Biofunctional Textiles for Aging Skin
Abstract
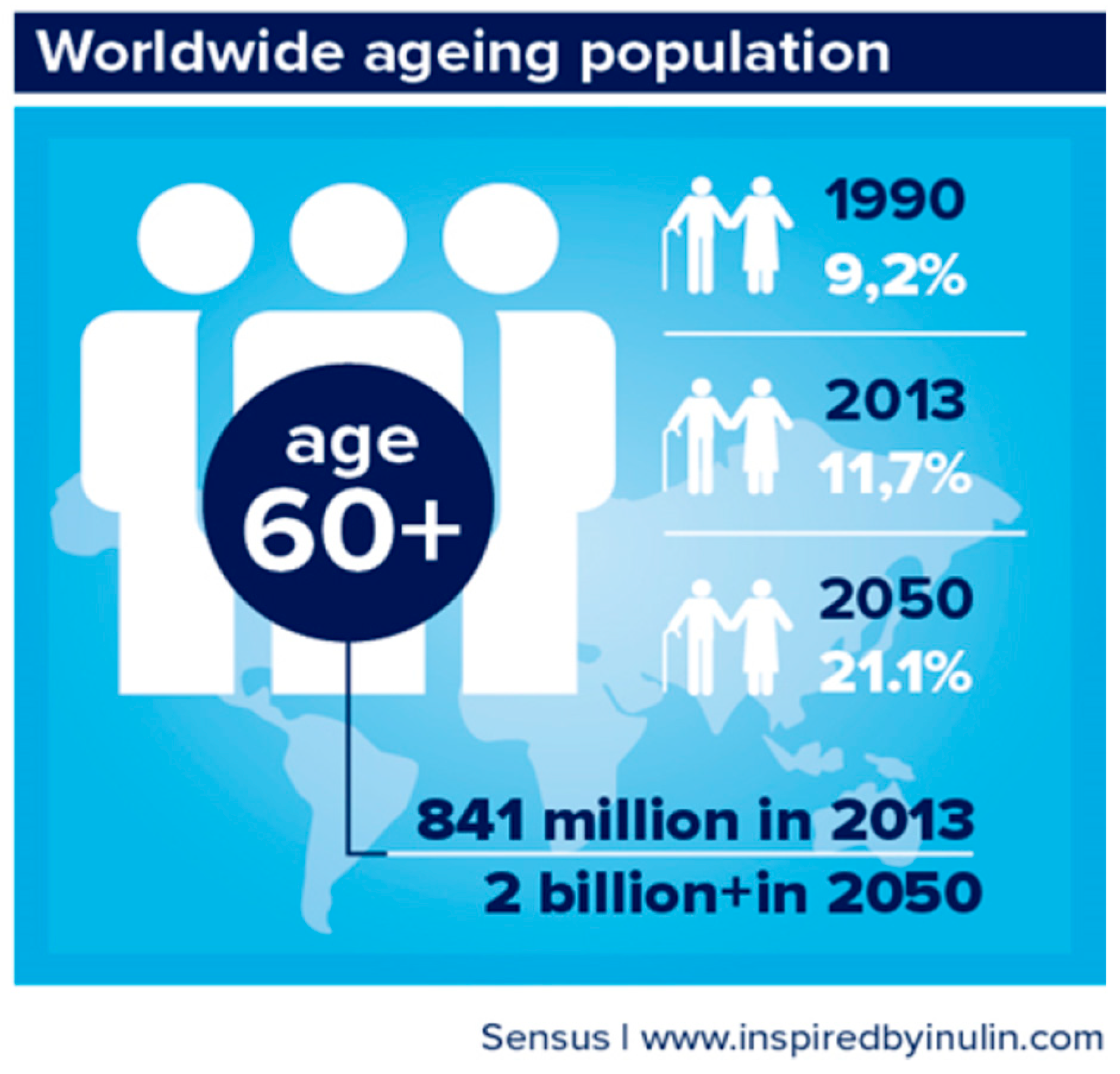
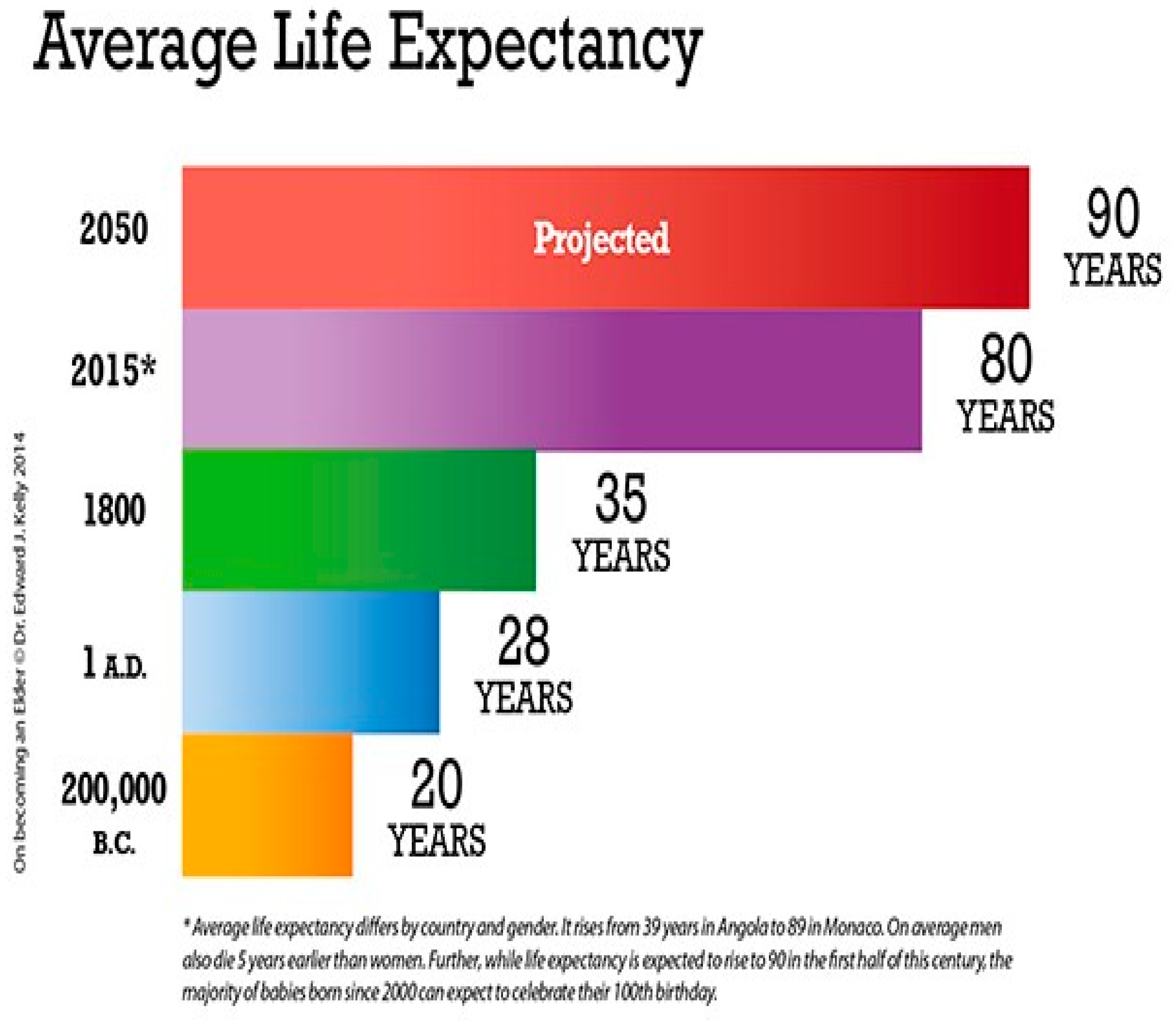
1. Introduction
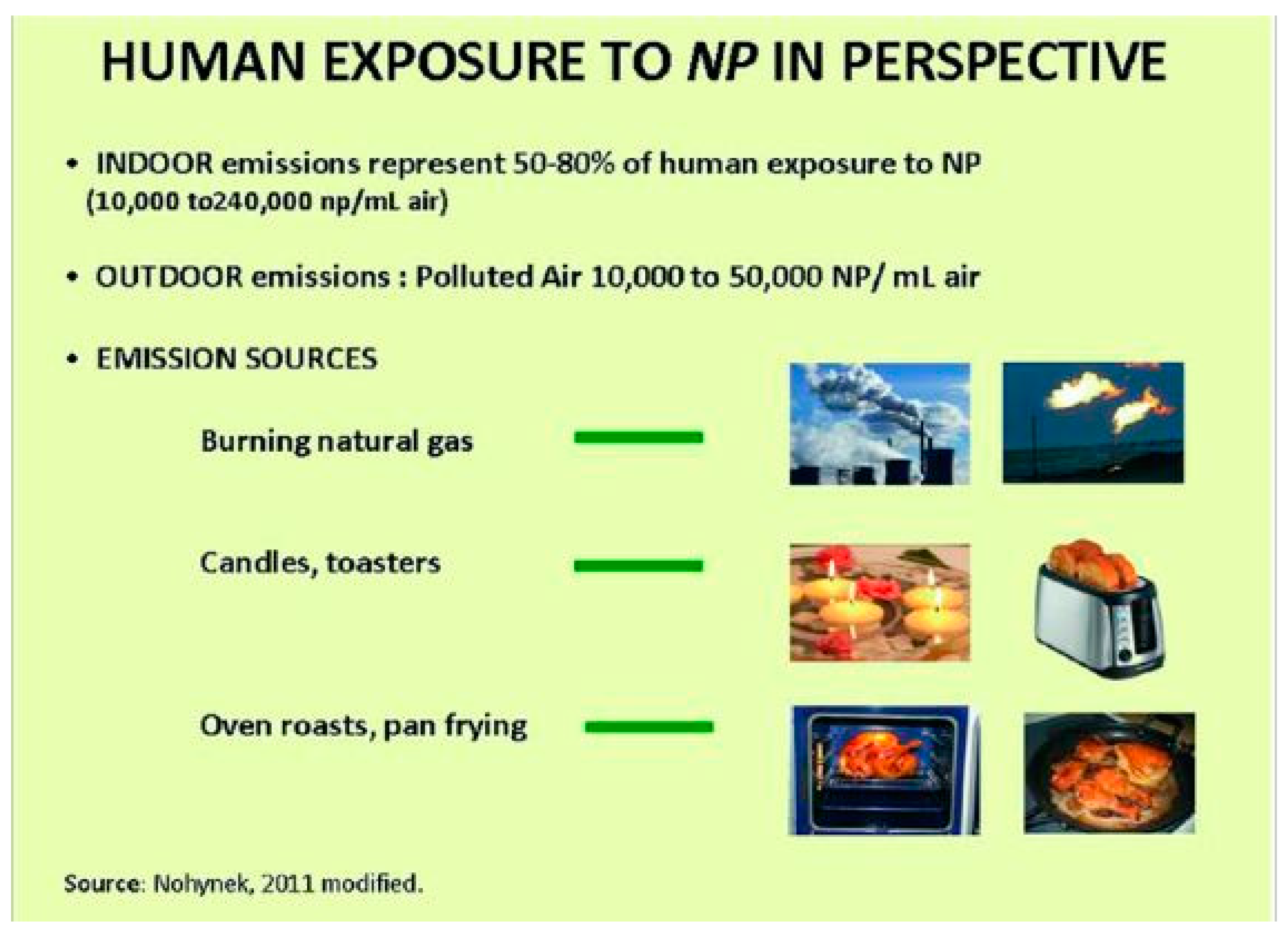
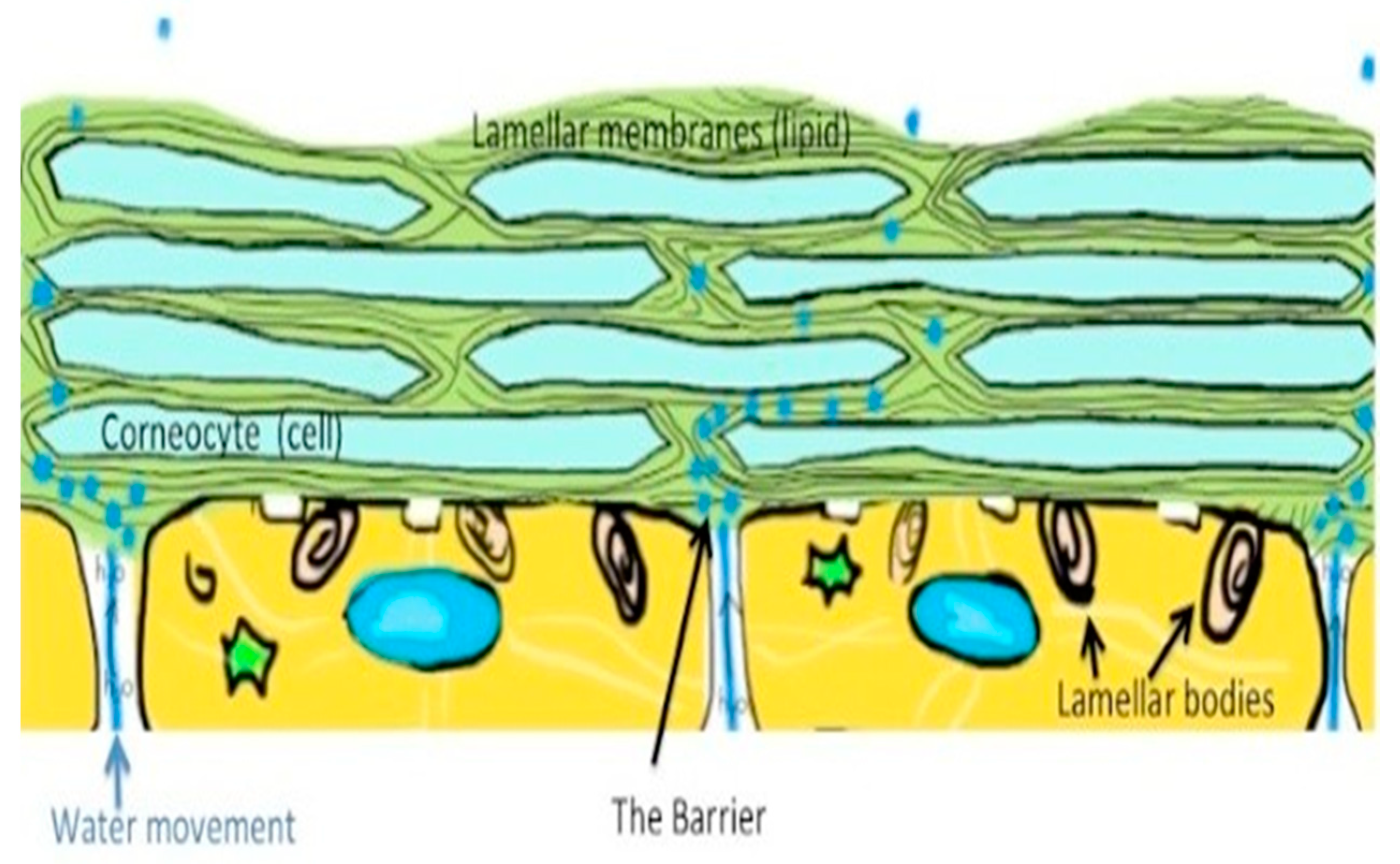
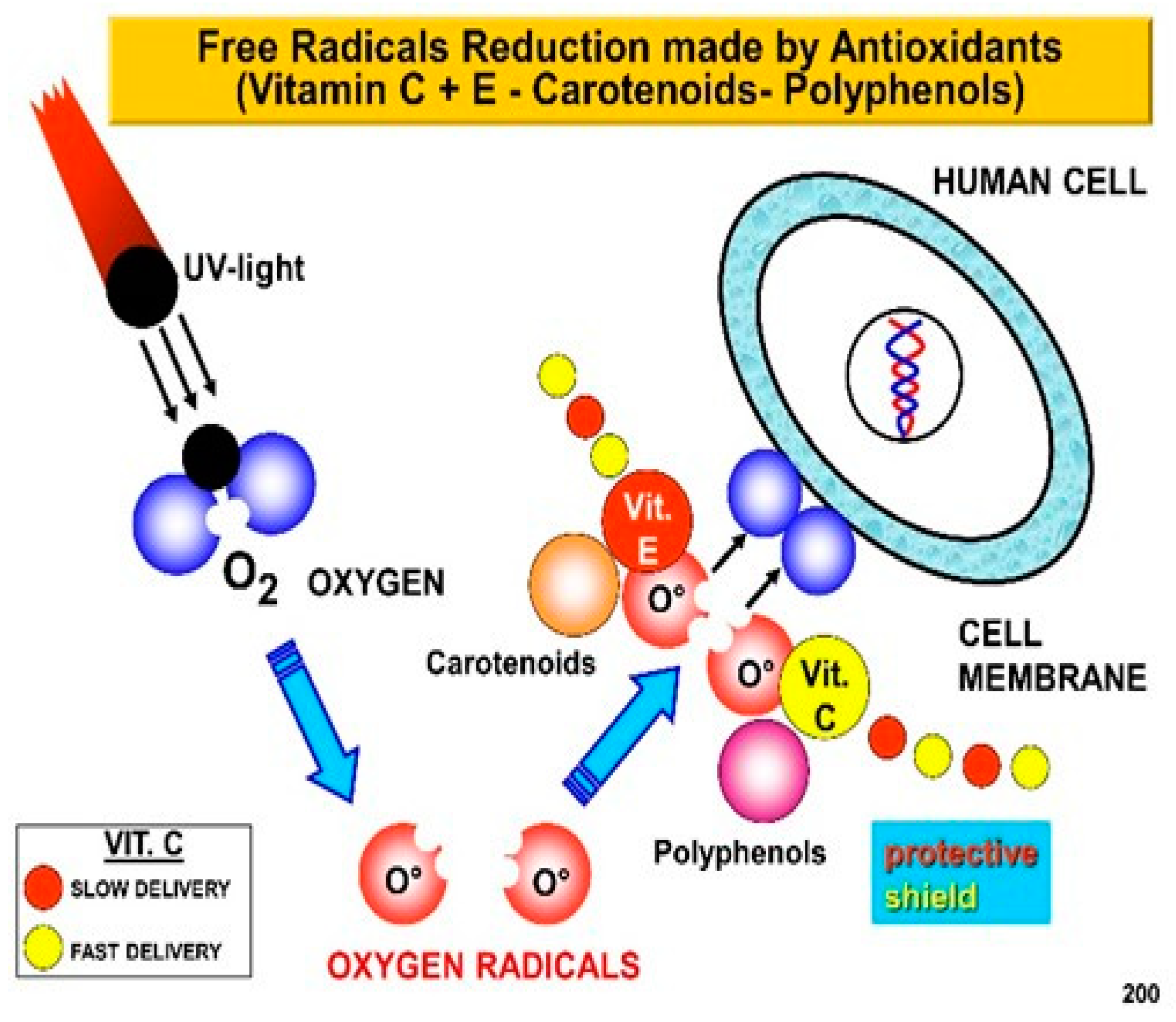
2. Skin
3. Skin Dressing
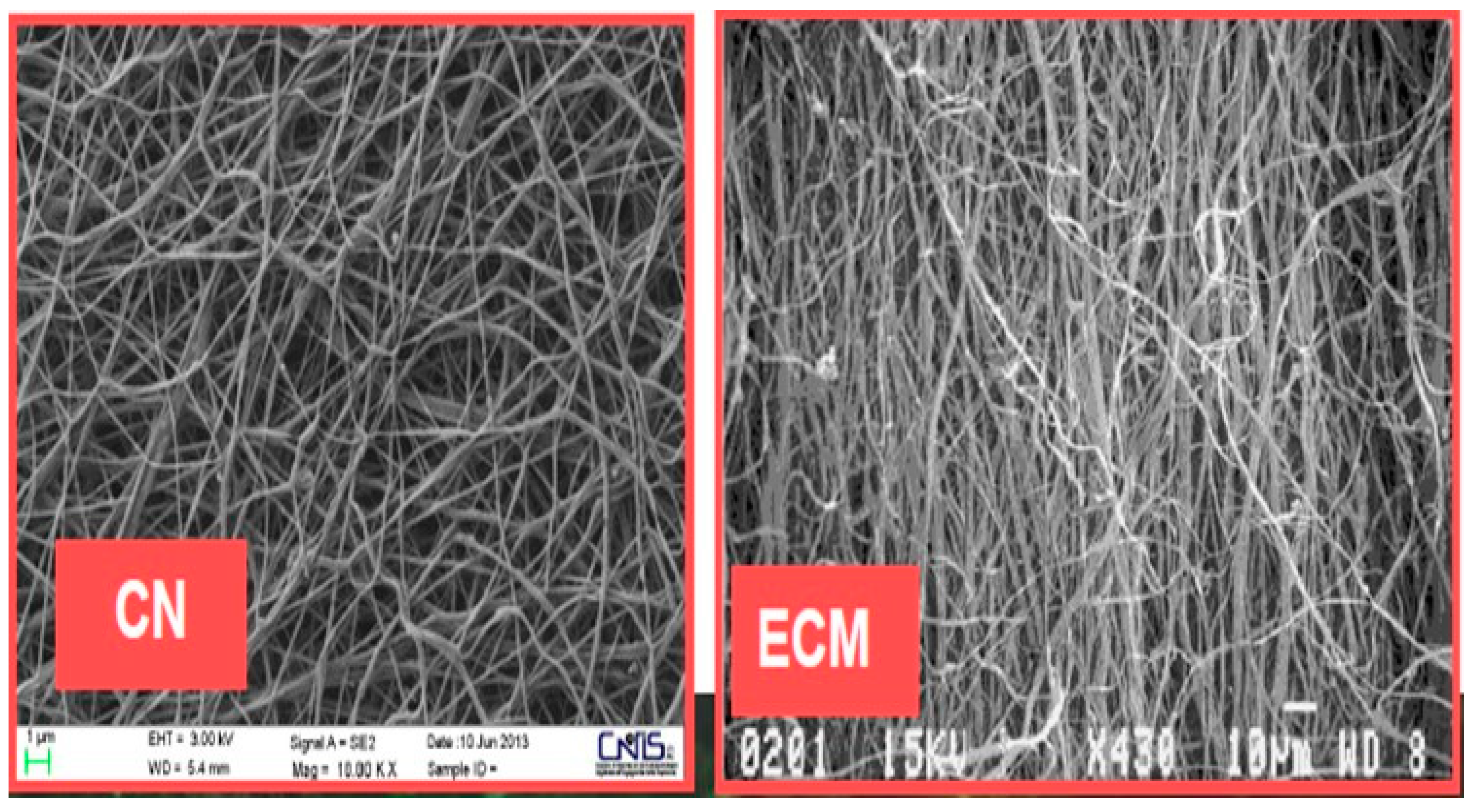
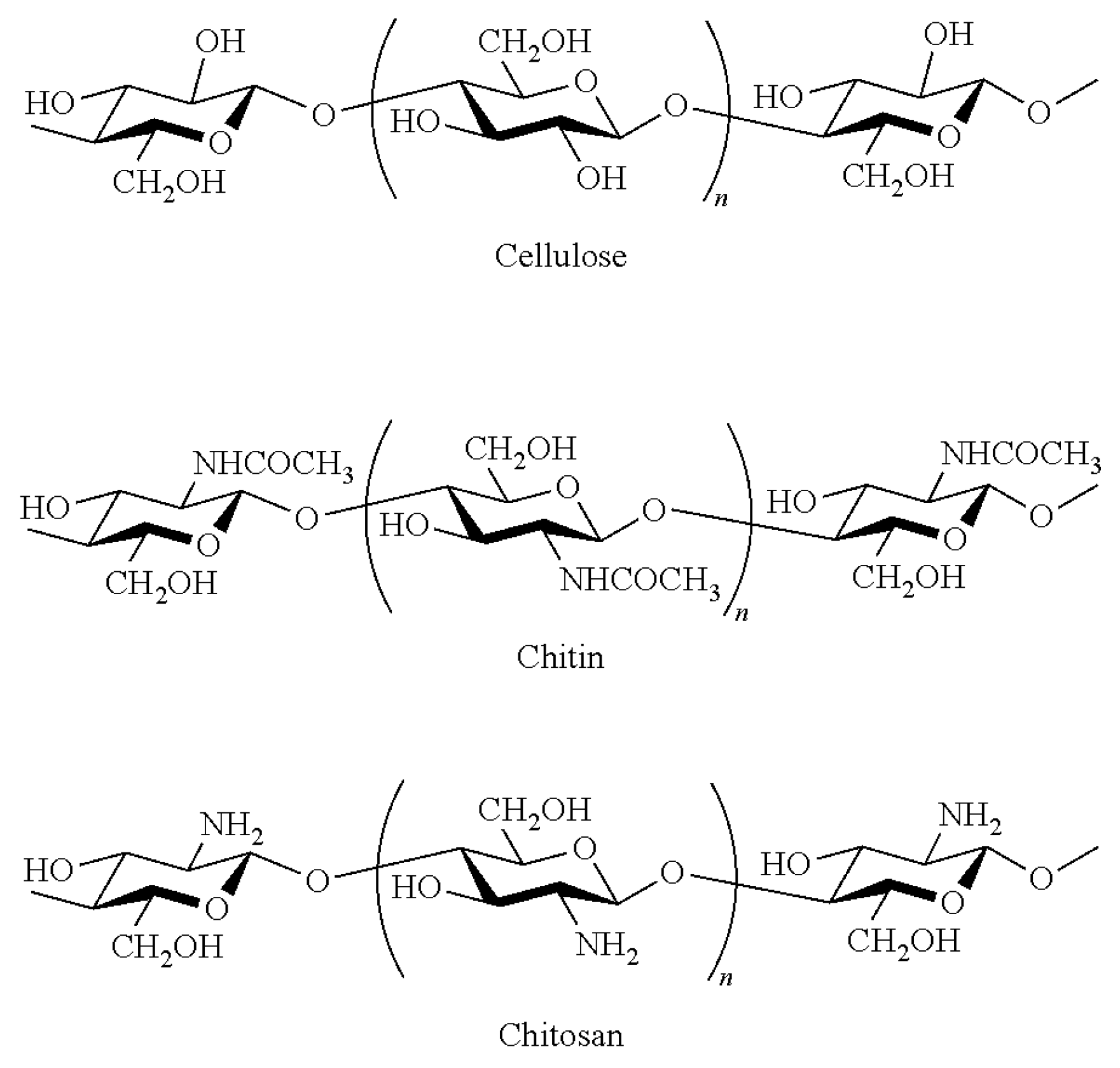
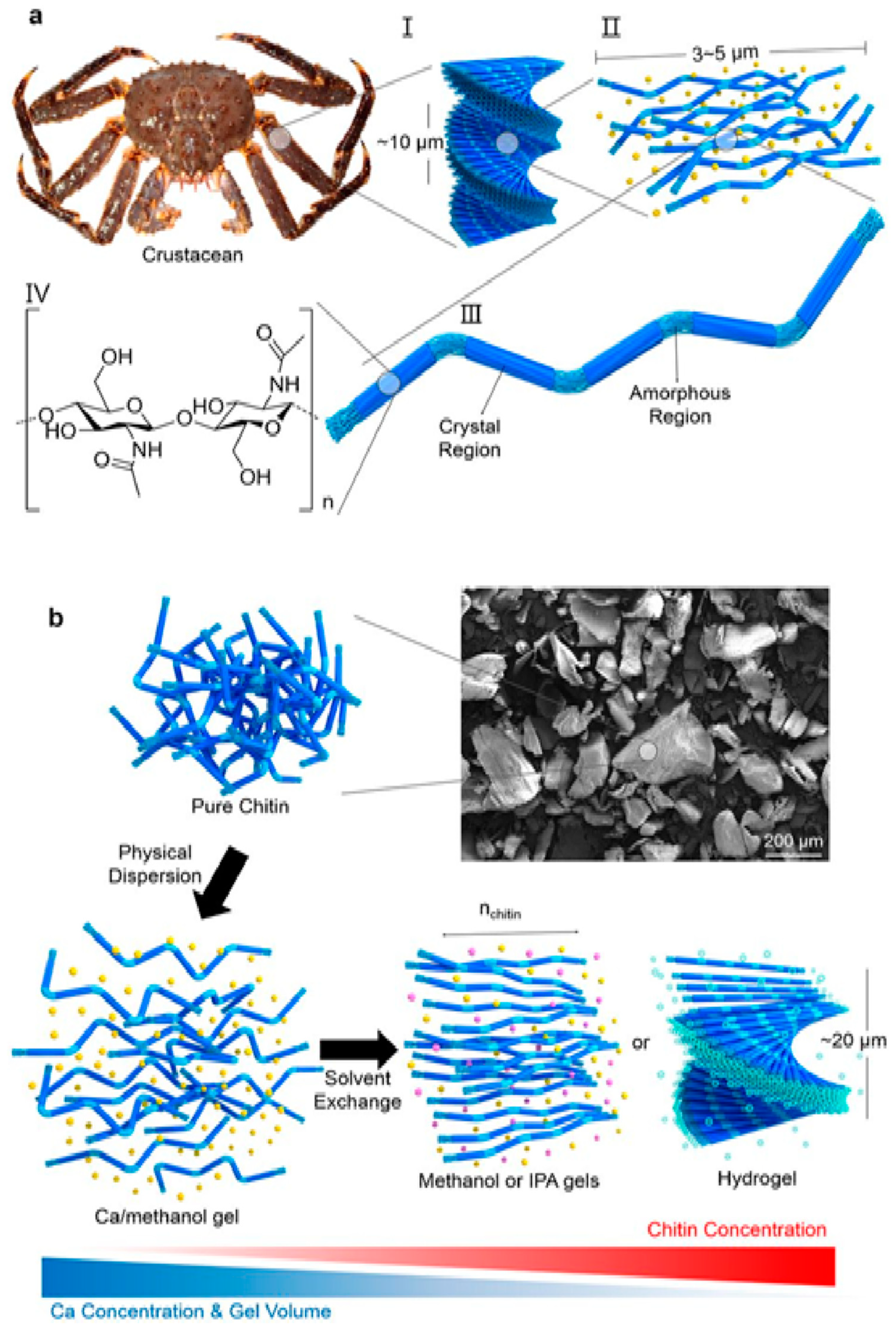
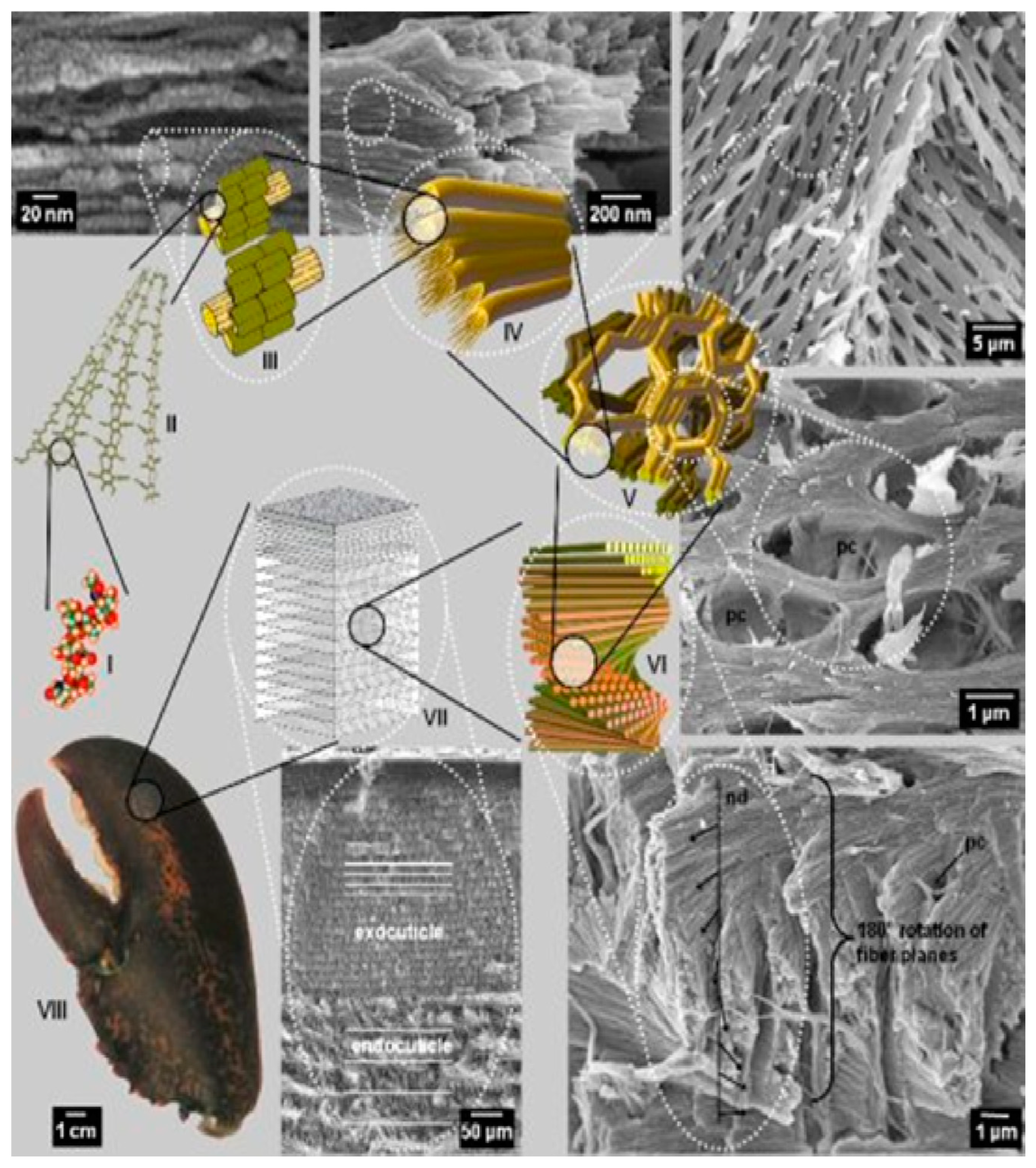
4. Chitin Nano-Fibrils
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- UN. The World Population Prospects: 2015 Revision; United Nations: New York, NY, USA, 2016; Available online: https://www.un.org/en/development/desa/publications/world-population-prospects-2015-revision.html (accessed on 11 April 2019).
- Zuo, W.; Jiang, S.; Guo, Z.; Feldman, M.W.; Tuljapurkar, S. Advancing front of old-age human survival. Proc. Natl. Acad. Sci. USA 2018, 115, 11209–11214. [Google Scholar] [CrossRef]
- Kligman, A.M.; Graham, J.A. Cosmetic Make-Over in Elderly Women. In A New Look at the Old Skin: A Challenge to Cosmetology; Morganti, P., Montagna, W., Eds.; International Ediemme: Rome, Italy, 1986; pp. 197–201. [Google Scholar]
- Jacinto, A.; Martinez-Arias, A.; Martin, P. Mechanism of Epithelial fusion and repair. Nat. Cell Biol. 2001, 3, E117–E123. [Google Scholar] [CrossRef] [PubMed]
- Dabrowska, A.K.; Spano, F.; Derler, S.; Adlhart, C.; Spencer, N.D. The relationship between skin function barrier properties and body-dependent factors. Skin Res. Technol. 2018, 24, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Athar, M.; Agarwal, R.; Bichers, D.R.; Mukhtar, H. Role of Reactive Oxygen Species in Skin. In Pharmacology of Skin; Mukhtar, H., Ed.; CRC Press: Boca Raton, FL, USA, 1992; pp. 269–279. [Google Scholar]
- Elias, P.M.; Menon, G.K. Structural and lipid biochemical correlates of the epidermal permeability barrier. Adv. Lipid Res. 1991, 24, 1–260000. [Google Scholar]
- Rinnerthaler, M.; Duschl, J.; Steinbacher, P.; Salzmann, M.; Bischof, J.; Schuller, M.; Wimmer, H.; Peer, T.; Bauer, J.M.; Richter, K. Age-related changes in the composition of the cornfield envelope in human skin. Exp. Dermatol. 2013, 22, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Kramer, U.; Schikowski, T. Recent Demographic Changes and Consequences for Dermatology. In Skin Aging; Gilchrest, B.A., Krutmann, J., Eds.; Springer: Berlin, Germany, 2006; pp. 1–8. [Google Scholar]
- Thiele, J.; Barland, C.O.; Ghadially, R.; Elias, P.M. Permeability and Antioxidant Barriers in Aged Epidermis. In Skin Aging; Gilchrest, B.A., Krutmann, J., Eds.; Springer: Berlin, Germany, 2006; pp. 65–79. [Google Scholar]
- Palombo, P.; Fabrizi, G.; Ruocco, V.; Ruocco, M.; Fluhr, J.; Roberts, R.; Morganti, P. Beneficial long-term effects of combined oral/topical antioxidant treatment with carotenoids Lutein and zeaxanthjn on human skin: A doubleblinded, placebo-controlled study in humans. Skin Pharmacol. Physiol. 2007, 20, 199–210. [Google Scholar] [CrossRef]
- Biagini, G.; Zizzi, A.; Giantomassi, F.; Orlando, F.; Lucorini, G.; Mattioli-Belmonte, M.; Tucci, M.G.; Morganti, P. Cutaneous Absorption of Nanostructurated Chitin Associated with Natural sinergie tic Molecules (Lutein). J. Appl. Cosmetol. 2008, 26, 69–80. [Google Scholar]
- Rosen, M. Delivery System Handbook for Personal Care and Cosmetic Products, 1st ed.; Rosen, M., Ed.; Elsevier: New York, NY, USA, 2005. [Google Scholar]
- Morganti, P. Use of Chitin Nanofibriks from Biomass for an Innovative Bioeconomy. In Nanofabrication Using Nanomaterials; Ebothe, J., Ahmed, W., Eds.; One Central Press: Manchester, UK, 2016; pp. 1–22. [Google Scholar]
- Morganti, P.; Carezzi, F.; Del Ciotto, P.; Morganti, G.; Nunziata, M.L.; Gao, X.H.; Chen, H.-D.; Tischenko, G.; Yudin, V.E. Chitin Nanofibrils: A Natural Multifunctional Polymer—Physicochemical characteristics, effectiveness and safeness. In Nanobiotechnology; Phoenix, D.A., Ahmed, W., Eds.; One Central Press: Manchester, UK, 2014; pp. 1–31. [Google Scholar]
- Mishra, A.K. Nanomedicine for Drug Delivery and Therapeutics; Scrivener Publishing and John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Kim, Y.; Ko, H.; Kwon, I.K.; Shin, K. Extracellular Matrix Revisited: Roles in Tissue Engineering. Int. Neurourol. J. 2016, 20 (Suppl. S1), S23–S29. [Google Scholar] [CrossRef]
- Garg, T.; Singh, O.; Arora, S.; Murthy, R. Scaffold: A novel carrier for cell and drug delivery. Crit. Rev. Ther. Drug Carrier Syst. 2010, 29, 1–63. [Google Scholar] [CrossRef]
- Morganti, P.; Febo, P. Problem & Solution for Biodegrading Baby Diapers. Eurocosmetics 2017, 24, 44–46. [Google Scholar]
- Morganti, P.; Coltelli, M.B.; Danti, S.; Bugnicourt, E. The skin: Goal of the EU PolyBioSkin project. Glob. Res. J. Pharm. Pharmacol. 2017, 2, 7–13. [Google Scholar]
- Morganti, P. Biodegradable polymers for a better future. J. Appl. Cosmetol. 2017, 35, 35–40. [Google Scholar]
- Morganti, P.; Coltelli, M.B.; Danti, S. Biobased tissues for innovative cosmetic products: PolyBioSkin asan EU Research Project. Glob. J. Nanomed. 2018, 3, 1–6. [Google Scholar]
- Morganti, P.; Coltelli, M.B.; Danti, S. Tessuti naturali per cosmetici innovativi: Un progetto di ricerca europeo. ICF 2018, 9, 38–43. [Google Scholar]
- Morganti, P. Natural Products Work in Multiple Ways. In Nutritional Cosmetics; Tabor, A., Blair, R., Eds.; William Andrew Publisher: Oxford, UK, 2009. [Google Scholar]
- Synowiecki, J.; Al-Khateeb, N.A. Production, properties, and some new applications of chitin and its Derivatives. Crit. Rev. Food Sci. Nutr. 2003, 43, 145–171. [Google Scholar] [CrossRef] [PubMed]
- Roberts, G. Chitin Chemistry; MacMillan: London, UK, 1998. [Google Scholar]
- Nishino, T.; Matsui, R.; Nakamae, K. Elastic Modulus of the Crystalline Regions of Chitin and Chitosan. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 1191–1196. [Google Scholar] [CrossRef]
- Tao, Q.; Devendra, V.; Milad, A.; Vikas, T. Influencr of Interfacial Interactions on Deformation Mechanisms and Interfacial Viscosity in alpha-Chitin-calcite Interfaces. Acta Biomater. 2015, 25, 325–338. [Google Scholar] [CrossRef]
- Oh, D.X.; Cha, Y.J.; Nguyen, H.L.; Je, H.H.; Jho, Y.S.; Hwang, D.S.; Yoon, D.K. Chiral nematic self-assembly of minimally surface damaged chitin Nanofibrils and its load bearing functions. Sci. Rep. 2006, 6, 23245. [Google Scholar] [CrossRef]
- Fabrius, H.O.; Sachs, C.; Triguero, R.P.; Raabe, D. Chitin-based ultrastructure influence of structural principles on the mechanics of a biological fiber-based composite material organization: The exoskeleton of the lobster Homarus americanus. Adv. Mater. 2009, 22, 391–400. [Google Scholar] [CrossRef]
- Ribadito, M. Chitin and Chitosan properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar]
- Mike’sovaa, J.; Haseka, J.; Tischenko, G.; Morganti, P. Rheological study of chitosan acetate solutions containing chitin Nanofibrils. Carbohydr. Polym. 2014, 32, 3–19. [Google Scholar]
- Yudin, V.E.; Dobrovolskaya, I.P.; Neelov, I.M.; Dresvyanina, E.N.; Poproyadukhin, P.V.; Ivan’kova, E.M.; Elokhovskii, V.Y.; Kasatkin, I.A.; Okrugin, B.M.; Morganti, P. Wet spinning of fibers made of chitosan and chitin Nanofibrils. Carbohydr. Polym. 2014, 108, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Tishchenko, G.; Morganti, P.; Stoller, M.; Kelnar, I.; Mikesova, J.; Kovarova, J.; Hasek, J.; Kuzel, R.; Netopilik, M.; Kapralkova, L.; et al. Chitin nanofibrils-Chitosan composite films: Characterization and properties. In Green Bio-Economy and Bionanotechnology for a More Sustainable Environment; Morganti, P., Ed.; MDPI: Basel, Switzerland, 2018. [Google Scholar]
- Aranaz, I.; Acosts, N.; Civera, C.; Elorza, B.; Mingo, J.; Castro, C.; de los Llanos Gandia, M.; Caballero, A.H. Cosmetics and Cosmeceuticals Appications of Chitin, Chitosan and Their Derivatives. Polymers 2018, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Morganti, P.; Palombo, M.; Carezzi, F.; Nunziata, M.L.; Morganti, G.; Cardillo, M.; Chianese, A. Green Nanotechnology Bioeconomy serving natural beauty masks to save the Environment. Cosmetics 2016, 3, 41. [Google Scholar] [CrossRef]
- Malafeev, K.V.; Moskalyuk, O.A.; Yudin, V.E.; Morganti, P.; Ivan’Kova, E.M.; Popova, E.N.; Elokhovskii, Y.U. Biodegradable polylactide/chitin composite fibers: Processing, structure, and mechanical properties. J. Appl. Cosmetol. 2017, 35, 163–173. [Google Scholar]
- Morganti, P.; Del Ciotto, P.; Stoller, M.; Chianese, A. Antibacterial and antiinflammatory Green Nanocomposites. Chem. Eng. Trans. 2016, 47, 62–66. [Google Scholar]
- Morganti, P.; Stoller, M. Chitin and Lignin: Natural Ingredients from Waste Materials to Make Innovative and Healthy Products for Humans and Plant. Chem. Eng. Trans. 2017, 60, 319–324. [Google Scholar]
- Morganti, P.; Fusco, A.; Paoletti, I.; Perfetto, B.; Del Ciotto, P.; Palombo, M.; Chianese, A.; Baronin, A.; Donnarumma, G. Antiinflammatory, Immunomodulatory, and Tissue Repair activity on Human Keratinocytes by a Green Innovative Nanocomposites. Materials 2017, 10, 843. [Google Scholar] [CrossRef]
- Morganti, P. Bionanotechnology to Save the Environment. Plant and Fishery’s Biomass as Alternative to Petrol; MDPI: Basel, Switzerland, 2019. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morganti, P.; Morganti, G.; Colao, C. Biofunctional Textiles for Aging Skin. Biomedicines 2019, 7, 51. https://doi.org/10.3390/biomedicines7030051
Morganti P, Morganti G, Colao C. Biofunctional Textiles for Aging Skin. Biomedicines. 2019; 7(3):51. https://doi.org/10.3390/biomedicines7030051
Chicago/Turabian StyleMorganti, Pierfrancesco, Gianluca Morganti, and Claudia Colao. 2019. "Biofunctional Textiles for Aging Skin" Biomedicines 7, no. 3: 51. https://doi.org/10.3390/biomedicines7030051
APA StyleMorganti, P., Morganti, G., & Colao, C. (2019). Biofunctional Textiles for Aging Skin. Biomedicines, 7(3), 51. https://doi.org/10.3390/biomedicines7030051




