Glioma FMISO PET/MR Imaging Concurrent with Antiangiogenic Therapy: Molecular Imaging as a Clinical Tool in the Burgeoning Era of Personalized Medicine
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
3.1. Utilization of Medical Imaging to Influence Personalized Medicine
3.2. The Role of Molecular Imaging in Personalized Medicine: Diagnosis
3.3. The Role of Molecular Imaging in Personalized Medicine: Prognosis
3.4. The Role of Molecular Imaging in Personalized Medicine: Monitoring of Targeted Therapy
3.5. Advantages of Simultaneous PET/MR Imaging
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Issa, A.M. Personalized Medicine and the Practice of Medicine in the 21st Century. McGill J. Med. 2007, 10, 53–57. [Google Scholar] [PubMed]
- Zurawska, U.; Baribeau, D.A.; Giilck, S.; Victor, C.; Gandhi, S.; Florescu, A.; Verma, S. Outcomes of her2-positive early-stage breast cancer in the trastuzumab era: A population-based study of Canadian patients. Curr. Oncol. 2013, 20, e539–e545. [Google Scholar] [CrossRef] [PubMed]
- Panoff, J.E.; Hurley, J.; Takita, C.; Reis, I.M.; Zhao, W.; Sujoy, V.; Gomez, C.R.; Jorda, M.; Koniaris, L.; Wright, J.L. Risk of locoregional recurrence by receptor status in breast cancer patients receiving modern systemic therapy and post-mastectomy radiation. Breast Cancer Res. Treat. 2011, 128, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Gabos, Z.; Thoms, J.; Ghosh, S.; Hanson, J.; Deschênes, J.; Sabri, S.; Abdulkarim, B. The association between biological subtype and locoregional recurrence in newly diagnosed breast cancer. Breast Cancer Res. Treat. 2010, 124, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.S.; Ma, C.D.; Wu, J.Y.; Yang, W.T.; Lu, H.F.; Wu, J.; Lu, J.S.; Shao, Z.M.; Shen, Z.Z.; Shen, K.W. Molecular subtype approximated by quantitative estrogen receptor, progesterone receptor and her2 can predict the prognosis of breast cancer. Tumori 2010, 96, 103–110. [Google Scholar] [PubMed]
- Arvold, N.D.; Taghian, A.G.; Niemierko, A.; Raad, R.F.A.; Sreedhara, M.; Nguyen, P.L.; Bellon, J.R.; Wong, J.S.; Smith, B.L.; Harris, J.R. Age, breast cancer subtype approximation, and local recurrence after breast-conserving therapy. J. Clin. Oncol. 2011, 29, 3885–3891. [Google Scholar] [CrossRef] [PubMed]
- Voduc, K.D.; Cheang, M.C.; Tyldesley, S.; Gelmon, K.; Nielsen, T.O.; Kennecke, H. Breast cancer subtypes and the risk of local and regional relapse. J. Clin. Oncol. 2010, 28, 1684–1691. [Google Scholar] [CrossRef] [PubMed]
- Cancello, G.; Maisonneuve, P.; Rotmensz, N.; Viale, G. Prognosis in women with small (T1mic,T1a,T1b) node-negative operable breast cancer by immunohistochemically selected subtypes. Breast Cancer Res. Treat. 2011, 127, 713–720. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.M.; Cole, S.R.; Tse, C.K.; Perou, C.M.; Carey, L.A.; Foulkes, W.D.; Dressler, L.G.; Geradts, J.; Millikan, R.C. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clin. Cancer Res. 2010, 16, 6100–6110. [Google Scholar] [CrossRef] [PubMed]
- Haque, R.; Ahmed, S.A.; Inzhakova, G.; Shi, J.; Avila, C.; Polikoff, J.; Bernstein, L.; Enger, S.M.; Press, M.F. Impact of breast cancer subtypes and treatment on survival: An analysis spanning two decades. Cancer Epidemiol. Biomarkers Prev. 2012, 21, 1848–1855. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, M.M. Trastuzumab, a recombinant dna-derived humanized monoclonal antibody, a novel agent for the treatment of metastatic breast cancer. Clin. Ther. 1999, 21, 309–318. [Google Scholar] [CrossRef]
- Cobleigh, M.A.; Vogel, C.L.; Tripathy, D.; Robert, N.J.; Scholl, S.; Fehrenbacher, L.; Wolter, J.M.; Paton, V.; Shak, S.; Lieberman, G.; et al. Multinational study of the efficacy and safety of humanized anti-her2 monoclonal antibody in women who have her2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J. Clin. Oncol. 1999, 17, 2639–2648. [Google Scholar] [PubMed]
- Mass, R.D.; Press, M.F.; Anderson, S.; Cobleigh, M.A.; Vogel, C.L.; Dybdal, N.; Leiberman, G.; Slamon, D.J. Evaluation of clinical outcomes according to her2 detection by fluorescence in situ hybridization in women with metastatic breast cancer treated with trastuzumab. Clin. Breast Cancer 2005, 6, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Leyland–Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of chemotherapy plus a monoclonal antibody against her2 for metastatic breast cancer that overexpresses her2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.L.; Cobleigh, M.A.; Tripathy, D.; Gutheil, J.C.; Harris, L.N.; Fehrenbacher, L.; Slamon, D.J.; Murphy, M.; Novotny, W.F.; Burchmore, M.; et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of her2-overexpressing metastatic breast cancer. J. Clin. Oncol. 2002, 20, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Muzi, M.; Peterson, L.M.; O’Sullivan, J.N.; Fink, J.R.; Rajendran, J.G.; McLaughlin, L.J.; Muzi, J.P.; Mankoff, D.A.; Krohn, K.A. 18F-Fluoromisonidazole Quantification of Hypoxia in Human Cancer Patients Using Image-Derived Blood Surrogate Tissue Reference Regions. J. Nucl. Med. 2015, 56, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Toyonaga, T.; Hirata, K.; Yamaguchi, S.; Hatanaka, K.C.; Yuzawa, S.; Manabe, O.; Kobayashi, K.; Watanabe, S.; Shiga, T.; Terasaka, S.; et al. 18F-fluoromisonidazole positron emission tomography can predict pathological necrosis of brain tumors. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Hirata, K.; Terasaka, S.; Shiga, T.; Hattori, N.; Magota, K.; Kobayashi, H.; Yamaguchi, S.; Houkin, K.; Tanaka, S.; Kuge, Y.; et al. 18F-Fluoromisonidazole positron emission tomography may differentiate glioblastoma multiforme from less malignant gliomas. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Barajas, R.F., Jr.; Pampaloni, M.H.; Clarke, J.L.; Seo, Y.; Savic, D.; Hawkins, R.A.; Behr, S.C.; Chang, S.M.; Berger, M.; Dillon, W.P.; et al. Assessing Biological Response to Bevacizumab Using 18F-Fluoromisonidazole PET/MR Imaging in a Patient with Recurrent Anaplastic Astrocytoma. Case Rep. Radiol. 2015, 2015, 731361. [Google Scholar] [CrossRef] [PubMed]
- Barajas, R.F., Jr.; Butowski, N.A.; Phillips, J.J.; Aghi, M.K.; Berger, M.S.; Chang, S.M.; Cha, S. The Development of Reduced Diffusion Following Bevacizumab Therapy Identifies Regions of Recurrent Disease in Patients with High-grade Glioma. Acad. Radiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Barajas, R.F., Jr.; Phillips, J.J.; Parvataneni, R.; Molinaro, A.; Essock-Burns, E.; Bourne, G.; Parsa, A.T.; Aghi, M.K.; McDermott, M.W.; Berger, M.S.; et al. Regional variation in histopathologic features of tumor specimens from treatment-naive glioblastoma correlates with anatomic and physiologic MR Imaging. Neuro Oncol. 2012, 14, 942–954. [Google Scholar] [CrossRef] [PubMed]
- Barajas, R.F., Jr.; Hodgson, J.G.; Chang, J.S.; Vandenberg, S.R.; Yeh, R.F.; Parsa, A.T.; McDermott, M.W.; Berger, M.S.; Dillon, W.P.; Cha, S. Glioblastoma multiforme regional genetic and cellular expression patterns: Influence on anatomic and physiologic MR imaging. Radiology 2010, 254, 564–576. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Macdonald, D.R.; Reardon, D.A.; Cloughesy, T.F.; Sorensen, A.G.; Galanis, E.; Degroot, J.; Wick, W.; Gilbert, M.R.; Lassman, A.B.; et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J. Clin. Oncol. 2010, 28, 1963–1972. [Google Scholar] [CrossRef] [PubMed]
- Brandsma, D.; Stalpers, L.; Taal, W.; Sminia, P.; van den Bent, M.J. Clinical features, mechanisms, and management of pseudoprogression in malignant glioma. Lancet Oncol. 2008, 9, 453–461. [Google Scholar] [CrossRef]
- Brandes, A.A.; Tosoni, A.; Spagnolli, F.; Frezza, G.; Leonardi, M.; Calbucci, F.; Franceschi, E. Disease progression or pseudoprogression after concomitant radiochemotherapy treatment: Pitfalls in neurooncology. Neuro Oncol. 2008, 10, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Barajas, R.F., Jr.; Chang, J.S.; Segal, M.R.; Parsa, A.T.; McDermott, M.W.; Berger, M.S.; Cha, S. Differentiation of recurrent glioblastoma multiforme from radiation necrosis after external beam radiation therapy with dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology 2009, 253, 486–496. [Google Scholar] [CrossRef] [PubMed]
- La Fougere, C.; Suchorska, B.; Bartenstein, P.; Kreth, F.W.; Tonn, J.C. Molecular imaging of gliomas with PET: Opportunities and limitations. Neuro Oncol. 2011, 13, 806–819. [Google Scholar] [CrossRef] [PubMed]
- Albert, N.L.; Weller, M.; Suchorska, B.; Galldiks, N.; Soffietti, R.; Kim, M.M.; la Fougère, C.; Pope, W.; Law, I.; Arbizu, J.; et al. Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Silverman, D.H.; Delaloye, S.; Czernin, J.; Kamdar, N.; Pope, W.; Satyamurthy, N.; Schiepers, C.; Cloughesy, T. 18F-FDOPA PET imaging of brain tumors: Comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. J. Nucl. Med. 2006, 47, 904–911. [Google Scholar] [PubMed]
- Galldiks, N.; Dunkl, V.; Stoffels, G.; Hutterer, M.; Rapp, M.; Sabel, M.; Reifenberger, G.; Kebir, S.; Dorn, F.; Blau, T.; et al. Diagnosis of pseudoprogression in patients with glioblastoma using O-(2-[18F]fluoroethyl)-l-tyrosine PET. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, K.; Czernin, J.; Cloughesy, T.; Lai, A.; Pomykala, K.L.; Benz, M.R.; Buck, A.K.; Phelps, M.E.; Chen, W. Comparison of visual and semiquantitative analysis of 18F-FDOPA-PET/CT for recurrence detection in glioblastoma patients. Neuro Oncol. 2014, 16, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Walter, F.; Cloughesy, T.; Walter, M.A.; Lai, A.; Nghiemphu, P.; Wagle, N.; Fueger, B.; Satyamurthy, N.; Phelps, M.E.; Czernin, J. Impact of 3,4-dihydroxy-6–18F-fluoro-l-phenylalanine PET/CT on managing patients with brain tumors: The referring physician’s perspective. J. Nucl. Med. 2012, 53, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Galldiks, N.; Stoffels, G.; Filss, C.; Rapp, M.; Blau, T.; Tscherpel, C.; Ceccon, G.; Dunkl, V.; Weinzierl, M.; Stoffel, M.; et al. The use of dynamic O-(2–18F-fluoroethyl)-l-tyrosine PET in the diagnosis of patients with progressive and recurrent glioma. Neuro Oncol. 2015, 17, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Tate, M.C.; Aghi, M.K. Biology of angiogenesis and invasion in glioma. Neurotherapeutics 2009, 6, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R. Brain tumor hypoxia: tumorigenesis, angiogenesis, imaging, pseudopreogression, and as a therapeutic target. J. Neurooncol. 2009, 92, 317–335. [Google Scholar] [CrossRef] [PubMed]
- Hockel, M.; Vaupel, P. Biological consequences of tumor hypoxia. Semin. Oncol. 2001, 28, 36–41. [Google Scholar] [CrossRef]
- Dachs, G.U.; Tozer, G.M. Hypoxia modulated gene expression: angiogenesis, metastasis and therapeutic exploitation. Eur. J. Cancer 2000, 36, 1649–1660. [Google Scholar] [CrossRef]
- Cosse, J.P.; Michiels, C. Tumour hypoxia affects the responsiveness of cancer cells to chemotherapy and promotes cancer progression. Anticancer Agents Med. Chem. 2008, 8, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Jerabek, P.A.; Patrick, T.B.; Kilbourn, M.R.; Dischino, D.D.; Welch, M.J. Synthesis and biodistribution of 18F-labeled fluoronitroimidazoles: Potential in vivo markers of hypoxic tissue. Int. J. Rad. Appl. Instrum. A 1986, 37, 599–605. [Google Scholar] [CrossRef]
- Rasey, J.S.; Koh, W.J.; Grierson, J.R.; Grunbaum, Z.; Krohn, K.A. Radiolabelled fluoromisonidazole as an imaging agent for tumor hypoxia. Int. J. Radiat. Oncol. Biol. Phys. 1989, 17, 985–991. [Google Scholar] [CrossRef]
- Rasey, J.S.; Grunbaum, Z.; Magee, S.; Nelson, N.J.; Olive, P.L.; Durand, R.E.; Krohn, K.A. Characterization of radiolabeled fluoromisonidazole as a probe for hypoxic cells. Radiat. Res. 1987, 111, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.M.; Rasey, J.S.; Spence, A.M.; Shaw, D.W.; Krohn, K.A. Binding of the hypoxia tracer [3H]misonidazole in cerebral ischemia. Stroke 1987, 18, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Valk, P.E.; Mathis, C.A.; Prados, M.D.; Gilbert, J.C.; Budinger, T.F. Hypoxia in human gliomas: Demonstration by PET with fluorine-18-fluoromisonidazole. J. Nucl. Med. 1992, 33, 2133–2137. [Google Scholar] [PubMed]
- Casciari, J.J.; Chin, L.K.; Livesey, J.C.; Boyles, D.; Steen, R.G.; Rasey, J.S. Growth rate, labeling index, and radiation survival of cells grown in the Matrigel thread in vitro tumor model. In Vitro Cell. Dev. Biol. Anim. 1995, 31, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.J.; Rasey, J.S.; Evans, M.L.; Grierson, J.R.; Lewellen, T.K.; Graham, M.M.; Krohn, K.A.; Griffin, T.W. Imaging of hypoxia in human tumors with [F-18]fluoromisonidazole. Int. J. Radiat. Oncol. Biol. Phys. 1992, 22, 199–212. [Google Scholar] [CrossRef]
- Rajendran, J.G.; Krohn, K.A. F-18 fluoromisonidazole for imaging tumor hypoxia: Imaging the microenvironment for personalized cancer therapy. Semin. Nucl. Med. 2015, 452, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Cher, L.M.; Murone, C.; Lawrentschuk, N.; Ramdave, S.; Papenfuss, A.; Hannah, A.; O’Keefe, G.J.; Sachinidis, J.I.; Berlangieri, S.U.; Fabinyi, G.; et al. Correlation of hypoxic cell fraction and angiogenesis with glucose metabolic rate in gliomas using 18F-fluoromisonidazole, 18F-FDG PET, and immunohistochemical studies. J. Nucl. Med. 2006, 47, 410–418. [Google Scholar] [PubMed]
- Swanson, K.R.; Chakraborty, G.; Wang, C.H.; Rockne, R.; Harpold, H.L.; Muzi, M.; Adamsen, T.C.; Krohn, K.A.; Spence, A.M. Complementary but distinct roles for MRI and 18F-Fluoromisonidazole PET in the assessment of human glioblastomas. J. Nucl. Med. 2009, 50, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Spence, A.M.; Muzi, M.; Swanson, K.R.; O’Sullivan, F.; Rockhill, J.K.; Rajendran, J.G.; Adamsen, T.C.; Link, J.M.; Swanson, P.E.; Yagle, K.J.; et al. Regional hypoxia in glioblastoma multiforme quantified with [18F]fluoromisonidazole positron emission tomography before radiotherapy: Correlation with time to progression and survival. Clin. Cancer Res. 2008, 14, 2623–2630. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, M.; Yamane, T.; Shinohara, S.; Fujiwara, K.; Hori, S.Y.; Tona, Y.; Yamazaki, H.; Naito, Y.; Senda, M. 18F-fluoromisonidazole positron emission tomography before treatment is a predictor of radiotherapy outcome and survival prognosis in patients with head and neck squamous cell carcinoma. Ann. Nucl. Med. 2011, 25, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Eschmann, S.M.; Paulsen, F.; Reimold, M.; Dittmann, H.; Welz, S.; Reischl, G.; Machulla, H.J.; Bares, R. Prognostic impact of hypoxia imaging with 18F-misonidazole PET in non-small cell lung cancer and head and neck cancer before radiotherapy. J. Nucl. Med. 2005, 46, 253–260. [Google Scholar] [PubMed]
- Koh, W.J.; Bergman, K.S.; Rasey, J.S.; Peterson, L.M.; Evans, M.L.; Graham, M.M.; Grierson, J.R.; Lindsley, K.L.; Lewellen, T.K.; Krohn, K.A.; et al. Evaluation of oxygenation status during fractionated radiotherapy in human nonsmall cell lung cancers using [F-18]fluoromisonidazole positron emission tomography. Int. J. Radiat. Oncol. Biol. Phys. 1995, 33, 391–398. [Google Scholar] [CrossRef]
- Askoxylakis, V.; Dinkel, J.; Eichinger, M.; Stieltjes, B.; Sommer, G.; Strauss, L.G.; Dimitrakopoulou-Strauss, A.; Kopp-Schneider, A.; Haberkorn, U.; Huber, P.E.; et al. Multimodal hypoxia imaging and intensity modulated radiation therapy for unresectable non-small-cell lung cancer: The HIL trial. Radiat. Oncol. 2012, 7, 157. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Lei, L.; Xu, J.; Sun, Y.; Zhang, Y.; Wang, X.; Pan, L.; Shao, Z.; Zhang, Y.; Liu, G. 18F-fluoromisonidazole PET/CT: A potential tool for predicting primary endocrine therapy resistance in breast cancer. J. Nucl. Med. 2013, 54, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Eary, J.F.; Link, J.M.; Muzi, M.; Conrad, E.U.; Mankoff, D.A.; White, J.K.; Krohn, K.A. Multiagent PET for risk characterization in sarcoma. J. Nucl. Med. 2011, 52, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Jansen, N.L.; Suchorska, B.; Wenter, V.; Schmid-Tannwald, C.; Todica, A.; Eigenbrod, S.; Niyazi, M.; Tonn, J.C.; Bartenstein, P.; Kreth, F.W.; et al. Prognostic significance of dynamic 18F-FET PET in newly diagnosed astrocytic high-grade glioma. J. Nucl. Med. 2015, 56, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Floeth, F.W.; Sabel, M.; Stoffels, G.; Pauleit, D.; Hamacher, K.; Steiger, H.J.; Langen, K.J. Prognostic value of 18F-fluoroethyl-l-tyrosine PET and MRI in small nonspecific incidental brain lesions. J. Nucl. Med. 2008, 49, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Jansen, N.L.; Suchorska, B.; Wenter, V.; Eigenbrod, S.; Schmid-Tannwald, C.; Zwergal, A.; Niyazi, M.; Drexler, M.; Bartenstein, P.; Schnell, O.; et al. Dynamic 18F-FET PET in newly diagnosed astrocytic low-grade glioma identifies high-risk patients. J. Nucl. Med. 2014, 55, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Thon, N.; Kunz, M.; Lemke, L.; Jansen, N.L.; Eigenbrod, S.; Kreth, S.; Lutz, J.; Egensperger, R.; Giese, A.; Herms, J.; et al. Dynamic F-FET PET in suspected WHO grade II gliomas defines distinct biological subgroups with different clinical courses. Int. J. Cancer 2015, 136, 2132–2145. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [PubMed]
- Jain, R.K.; di Tomaso, E.; Duda, D.G.; Loeffler, J.S.; Sorensen, A.G.; Batchelor, T.T. Angiogenesis in brain tumours. Nat. Rev. Neurosci. 2007, 8, 610–622. [Google Scholar] [CrossRef] [PubMed]
- Brem, S.; Cotran, R.; Folkman, J. Tumor angiogenesis: A quantitative method for histologic grading. J. Natl. Cancer Inst. 1972, 48, 347–356. [Google Scholar] [PubMed]
- Dewhirst, M.W.; Cao, Y.; Moeller, B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat. Rev. Cancer 2008, 8, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Salmaggi, A.; Eoli, M.; Frigerio, S.; Silvani, A.; Gelati, M.; Corsini, E.; Broggi, G.; Boiardi, A. Intracavitary VEGF, bFGF, IL-8, IL-12 levels in primary and recurrent malignant glioma. J. Neurooncol. 2003, 62, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Pore, N.; Liu, S.; Haas-Kogan, D.A.; O’Rourke, D.M.; Maity, A. PTEN mutation and epidermal growth factor receptor activation regulate vascular endothelial growth factor (VEGF) mRNA expression in human glioblastoma cells by transactivating the proximal VEGF promoter. Cancer Res. 2003, 63, 236–241. [Google Scholar] [PubMed]
- Kaur, B.; Khwaja, F.W.; Severson, E.A.; Matheny, S.L.; Brat, D.J.; Van Meir, E.G. Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis. Neuro Oncol. 2005, 7, 134–153. [Google Scholar] [CrossRef] [PubMed]
- Barajas, R.F., Jr.; Phillips, J.J.; Vandenberg, S.R.; McDermott, M.W.; Berger, M.S.; Dillon, W.P.; Cha, S. Pro-angiogenic cellular and genomic expression patterns within glioblastoma influences dynamic susceptibility weighted perfusion MRI. Clin. Radiol. 2015, 70, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Vredenburgh, J.J.; Desjardins, A.; Herndon, J.E., 2nd; Dowell, J.M.; Reardon, D.A.; Quinn, J.A.; Rich, J.N.; Sathornsumetee, S.; Gururangan, S.; Wagner, M.; et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin. Cancer Res. 2007, 13, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Vredenburgh, J.J.; Desjardins, A.; Herndon, J.E., 2nd; Marcello, J.; Reardon, D.A.; Quinn, J.A.; Rich, J.N.; Sathornsumetee, S.; Gururangan, S.; Sampson, J.; et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J. Clin. Oncol. 2007, 25, 4722–4729. [Google Scholar] [CrossRef] [PubMed]
- Bokstein, F.; Shpigel, S.; Blumenthal, D.T. Treatment with bevacizumab and irinotecan for recurrent high-grade glial tumors. Cancer 2008, 112, 2267–2273. [Google Scholar] [CrossRef] [PubMed]
- Hovey, E.J.; Field, K.M.; Rosenthal, M.; Nowak, A.K.; Cher, L.; Wheeler, H.; Barnes, E.; Phal, P.; Livingstone, A.; Sawkins, K.; et al. Continuing or ceasing bevacizumab at disease progression: Results from the CABARET study, a prospective randomized phase II trial in patients with recurrent glioblastoma. In Proceedings of the 2015 ASCO Annual Meeting, Chicago, IL, USA, 29 May–2 June 2015.
- Wick, W.; Brandes, A.A.; Gorlia, T.; Bendszus, M.; Sahm, F.; Taal, W.; Taphoorn, M.; Domont, J.; Idbaih, A.; Campone, M.; et al. LB-05PHASE III trial exploring the combination of bevacizumab and lomustine in patients with first recurrence of a glioblastoma: The eortc 26101 trial. Neuro Oncol. 2015, 17 (suppl. 5), v1. [Google Scholar] [CrossRef]
- Tong, R.T.; Boucher, Y.; Kozin, S.V.; Winkler, F.; Hicklin, D.J.; Jain, R.K. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004, 64, 3731–3736. [Google Scholar] [CrossRef] [PubMed]
- Kreisl, T.N.; Zhang, W.; Odia, Y.; Shih, J.H.; Butman, J.A.; Hammoud, D.; Iwamoto, F.M.; Sul, J.; Fine, H.A. A phase II trial of single-agent bevacizumab in patients with recurrent anaplastic glioma. Neuro Oncol. 2011, 13, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Hutterer, M.; Nowosielski, M.; Putzer, D.; Waitz, D.; Tinkhauser, G.; Kostron, H.; Muigg, A.; Virgolini, I.J.; Staffen, W.; Trinka, E.; et al. O-(2–18F-fluoroethyl)-l-tyrosine PET predicts failure of antiangiogenic treatment in patients with recurrent high-grade glioma. J. Nucl. Med. 2011, 52, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Galldiks, N.; Rapp, M.; Stoffels, G.; Fink, G.R.; Shah, N.J.; Coenen, H.H.; Sabel, M.; Langen, K.J. Response assessment of bevacizumab in patients with recurrent malignant glioma using [18F]fluoroethyl-l-tyrosine PET in comparison to MRI. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenberg, J.; Czernin, J.; Cloughesy, T.F.; Ellingson, B.M.; Pope, W.B.; Geist, C.; Dahlbom, M.; Silverman, D.H.; Satyamurthy, N.; Phelps, M.E.; et al. 3′-deoxy-3′-18F-fluorothymidine PET and MRI for early survival predictions in patients with recurrent malignant glioma treated with bevacizumab. J. Nucl. Med. 2012, 53, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Gerstner, E.R.; Zhang, Z.; Fink, J.R.; Muzi, M.; Hanna, L.; Greco, E.; Prah, M.; Schmainda, K.; Mintz, A.; Kostakoglu, L.; et al. ACRIN 6684: Assessment of Tumor Hypoxia in Newly Diagnosed Glioblastoma Using 18F-FMISO PET and MRI. Clin. Cancer Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Tamura, R.; Tanaka, T.; Miyake, K.; Tabei, Y.; Ohara, K.; Sampetrean, O.; Kono, M.; Mizutani, K.; Yamamoto, Y.; Murayama, Y.; et al. Histopathological investigation of glioblastomas resected under bevacizumab treatment. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Poisson, L.; Narang, J.; Scarpace, L.; Rosenblum, M.L.; Rempel, S.; Mikkelsen, T. Correlation of perfusion parameters with genes related to angiogenesis regulation in glioblastoma: A feasibility study. Am. J. Neuroradiol. 2012, 33, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Vidiri, A.; Pace, A.; Fabi, A.; Maschio, M.; Latagliata, G.M.; Anelli, V.; Piludu, F.; Carapella, C.M.; Giovinazzo, G.; Marzi, S. Early perfusion changes in patients with recurrent high-grade brain tumor treated with Bevacizumab: Preliminary results by a quantitative evaluation. J. Exp. Clin. Cancer Res. 2012, 31, 33. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, J.; Gatidis, S.; Schmidt, H.; Gückel, B.; Bezrukov, I.; Pfannenberg, C.A.; Reimold, M.; Ebinger, M.; Fuchs, J.; Claussen, C.D.; et al. Simultaneous Whole-Body PET/MR Imaging in Comparison to PET/CT in Pediatric Oncology: Initial Results. Radiology 2014, 273, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Lyons, K.; Seghers, V.; Sorensen, J.I.; Zhang, W.; Paldino, M.J.; Krishnamurthy, R.; Rohren, E.M. Comparison of Standardized Uptake Values in Normal Structures between PET/CT and PET/MRI in a Tertiary Pediatric Hospital: A Prospective Study. Am. J. Roentgenol. 2015, 205, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Malone, I.; Ansorge, R.; Williams, G. Attenuation Correction Methods Suitable for Brain Imaging with a PET/MRI Scanner: A Comparison of Tissue Atlas and Template Attenuation Map Approaches. J. Nucl. Med. 2011, 52, 1142–1114. [Google Scholar] [CrossRef] [PubMed]
- Spick, C.; Herrmann, K.; Czernin, J. 18F-FDG PET/CT and PET/MRI Perform Equally Well in Cancer: Evidence from Studies on More Than 2300 Patients. J. Nucl. Med. 2016, 57, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Kershah, S.; Partovi, S.; Traughber, B.J.; Muzic, R.F., Jr.; Schluchter, M.D.; O’Donnell, J.K.; Faulhaber, P. Comparison of standardized uptake values in normal structures between PET/CT and PET/MRI in an oncology patient population. Mol. Imaging Biol. 2013, 15, 776–785. [Google Scholar] [CrossRef] [PubMed]

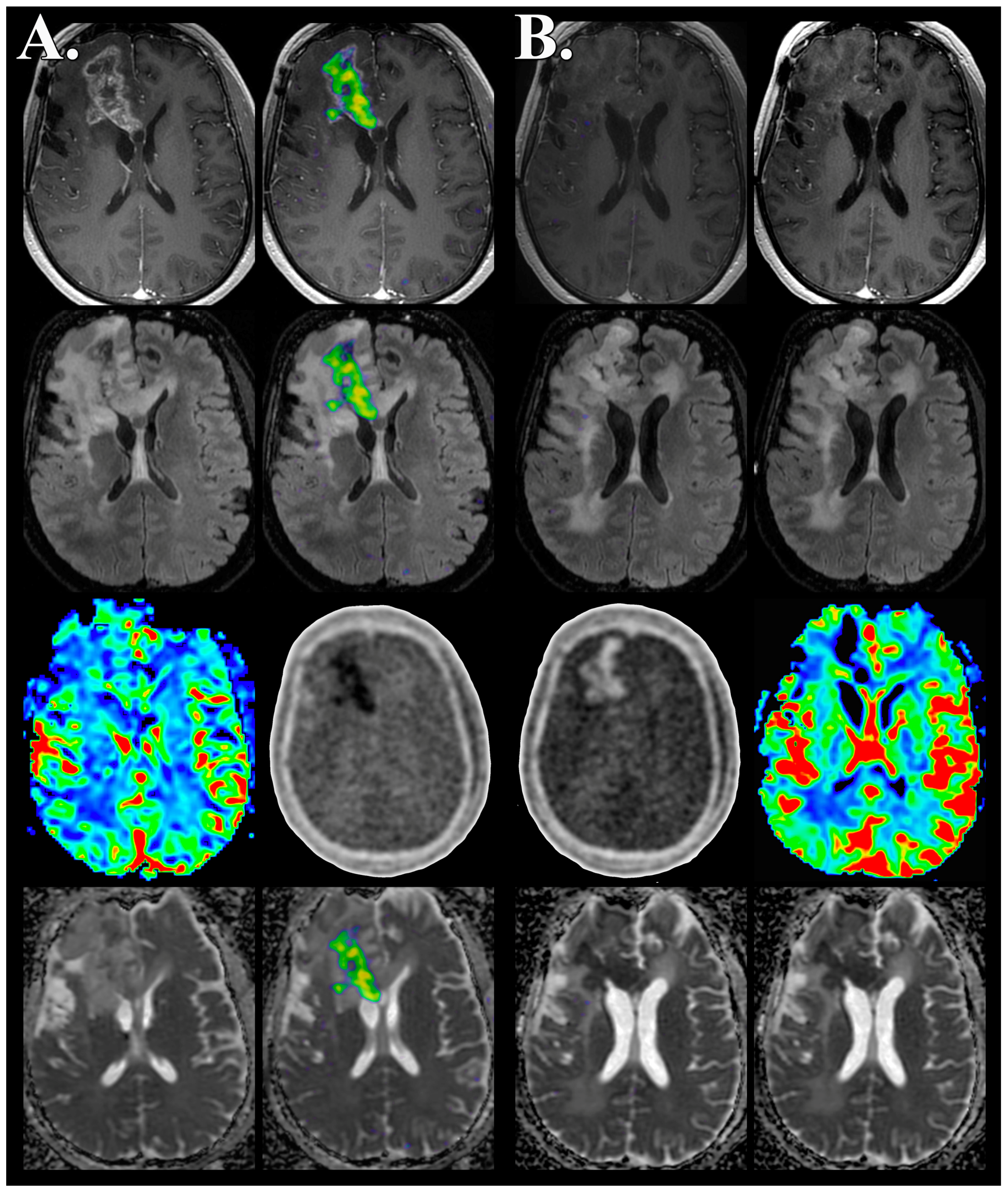
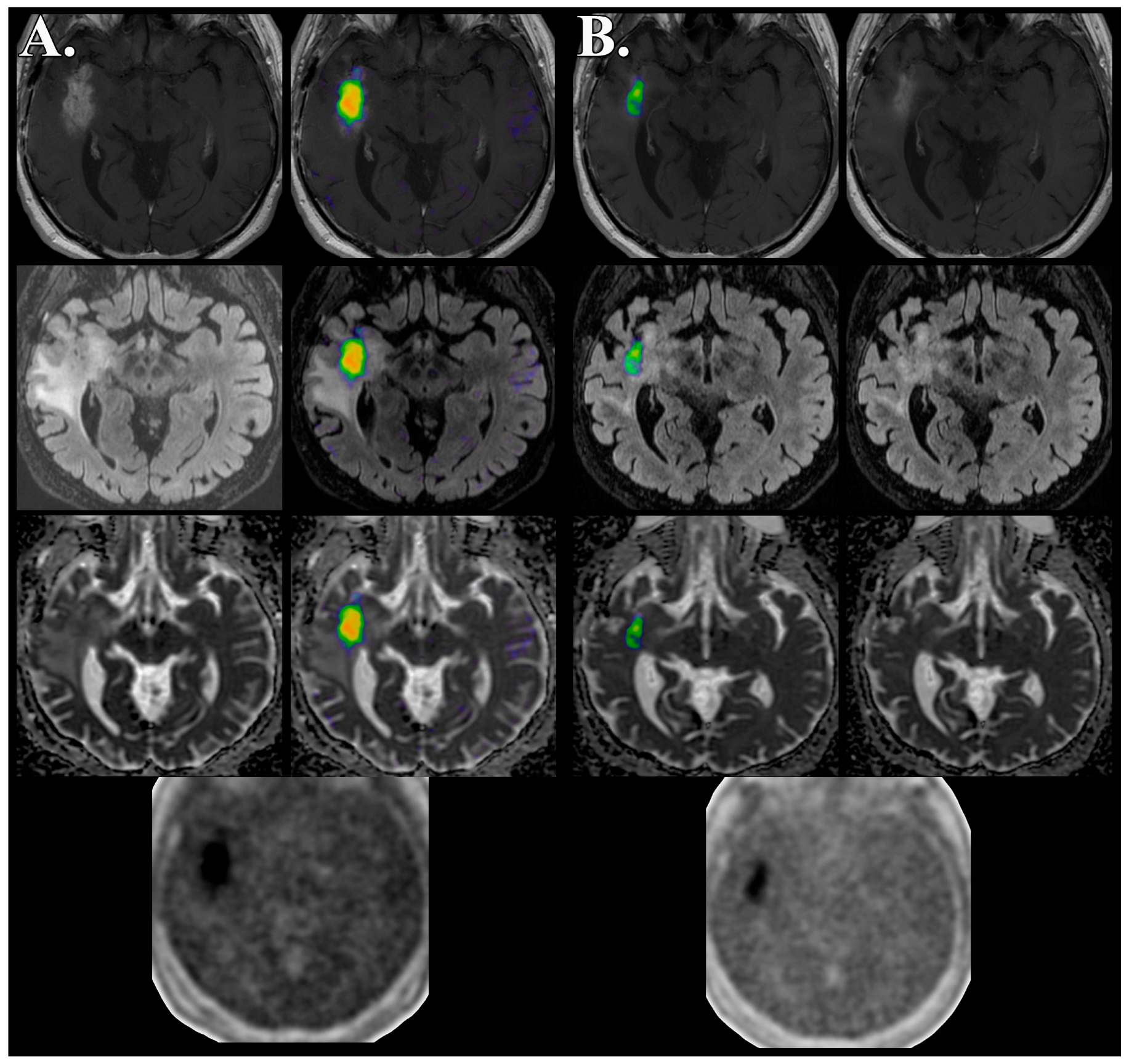
| Patient # | Age/Sex | Initial Dx | Molecular Profile | FMISO PET/MRI Time | Clinical Outcome |
|---|---|---|---|---|---|
| 1 | 61/M | AA | IDH Wild | 36 | Recurrence |
| 2 | 43/M | OA | IDH Mutant, 1P/19Q intact | 100 | Recurrence |
| 3 | 65/M | AA | IDH Wild | 46 | Recurrence |
| 4 | 52/W | AA | NOS | 155 | Pseudoprogression |
| Patient # | 1 | 2 | 3 | 4 | Mean | Standard Deviation |
|---|---|---|---|---|---|---|
| Hypoxic Volume | −19.2 | −97.1 | −86.5 | 0 | −50.75 | 48.25 |
| Hypoxic Volume T/Bmean | −3.06 | −18.2 | 5.78 | NA | −5.16 | 10.23 |
| Hypoxic Volume T/Bmax | −13.1 | −31.1 | −28.9 | NA | −24.37 | 14.58 |
| FLAIR Volume | −59.9 | 3.26 | 7.32 | −14.1 | −15.86 | 30.80 |
| FLAIR T/Bmean | 2.51 | −22.6 | −1.09 | 14.1 | −1.77 | 15.32 |
| FLAIR T/Bmax | −27.9 | −46.7 | −51.4 | 62.1 | −15.98 | 53.03 |
| Enhancing Volume | −80.4 | −96.9 | −95.8 | −41.4 | −78.63 | 25.93 |
| Enhancing T/Bmean | 34.1 | −33.3 | −1.92 | 11.5 | 2.60 | 28.17 |
| Enhancing T/Bmax | −27.9 | −46.7 | −51.4 | 62.1 | −15.98 | 53.03 |
| Lesion rCBV | −22.0 | −50.1 | −46.2 | −0.04 | −30.5 | 17.3 |
| Lesion rCBF | −18.4 | −12.1 | −0.04 | −0.01 | −0.22 | 0.08 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barajas, R.F.; Krohn, K.A.; Link, J.M.; Hawkins, R.A.; Clarke, J.L.; Pampaloni, M.H.; Cha, S. Glioma FMISO PET/MR Imaging Concurrent with Antiangiogenic Therapy: Molecular Imaging as a Clinical Tool in the Burgeoning Era of Personalized Medicine. Biomedicines 2016, 4, 24. https://doi.org/10.3390/biomedicines4040024
Barajas RF, Krohn KA, Link JM, Hawkins RA, Clarke JL, Pampaloni MH, Cha S. Glioma FMISO PET/MR Imaging Concurrent with Antiangiogenic Therapy: Molecular Imaging as a Clinical Tool in the Burgeoning Era of Personalized Medicine. Biomedicines. 2016; 4(4):24. https://doi.org/10.3390/biomedicines4040024
Chicago/Turabian StyleBarajas, Ramon F., Kenneth A. Krohn, Jeanne M. Link, Randall A. Hawkins, Jennifer L. Clarke, Miguel H. Pampaloni, and Soonmee Cha. 2016. "Glioma FMISO PET/MR Imaging Concurrent with Antiangiogenic Therapy: Molecular Imaging as a Clinical Tool in the Burgeoning Era of Personalized Medicine" Biomedicines 4, no. 4: 24. https://doi.org/10.3390/biomedicines4040024
APA StyleBarajas, R. F., Krohn, K. A., Link, J. M., Hawkins, R. A., Clarke, J. L., Pampaloni, M. H., & Cha, S. (2016). Glioma FMISO PET/MR Imaging Concurrent with Antiangiogenic Therapy: Molecular Imaging as a Clinical Tool in the Burgeoning Era of Personalized Medicine. Biomedicines, 4(4), 24. https://doi.org/10.3390/biomedicines4040024





