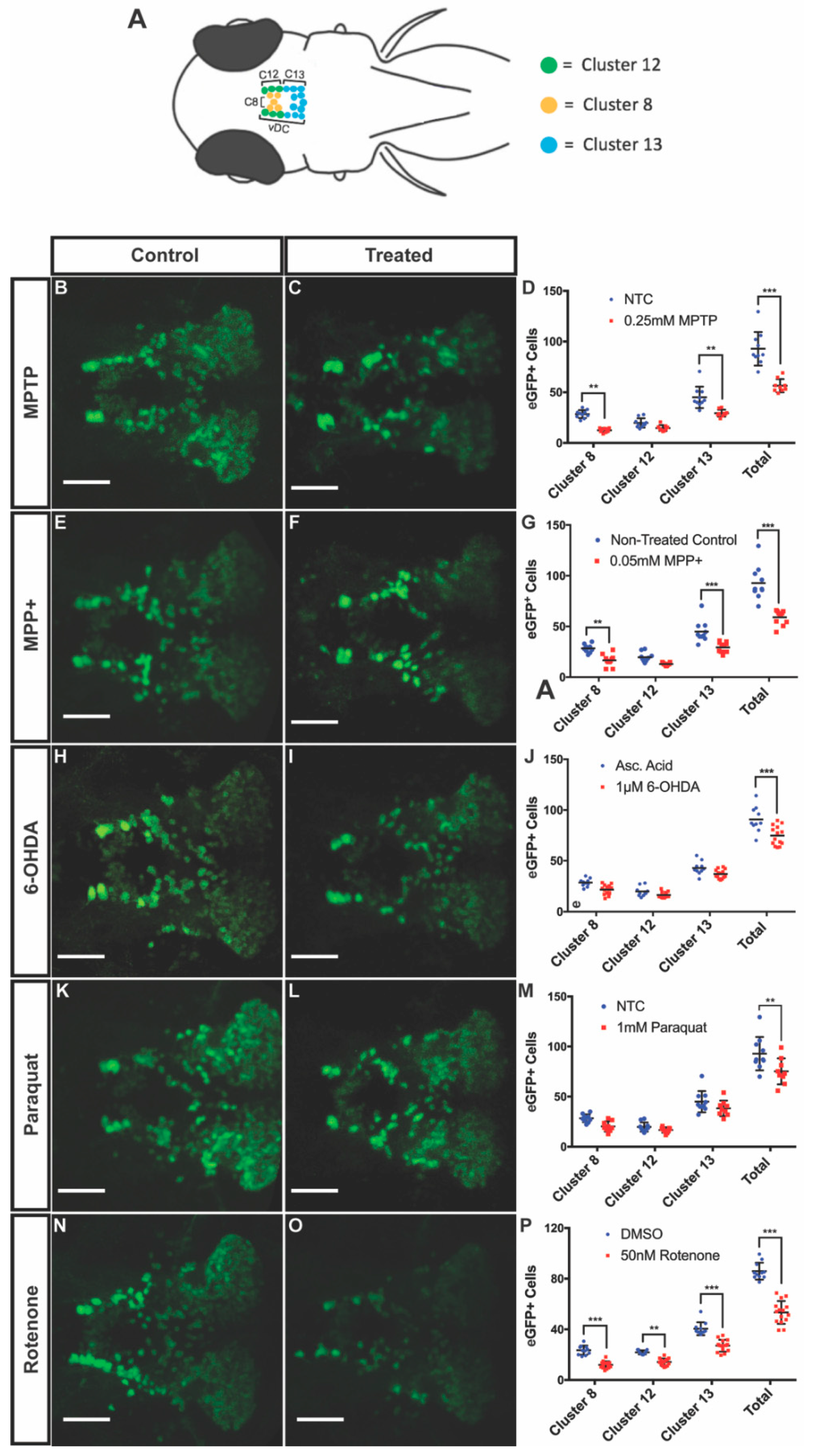
3.2. DAnergic Neuronal Loss in the Ventral Diencephalon (vDC)
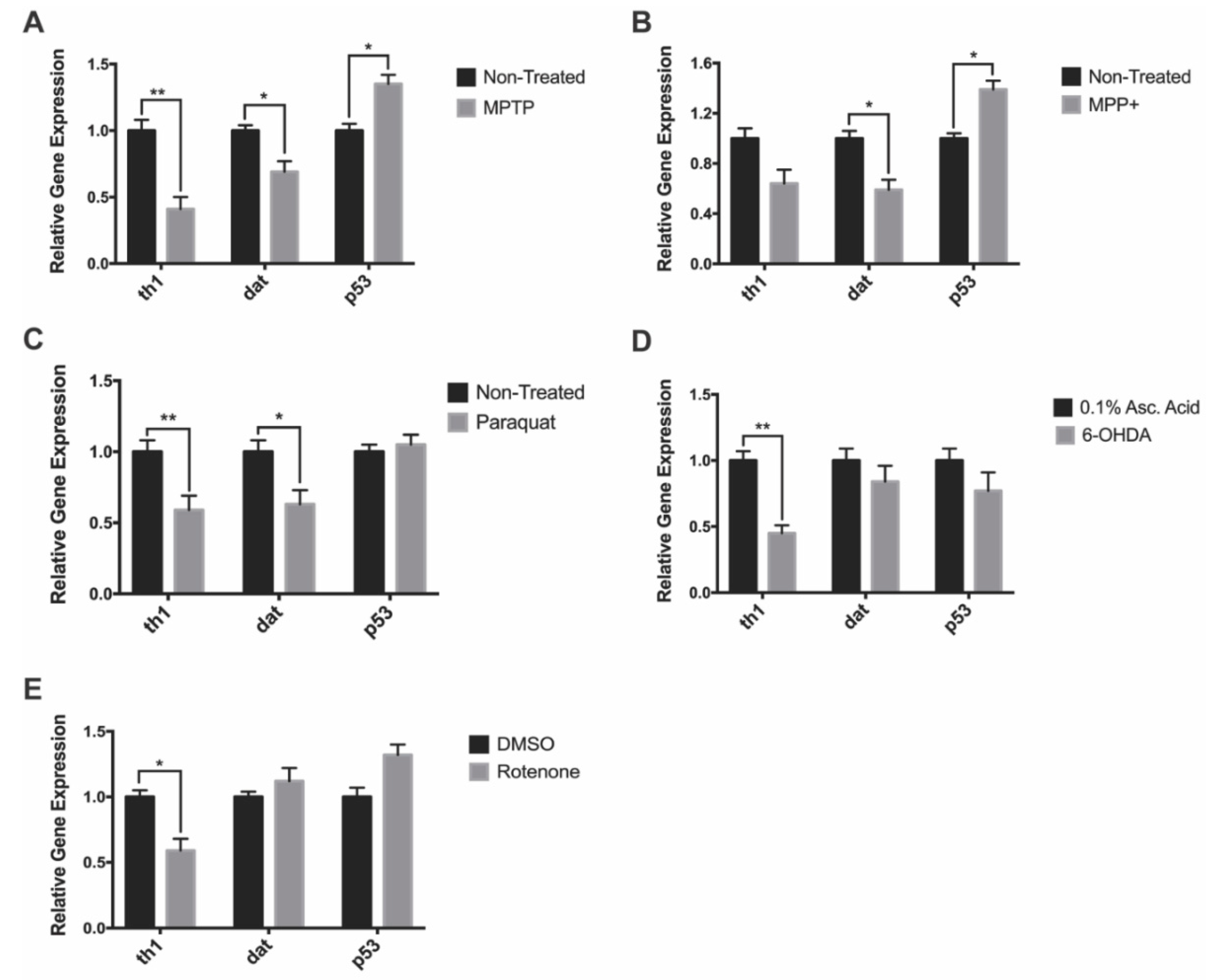
3.3. Gene Expression
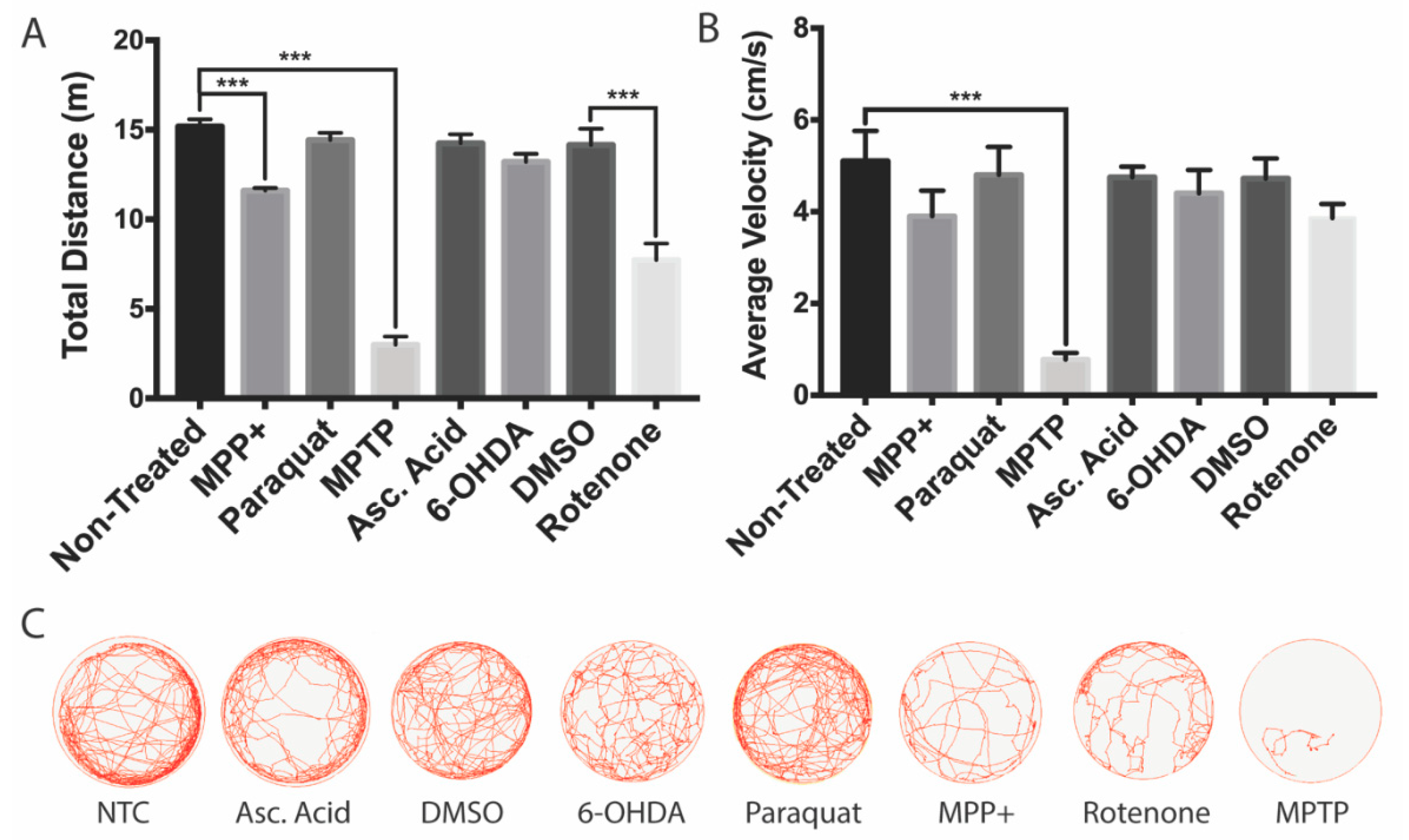
3.4. Effects on Locomotion
4. Discussion
4.1. MPTP and MPP+
4.2. Paraquat
4.3. 6-OHDA
4.4. Rotenone
4.5. Comparison of Effectiveness between Tested Neurotoxins
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PD | Parkinson’s disease |
| hpf | Hour(s) post fertilization |
| dpf | Day(s) post fertilization |
| dpt | Day(s) post treatment |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| MPP+ | 1-methyl-4-phenyl-pyridinium |
| Paraquat | Methyl viologen dichloride hydrate |
| 6-OHDA | 6-hydroxydopamine |
| DMSO | Dimethyl sulfoxide |
| LC50 | Median lethal dose |
| DAnergic | Dopaminergic |
| vDC | Ventral diencephalon |
| BBB | Blood-brain barrier |
| th | Tyrosine hydroxylase |
| dat | Dopamine transporter |
| eGFP | Enhanced green fluorescent protein |
| SEM | Standard error of the mean |
| MAO-B | Monoamine oxidase B |
| ETC | Electron transport chain |
| mRNA | Messenger ribonucleic acid |
| ROS | Reactive oxygen species |
References
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, C.; Westenberger, A. Genetics of Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a008888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dexter, D.T.; Jenner, P. Parkinson disease: From pathology to molecular disease mechanisms. Free Radic. Biol. Med. 2013, 62, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Meredith, G.E.; Rademacher, D.J. MPTP mouse models of Parkinson’s disease: An update. J. Parkinsons. Dis. 2011, 1, 19–33. [Google Scholar] [CrossRef] [Green Version]
- Blesa, J.; Phani, S.; Jackson-Lewis, V.; Przedborski, S. Classic and new animal models of Parkinson’s disease. J. Biomed. Biotechnol. 2012, 2012, 845618. [Google Scholar] [CrossRef]
- Bhurtel, S.; Katila, N.; Srivastav, S.; Neupane, S.; Choi, D.-Y. Mechanistic comparison between MPTP and rotenone neurotoxicity in mice. Neurotoxicology 2019, 71, 113–121. [Google Scholar] [CrossRef]
- Przedborski, S.; Tieu, K.; Perier, C.; Vila, M. MPTP as a mitochondrial neurotoxic model of Parkinson’s disease. J. Bioenerg. Biomembr. 2004, 36, 375–379. [Google Scholar] [CrossRef]
- Lam, C.S.; Korzh, V.; Strahle, U. Zebrafish embryos are susceptible to the dopaminergic neurotoxin MPTP. Eur. J. Neurosci. 2005, 21, 1758–1762. [Google Scholar] [CrossRef]
- Best, J.D.; Alderton, W.K. Zebrafish: An in vivo model for the study of neurological diseases. Neuropsychiatr. Dis. Treat. 2008, 4, 567–576. [Google Scholar] [CrossRef] [Green Version]
- Bretaud, S.; Lee, S.; Guo, S. Sensitivity of zebrafish to environmental toxins implicated in Parkinson’s disease. Neurotoxicol. Teratol. 2004, 26, 857–864. [Google Scholar] [CrossRef]
- Stewart, A.M.; Braubach, O.; Spitsbergen, J.; Gerlai, R.; Kalueff, A. V Zebrafish models for translational neuroscience research: From tank to bedside. Trends Neurosci. 2014, 37, 264–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Y.; Guo, Q.; Shan, M.; Wu, Y.; Huang, S.; Zhao, H.; Hong, H.; Yang, M.; Yang, X.; Ren, L.; et al. Spatial and Temporal Distribution of Dopaminergic Neurons during Development in Zebrafish. Front. Neuroanat. 2016, 10, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holzschuh, J.; Ryu, S.; Aberger, F.; Driever, W. Dopamine transporter expression distinguishes dopaminergic neurons from other catecholaminergic neurons in the developing zebrafish embryo. Mech. Dev. 2001, 101, 237–243. [Google Scholar] [CrossRef]
- Sallinen, V.; Torkko, V.; Sundvik, M.; Reenilä, I.; Khrustalyov, D.; Kaslin, J.; Panula, P. MPTP and MPP+ target specific aminergic cell populations in larval zebrafish. J. Neurochem. 2009, 108, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.; Diekmann, H.; Goldsmith, P. Functional characterisation of the maturation of the blood-brain barrier in larval zebrafish. PLoS ONE 2013, 8, e77548. [Google Scholar] [CrossRef]
- Sillitoe, R.V.; Vogel, M.W. Desire, Disease, and the Origins of the Dopaminergic System. Schizophr. Bull. 2008, 34, 212–219. [Google Scholar] [CrossRef] [Green Version]
- Santana, C.; Rodriguez, M.; Afonso, D.; Arevalo, R. Dopaminergic neuron development in rats: Biochemical study from prenatal life to adulthood. Brain Res. Bull. 1992, 29, 7–13. [Google Scholar] [CrossRef]
- Lidow, M.S. D1- and D2 dopaminergic receptors in the developing cerebral cortex of macaque monkey: A film autoradiographic study. Neuroscience 1995, 65, 439–452. [Google Scholar] [CrossRef]
- Tieu, K. A guide to neurotoxic animal models of Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2011, 1, a009316. [Google Scholar] [CrossRef]
- Hare, D.J.; Adlard, P.A.; Doble, P.A.; Finkelstein, D.I. Metallobiology of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity. Metallomics 2013, 5, 91–109. [Google Scholar] [CrossRef] [Green Version]
- Bromilow, R.H. Paraquat and sustainable agriculture. Pest. Manag. Sci. 2004, 60, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.R.; Quan, Y.; Sherer, T.B.; Greenamyre, J.T.; Miller, G.W. Paraquat Neurotoxicity is Distinct from that of MPTP and Rotenone. Toxicol. Sci. 2005, 88, 193–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhillon, A.S.; Tarbutton, G.L.; Levin, J.L.; Plotkin, G.M.; Lowry, L.K.; Nalbone, J.T.; Shepherd, S. Pesticide/Environmental Exposures and Parkinson’s Disease in East Texas. J. Agromedicine 2008, 13, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Rappold, P.M.; Cui, M.; Chesser, A.S.; Tibbett, J.; Grima, J.C.; Duan, L.; Sen, N.; Javitch, J.A.; Tieu, K. Paraquat neurotoxicity is mediated by the dopamine transporter and organic cation transporter-3. Proc. Natl. Acad. Sci. USA 2011, 108, 20766–20771. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Souders, C., II; Zhao, Y.; Martyniuk, C. Exposure to paraquat affects mitochondrial bioenergetics, dopamine system expression, and locomotor activity in zebrafish (Danio rerio). Chemosphere 2017, 191, 106–117. [Google Scholar] [CrossRef]
- Nellore, J.; P, N. Paraquat exposure induces behavioral deficits in larval zebrafish during the window of dopamine neurogenesis. Toxicol. Reports 2015, 2, 950–956. [Google Scholar] [CrossRef] [Green Version]
- Bortolotto, J.; Cognato, G.; Rilo Christoff, R.; Nery, L.; Leite, C.; Kist, L.; Bogo, M.; Vianna, M.; Bonan, C. Long-Term Exposure to Paraquat Alters Behavioral Parameters and Dopamine Levels in Adult Zebrafish (Danio Rerio). Zebrafish 2014, 11, 142–153. [Google Scholar] [CrossRef]
- Reader, T.; Dewar, K.M. Review article Effects of denervation and hyperinnervation on dopamine and serotonin systems in the rat neostriatum: Implications for human Parkinsons disease. Neurochem. Int. 1999, 34, 1–21. [Google Scholar] [CrossRef]
- Dauer, W.; Przedborski, S. Parkinson’s Disease: Mechanisms and Models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef] [Green Version]
- Bové, J.; Perier, C. Neurotoxin-based models of Parkinson’s disease. Neuroscience 2012, 211, 51–76. [Google Scholar] [CrossRef]
- Fulceri, F.; Biagioni, F.; Lenzi, P.; Falleni, A.; Gesi, M.; Ruggieri, S.; Fornai, F. Nigrostriatal Damage with 6-OHDA. Ann. N. Y. Acad. Sci. 2006, 1074, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Nanda, N.B.P.; Das, P.C.; Jena, J. Use of Rotenone as Piscicide: Toxicity Levels in a Few Common Freshwater Predatory and Weed Fishes. J. Appl. Aquac. 2009, 21, 241–249. [Google Scholar] [CrossRef]
- Ott, K.C.; Rotenone, D.P. A Brief Review of its Chemistry, Environmental Fate, and the Toxicity of Rotenone Formulations. Available online: https://pdfs.semanticscholar.org/2944/9a8fda74b7bb956f78aca6169b14b44bdb14.pdf (accessed on 23 April 2006).
- Cabezas, R.; Fidel, M.; Torrente, D.; Santos El-Bach, R.; Morales, L.; Gonzalez, J.; Barreto, G.E. Astrocytes Role in Parkinson: A Double-Edged Sword. In Neurodegenerative Diseases; IntechOpen: London, UK, 2013. [Google Scholar]
- Sinha, P.; Ghosh, N.; Mitra, S.; Bhattacharyya, A. Neuroinflammation during Parkinson’s Disease: Key Cells and Molecules Involved in It. Inflamm. Common Link Brain Pathol. 2016, 185–208. [Google Scholar]
- Martel, S.; Keow, J.Y.; Ekker, M. Rotenone Neurotoxicity Causes Dopamine Neuron Loss in Zebrafish. Univ. Ottawa J. Med. 2015, 5, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Xi, Y.; Yu, M.; Godoy, R.; Hatch, G.; Poitras, L.; Ekker, M. Transgenic zebrafish expressing green fluorescent protein in dopaminergic neurons of the ventral diencephalon. Dev. Dyn. 2011, 240, 2539–2547. [Google Scholar] [CrossRef]
- Keow, J. Physiological and Behavioral Changes in a Rotenone Model of Dopamine Neurotoxicity and Neurodegeneration in Zebrafish. Ph.D Thesis, University of Ottawa, Ottawa, ON, Canada, 2016. [Google Scholar]
- Barreto-Valer, K.; López-Bellido, R.; Macho Sánchez-Simón, F.; Rodríguez, R.E. Modulation by Cocaine of Dopamine Receptors through miRNA-133b in Zebrafish Embryos. PLoS ONE 2012, 7, e52701. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-C.; Semenova, S.; Rozov, S.; Sundvik, M.; Bonkowsky, J.L.; Panula, P. A Novel Developmental Role for Dopaminergic Signaling to Specify Hypothalamic Neurotransmitter Identity. J. Biol. Chem. 2016, 291, 21880–21892. [Google Scholar] [CrossRef] [Green Version]
- Tang, R.; Dodd, A.; Lai, D.; McNabb, W.C.; Love, D.R. Validation of Zebrafish (Danio rerio) Reference Genes for Quantitative Real-time RT-PCR Normalization. Acta Biochim. Biophys. Sin. 2007, 39, 384–390. [Google Scholar] [CrossRef] [Green Version]
- Vaz, R.L.; Outeiro, T.F.; Ferreira, J.J. Zebrafish as an Animal Model for Drug Discovery in Parkinson’s Disease and Other Movement Disorders: A Systematic Review. Front. Neurol. 2018, 9, 347. [Google Scholar] [CrossRef]
- Feng, C.; Chen, C. Effects of 6-Hydroxydopamine Exposure on Motor Activity and Biochemical Expression in Zebrafish (Danio Rerio) Larvae. Zebrafish 2014, 11, 227–239. [Google Scholar] [CrossRef] [Green Version]
- Barbeau, A.; Dallaire, L.; Buu, N.T.; Poirier, J.; Rucinska, E. Comparative behavioral, biochemical and pigmentary effects of MPTP, MPP+ and paraquat in rana pipiens. Life Sci. 1985, 37, 1529–1538. [Google Scholar] [CrossRef]
- Matsui, H.; Taniguchi, Y.; Inoue, H.; Uemura, K.; Takeda, S.; Takahashi, R. A chemical neurotoxin, MPTP induces Parkinson’s disease like phenotype, movement disorders and persistent loss of dopamine neurons in medaka fish. Neurosci. Res. 2009, 65, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Farrell, T.C.; Cario, C.L.; Milanese, C.; Vogt, A.; Jeong, J.-H.; Burton, E.A. Evaluation of spontaneous propulsive movement as a screening tool to detect rescue of Parkinsonism phenotypes in zebrafish models. Neurobiol. Dis. 2011, 44, 9–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reczek, C.R.; Birsoy, K.; Kong, H.; Martínez-Reyes, I.; Wang, T.; Gao, P.; Sabatini, D.M.; Chandel, N.S. A CRISPR screen identifies a pathway required for paraquat-induced cell death. Nat. Chem. Biol. 2017, 13, 1274. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Ohtaki, K.; Matsubara, K.; Aoyama, K.; Uezono, T.; Saito, O.; Suno, M.; Ogawa, K.; Hayase, N.; Kimura, K.; et al. Carrier-mediated processes in blood–brain barrier penetration and neural uptake of paraquat. Brain Res. 2001, 906, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Casarejos, M.J.; Canals, S.; De Bernardo, S.; Mena, M.A.; Rodrı, E. Thiolic antioxidants protect from nitric oxide-induced toxicity in fetal midbrain cultures. Neuropharmacology 2002, 43, 877–888. [Google Scholar]
- Riederer, P.; Sofic, E.; Rausch, W.; Schmidt, B.; Reynolds, G.P.; Jellinger, K.; Youdim, M.B.H. Transition Metals, Ferritin, Glutathione, and Ascorbic Acid in Parkinsonian Brains. J. Neurochem. 1989, 52, 515–520. [Google Scholar] [CrossRef]
- Swarnkar, S.; Singh, S.; Mathur, R.; Patro, I.; Nath, C. A study to correlate rotenone induced biochemical changes and cerebral damage in brain areas with neuromuscular coordination in rats. Toxicology 2010, 272, 17–22. [Google Scholar] [CrossRef]
- Coulom, H.; Birman, S. Chronic exposure to rotenone models sporadic Parkinson’s disease in Drosophila melanogaster. J. Neurosci. 2004, 24, 10993–10998. [Google Scholar] [CrossRef] [Green Version]
- Betarbet, R.; Sherer, T.B.; MacKenzie, G.; Garcia-Osuna, M.; Panov, A.V.; Greenamyre, J.T. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat. Neurosci. 2000, 3, 1301–1306. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, J.-D.; Song, L.-K.; Li, J.; Chu, S.-F.; Yuan, Y.-H.; Chen, N.-H. Environment-contact administration of rotenone: A new rodent model of Parkinson’s disease. Behav. Brain Res. 2015, 294, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Kastenhuber, E.; Kratochwil, C.F.; Ryu, S.; Schweitzer, J.; Driever, W. Genetic dissection of dopaminergic and noradrenergic contributions to catecholaminergic tracts in early larval zebrafish. J. Comp. Neurol. 2010, 518, 439–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Primer | Forward Sequence (5′–3′) | Reverse Sequence (5′–3′) | Reference |
|---|---|---|---|
| dat | AGACATCTGGGAAGGTGGTG | ACCTGAGCATCATACAGGCG | [39] |
| th1 | GACGGAAGATGATCGGAGACA | CCGCCATGTTCCGATTTCT | [40] |
| p53 | ATATCCTGGCGAACATTTGG | ACGTCCACCACCACCATTTGAAC | |
| ywhaz | TCTGCAATGATGTGTTGGAGC | TCAATGGTTGCTTTCTTGTCGTC | [41] |
| rpl13a | TCTGGAGGACTGTAAGAGGTATGC | AGACGCACAATCTTGAGAGCAG | |
| ef1a | CTGGAGGCCAGCTCAAACAT | ATCAAGAAGAGTAGTACCGCTAGCATTAC |
| Drug | LC50 Concentration | Locomotion | Global vDC DAnergic Loss (%) | Morphological Defects | Other Effects |
|---|---|---|---|---|---|
| MPTP | 0.25 mM | Severe | 39% | Cardiac and tail curvature (9/20) | Unresponsive to touch |
| MPP+ | 0.05 mM | Moderate | 35% | None of significance | Unresponsive to touch |
| Paraquat | 1 mM | None | 16% | Stunted development (3/20) | None of significance |
| 6-OHDA | 1 µM | None | 18% | Cardiac defect (2/20) | None of significance |
| Rotenone | 50 nM | Moderate | 36% | Cardiac defect (4/20) | None of significance |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalyn, M.; Hua, K.; Mohd Noor, S.; Wong, C.E.D.; Ekker, M. Comprehensive Analysis of Neurotoxin-Induced Ablation of Dopaminergic Neurons in Zebrafish Larvae. Biomedicines 2020, 8, 1. https://doi.org/10.3390/biomedicines8010001
Kalyn M, Hua K, Mohd Noor S, Wong CED, Ekker M. Comprehensive Analysis of Neurotoxin-Induced Ablation of Dopaminergic Neurons in Zebrafish Larvae. Biomedicines. 2020; 8(1):1. https://doi.org/10.3390/biomedicines8010001
Chicago/Turabian StyleKalyn, Michael, Khang Hua, Suzita Mohd Noor, Chee Ern David Wong, and Marc Ekker. 2020. "Comprehensive Analysis of Neurotoxin-Induced Ablation of Dopaminergic Neurons in Zebrafish Larvae" Biomedicines 8, no. 1: 1. https://doi.org/10.3390/biomedicines8010001
APA StyleKalyn, M., Hua, K., Mohd Noor, S., Wong, C. E. D., & Ekker, M. (2020). Comprehensive Analysis of Neurotoxin-Induced Ablation of Dopaminergic Neurons in Zebrafish Larvae. Biomedicines, 8(1), 1. https://doi.org/10.3390/biomedicines8010001





