A High-Throughput Assay for Congenital and Age-Related Eye Diseases in Zebrafish
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Animal Care
2.3. Microinjection
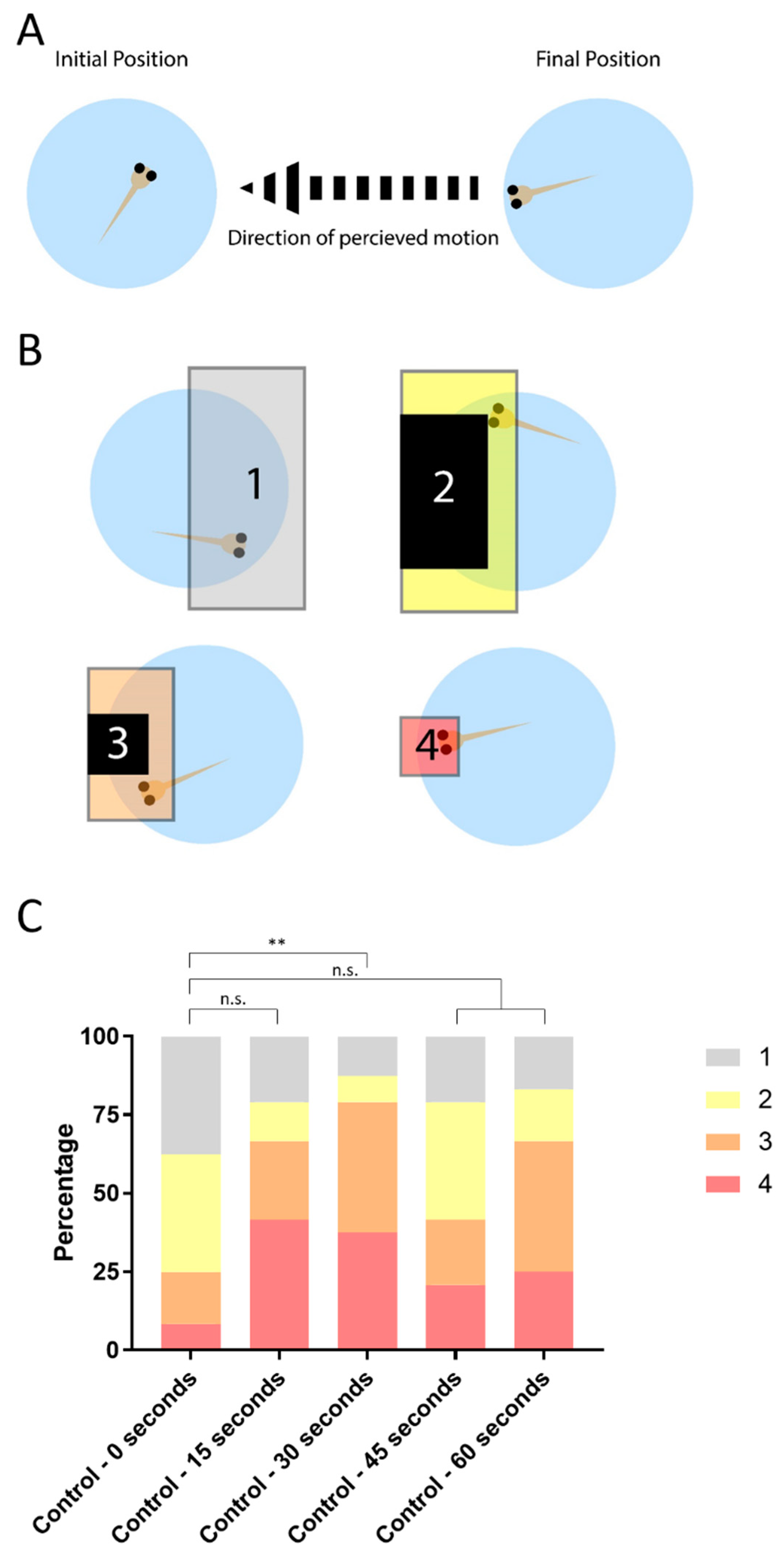
2.4. Automated Startle Response
2.5. OptoMotor Response Android Application
2.6. Statistical Analysis
2.7. Whole Mount In Situ Hybridization
2.8. Retinal Histology and Flourescent Microscopy
3. Results
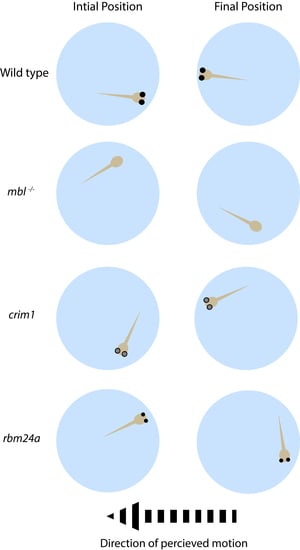
3.1. OptoMotor Assay for Visual Accuity
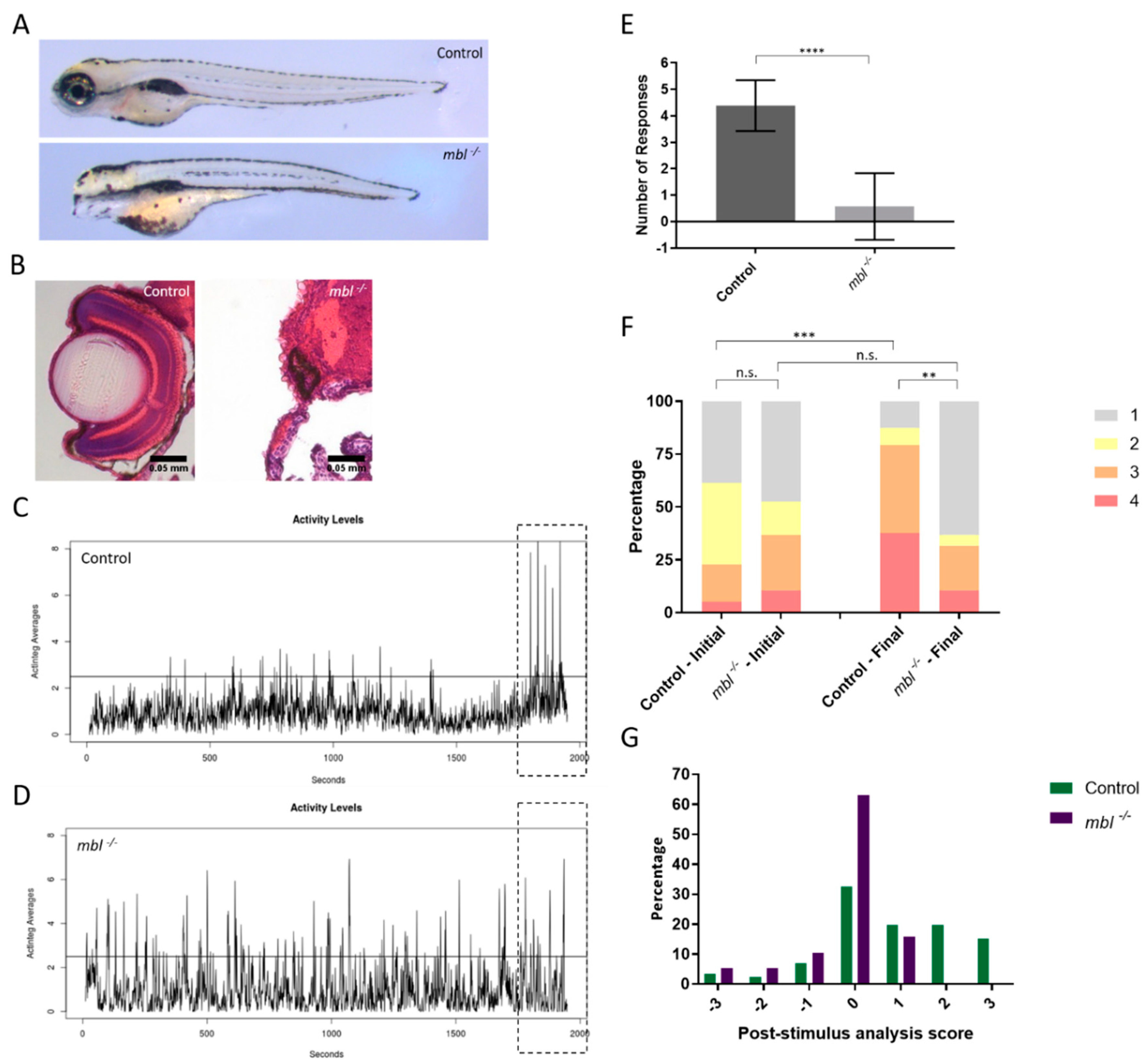
3.2. Establishing the Masterblind Line of Fish as a Negative Control in Visual Studies
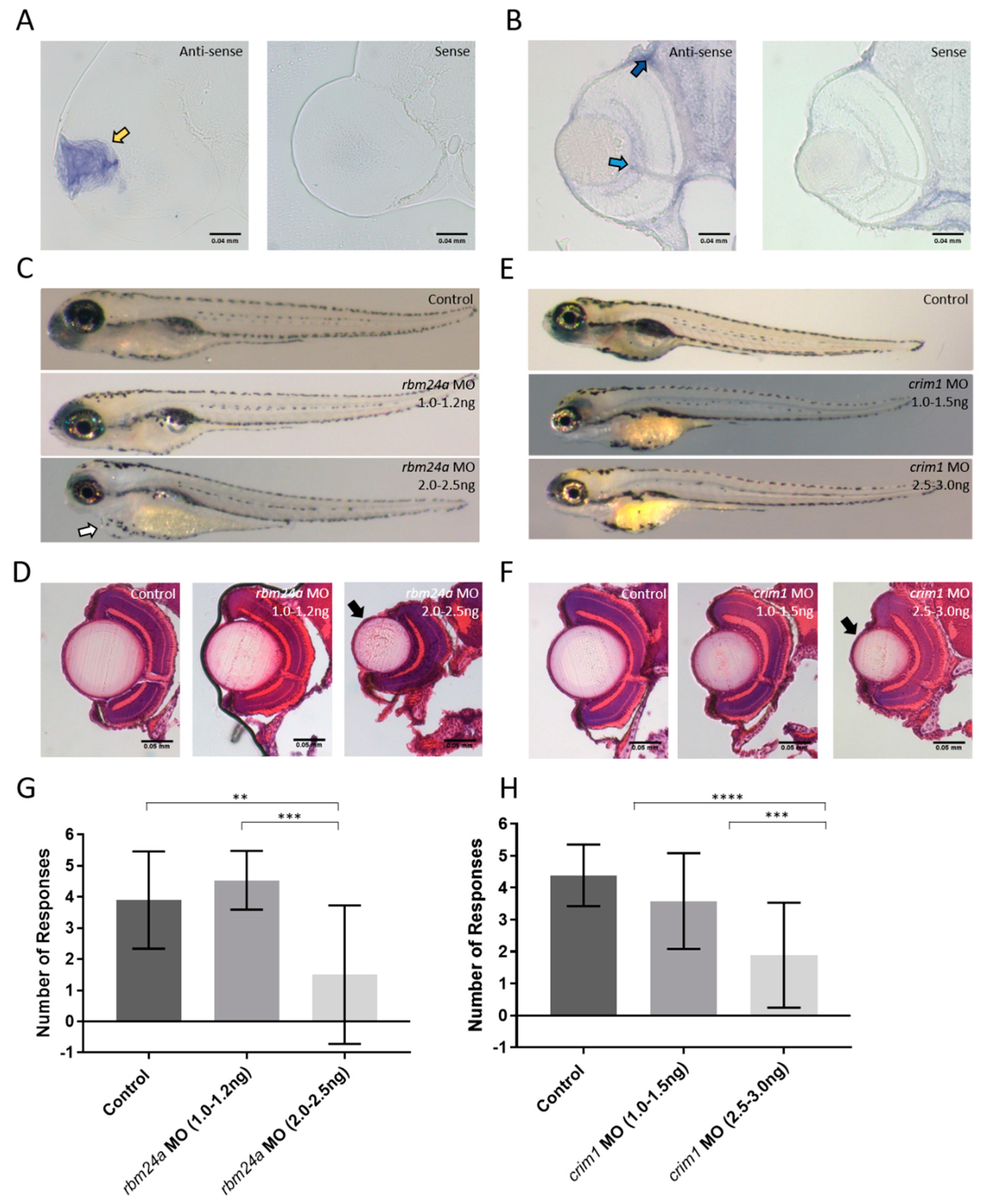
3.3. Candidate Gene Selection: crim1 and rbm24a
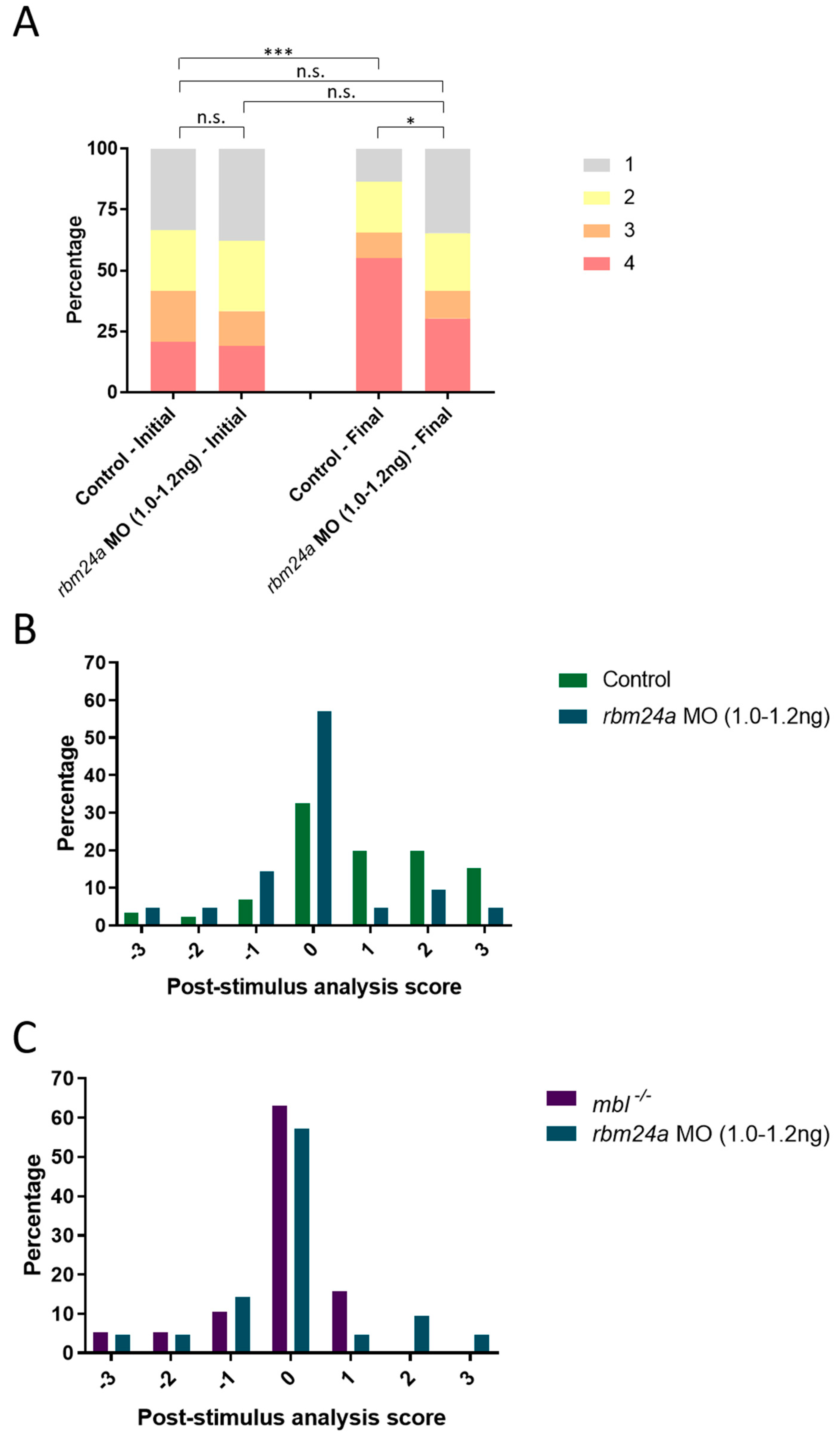
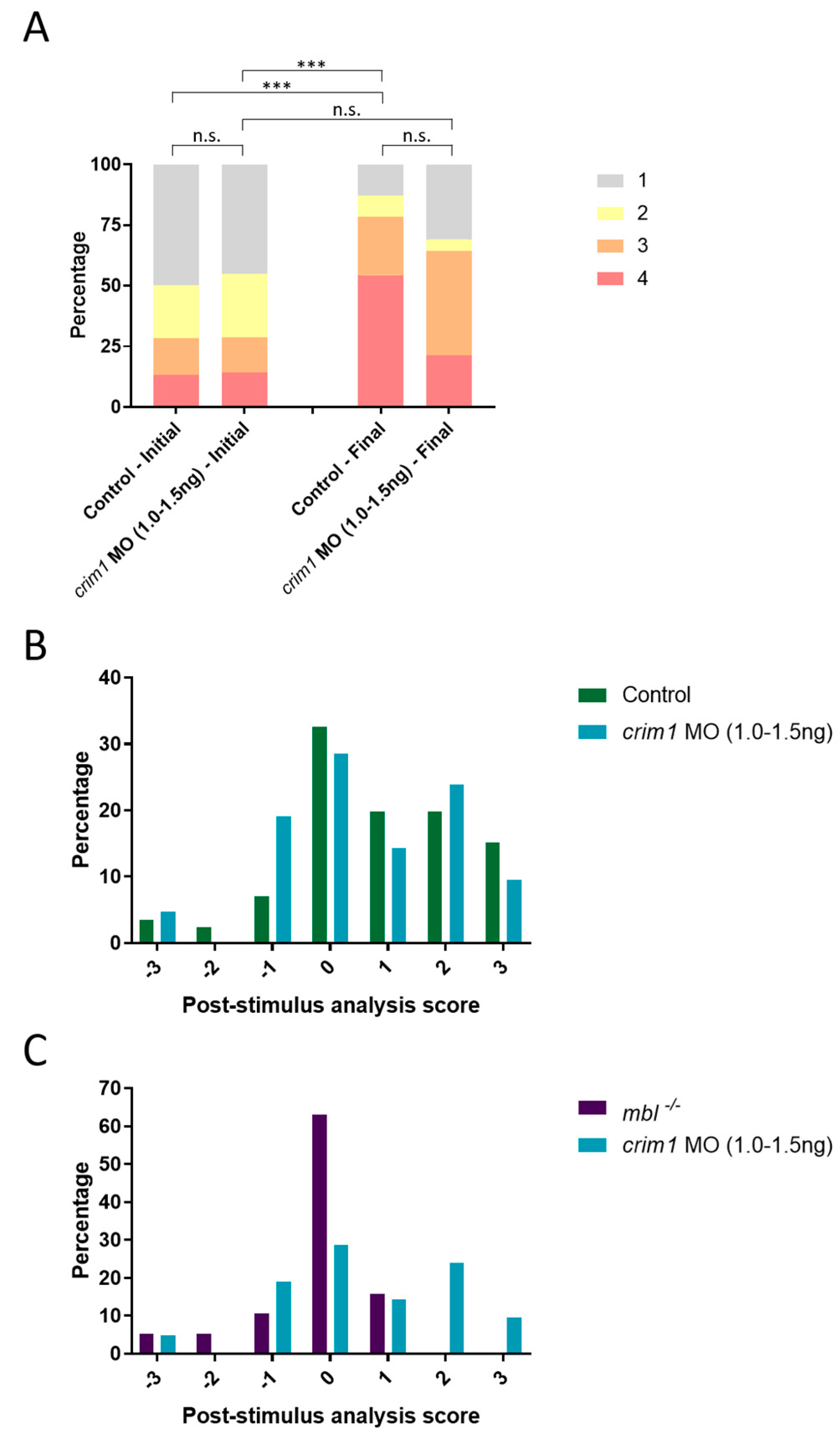
3.4. Analysis of rbm24a and crim1 Morphant Phenotypes and Response to VIZN
3.5. rbm24a Morphants Are Visually Impaired by OMR
3.6. crim1 Morphants Are Visually Normal by OMR
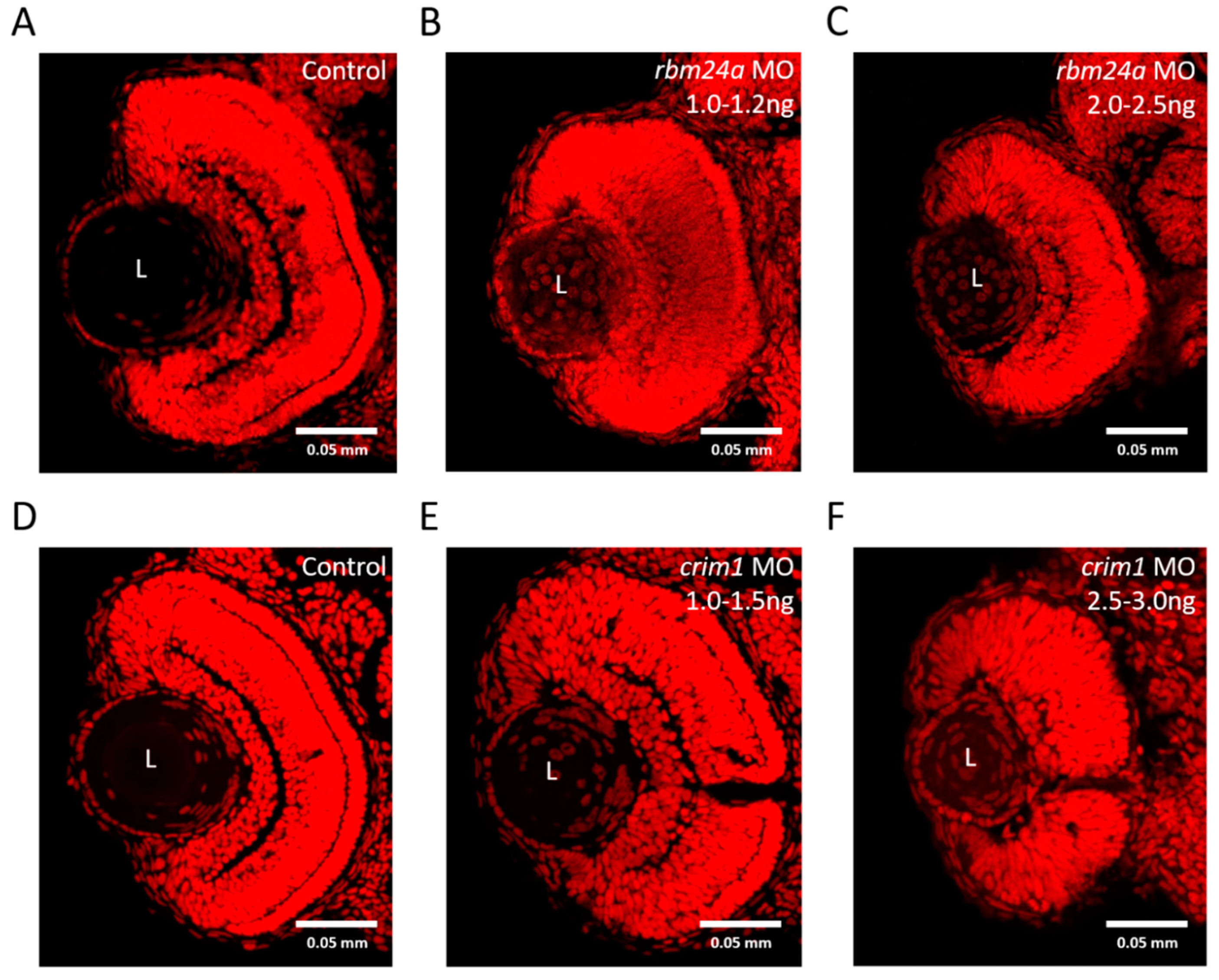
3.7. rbm24a and crim1 Morphants Have Congential Eye Defects
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wikler, K.C.; Rakic, P. Distribution of photoreceptor subtypes in the retina of diurnal and nocturnal primates. J. Neurosci. 1990, 10, 3390–3401. [Google Scholar] [CrossRef]
- Young, R.W. Cell differentiation in the retina of the mouse. Anat. Rec. 1985, 212, 199–205. [Google Scholar] [CrossRef]
- Bassett, E.A.; Wallace, V.A. Cell fate determination in the vertebrate retina. Trends Neurosci. 2012, 35, 565–573. [Google Scholar] [CrossRef]
- Chhetri, J.; Jacobson, G.; Gueven, N. Zebrafish—On the move towards ophthalmological research. Eye 2014, 28, 367. [Google Scholar] [CrossRef]
- Menger, G.J.; Koke, J.R.; Cahill, G.M. Diurnal and circadian retinomotor movements in zebrafish. Vis. Neurosci. 2005, 22, 203–209. [Google Scholar] [CrossRef]
- Richardson, R.; Tracey-White, D.; Webster, A.; Moosajee, M. The zebrafish eye—A paradigm for investigating human ocular genetics. Eye 2017, 31, 68. [Google Scholar] [CrossRef]
- Scott, C.A.; Marsden, A.N.; Slusarski, D.C. Automated, high-throughput, in vivo analysis of visual function using the zebrafish. Dev. Dyn. 2016, 245, 605–613. [Google Scholar] [CrossRef]
- Collewijn, H. Optokinetic eye movements in the rabbit: Input-output relations. Vis. Res. 1969, 9, 117–132. [Google Scholar] [CrossRef]
- Gresty, M. Eye, head and body movements of the guinea pig in response to optokinetic stimulation and sinusoidal oscillation in yaw. Pflügers Arch. 1975, 353, 201–214. [Google Scholar] [CrossRef]
- Dieringer, N.; Precht, W.; Blight, A. Resetting fast phases of head and eye and their linkage in the frog. Exp. Brain Res. 1982, 47, 407–416. [Google Scholar] [CrossRef]
- Anstis, S.; Hutahajan, P.; Cavanagh, P. Optomotor test for wavelength sensitivity in guppyfish (Poecilia reticulata). Vis. Res. 1998, 38, 45–53. [Google Scholar] [CrossRef]
- Cameron, D.J.; Rassamdana, F.; Tam, P.; Dang, K.; Yanez, C.; Ghaemmaghami, S.; Dehkordi, M.I. The optokinetic response as a quantitative measure of visual acuity in zebrafish. J. Vis. Exp. JoVE 2013, 80, e50832. [Google Scholar] [CrossRef]
- Brockerhoff, S.E.; Hurley, J.B.; Janssen-Bienhold, U.; Neuhauss, S.; Driever, W.; Dowling, J.E. A behavioral screen for isolating zebrafish mutants with visual system defects. Proc. Natl. Acad. Sci. USA 1995, 92, 10545–10549. [Google Scholar] [CrossRef]
- Easter, S.S., Jr.; Nicola, G.N. The development of vision in the zebrafish (Danio rerio). Dev. Biol. 1996, 180, 646–663. [Google Scholar] [CrossRef]
- Orger, M.B.; Smear, M.C.; Anstis, S.M.; Baier, H. Perception of Fourier and non-Fourier motion by larval zebrafish. Nat. Neurosci. 2000, 3, 1128. [Google Scholar] [CrossRef]
- Neuhauss, S.C.; Biehlmaier, O.; Seeliger, M.W.; Das, T.; Kohler, K.; Harris, W.A.; Baier, H. Genetic disorders of vision revealed by a behavioral screen of 400 essential loci in zebrafish. J. Neurosci. 1999, 19, 8603–8615. [Google Scholar] [CrossRef]
- Verma, A.S.; FitzPatrick, D.R. Anophthalmia and microphthalmia. Orphanet J. Rare Dis. 2007, 2, 47. [Google Scholar] [CrossRef]
- Campbell, H.; Holmes, E.; MacDonald, S.; Morrison, D.; Jones, I. A capture-recapture model to estimate prevalence of children born in Scotland with developmental eye defects. J. Cancer Epidemiol. Prev. 2002, 7, 21–28. [Google Scholar] [CrossRef]
- Dash, S.; Lachke, S.A. Identification of a new RNA binding protein Rbm24 linked to anophthalmia, microphthalmia and lens defects. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3063. [Google Scholar]
- Yang, J.; Hung, L.-H.; Licht, T.; Kostin, S.; Looso, M.; Khrameeva, E.; Bindereif, A.; Schneider, A.; Braun, T. RBM24 is a major regulator of muscle-specific alternative splicing. Dev. Cell 2014, 31, 87–99. [Google Scholar] [CrossRef]
- Xu, E.; Zhang, J.; Zhang, M.; Jiang, Y.; Cho, S.-J.; Chen, X. RNA-binding protein RBM24 regulates p63 expression via mRNA stability. Mol. Cancer Res. 2014, 12, 359–369. [Google Scholar] [CrossRef]
- Zhang, T.; Lin, Y.; Liu, J.; Zhang, Z.G.; Fu, W.; Guo, L.Y.; Pan, L.; Kong, X.; Zhang, M.K.; Lu, Y.H. Rbm24 regulates alternative splicing switch in embryonic stem cell cardiac lineage differentiation. Stem Cells 2016, 34, 1776–1789. [Google Scholar] [CrossRef]
- Dash, S.; Siddam, A.D.; Barnum, C.E.; Janga, S.C.; Lachke, S.A. RNA-binding proteins in eye development and disease: Implication of conserved RNA granule components. Wiley Interdiscip. Rev. RNA 2016, 7, 527–557. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, M.; Qian, Y.; Xu, E.; Zhang, J.; Chen, X. Rbm24, an RNA-binding protein and a target of p53, regulates p21 expression via mRNA stability. J. Biol. Chem. 2014, 289, 3164–3175. [Google Scholar] [CrossRef]
- Maragh, S.; Miller, R.A.; Bessling, S.L.; McGaughey, D.M.; Wessels, M.W.; de Graaf, B.; Stone, E.A.; Bertoli-Avella, A.M.; Gearhart, J.D.; Fisher, S. Identification of RNA binding motif proteins essential for cardiovascular development. BMC Dev. Biol. 2011, 11, 62. [Google Scholar] [CrossRef]
- Poon, K.L.; Tan, K.T.; Wei, Y.Y.; Ng, C.P.; Colman, A.; Korzh, V.; Xu, X.Q. RNA-binding protein RBM24 is required for sarcomere assembly and heart contractility. Cardiovasc. Res. 2012, 94, 418–427. [Google Scholar] [CrossRef]
- Oberleitner, S. Seb4–an RNA-Binding Protein as a Novel Regulator of Myogenesis during Early Development in Xenopus Laevis. Ph.D. Thesis, LMU, Munich, Germany, 2008. [Google Scholar]
- Lachke, S.A.; Ho, J.W.; Kryukov, G.V.; O’Connell, D.J.; Aboukhalil, A.; Bulyk, M.L.; Park, P.J.; Maas, R.L. iSyTE: Integrated Systems Tool for Eye gene discovery. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1617–1627. [Google Scholar] [CrossRef]
- Grifone, R.; Xie, X.; Bourgeois, A.; Saquet, A.; Duprez, D.; Shi, D.-L. The RNA-binding protein Rbm24 is transiently expressed in myoblasts and is required for myogenic differentiation during vertebrate development. Mech. Dev. 2014, 134, 1–15. [Google Scholar] [CrossRef]
- Maragh, S.; Miller, R.A.; Bessling, S.L.; Wang, G.; Hook, P.W.; McCallion, A.S. Rbm24a and Rbm24b are required for normal somitogenesis. PLoS ONE 2014, 9, e105460. [Google Scholar] [CrossRef]
- Asbell, P.A.; Dualan, I.; Mindel, J.; Brocks, D.; Ahmad, M.; Epstein, S. Age-related cataract. Lancet 2005, 365, 599–609. [Google Scholar] [CrossRef]
- Messina-Baas, O.; Cuevas-Covarrubias, S.A. Inherited Congenital Cataract: A Guide to Suspect the Genetic Etiology in the Cataract Genesis. Mol. Syndromol. 2017, 8, 58–78. [Google Scholar] [CrossRef]
- Shiels, A.; Hejtmancik, J.F. Mutations and mechanisms in congenital and age-related cataracts. Exp. Eye Res. 2017, 156, 95–102. [Google Scholar] [CrossRef]
- François, J. Genetics of cataract. Ophthalmologica 1982, 184, 61–71. [Google Scholar] [CrossRef]
- Haargaard, B.; Wohlfahrt, J.; Rosenberg, T.; Fledelius, H.C.; Melbye, M. Risk factors for idiopathic congenital/infantile cataract. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3067–3073. [Google Scholar] [CrossRef]
- Merin, S. Inherited cataracts. In Inherited Eye Disease: Diagnosis and Clinical Management; Marcel Dekker: New York, NY, USA, 1991; pp. 86–120. [Google Scholar]
- Zhang, Y.; Fan, J.; Ho, J.W.; Hu, T.; Kneeland, S.C.; Fan, X.; Xi, Q.; Sellarole, M.A.; de Vries, W.N.; Lu, W. Crim1 regulates integrin signaling in murine lens development. Development 2016, 143, 356–366. [Google Scholar] [CrossRef]
- Toker, E.; Elcioglu, N.; Ozcan, E.; Yenice, O.; Ogut, M. Colobomatous macrophthalmia with microcornea syndrome: Report of a new pedigree. Am. J. Med. Genet. Part A 2003, 121, 25–30. [Google Scholar] [CrossRef]
- Beleggia, F.; Li, Y.; Fan, J.; Elcioğlu, N.H.; Toker, E.; Wieland, T.; Maumenee, I.H.; Akarsu, N.A.; Meitinger, T.; Strom, T.M. CRIM1 haploinsufficiency causes defects in eye development in human and mouse. Hum. Mol. Genet. 2015, 24, 2267–2273. [Google Scholar] [CrossRef]
- Tam, O.H.; Pennisi, D.; Wilkinson, L.; Little, M.H.; Wazin, F.; Wan, V.L.; Lovicu, F.J. Crim1 is required for maintenance of the ocular lens epithelium. Exp. Eye Res. 2018, 170, 58–66. [Google Scholar] [CrossRef]
- Iyer, S.; Pennisi, D.J.; Piper, M. Crim1-, a regulator of developmental organogenesis. Histol. Histopathol. 2016, 31, 1049–1057. [Google Scholar]
- Schneider, I.; Houston, D.W.; Rebagliati, M.R.; Slusarski, D.C. Calcium fluxes in dorsal forerunner cells antagonize β-catenin and alter left-right patterning. Development 2008, 135, 75–84. [Google Scholar] [CrossRef]
- Kinna, G.; Kolle, G.; Carter, A.; Key, B.; Lieschke, G.J.; Perkins, A.; Little, M.H. Knockdown of zebrafish crim1 results in a bent tail phenotype with defects in somite and vascular development. Mech. Dev. 2006, 123, 277–287. [Google Scholar] [CrossRef]
- Heon, E.; Kim, G.; Qin, S.; Garrison, J.E.; Tavares, E.; Vincent, A.; Nuangchamnong, N.; Scott, C.A.; Slusarski, D.C.; Sheffield, V.C. Mutations in C8ORF37 cause Bardet Biedl syndrome (BBS21). Hum. Mol. Genet. 2016, 25, 2283–2294. [Google Scholar] [CrossRef]
- DeLuca, A.P.; Whitmore, S.S.; Barnes, J.; Sharma, T.P.; Westfall, T.A.; Scott, C.A.; Weed, M.C.; Wiley, J.S.; Wiley, L.A.; Johnston, R.M. Hypomorphic mutations in TRNT1 cause retinitis pigmentosa with erythrocytic microcytosis. Hum. Mol. Genet. 2015, 25, 44–56. [Google Scholar] [CrossRef]
- Haffter, P.; Granato, M.; Brand, M.; Mullins, M.C.; Hammerschmidt, M.; Kane, D.A.; Odenthal, J.; Van Eeden, F.; Jiang, Y.-J.; Heisenberg, C.-P. The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development 1996, 123, 1–36. [Google Scholar]
- Heisenberg, C.-P.; Houart, C.; Take-uchi, M.; Rauch, G.-J.; Young, N.; Coutinho, P.; Masai, I.; Caneparo, L.; Concha, M.L.; Geisler, R. A mutation in the Gsk3–binding domain of zebrafish Masterblind/Axin1 leads to a fate transformation of telencephalon and eyes to diencephalon. Genes Dev. 2001, 15, 1427–1434. [Google Scholar] [CrossRef]
- Van de Water, S.; van de Wetering, M.; Joore, J.; Esseling, J.; Bink, R.; Clevers, H.; Zivkovic, D. Ectopic Wnt signal determines the eyeless phenotype of zebrafish masterblind mutant. Development 2001, 128, 3877–3888. [Google Scholar]
- Severi, K.E.; Böhm, U.L.; Wyart, C. Investigation of hindbrain activity during active locomotion reveals inhibitory neurons involved in sensorimotor processing. Sci. Rep. 2018, 8, 13615. [Google Scholar] [CrossRef]
- Greiling, T.M.; Clark, J.I. Early lens development in the zebrafish: A three-dimensional time-lapse analysis. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2009, 238, 2254–2265. [Google Scholar] [CrossRef]
- Vihtelic, T.S.; Yamamoto, Y.; Springer, S.S.; Jeffery, W.R.; Hyde, D.R. Lens opacity and photoreceptor degeneration in the zebrafish lens opaque mutant. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2005, 233, 52–65. [Google Scholar]
- Goishi, K.; Shimizu, A.; Najarro, G.; Watanabe, S.; Rogers, R.; Zon, L.I.; Klagsbrun, M. αA-crystallin expression prevents γ-crystallin insolubility and cataract formation in the zebrafish cloche mutant lens. Development 2006, 133, 2585–2593. [Google Scholar] [CrossRef]
- Cheng, F.; Pan, Y.; Lu, Y.-M.; Zhu, L.; Chen, S. RNA-binding protein Dnd1 promotes breast cancer apoptosis by stabilizing the Bim mRNA in a miR-221 binding site. Biomed. Res. Int. 2017, 2017, 9596152. [Google Scholar] [CrossRef]
- Lin, X.; Shen, J.; Peng, D.; He, X.; Xu, C.; Chen, X.; Tanyi, J.L.; Montone, K.; Fan, Y.; Huang, Q. RNA-binding protein LIN28B inhibits apoptosis through regulation of the AKT2/FOXO3A/BIM axis in ovarian cancer cells. Signal Transduct. Target. Ther. 2018, 3, 23. [Google Scholar] [CrossRef]
- Talwar, S.; Balasubramanian, S.; Sundaramurthy, S.; House, R.; Wilusz, C.J.; Kuppuswamy, D.; D’silva, N.; Gillespie, M.B.; Hill, E.G.; Palanisamy, V. Overexpression of RNA-binding protein CELF1 prevents apoptosis and destabilizes pro-apoptotic mRNAs in oral cancer cells. RNA Biol. 2013, 10, 277–286. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Fujimori-Tonou, N.; Yoshimura, Y.; Kishi, T.; Okamoto, H.; Masai, I. Mutation of DNA primase causes extensive apoptosis of retinal neurons through the activation of DNA damage checkpoint and tumor suppressor p53. Development 2008, 135, 1247–1257. [Google Scholar] [CrossRef]
- Pant, S.D.; March, L.D.; Famulski, J.K.; French, C.R.; Lehmann, O.J.; Waskiewicz, A.J. Molecular mechanisms regulating ocular apoptosis in zebrafish gdf6a mutants. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5871–5879. [Google Scholar] [CrossRef]
- Lorén, C.E.; Schrader, J.W.; Ahlgren, U.; Gunhaga, L. FGF signals induce Caprin2 expression in the vertebrate lens. Differentiation 2009, 77, 386–394. [Google Scholar] [CrossRef]
- Dash, S.; Dang, C.A.; Beebe, D.C.; Lachke, S.A. Deficiency of the RNA binding protein caprin2 causes lens defects and features of peters anomaly. Dev. Dyn. 2015, 244, 1313–1327. [Google Scholar] [CrossRef]
- Lachke, S.A.; Alkuraya, F.S.; Kneeland, S.C.; Ohn, T.; Aboukhalil, A.; Howell, G.R.; Saadi, I.; Cavallesco, R.; Yue, Y.; Tsai, A.C. Mutations in the RNA granule component TDRD7 cause cataract and glaucoma. Science 2011, 331, 1571–1576. [Google Scholar] [CrossRef]
- Raji, B.; Dansault, A.; Leemput, J.; de la Houssaye, G.; Vieira, V.; Kobetz, A.; Arbogast, L.; Masson, C.; Menasche, M.; Abitbol, M. The RNA-binding protein Musashi-1 is produced in the developing and adult mouse eye. Mol. Vis. 2007, 13, 1412–1427. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brastrom, L.K.; Scott, C.A.; Dawson, D.V.; Slusarski, D.C. A High-Throughput Assay for Congenital and Age-Related Eye Diseases in Zebrafish. Biomedicines 2019, 7, 28. https://doi.org/10.3390/biomedicines7020028
Brastrom LK, Scott CA, Dawson DV, Slusarski DC. A High-Throughput Assay for Congenital and Age-Related Eye Diseases in Zebrafish. Biomedicines. 2019; 7(2):28. https://doi.org/10.3390/biomedicines7020028
Chicago/Turabian StyleBrastrom, Lindy K., C. Anthony Scott, Deborah V. Dawson, and Diane C. Slusarski. 2019. "A High-Throughput Assay for Congenital and Age-Related Eye Diseases in Zebrafish" Biomedicines 7, no. 2: 28. https://doi.org/10.3390/biomedicines7020028
APA StyleBrastrom, L. K., Scott, C. A., Dawson, D. V., & Slusarski, D. C. (2019). A High-Throughput Assay for Congenital and Age-Related Eye Diseases in Zebrafish. Biomedicines, 7(2), 28. https://doi.org/10.3390/biomedicines7020028