Low High-Density Lipoprotein Cholesterol Predisposes to Coronary Artery Ectasia
Abstract
1. Introduction
2. Experimental Section
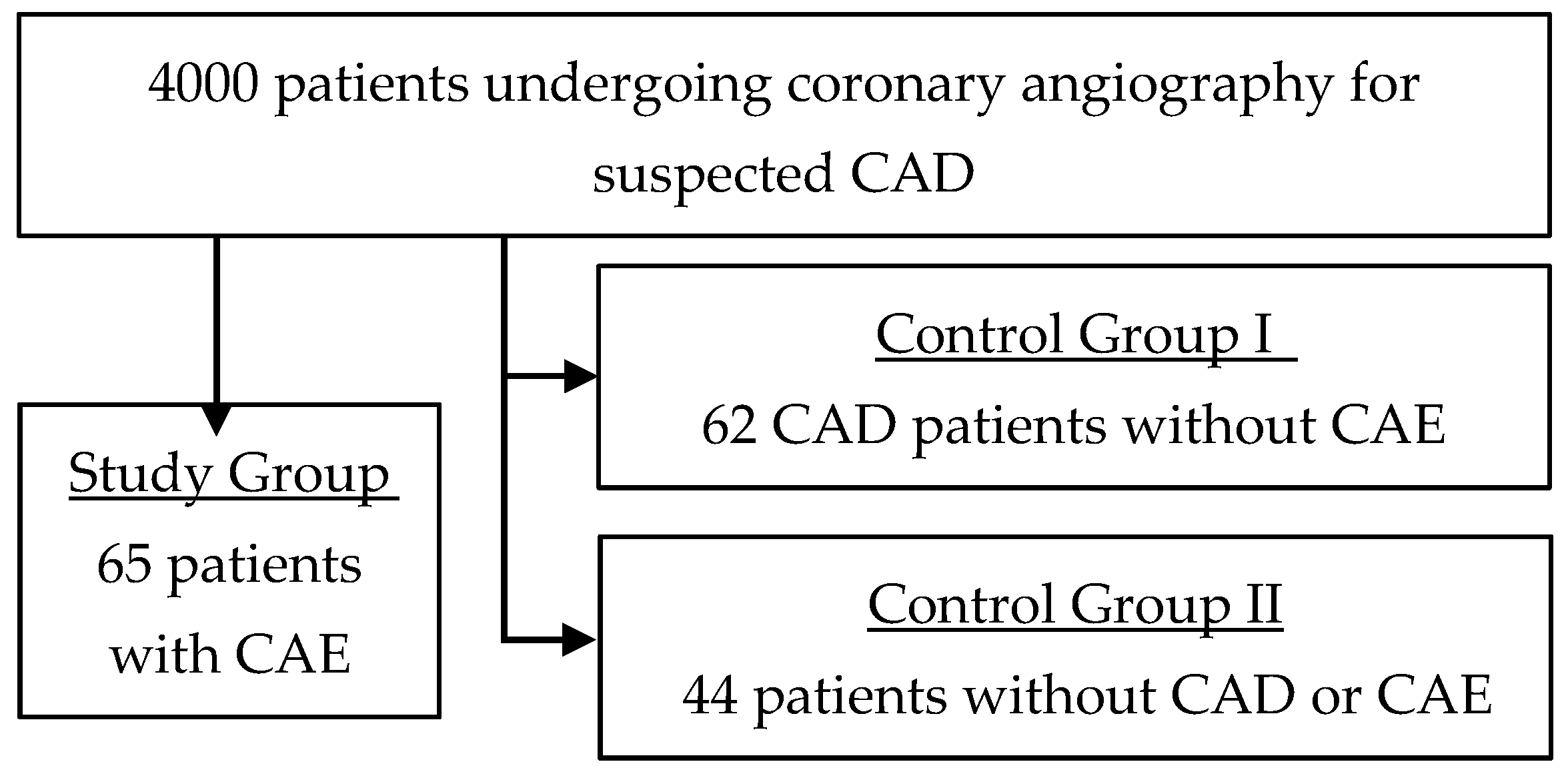
2.1. Study Population
2.2. Data Collection
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Coronary artery surgery study (CASS): A randomized trial of coronary artery bypass surgery. Survival data. Circulation 1983, 68, 939–950. [CrossRef] [PubMed]
- Sharma, S.N.; Kaul, U.; Sharma, S.; Wasir, H.S.; Manchanda, S.C.; Bahl, V.K.; Talwar, K.K.; Rajani, M.; Bhatia, M.L. Coronary arteriographic profile in young and old Indian patients with ischaemic heart disease: A comparative study. Indian Heart J. 1990, 42, 365–369. [Google Scholar] [PubMed]
- Hartnell, G.G.; Parnell, B.M.; Pridie, R.B. Coronary artery ectasia. Its prevalence and clinical significance in 4993 patients. Heart 1985, 54, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Antoniadis, A.P.; Chatzizisis, Y.S.; Giannoglou, G.D. Pathogenetic mechanisms of coronary ectasia. Int. J. Cardiol. 2008, 130, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Doi, T.; Kataoka, Y.; Noguchi, T.; Shibata, T.; Nakashima, T.; Kawakami, S.; Nakao, K.; Fujino, M.; Nagai, T.; Kanaya, T.; et al. Coronary Artery Ectasia Predicts Future Cardiac Events in Patients with Acute Myocardial Infarction. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 2350–2355. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, C.; Liu, W. Coronary artery ectasia presenting with thrombus embolization and acute myocardial infarction. Medicine (Baltimore) 2017, 96, e5976. [Google Scholar] [CrossRef] [PubMed]
- Baman, T.S.; Cole, J.H.; Devireddy, C.M.; Sperling, L.S. Risk factors and outcomes in patients with coronary artery aneurysms. Am. J. Cardiol. 2004, 93, 1549–1551. [Google Scholar] [CrossRef] [PubMed]
- Sudhir, K.; Ports, T.A.; Amidon, T.M.; Goldberger, J.J.; Bhushan, V.; Kane, J.P.; Yock, P.; Malloy, M.J. Increased Prevalence of Coronary Ectasia in Heterozygous Familial Hypercholesterolemia. Circulation 1995, 91, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Markis, J.E.; Joffe, C.D.; Cohn, P.F.; Feen, D.J.; Herman, M.V.; Gorlin, R. Clinical significance of coronary arterial ectasia. Am. J. Cardiol. 1976, 37, 217–222. [Google Scholar] [CrossRef]
- Qin, Y.; Tang, C.; Ma, C.; Yan, G. Risk factors for coronary artery ectasia and the relationship between hyperlipidemia and coronary artery ectasia. Coron. Artery. Dis. 2019, 30, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Abou Sherif, S.; Ozden Tok, O.; Taşköylü, Ö.; Goktekin, O.; Kilic, I.D. Coronary Artery Aneurysms: A Review of the Epidemiology, Pathophysiology, Diagnosis, and Treatment. Front. Cardiovasc. Med. 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Junker, R.; Heinrich, J.; Ulbrich, H.; Schulte, H.; Schönfeld, R.; Köhler, E.; Assmann, G. Relationship Between Plasma Viscosity and the Severity of Coronary Heart Disease. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 870–875. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liang, S.; Zhang, Y.; Gao, X.; Zhao, H.; Di, B.; Sheng, Q.; Liu, R. Is Coronary Artery Ectasia a Thrombotic Disease? Angiology 2019, 70, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Dahhan, A. Coronary Artery Ectasia in Atherosclerotic Coronary Artery Disease, Inflammatory Disorders, and Sickle Cell Disease. Cardiovasc. Ther. 2015, 33, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Aksu, T.; Uygur, B.; Kosar, M.D.; Guray, U.; Arat, N.; Korkmaz, S.; Colak, A. Coronary artery ectasia: Its frequency and relationship with atherosclerotic risk factors in patients undergoing cardiac catheterization. Anadolu. Kardiyol. Dergisi/Anatol. J. Cardiol. 2011, 11, 280–285. [Google Scholar] [CrossRef] [PubMed]
| Variable | Ectatic (n = 65) | Obstructive (n = 62) | Control (n = 44) | p-Value |
|---|---|---|---|---|
| Patient Characteristics | ||||
| Height, cm | 175.64 ± 6.62 | 170.32 ± 9.90 | 169.61 ± 10.34 | 0.04 |
| Weight, kg | 89.35 ± 12.97 | 86.14 ± 17.19 | 81.25 ± 19.24 | 0.17 |
| BMI | 28.96 ± 3.82 | 29.62 ± 4.92 | 28.22 ± 6.30 | 0.49 |
| Comorbidities | ||||
| Stable angina, n (%) | 5 (7.7) | 2 (3.2) | 10 (22.7) | 0.003 |
| Silent ischemia, n (%) | 3 (4.6) | 0 (0.0) | 2 (4.5) | 0.23 |
| Unstable angina, n (%) | 28 (43.1) | 18 (29.0) | 29 (65.9) | 0.001 |
| NSTEMI, n (%) | 13 (20.0) | 18 (29.0) | 4 (9.1) | 0.04 |
| STEMI, n (%) | 22 (33.8) | 29 (46.8) | 8 (18.2) | 0.001 |
| Hypertension, n (%) | 51 (78.5) | 48 (77.4) | 29 (69.0) | 0.50 |
| Diabetes, n (%) | 20 (30.8) | 18 (29.0) | 15 (35.7) | 0.76 |
| Dyslipidemia, n (%) | 54 (83.1) | 55 (88.7) | 34 (81.0) | 0.50 |
| Smoker, n (%) | 35 (53.8) | 37 (59.7) | 18 (42.9) | 0.23 |
| Prior of angina, n (%) | 2 (3.1) | 4 (6.5) | 1 (2.4) | 0.51 |
| Prior typical/atypical chest pain, n (%) | 1 (1.5) | 1 (1.6) | 2 (4.8) | 0.50 |
| Prior CAD, n (%) | 23 (35.4%) | 26 (41.9) | 7 (16.7) | 0.02 |
| Pharmacological Treatment | ||||
| Statins, n (%) | 51 (81.0) | 58 (96.7) | 27 (79.4) | 0.014 |
| Diuretics, n (%) | 15 (23.8) | 15 (25.0) | 9 (26.5) | 0.95 |
| Insulin, n (%) | 1 (1.6) | 1 (1.7) | 2 (5.9) | 0.37 |
| ACEi or ARB, n (%) | 39 (61.9) | 45 (75.0) | 20 (58.8) | 0.18 |
| Aspirin, n (%) | 55 (87.3) | 60 (100) | 23 (67.6) | 0.001 |
| CCB, n (%) | 6 (9.5) | 12 (20.0) | 8 (23.5) | 0.13 |
| Nitrates, n (%) | 7 (11.1) | 4 (6.7) | 2 (5.9) | 0.56 |
| Oral hypoglycaemic, n (%) | 9 (14.3) | 10 (16.7) | 9 (26.5) | 0.31 |
| Variable | Ectatic (n = 65) | Obstructive (n = 62) | Control (n = 44) | p-Value |
|---|---|---|---|---|
| Total cholesterol, mg/dL | 185.14 ± 55.44 | 185.51 ± 54.55 | 190.39 ± 37.76 | 0.85 |
| Triglyceride, mg/dL | 155.89 ± 87.07 | 184.26 ± 128.43 | 159.69 ± 114.34 | 0.34 |
| HDL-C, mg/dL | 40.13 ± 10.15 | 38.67 ± 10.16 | 45.52 ± 11.97 | 0.007 |
| LDL-C, mg/dL | 116.61 ± 51.86 | 110.37 ± 44.03 | 117.86 ± 32.66 | 0.67 |
| Total cholesterol/HDL-C ratio | 4.76 ± 1.2 | 4.92 ± 1.11 | 4.15 ± 0.77 | 0.001 |
| Triglyceride/HDL-C ratio | 3.78 ± 2.24 | 4.67 ± 2.65 | 3.43 ± 2.76 | 0.03 |
| LDL-C/HDL-C ratio | 3.02 ± 1.76 | 2.87 ± 1.53 | 2.66 ± 1.03 | 0.001 |
| Hemoglobin, g/dL | 14.09 ± 1.76 | 13.37 ± 1.57 | 13.49 ± 1.63 | 0.049 |
| Hematocrit (%) | 41.38 ± 5.00 | 39.33 ± 4.45 | 39.67 ± 4.35 | 0.04 |
| Creatinine, mg/dL | 1.20 ± 0.80 | 1.12 ± 0.63 | 1.23 ± 0.88 | 0.74 |
| Variable | Ectatic (n = 65) | Obstructive (n = 62) | Control (n = 44) | p-Value | |
|---|---|---|---|---|---|
| Rest LV function, n (%) | Normal | 28 (51.9) | 25 (51.0) | 27 (75.0) | 0.09 |
| Mild | 5 (9.3) | 10 (20.4) | 3 (8.3) | ||
| Moderate | 10 (18.5) | 9 (18.4) | 4 (11.1) | ||
| Severe | 11 (20.4) | 5 (10.2) | 2 (5.6) | ||
| Ejection fraction, mean ± SD | 48.55 ± 14.25 | 50.01 ± 11.28 | 55.94 ± 11.44 | 0.02 | |
| LV hypertrophy, n (%) | 20 (38.5) | 19 (33.3) | 9 (23.7) | 0.33 | |
| Mitral regurgitation, n (%) | Mild | 47 (87.0) | 58 (93.5) | 35 (92.1) | 0.45 |
| Moderate | 7 (13.0) | 4 (6.5) | 3 (7.9) | ||
| Aortic stenosis, n (%) | Mild | 51 (94.4) | 59 (95.2) | 36 (97.3) | 0.80 |
| Moderate | 3 (5.6) | 3 (4.8) | 1 (2.7) | ||
| Tricuspid regurgitation, n (%) | Mild | 51 (96.2) | 59 (95.2) | 37 (97.4) | 0.85 |
| Moderate | 2 (3.8) | 3 (4.8) | 1 (2.6) | ||
| Pulmonary pressure (mmHg), mean ± SD | 25.48 ± 6.54 | 25.86 ± 11.07 | 23.96 ± 7.08 | 0.63 | |
| ECG pathological findings, n (%) | 21 (35.6) | 4 (7.4) | 5 (11.9) | <0.001 | |
| Variable | p-Value | OR | 95% CI | |
|---|---|---|---|---|
| Lower | Upper | |||
| LDL-C/HDL-C ratio | 0.034 | 1.987 | 1.542 | 2.882 |
| HDL-C, mg/dL | 0.029 | 0.858 | 0.749 | 0.984 |
| Hemoglobin, g/dL | 0.021 | 2.060 | 1.114 | 3.809 |
| Statin use | 0.060 | 16.562 | 0.890 | 308.351 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jafari, J.; Daum, A.; Hamed, J.A.; Osherov, A.; Orlov, Y.; Yosefy, C.; Gallego-Colon, E. Low High-Density Lipoprotein Cholesterol Predisposes to Coronary Artery Ectasia. Biomedicines 2019, 7, 79. https://doi.org/10.3390/biomedicines7040079
Jafari J, Daum A, Hamed JA, Osherov A, Orlov Y, Yosefy C, Gallego-Colon E. Low High-Density Lipoprotein Cholesterol Predisposes to Coronary Artery Ectasia. Biomedicines. 2019; 7(4):79. https://doi.org/10.3390/biomedicines7040079
Chicago/Turabian StyleJafari, Jamal, Aner Daum, Jihad Abu Hamed, Azriel Osherov, Yan Orlov, Chaim Yosefy, and Enrique Gallego-Colon. 2019. "Low High-Density Lipoprotein Cholesterol Predisposes to Coronary Artery Ectasia" Biomedicines 7, no. 4: 79. https://doi.org/10.3390/biomedicines7040079
APA StyleJafari, J., Daum, A., Hamed, J. A., Osherov, A., Orlov, Y., Yosefy, C., & Gallego-Colon, E. (2019). Low High-Density Lipoprotein Cholesterol Predisposes to Coronary Artery Ectasia. Biomedicines, 7(4), 79. https://doi.org/10.3390/biomedicines7040079






