Abstract
Innate lymphoid cells (ILCs) are a group of immune cells of the lymphoid lineage that do not possess antigen specificity. The group includes natural killer (NK) cells, lymphoid tissue inducer (LTi) cells and the recently identified ILC1s, ILC2s and ILC3s. Although the role of NK cells in the context of cancer has been well established, the involvement of other ILC subsets in cancer progression and resistance is just emerging. Here, we review the literature on the role of the different ILC subsets in tumor immunity and discuss its implications for cancer treatment and monitoring.
1. Introduction
The immune system is specialized in the detection and eradication of tumor cells that have developed following a failure of intrinsic tumor-suppression mechanisms. In the initial phase of the anti-tumor immune response, tissue-resident innate immune cells, such as macrophages, are stimulated to produce pro-inflammatory cytokines and chemokines. This results in the attraction of other innate cells, including natural killer (NK) cells that can directly lyse transformed cells. In a later phase, dendritic cells (DCs) take up tumor antigens released by dying tumor cells and present those in the lymph node to naïve T cells, resulting in a wave of antigen-specific cytotoxic T lymphocytes (CTLs) and helper T cells that further aid tumor destruction. Increased understanding of all players involved in tumor immunity is important for the development of new, and improvement of current, cancer therapies. Recently, the family of innate cells to which NK cells belong, has been expanded to include newly identified members. These innate lymphoid cells (ILCs) are characterized by a classic lymphoid cell morphology, but lack lineage-specific markers and somatically rearranged antigen receptors. Based on their cytokine profiles and set of expressed transcription factors, they have been divided into three distinct subclasses: group 1 ILCs, group 2 ILCs and group 3 ILCs [1].
Group 1 ILCs are characterized by their ability to secrete interferon (IFN)-γ and include NK cells and ILC1s [1]. Upon activation, NK cells release perforin and granzyme molecules, allowing them to lyse targets like virus-infected or transformed cells [2]. In addition, they secrete pro-inflammatory cytokines. As their anti-tumor effector functions have been extensively reviewed elsewhere [3], they are out the scope of this review. ILC1s highly express the transcription factor T-bet and can be subdivided based on CD127 expression. Where CD127low ILC1s are mostly present in the intraepithelial layer [4], CD127high ILC1s are found in the lamina propria [5]. ILC1s respond to IL-12, IL-15 and IL-18, upon which they can secrete IFN-γ and tumor necrosis factor (TNF)-α [4,5,6]. Similar to T helper 1 (Th1) cells, ILC1s mediate immunity against intracellular bacteria and parasites [6,7]. However, they can play a pathogenic role in chronic inflammation, as numbers of ILC1s are elevated in the intestine of Crohn’s disease patients [4,5].
ILC2s make up group 2 ILCs and are dependent on transcription factor GATA-binding protein 3 for their development and maintenance [8,9]. They can be stimulated by IL-33, IL-25 or thymic stroma lymphopoietin (TSLP), alarmins secreted by epithelial cells upon cellular stress and tissue damage [10,11]. ILC2s release predominantly IL-5 and IL-13, but have also been reported to secrete IL-4, IL-9 and amphiregulin [12,13,14,15]. They are involved in tissue repair [15], anti-helminth immunity [12,13,16] and allergic inflammation [10,17,18]. ILC2s are found in lung, skin and gut, but small numbers of circulating ILC2s can also be detected in blood [10].
Group 3 ILCs, which include lymphoid tissue inducer (LTi) cells and ILC3s, are characterized by the expression of transcription factor RAR-related orphan receptor (ROR)γt [19,20,21]. These cells can be stimulated by IL-1β and IL-23 and mainly produce IL-17 and/or IL-22 [22,23,24]. Whereas LTi cells promote the formation of tertiary lymphoid structures [25], ILC3s are involved in antibacterial immunity, tissue homeostasis and repair and chronic inflammation [22,26,27,28]. ILC3s can be subdivided based on expression of certain natural cytotoxicity receptors (NCRs): NKp46 in mice and NKp44 in humans [29]. Engagement of NKp44 on human NCR+ ILC3s has been associated with a more pro-inflammatory cytokine response, including the release of TNF-α, as opposed to the release of IL-22 which is induced upon stimulation with cytokines like IL-23 [30]. NCR− ILC3s are mainly found in healthy skin [31], while NCR+ ILC3s are the predominant ILC subset in healthy intestine and tonsil [29,32].
Based on transcriptional activity, cytokine profiles and effector functions, it is clear that ILCs strongly resemble the different helper T cell subsets: ILC1s and Th1 cells, ILC2s and Th2 cells and ILC3s and Th17 cells. In a similar way, NK cells can be viewed as the innate counterpart of CTLs. The role of helper T cells, CTLs and NK cells in tumor resistance and progression has been largely established, but data about ILCs are limited. However, these often overlooked immune cells may play a critical role in tumor immunity. As they do not require antigen sensing for their activation, ILCs respond more rapidly than adaptive T cells. While NK cells circulate in the blood, the other ILCs reside in mucosa and mucosal-associated lymphoid tissues. These ILCs are therefore possibly among the first to respond to, or induce formation of, tissue-resident tumors. Their effects are mostly related to cytokines released upon activation and thus vary among the different subsets. Depending on the secreted cytokines and the specific tumor microenvironment, ILCs may either aid anti-tumor immune responses or promote tumor formation and growth. Here, we summarize each subset in the context of tumor immunology.
2. ILC1s in Tumor Immunity
The main effector function of activated ILC1s is the production of IFN-γ, while lower levels of TNF-α might also be produced. Although there is currently no direct evidence linking ILC1s with tumor immunity, the effect of their secreted cytokines has been extensively investigated.
IFN-γ acts directly on tumor cells to upregulate expression of major histocompatibility complex (MHC) class I, thereby increasing anti-tumor immune responses [33]. Furthermore, IFN-γ can inhibit tumor cell proliferation [34] and angiogenesis [35], while promoting apoptosis [36]. In addition to its direct anti-tumorigenic effects, IFN-γ also enhances activity of cytotoxic effector cells, including CTLs, NK cells and macrophages [37,38,39]. Moreover, it is an important cytokine for the polarization of CD4+ T cells into Th1 cells, that in turn contribute to anti-tumor CTL and macrophage responses [40,41]. However, it should be noted that IFN-γ may have pro-tumorigenic effects in other settings, where it is implicated to actually enhance proliferation and to mediate tumor resistance to CTL- and NK-mediated killing [42].
TNF-α has been shown to mediate anti-tumor immunity as well. It mediates recruitment and stimulation of macrophages and DCs, resulting in strong anti-tumor responses [43,44]. Furthermore, in vivo models demonstrate that TNF-α signaling is required for CTL generation and that TNF-α secreted by CTLs directly induces tumor rejection [45,46]. However, dysregulated TNF-α signaling can also promote tumor formation and growth. On a cellular level, TNF-α induces growth [47], angiogenesis [48] and migration [49]. It mediates epithelial-mesenchymal transition [50], leading to a loss of cell-cell contact and thereby enhanced migration, which is a hallmark of metastasis. Mouse models of colitis-associated colorectal cancer (CRC) and skin cancer show that blocking of TNF-α can prevent tumor formation [51,52].
Both IFN-γ and TNF-α can thus play a dual role in tumor immunity. It seems likely that ILC1s are able to promote anti-tumor immunity (Figure 1), though their role might be hampered in cancer patients. This was shown in acute myeloid leukemia patients, where circulating ILCs display a reduced capacity to produce IFN-γ and TNF-α [53]. The anti-tumor potential of ILC1s has been corroborated by a recent study describing ILC1-like cells expressing NK1.1, CD49a and CD103. Unlike CD127+ ILC1s, these cells express granzyme B and TRAIL and can efficiently lyse tumor cells, which was crucial for immunosurveillance in a mammary tumor model [54]. It remains to be established whether this cell type represents a new entry in the group 1 ILCs and whether an equivalent exists in humans. Thus, further research is required to elucidate the role and interactions of ILC1s in cancer.
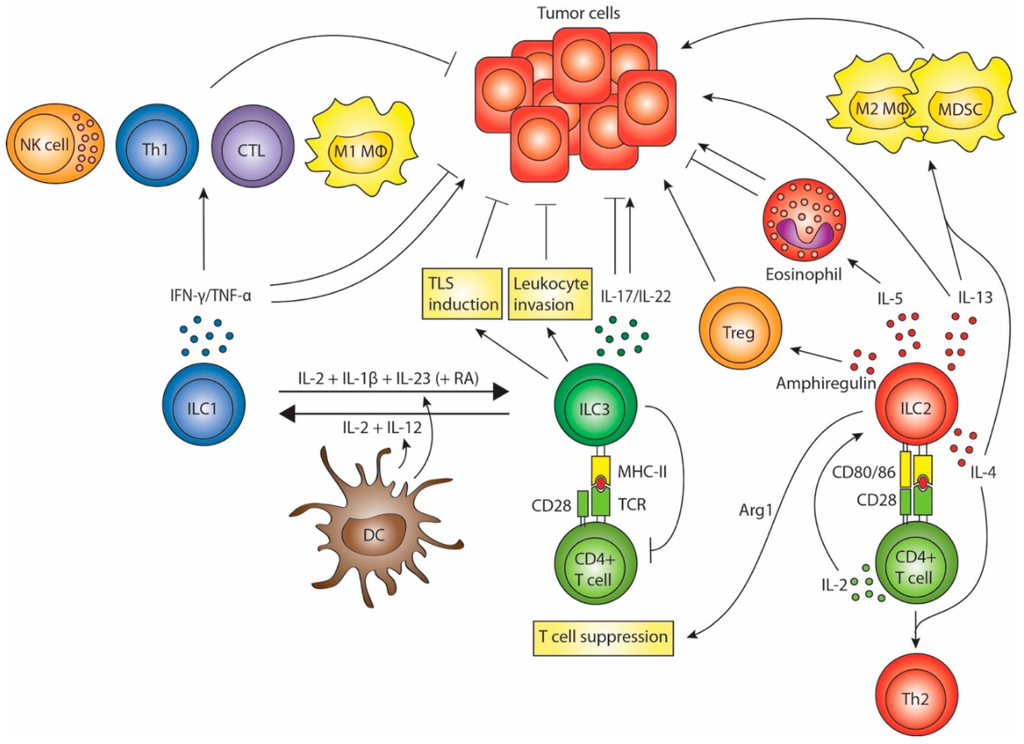
Figure 1.
ILC (Innate lymphoid cell) interactions in tumor immunity. ILC1s secrete IFN-γ and TNF-α, which can have anti- or pro-tumorigenic effects. ILC3s may promote tumor formation and progression by secreting IL-17 and IL-22 and possibly by suppressing T cell responses. Conversely, ILC3s might promote anti-tumor responses by enhancing leukocyte invasion, promoting tertiary lymphoid structure induction and through the anti-tumor effects of IL-17 and IL-22. ILC2s may contribute to tumor progression either directly through the tumorigenic effects of IL-13, or indirectly by stimulating M2 macrophages and myeloid-derived suppressor cells through IL-13 and IL-4. ILC2-associated eosinophilia could inhibit metastasis formation, but may also exert pro-tumorigenic effects. Production of amphiregulin and arginase-1 suggests that ILC2s may inhibit T cell responses either directly or via stimulation of regulatory T cells. Finally, ILC2s can skew CD4+ T cells to a Th2 phenotype in an indirect (via secretion of type 2 cytokines) or direct manner (via activation of naïve CD4+ T cells). Whether these effects of ILC2s on T cells hamper anti-tumor responses is not known. ILCs are functionally plastic. ILC1s can differentiate into ILC3s and vice versa, a process which is mediated by DCs.
3. ILC2s in Tumor Immunity
Where type 1 responses favor tumor immune surveillance, type 2 responses are generally associated with an environment promoting tumor formation and progression. The central role of IL-33 in inducing tumor-promoting type 2 responses has recently gained attention. Administration of IL-33 in a murine model of breast cancer resulted in increased tumor growth and development of metastases, which was correlated with increased intratumoral numbers of IL-13-producing ILCs, IL-13 receptor α 1-expressing myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs) [55]. IL-33 stimulates ILC2s to secrete large amounts of IL-13, which has been shown to activate tumor-promoting MDSCs and their production of anti-inflammatory transforming growth factor-β (TGF-β) [56]. In addition, IL-13 can polarize macrophages towards a pro-tumorigenic M2 phenotype [57]. Although direct evidence is currently lacking, it hence seems likely that ILC2s contribute to tumor immune evasion via IL-13-mediated stimulation of tumor-associated myeloid cells.
ILC2-derived IL-13 might also induce de novo malignant transformation. Patients suffering from liver cirrhosis have elevated serum levels of IL-33 [58]. In a mouse model of hepatic fibrosis, the effects of IL-33 were shown to be related to activation and proliferation of liver-resident ILC2s. Their production of IL-13 activated hepatic stellate cells, resulting in hepatic fibrosis [59]. Liver fibrosis is linked to an increased risk of developing hepatocellular cancer [60]. Since ILC2s appear to play a role in the development of fibrosis, it is possible that these cells are involved in the transition from fibrosis to hepatocellular cancer as well. More direct evidence for a role of ILC2s in carcinogenesis exists for cholangiocarcinoma. Administration of IL-33 in a mouse model of biliary atresia induced increased numbers of ILC2s that secreted IL-13, ultimately resulting in cholangiocyte hyperplasia [61].
ILC2s may have an anti-tumor effect as well. Besides secreting IL-13, activated ILC2s produce high amounts of IL-5. This cytokine acts on eosinophils, which can release a storm of various cytokines, cytotoxic granules, cationic proteins and other factors. IL-5-producing ILC2s were shown to inhibit the formation of lung metastases in mice via recruitment and activation of eosinophils [62]. Depletion of IL-5 in this model resulted in an increase of metastases. Nevertheless, eosinophils have also been implicated to promote tumor growth and are associated with a poor prognosis in several cancer types [63,64].
Furthermore, ILC2s express molecules that are associated with T cell suppression. An increased frequency of circulating ILC2s can be detected in the blood of gastric cancer patients [65]. This is accompanied by an increase in IL-33, IL-25, IL-5 and IL-4, but paradoxically a decrease of IL-13. Interestingly, mRNA levels of iNOS and arginase-1 (Arg1) are increased as well, which may indicate an increase in MDSCs and M2 macrophages. Arg1 is an enzyme that metabolizes l-arginine into urea and l-ornithine. When T cells are depleted of l-arginine, it results in expression of defective T cell receptors and a halt of their cell cycle progression [66,67]. Increased Arg1 activity in the tumor microenvironment by myeloid cells can therefore lead to tumor immune evasion [68]. Arg1 production in murine macrophages is induced by IL-4 and IL-13 [69,70], cytokines that can be produced by ILC2s. Moreover, ILC2s themselves constitutively express Arg1 under both homeostatic conditions and during helminth infections [71]. Hence, it is possible that ILC2s inhibit T cell responses in the tumor microenvironment via their Arg1 activity and upregulation of Arg1 in myeloid cells. ILC2s might also induce immune suppression via secretion of amphiregulin [72]. Amphiregulin enhances Treg activity in vivo and can thereby inhibit anti-tumor immune responses induced by DC vaccination [73].
Finally, ILC2s can alter adaptive immune responses by skewing naïve CD4+ T cells to a Th2 phenotype. In T cells, IL-4 induces STAT6-mediated expression of GATA-binding protein 3, the master transcription factor of Th2 cells [74]. Cytokines like IL-4 act as a polarizing signal (signal 3) during T cell priming. For naïve T cells to become effector cells however, they also require signal 1 (T cell receptor binding of antigen-presenting MHC) and signal 2 (co-stimulation). By providing a type 2 cytokine milieu, ILC2s can therefore steer differentiation of activated T cells [75]. In addition, ILC2s may also directly activate naïve T cells, by acting as antigen-presenting cells. ILC2s can express both MHC class II and co-stimulatory molecules, including OX40L, ICOS, CD80 and CD86 [75,76,77,78]. Culture of CD4+ T cells with antigen-pulsed ILC2s in vitro leads to T cell differentiation into Th2 cells and a blockade of Th1 differentiation [77,78]. ILC2-T cell crosstalk also leads to proliferation and increased cytokine secretion of ILC2s, which is mediated by T cell-derived IL-2 [77,78]. Moreover, depletion of ILC2 in vivo causes diminished Th2-mediated responses [18,77]. In one such model, ILC2-derived IL-13 was critical for migration of DCs from the lung to draining lymph nodes where they were able to activate and prime naïve T cells [18]. Thus, ILC2-mediated Th2 skewing can occur both locally and at a distance. Whether ILC2-induced Th2 skewing hampers adaptive anti-tumor responses remains to be established.
Collectively, these data mainly point towards an immunosuppressive role of ILC2s in tumor immunity (Figure 1) and they therefore seem an interesting target for cancer immunotherapies.
4. ILC3s in Tumor Immunity
The pathogenic role of ILC3s has almost exclusively been studied in the gut. This group of innate cells was recently shown to be involved in inflammatory bowel disease (IBD). IBD, which includes ulcerative colitis and Crohn’s disease, is characterized by chronic inflammation of the intestine. Critical in immune-mediated chronic inflammation is the IL-23/IL-17 cytokine axis [79]; IL-23, which is upregulated in active disease, mediates the release of the pro-inflammatory cytokine IL-17 from Th17 cells. IL-23 also acts on ILC3s to release IL-17 and this was essential for the development of bacteria-driven colitis in Rag−/− and T-bet−/−.Rag2−/− mice [26,80]. In support of these findings, the inflamed intestine of Crohn’s disease patients show increased expression of IL17A and IL17F among CD3− cells [81]. Interestingly, tissue-infiltrating neutrophils were identified as the main source of IL-23 in IBD [82]. Furthermore, IL-17 signaling can synergize with TNF-α to induce secretion of neutrophil-attracting chemokines by intestinal epithelial cells [80]. Hence, a positive feedback loop might be formed between IL-23-producing neutrophils and IL-17-producing Th17 cells and ILC3s. Long-standing inflammation can promote tumor formation and as such, patients suffering from IBD are at an increased risk of developing CRC [83]. This raises the question whether ILC3s are also involved in the formation of malignancies.
There is ample evidence that IL-23, the upstream mediator of ILC3s, is involved in carcinogenesis. Human colon tumors have elevated levels of IL-23 compared to healthy tissue [84]. Furthermore, mice deficient for IL-23 are resistant to tumor formation induced by chemical carcinogenesis and the growth of transplanted tumor cell lines was inhibited in mice deficient for the IL-23 receptor [84]. Blockade of the IL-23 receptor also inhibits bacterial-induced colonic tumor formation [85]. Transgenic expression of IL-23 in wild-type mice even results in adenoma formation independent of carcinogens [86]. In this particular model, IL-17-producing ILC3s were essential for the adenoma formation. In human CRC, IL-17 promotes angiogenesis via vascular endothelial growth factor (VEGF) production by tumor cells and is correlated with a poor prognosis [87]. The association between IL-17 and angiogenesis has also been established in other human cancers, including breast [88], lung [89], pancreatic [90], hepatocellular [91] and gastric cancer [92]. In addition to its angiogenic effects, IL-17 may also induce MDSCs and their recruitment at the tumor site, as demonstrated by experiments performed in tumor-bearing mice [93].
ILC3s may also release large amounts of IL-22 upon stimulation by IL-23. Under homeostatic conditions, IL-22 is inhibited by IL-22 binding protein (IL-22-BP), a soluble receptor secreted by immature DCs [94]. During cellular damage, IL-22BP is downregulated, allowing IL-22 to induce tissue repair. However, aberrant proliferation induced by IL-22 may promote tumor growth [95]. Moreover, in a colitis-associated colon cancer model, IL-22BP-deficient mice showed accelerated tumor development and an increase in quantity and size of tumors compared to wild type control mice, suggesting an important role for IL-22 in colitis-associated CRC [96]. In line with these results, IL-22-producing ILC3s were shown to promote tumor growth in a mouse model of bacteria-induced CRC, as depletion of IL-17+ IL-22+ ILC3s prevented the development of malignancies [97]. In this model, IL-17 only played a minor role in promoting tumor growth. Blocking of IL-17 merely reduced inflammation, while blocking of IL-22 led to a significant decrease in tumor burden. IL-22 produced by ILC3s may play an important role in human CRC as well, as both IL-22+ CD3+ and IL-22+ CD3− cells can be detected within CRC tumors [97].
Similar to ILC2s, NCR− ILC3s can present antigen on MHC class II to T cells. However, only splenic ILC3s are able to induce T cell responses [98]. In contrast, intestinal ILC3s lack co-stimulatory molecules and thus limit T cell responses [99]. Instead, these intestinal ILC3s induce T cell death via outcompeting the T cells for IL-2. This mechanism of T cell inhibition is important for maintaining intestinal tolerance: mice with ILC3s lacking MHC class II have increased intestinal inflammation due to T cell responses against commensal bacteria [99]. Whether these activities also contribute to tumor immune evasion in the gut is currently unknown.
In sharp contrast, ILC3-produced IL-17 and IL-22 may also promote tumor immunity in other settings. IL-17 was shown to boost tumor-antigen specific CTL responses in mice engrafted with hematopoietic tumors [100]. In addition, IL-17-deficient mice inoculated with a colon cancer cell line had increased tumor growth and metastasis compared to wild type control mice, which was correlated with decreased NK and tumor-specific T cell responses [101]. Furthermore, IL-22 was shown to reduce tumor growth in a breast cancer model [102]. In addition, although prolonged IL-22 production promotes tumor growth in a colitis-associated colon cancer model, it is suggested that IL-22 in the early phase of colitis actually protects against tumor formation [96].
A study by Carrega et al. showed more direct evidence that ILC3s may exhibit anti-tumorigenic effects [103]. The authors found that, while only low numbers of NCR+ ILC3s are present in healthy lung tissue, a higher frequency is present in non-small cell lung cancer. NCR+ ILC3s especially accumulated on the edge of intratumoral tertiary lymphoid structures (TLSs), which are associated with favorable prognosis. Moreover, NCR+ ILC3s were correlated with increased TLS density and less advanced tumor stage, suggesting that via induction of TLSs they promote tumor immunity. Importantly, the NCR+ ILC3s can recognize and interact with both tumor cells and tumor-associated fibroblasts via NKp44. This results in significant secretion of TNF-α and IL-8, but only low amounts of IL-22 and IL-2. NCR+ ILC3s in mice have also been linked to tumor suppression. Administration of IL-12 in a melanoma model led to a reduction of tumor growth, which was mediated by NCR+ ILC3s [104]. The mechanism behind this tumor suppression involved an upregulation of adhesion molecules in the tumor microenvironment, leading to enhanced leukocyte invasion. Group 3 ILCs were also crucial for the anti-tumor effects of a combination of chemotherapy and antibodies directed against a tumor antigen in a melanoma mouse model deficient for adaptive immunity [105]. In this model, CD90+ NK1.1− ILCs expressing RORγt promoted macrophage infiltration, which was suggested to be the cause of the tumor growth arrest.
LTi cells are phenotypically very similar to ILC3s. These cells are crucial for the development of the lymphatic system in the embryonic state, but remain present during adult life and are implicated to help maintain lymphoid structures [23]. They can take part in innate immune responses, as LTi cells were critical for resolving an infection of Citrobacter rodentium in mice [106]. Their role in tumor immunity is not clear and research is hampered by the lack of a good definition of this cell type. However, one study linked LTi cells with tumor immune evasion [107]. This study showed that melanoma cells overexpressing chemokine CCL21 promotes attraction of Tregs, MDSCs and naïve T cells and induces lymphoid-like stroma, resulting in enhanced tumor growth. CCL21 overexpression in LTi-depleted mice did not enhance tumor growth, suggesting that LTi cells play a role in CCL21-dependent immunological tolerance.
Altogether, the ambiguous role of the ILC3s in tumor immunity shows similarities with that of its adaptive counterpart, the Th17 cell. Whether the effect of ILC3s is tumorigenic or anti-tumorigenic seems to depend on the specific subset, the timing of their responses and the specific tumor microenvironment (Figure 1). Especially in inflammation-associated cancers, prolonged ILC3-mediated responses seem to promote tumor progression.
5. ILC Plasticity and Crosstalk with DCs
Not only do ILCs act directly on tumor and tumor-associated cells, they also possess the ability to steer adaptive T cell responses. Another cell type that should not be neglected in ILC biology is the DC. DCs form a crucial link between the adaptive and innate immune system [108]. By acting as professional antigen-presenting cells, they initiate antigen-specific T and B cell responses and are therefore essential for induction of a long-lasting anti-tumor immune response. Besides, DCs also interact with innate and innate-like immune cells such as NK cells, natural killer T cells and γδ T cells and these interactions might be utilized to improve upon current anti-cancer immunotherapies [109]. Evidence is now accumulating that DCs interact with ILCs as well, especially in the gut.
NCR+ ILC3s form the main ILC population present in healthy intestine. In contrast, the inflamed intestine of Crohn’s disease patients mostly contains CD127+ ILC1s. Bernink et al. have shown that this discrepancy is due to ILC plasticity [32]. The differentiation of ILC3s into CD127+ ILC1s in vitro can be mediated by either CD14+ DCs or addition of IL-12 [5,32]. It is likely that this process occurs in a physiological setting as well, as greater numbers of CD14+ DCs are present in inflamed tissue of Crohn’s disease patients and they show increased expression of IL12A [32]. This plasticity is reversible, as CD127+ ILC1s differentiate into NCR+ ILC3s when stimulated with IL-2, IL-1β and IL-23 [32]. Retinoic acid further enhances the conversion. These findings indicate a role of DC-induced ILC plasticity in gut immunity and homeostasis. An infection in the gut may cause an influx of CD14+ inflammatory DCs that release IL-12, resulting in the conversion of ILC3s into ILC1s. These ILC1s subsequently amplify inflammatory type 1 immune responses and aid in clearing the infection. When the infection is resolved, CD14+ DCs leave and tissue-resident CD14− DCs can convert CD127+ ILC1s back into NCR+ ILC3s via secretion of IL-23 and retinoic acid. Taken together, DCs can control the fate of ILCs by exposing them to a certain cytokine milieu (Figure 1).
In Crohn’s disease, ILCs take part in an inflammatory feedback loop. Loss of mucosal integrity and exposure to microbial gut flora leads to a state of chronic inflammation. On one hand, this causes prolonged activation of ILC3s, resulting in secretion of high levels of IL-17 that further aggravate the inflammatory response [81]. On the other hand, it results in a persistent increase in IFN-γ-producing ILC1s that too enhance inflammatory responses and may therefore contribute to the disease [5]. With this in mind, Bernink et al. proposed that forcing a switch of IFN-γ-producing ILC1s to IL-22-producing ILC3s holds therapeutic effect in Crohn’s disease [32]. Consequently, this might also prevent the development of inflammation-associated tumors in those patients. One can speculate that in certain cancers, an imbalance between ILC subsets exists as well. Contrary to the chronic inflammatory status in Crohn’s disease, most tumor microenvironments dampen immune responses and thereby evade immune detection. Hence in this setting, an increase in IFN-γ-producing ILC1s might be beneficial.
In vitro and in vivo experiments have shown that colonic DCs are an important source of IL-23, the main activator of ILC3s and LTi cells [80,110,111]. However, ILCs might also activate DCs. In the murine gut, ILC3s were identified as the main producers of GM-CSF, which they release upon stimulation by macrophage-derived IL-1β [28]. GM-CSF is required for optimal differentiation and function of tissue-resident macrophages and DCs. Consequently, mice deficient for GM-CSF have reduced numbers and activity of macrophages and DCs [28]. This indicates that crosstalk between DCs and ILCs is bidirectional.
6. ILCs in Cancer Treatment
Classic ways to treat cancer include surgery, radiation and chemotherapy. In recent years, much progression has been made in what now is the fourth pillar of cancer therapy: immunotherapy. Cancer is now seen as a disease arising from inadequate resolving of neoplastic cells by the immune system. Tumor cells do not only evolve ways to evade the immune system themselves, via up- and downregulation of membrane molecules and secretion of soluble factors, but often create and depend on a mesh of other cells that help them in suppressing the immune system. Characterization of this tumor microenvironment has therefore become a useful tool for monitoring disease progression and predicting clinical outcome. Since different ILC subsets can exert either pro- or anti-tumorigenic effects, their detection in patient blood and/or biopsies might contribute to a better assessment of disease status. There are however some drawbacks to implementing ILC detection in disease monitoring. The presence of ILCs in peripheral blood is very low. They make up only 0.01% to 0.1% of all circulating lymphocytes and most of these ILCs are ILC2s [112]. Still, changes in these percentages may be detected in certain cancer types. It was reported that in acute myeloid leukemia, numbers of ILCs are decreased and show a relative increase of ILC1s and decrease of NCR+ ILC3s [53]. More research is needed to determine if changes in ILC populations have a prognostic value. However, since higher numbers of ILCs reside in mucosal tissues, detection of ILCs in patient biopsies seems preferable over detecting them in blood. Yet obtaining multiple biopsies during the course of the disease puts a big burden on patients. Another hurdle to overcome is the amount of markers that are needed for ILC identification, which can be a limitation in particular for immunohistochemistry. Moreover, multiple different names and markers have been used in different studies for characterization of ILCs [1]. Advances in this field will therefore require the development of standardized protocols for ILC detection.
Besides their potential use as a prognostic marker, ILCs also pose as interesting targets for immunotherapy. Adoptive transfer of ILC subsets that exert anti-tumorigenic effects, or depletion or blocking of ILC subsets that promote tumor induction or progression can be two possible therapeutic strategies. The plasticity of ILCs could also be exploited to tip the balance between certain ILC subsets. In settings were ILC3s contribute to tumor development and progression, their conversion into IFN-γ-secreting ILC1s might be a method to improve tumor rejection. Cytokine injections could be one way to promote ILC polarization. However, administration of cytokines can have severe side effects, as with IL-2 immunotherapy is shown [113]. Another method could be DC vaccination, considering that DCs can induce phenotype switching of ILCs and mediate ILC activation. For this purpose, the injected DCs should be engineered in such a way that they secrete factors that mediate the generation and activation of the ILC subset of interest. Blocking factors that polarize ILCs in a disease-promoting subset should also be considered. Retinoic acid blocking in certain cancers might, among other effects, inhibit conversion of ILC1s into pathogenic ILC3s. Interestingly, blocking of the retinoic acid receptor in a mouse model of melanoma increases efficacy of DC vaccination [114]. This is caused by a reduction in Tregs and an increase in IL-12 production by DCs. Whether ILCs play a role in this model has not been examined. Still, it might be worthwhile to investigate whether therapies targeting ILCs can improve current treatment modalities.
One of the most significant breakthroughs in tumor immunotherapy is the use of the so-called checkpoint inhibitors, antibodies directed against immunomodulatory molecules, such as CTLA-4 or PD-1 [115]. Tumor and tumor-associated cells often overexpress ligands for immunomodulatory receptors, thereby holding back effective T cell responses. Checkpoint inhibitor-based therapy aims at removing these breaks. There is rational for combining this with strategies targeting ILCs. The use of checkpoint inhibitors brings the immune system in a more activated state, thus making it more susceptible to the effects of cytokines released by ILCs. Moreover, the potential role of certain ILC subsets to promote leukocyte invasion and lymphoid structure formation or maintenance could be utilized to enhance access of activated effector cells to the tumor site. This potential application of ILCs should therefore warrant further investigation. Whether ILCs themselves are also directly affected by checkpoint inhibitors is not known. However, it has been reported that NK cells of multiple myeloma patients can express PD-1 and that anti-PD-1 antibodies enhance the effector functions of these NK cells [116].
7. Concluding Remarks
Since the discovery of tissue-resident ILCs, it has become clear that these cells play an important role in mucosal immunity and are involved in immune-mediated diseases such as IBD. Much less is currently known about their involvement in cancer. Limited data suggests that these ILCs can promote tumorigenesis, mediate tumor maintenance or contribute to anti-tumor immune responses, depending on the specific ILC subset and the microenvironment. ILCs show plasticity in their phenotype and can be polarized by DCs in a cytokine-dependent manner. This plasticity can possibly be exploited in future cancer therapies. However, more clinical research is needed to define the effects of ILCs as well as their usefulness as prognostic markers or therapeutic targets in cancer treatment.
Acknowledgments
This work was supported by NWO-VICI 91814655 to I. Jolanda M. de Vries.
Author Contributions
Writing and review of the manuscript: Jasper J. P. van Beek, Anne W. J. Martens, Ghaith Bakdash and I. Jolanda M. de Vries.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Spits, H.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate lymphoid cells—A proposal for uniform nomenclature. Nat. Rev. Immunol. 2013, 13, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Ugolini, S.; Blaise, D.; Chabannon, C.; Brossay, L. Targeting natural killer cells and natural killer T cells in cancer. Nat. Rev. Immunol. 2012, 12, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, A.; Vermi, W.; Lee, J.S.; Lonardi, S.; Gilfillan, S.; Newberry, R.D.; Cella, M.; Colonna, M. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-γ-producing cells. Immunity 2013, 38, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Bernink, J.H.; Peters, C.P.; Munneke, M.; te Velde, A.A.; Meijer, S.L.; Weijer, K.; Hreggvidsdottir, H.S.; Heinsbroek, S.E.; Legrand, N.; Buskens, C.J.; et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat. Immunol. 2013, 14, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Klose, C.S.N.; Flach, M.; Möhle, L.; Rogell, L.; Hoyler, T.; Ebert, K.; Fabiunke, C.; Pfeifer, D.; Sexl, V.; Fonseca-Pereira, D.; et al. Differentiation of Type 1 ILCs from a Common Progenitor to All Helper-like Innate Lymphoid Cell Lineages. Cell 2014, 157, 340–356. [Google Scholar] [CrossRef] [PubMed]
- Klose, C.S.N.; Kiss, E.A.; Schwierzeck, V.; Ebert, K.; Hoyler, T.; D’Hargues, Y.; Göppert, N.; Croxford, A.L.; Waisman, A.; Tanriver, Y.; et al. A T-bet gradient controls the fate and function of CCR6-RORγt+ innate lymphoid cells. Nature 2013, 494, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Hoyler, T.; Klose, C.S.N.; Souabni, A.; Turqueti-Neves, A.; Pfeifer, D.; Rawlins, E.L.; Voehringer, D.; Busslinger, M.; Diefenbach, A. The Transcription Factor GATA-3 Controls Cell Fate and Maintenance of Type 2 Innate Lymphoid Cells. Immunity 2012, 37, 634–648. [Google Scholar] [CrossRef] [PubMed]
- Mjösberg, J.; Bernink, J.; Golebski, K.; Karrich, J.J.; Peters, C.P.; Blom, B.; te Velde, A.A.; Fokkens, W.J.; van Drunen, C.M.; Spits, H. The Transcription Factor GATA3 Is Essential for the Function of Human Type 2 Innate Lymphoid Cells. Immunity 2012, 37, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Mjösberg, J.M.; Trifari, S.; Crellin, N.K.; Peters, C.P.; van Drunen, C.M.; Piet, B.; Fokkens, W.J.; Cupedo, T.; Spits, H. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat. Immunol. 2011, 12, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Siracusa, M.C.; Saenz, S.A.; Noti, M.; Monticelli, L.A.; Sonnenberg, G.F.; Hepworth, M.R.; Van Voorhees, A.S.; Comeau, M.R.; Artis, D. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci. Transl. Med. 2013, 5, 170ra16. [Google Scholar] [CrossRef] [PubMed]
- Fallon, P.G.; Ballantyne, S.J.; Mangan, N.E.; Barlow, J.L.; Dasvarma, A.; Hewett, D.R.; McIlgorm, A.; Jolin, H.E.; McKenzie, A.N.J. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J. Exp. Med. 2006, 203, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- Moro, K.; Yamada, T.; Tanabe, M.; Takeuchi, T.; Ikawa, T.; Kawamoto, H.; Furusawa, J.-I.; Ohtani, M.; Fujii, H.; Koyasu, S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature 2010, 463, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, C.; Hirota, K.; Stieglitz, B.; Van Snick, J.; Tolaini, M.; Lahl, K.; Sparwasser, T.; Helmby, H.; Stockinger, B. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat. Immunol. 2011, 12, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Monticelli, L.A.; Sonnenberg, G.F.; Abt, M.C.; Alenghat, T.; Ziegler, C.G.K.; Doering, T.A.; Angelosanto, J.M.; Laidlaw, B.J.; Yang, C.Y.; Sathaliyawala, T.; et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat. Immunol. 2011, 12, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Price, A.E.; Liang, H.-E.; Sullivan, B.M.; Reinhardt, R.L.; Eisley, C.J.; Erle, D.J.; Locksley, R.M. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc. Natl. Acad. Sci. USA 2010, 107, 11489–11494. [Google Scholar] [CrossRef] [PubMed]
- Halim, T.Y.F.; Krauß, R.H.; Sun, A.C.; Takei, F. Lung Natural Helper Cells Are a Critical Source of Th2 Cell-Type Cytokines in Protease Allergen-Induced Airway Inflammation. Immunity 2012, 36, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Halim, T.Y.F.; Steer, C.A.; Mathä, L.; Gold, M.J.; Martinez-Gonzalez, I.; McNagny, K.M.; McKenzie, A.N.J.; Takei, F. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity 2014, 40, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Eberl, G.; Marmon, S.; Sunshine, M.-J.; Rennert, P.D.; Choi, Y.; Littman, D.R. An essential function for the nuclear receptor RORγt in the generation of fetal lymphoid tissue inducer cells. Nat. Immunol. 2004, 5, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Sanos, S.L.; Bui, V.L.; Mortha, A.; Oberle, K.; Heners, C.; Johner, C.; Diefenbach, A. RORγt and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat. Immunol. 2009, 10, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Vonarbourg, C.; Mortha, A.; Bui, V.L.; Hernandez, P.P.; Kiss, E.A.; Hoyler, T.; Flach, M.; Bengsch, B.; Thimme, R.; Hölscher, C.; et al. Regulated expression of nuclear receptor RORγt confers distinct functional fates to NK cell receptor-expressing RORγt(+) innate lymphocytes. Immunity 2010, 33, 736–751. [Google Scholar] [CrossRef] [PubMed]
- Satoh-Takayama, N.; Vosshenrich, C.A.J.; Lesjean-Pottier, S.; Sawa, S.; Lochner, M.; Rattis, F.; Mention, J.-J.; Thiam, K.; Cerf-Bensussan, N.; Mandelboim, O.; et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity 2008, 29, 958–970. [Google Scholar] [CrossRef] [PubMed]
- Cupedo, T.; Crellin, N.K.; Papazian, N.; Rombouts, E.J.; Weijer, K.; Grogan, J.L.; Fibbe, W.E.; Cornelissen, J.J.; Spits, H. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat. Immunol. 2009, 10, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.; Becknell, B.; Freud, A.G.; McClory, S.; Briercheck, E.; Yu, J.; Mao, C.; Giovenzana, C.; Nuovo, G.; Wei, L.; et al. Interleukin-1beta selectively expands and sustains interleukin-22+ immature human natural killer cells in secondary lymphoid tissue. Immunity 2010, 32, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Mebius, R.E.; Rennert, P.; Weissman, I.L. Developing lymph nodes collect CD4+CD3- LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity 1997, 7, 493–504. [Google Scholar] [CrossRef]
- Buonocore, S.; Ahern, P.P.; Uhlig, H.H.; Ivanov, I.I.; Littman, D.R.; Maloy, K.J.; Powrie, F. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 2010, 464, 1371–1375. [Google Scholar] [CrossRef] [PubMed]
- Sonnenberg, G.F.; Monticelli, L.A.; Alenghat, T.; Fung, T.C.; Hutnick, N.A.; Kunisawa, J.; Shibata, N.; Grunberg, S.; Sinha, R.; Zahm, A.M.; et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science 2012, 336, 1321–1325. [Google Scholar] [CrossRef] [PubMed]
- Mortha, A.; Chudnovskiy, A.; Hashimoto, D.; Bogunovic, M.; Spencer, S.P.; Belkaid, Y.; Merad, M. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science 2014, 343, 1249288. [Google Scholar] [CrossRef] [PubMed]
- Hoorweg, K.; Peters, C.P.; Cornelissen, F.; Aparicio-Domingo, P.; Papazian, N.; Kazemier, G.; Mjösberg, J.M.; Spits, H.; Cupedo, T. Functional Differences between Human NKp44− and NKp44+ RORC+ Innate Lymphoid Cells. Front. Immunol. 2012, 3, 72. [Google Scholar] [CrossRef] [PubMed]
- Glatzer, T.; Killig, M.; Meisig, J.; Ommert, I.; Luetke-Eversloh, M.; Babic, M.; Paclik, D.; Blüthgen, N.; Seidl, R.; Seifarth, C.; et al. RORγt+ Innate Lymphoid Cells Acquire a Proinflammatory Program upon Engagement of the Activating Receptor NKp44. Immunity 2013, 38, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Villanova, F.; Flutter, B.; Tosi, I.; Grys, K.; Sreeneebus, H.; Perera, G.K.; Chapman, A.; Smith, C.H.; Di Meglio, P.; Nestle, F.O. Characterization of innate lymphoid cells in human skin and blood demonstrates increase of NKp44+ ILC3 in psoriasis. J. Investig. Dermatol. 2014, 134, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Bernink, J.H.; Krabbendam, L.; Germar, K.; de Jong, E.; Gronke, K.; Kofoed-Nielsen, M.; Munneke, J.M.; Hazenberg, M.D.; Villaudy, J.; Buskens, C.J.; et al. Interleukin-12 and -23 Control Plasticity of CD127+ Group 1 and Group 3 Innate Lymphoid Cells in the Intestinal Lamina Propria. Immunity 2015, 43, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Martini, M.; Testi, M.G.; Pasetto, M.; Picchio, M.C.; Innamorati, G.; Mazzocco, M.; Ugel, S.; Cingarlini, S.; Bronte, V.; Zanovello, P.; et al. IFN-gamma-mediated upmodulation of MHC class I expression activates tumor-specific immune response in a mouse model of prostate cancer. Vaccine 2010, 28, 3548–3557. [Google Scholar] [CrossRef] [PubMed]
- Bromberg, J.F.; Horvath, C.M.; Wen, Z.; Schreiber, R.D.; Darnell, J.E. Transcriptionally active Stat1 is required for the antiproliferative effects of both interferon alpha and interferon gamma. Proc. Natl. Acad. Sci. USA 1996, 93, 7673–7678. [Google Scholar] [CrossRef] [PubMed]
- Beatty, G.; Paterson, Y. IFN-gamma-dependent inhibition of tumor angiogenesis by tumor-infiltrating CD4+ T cells requires tumor responsiveness to IFN-gamma. J. Immunol. 2001, 166, 2276–2282. [Google Scholar] [CrossRef] [PubMed]
- Detjen, K.M.; Farwig, K.; Welzel, M.; Wiedenmann, B.; Rosewicz, S. Interferon γ inhibits growth of human pancreatic carcinoma cells via caspase-1 dependent induction of apoptosis. Gut 2001, 49, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Whitmire, J.K.; Tan, J.T.; Whitton, J.L. Interferon-gamma acts directly on CD8+ T cells to increase their abundance during virus infection. J. Exp. Med. 2005, 201, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Reiter, Z. Interferon—A major regulator of natural killer cell-mediated cytotoxicity. J. Interferon Res. 1993, 13, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Chakravarty, S.D.; Ivashkiv, L.B. Regulation of interferon and Toll-like receptor signaling during macrophage activation by opposing feedforward and feedback inhibition mechanisms. Immunol. Rev. 2008, 226, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Haabeth, O.A.W.; Lorvik, K.B.; Hammarström, C.; Donaldson, I.M.; Haraldsen, G.; Bogen, B.; Corthay, A. Inflammation driven by tumour-specific Th1 cells protects against B-cell cancer. Nat. Commun. 2011, 2, 240. [Google Scholar] [CrossRef] [PubMed]
- Hoepner, S.; Loh, J.M.S.; Riccadonna, C.; Derouazi, M.; Maroun, C.Y.; Dietrich, P.-Y.; Walker, P.R. Synergy between CD8 T cells and Th1 or Th2 polarised CD4 T cells for adoptive immunotherapy of brain tumours. PLoS ONE 2013, 8, e63933. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, M.R.; Merlino, G. The two faces of interferon-γ in cancer. Clin. Cancer Res. 2011, 17, 6118–6124. [Google Scholar] [CrossRef] [PubMed]
- Villeneuve, J.; Tremblay, P.; Vallières, L. Tumor necrosis factor reduces brain tumor growth by enhancing macrophage recruitment and microcyst formation. Cancer Res. 2005, 65, 3928–3936. [Google Scholar] [CrossRef] [PubMed]
- Larmonier, N.; Cathelin, D.; Larmonier, C.; Nicolas, A.; Merino, D.; Janikashvili, N.; Audia, S.; Bateman, A.; Thompson, J.; Kottke, T.; et al. The inhibition of TNF-α anti-tumoral properties by blocking antibodies promotes tumor growth in a rat model. Exp. Cell Res. 2007, 313, 2345–2355. [Google Scholar] [CrossRef] [PubMed]
- Calzascia, T.; Pellegrini, M.; Hall, H.; Sabbagh, L.; Ono, N.; Elford, A.R.; Mak, T.W.; Ohashi, P.S. TNF-alpha is critical for antitumor but not antiviral T cell immunity in mice. J. Clin. Investig. 2007, 117, 3833–3845. [Google Scholar] [PubMed]
- Dace, D.S.; Chen, P.W.; Niederkorn, J.Y. CD8+ T cells circumvent immune privilege in the eye and mediate intraocular tumor rejection by a TNF-alpha-dependent mechanism. J. Immunol. 2007, 178, 6115–6122. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Boyer, C.M.; Whitaker, R.S.; Berchuck, A.; Wiener, J.R.; Weinberg, J.B.; Bast, R.C. Tumor necrosis factor alpha as an autocrine and paracrine growth factor for ovarian cancer: Monokine induction of tumor cell proliferation and tumor necrosis factor alpha expression. Cancer Res. 1993, 53, 1939–1944. [Google Scholar] [PubMed]
- Leibovich, S.J.; Polverini, P.J.; Shepard, H.M.; Wiseman, D.M.; Shively, V.; Nuseir, N. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-alpha. Nature 1987, 329, 630–632. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.A.; Arnott, C.H.; Robinson, S.C.; Moore, R.J.; Thompson, R.G.; Marshall, J.F.; Balkwill, F.R. TNF-alpha regulates epithelial expression of MMP-9 and integrin alphavbeta6 during tumour promotion. A role for TNF-alpha in keratinocyte migration? Oncogene 2004, 23, 6954–6966. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, H.-S.; Zhou, B.-H.; Li, C.-L.; Zhang, F.; Wang, X.-F.; Zhang, G.; Bu, X.-Z.; Cai, S.-H.; Du, J. Epithelial-mesenchymal transition (EMT) induced by TNF-α requires AKT/GSK-3β-mediated stabilization of snail in colorectal cancer. PLoS ONE 2013, 8, e56664. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.A.; Moore, R.J.; Arnott, C.H.; East, N.; Thompson, R.G.; Scallon, B.J.; Shealy, D.J.; Balkwill, F.R. An anti-tumor necrosis factor-alpha antibody inhibits the development of experimental skin tumors. Mol. Cancer Ther. 2003, 2, 445–451. [Google Scholar] [PubMed]
- Popivanova, B.K.; Kitamura, K.; Wu, Y.; Kondo, T.; Kagaya, T.; Kaneko, S.; Oshima, M.; Fujii, C.; Mukaida, N. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J. Clin. Investig. 2008, 118, 560–570. [Google Scholar] [PubMed]
- Trabanelli, S.; Curti, A.; Lecciso, M.; Salomé, B.; Riether, C.; Ochsenbein, A.; Romero, P.; Jandus, C. CD127+ innate lymphoid cells are dysregulated in treatment naïve acute myeloid leukemia patients at diagnosis. Haematologica 2015, 100, e257–e260. [Google Scholar] [CrossRef] [PubMed]
- Dadi, S.; Chhangawala, S.; Whitlock, B.M.; Franklin, R.A.; Luo, C.T.; Oh, S.A.; Toure, A.; Pritykin, Y.; Huse, M.; Leslie, C.S.; et al. Cancer Immunosurveillance by Tissue-Resident Innate Lymphoid Cells and Innate-like T Cells. Cell 2016, 164, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, I.P.; Pejnovic, N.N.; Radosavljevic, G.D.; Pantic, J.M.; Milovanovic, M.Z.; Arsenijevic, N.N.; Lukic, M.L. Interleukin-33/ST2 axis promotes breast cancer growth and metastases by facilitating intratumoral accumulation of immunosuppressive and innate lymphoid cells. Int. J. Cancer 2014, 134, 1669–1682. [Google Scholar] [CrossRef] [PubMed]
- Terabe, M.; Matsui, S.; Park, J.-M.; Mamura, M.; Noben-Trauth, N.; Donaldson, D.D.; Chen, W.; Wahl, S.M.; Ledbetter, S.; Pratt, B.; et al. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: Abrogation prevents tumor recurrence. J. Exp. Med. 2003, 198, 1741–1752. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, M.; Hardaway, J.C.; Guloglu, F.B.; Miller, M.M.; Hoeman, C.M.; Zaghouani, A.A.; Wan, X.; Rowland, L.M.; Cascio, J.A.; Sherman, M.P.; et al. IL-13Rα1 is a surface marker for M2 macrophages influencing their differentiation and function. Eur. J. Immunol. 2014, 44, 842–855. [Google Scholar] [CrossRef] [PubMed]
- Marvie, P.; Lisbonne, M.; L’helgoualc'h, A.; Rauch, M.; Turlin, B.; Preisser, L.; Bourd-Boittin, K.; Théret, N.; Gascan, H.; Piquet-Pellorce, C.; et al. Interleukin-33 overexpression is associated with liver fibrosis in mice and humans. J. Cell. Mol. Med. 2010, 14, 1726–1739. [Google Scholar] [CrossRef] [PubMed]
- Mchedlidze, T.; Waldner, M.; Zopf, S.; Walker, J.; Rankin, A.L.; Schuchmann, M.; Voehringer, D.; McKenzie, A.N.J.; Neurath, M.F.; Pflanz, S.; et al. Interleukin-33-Dependent Innate Lymphoid Cells Mediate Hepatic Fibrosis. Immunity 2013, 39, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Fattovich, G.; Stroffolini, T.; Zagni, I.; Donato, F. Hepatocellular Carcinoma in Cirrhosis: Incidence and Risk Factors. Gastroenterology 2004, 127, S35–S50. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Razumilava, N.; Gores, G.J.; Walters, S.; Mizuochi, T.; Mourya, R.; Bessho, K.; Wang, Y.-H.; Glaser, S.S.; Shivakumar, P.; et al. Biliary repair and carcinogenesis are mediated by IL-33-dependent cholangiocyte proliferation. J. Clin. Investig. 2014, 124, 3241–3251. [Google Scholar] [CrossRef] [PubMed]
- Ikutani, M.; Yanagibashi, T.; Ogasawara, M.; Tsuneyama, K.; Yamamoto, S.; Hattori, Y.; Kouro, T.; Itakura, A.; Nagai, Y.; Takaki, S.; et al. Identification of innate IL-5-producing cells and their role in lung eosinophil regulation and antitumor immunity. J. Immunol. 2012, 188, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Zaynagetdinov, R.; Sherrill, T.P.; Gleaves, L.A.; McLoed, A.G.; Saxon, J.A.; Habermann, A.C.; Connelly, L.; Dulek, D.; Peebles, R.S.; Fingleton, B.; et al. Interleukin-5 facilitates lung metastasis by modulating the immune microenvironment. Cancer Res. 2015, 75, 1624–1634. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Liu, L.-B.; Shang, W.-Q.; Chang, K.-K.; Meng, Y.-H.; Mei, J.; Yu, J.-J.; Li, D.-J.; Li, M.-Q. The infiltration and functional regulation of eosinophils induced by TSLP promote the proliferation of cervical cancer cell. Cancer Lett. 2015, 364, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Bie, Q.; Zhang, P.; Su, Z.; Zheng, D.; Ying, X.; Wu, Y.; Yang, H.; Chen, D.; Wang, S.; Xu, H. Polarization of ILC2s in peripheral blood might contribute to immunosuppressive microenvironment in patients with gastric cancer. J. Immunol. Res. 2014, 2014, 923135. [Google Scholar] [CrossRef] [PubMed]
- Zea, A.H.; Rodriguez, P.C.; Culotta, K.S.; Hernandez, C.P.; DeSalvo, J.; Ochoa, J.B.; Park, H.-J.; Zabaleta, J.; Ochoa, A.C. l-Arginine modulates CD3ζ expression and T cell function in activated human T lymphocytes. Cell. Immunol. 2004, 232, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.C.; Quiceno, D.G.; Ochoa, A.C. l-arginine availability regulates T-lymphocyte cell-cycle progression. Blood 2007, 109, 1568–1573. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.C.; Quiceno, D.G.; Zabaleta, J.; Ortiz, B.; Zea, A.H.; Piazuelo, M.B.; Delgado, A.; Correa, P.; Brayer, J.; Sotomayor, E.M.; et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004, 64, 5839–5849. [Google Scholar] [CrossRef] [PubMed]
- Munder, M.; Eichmann, K.; Modolell, M. Alternative metabolic states in murine macrophages reflected by the nitric oxide synthase/arginase balance: Competitive regulation by CD4+ T cells correlates with Th1/Th2 phenotype. J. Immunol. 1998, 160, 5347–5354. [Google Scholar] [PubMed]
- Bronte, V.; Serafini, P.; De Santo, C.; Marigo, I.; Tosello, V.; Mazzoni, A.; Segal, D.M.; Staib, C.; Lowel, M.; Sutter, G.; et al. IL-4-induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. J. Immunol. 2003, 170, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Bando, J.K.; Nussbaum, J.C.; Liang, H.; Locksley, R.M. Type 2 innate lymphoid cells constitutively express arginase-I in the naive and inflamed lung. J. Leukoc. Biol. 2013, 94, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Monticelli, L.A.; Osborne, L.C.; Noti, M.; Tran, S.V.; Zaiss, D.M.W.; Artis, D. IL-33 promotes an innate immune pathway of intestinal tissue protection dependent on amphiregulin-EGFR interactions. Proc. Natl. Acad. Sci. USA 2015, 112, 10762–10767. [Google Scholar] [CrossRef] [PubMed]
- Zaiss, D.M.W.; van Loosdregt, J.; Gorlani, A.; Bekker, C.P.J.; Gröne, A.; Sibilia, M.; van Bergen en Henegouwen, P.M.P.; Roovers, R.C.; Coffer, P.J.; Sijts, A.J.A.M. Amphiregulin enhances regulatory T cell-suppressive function via the epidermal growth factor receptor. Immunity 2013, 38, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Tanaka, T.; Shi, W.; Matsumoto, M.; Minami, M.; Kashiwamura, S.; Nakanishi, K.; Yoshida, N.; Kishimoto, T.; Akira, S. Essential role of Stat6 in IL-4 signalling. Nature 1996, 380, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Drake, L.Y.; Iijima, K.; Kita, H. Group 2 innate lymphoid cells and CD4+ T cells cooperate to mediate type 2 immune response in mice. Allergy 2014, 69, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Neill, D.R.; Wong, S.H.; Bellosi, A.; Flynn, R.J.; Daly, M.; Langford, T.K.A.; Bucks, C.; Kane, C.M.; Fallon, P.G.; Pannell, R.; et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 2010, 464, 1367–1370. [Google Scholar] [CrossRef] [PubMed]
- Oliphant, C.J.; Hwang, Y.Y.; Walker, J.A.; Salimi, M.; Wong, S.H.; Brewer, J.M.; Englezakis, A.; Barlow, J.L.; Hams, E.; Scanlon, S.T.; et al. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity 2014, 41, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Mirchandani, A.S.; Besnard, A.-G.; Yip, E.; Scott, C.; Bain, C.C.; Cerovic, V.; Salmond, R.J.; Liew, F.Y. Type 2 innate lymphoid cells drive CD4+ Th2 cell responses. J. Immunol. 2014, 192, 2442–2448. [Google Scholar] [CrossRef] [PubMed]
- Cătană, C.-S.; Berindan Neagoe, I.; Cozma, V.; Magdaş, C.; Tăbăran, F.; Dumitraşcu, D.L. Contribution of the IL-17/IL-23 axis to the pathogenesis of inflammatory bowel disease. World J. Gastroenterol. 2015, 21, 5823–5830. [Google Scholar] [PubMed]
- Ermann, J.; Staton, T.; Glickman, J.N.; de Waal Malefyt, R.; Glimcher, L.H. Nod/Ripk2 signaling in dendritic cells activates IL-17A-secreting innate lymphoid cells and drives colitis in T-bet–/–.Rag2–/– (TRUC) mice. Proc. Natl. Acad. Sci. USA 2014, 111, E2559–E2566. [Google Scholar] [CrossRef] [PubMed]
- Geremia, A.; Arancibia-Cárcamo, C.V.; Fleming, M.P.P.; Rust, N.; Singh, B.; Mortensen, N.J.; Travis, S.P.L.; Powrie, F. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J. Exp. Med. 2011, 208, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Kvedaraite, E.; Lourda, M.; Ideström, M.; Chen, P.; Olsson-Åkefeldt, S.; Forkel, M.; Gavhed, D.; Lindforss, U.; Mjösberg, J.; Henter, J.-I.; et al. Tissue-infiltrating neutrophils represent the main source of IL-23 in the colon of patients with IBD. Gut 2015. [Google Scholar] [CrossRef] [PubMed]
- Triantafillidis, J.K.; Nasioulas, G.; Kosmidis, P.A. Colorectal cancer and inflammatory bowel disease: Epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies. Anticancer Res. 2009, 29, 2727–2737. [Google Scholar] [PubMed]
- Langowski, J.L.; Zhang, X.; Wu, L.; Mattson, J.D.; Chen, T.; Smith, K.; Basham, B.; McClanahan, T.; Kastelein, R.A.; Oft, M. IL-23 promotes tumour incidence and growth. Nature 2006, 442, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Rhee, K.-J.; Albesiano, E.; Rabizadeh, S.; Wu, X.; Yen, H.-R.; Huso, D.L.; Brancati, F.L.; Wick, E.; McAllister, F.; et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat. Med. 2009, 15, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Chan, I.H.; Jain, R.; Tessmer, M.S.; Gorman, D.; Mangadu, R.; Sathe, M.; Vives, F.; Moon, C.; Penaflor, E.; Turner, S.; et al. Interleukin-23 is sufficient to induce rapid de novo gut tumorigenesis, independent of carcinogens, through activation of innate lymphoid cells. Mucosal Immunol. 2014, 7, 842–856. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Duan, Y.; Cheng, X.; Chen, X.; Xie, W.; Long, H.; Lin, Z.; Zhu, B. IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma. Biochem. Biophys. Res. Commun. 2011, 407, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Mulcahy, L.A.; Mohammed, R.A.; Lee, A.H.; Franks, H.A.; Kilpatrick, L.; Yilmazer, A.; Paish, E.C.; Ellis, I.O.; Patel, P.M.; et al. IL-17 expression by breast-cancer-associated macrophages: IL-17 promotes invasiveness of breast cancer cell lines. Breast Cancer Res. 2008, 10, R95. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Shen, J.; Cao, J.; Zhou, Y.; Shang, L.; Jin, S.; Cao, S.; Che, D.; Liu, F.; Yu, Y. Interleukin-17 promotes angiogenesis by stimulating VEGF production of cancer cells via the STAT3/GIV signaling pathway in non-small-cell lung cancer. Sci. Rep. 2015, 5, 16053. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Fei, M.; Wu, Y.; Zheng, D.; Wan, D.; Wang, L.; Li, D. Distribution and clinical significance of Th17 cells in the tumor microenvironment and peripheral blood of pancreatic cancer patients. Int. J. Mol. Sci. 2011, 12, 7424–7437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-P.; Yan, J.; Xu, J.; Pang, X.-H.; Chen, M.-S.; Li, L.; Wu, C.; Li, S.-P.; Zheng, L. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J. Hepatol. 2009, 50, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yang, T.; Liu, X.; Guo, J.N.; Xie, T.; Ding, Y.; Lin, M.; Yang, H. IL-17 promotes tumor angiogenesis through Stat3 pathway mediated upregulation of VEGF in gastric cancer. Tumor Biol. 2015. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Li, H.; Yusuf, N.; Elmets, C.A.; Li, J.; Mountz, J.D.; Xu, H. IL-17 promotes tumor development through the induction of tumor promoting microenvironments at tumor sites and myeloid-derived suppressor cells. J. Immunol. 2010, 184, 2281–2288. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.C.J.; Bériou, G.; Heslan, M.; Chauvin, C.; Utriainen, L.; Aumeunier, A.; Scott, C.L.; Mowat, A.; Cerovic, V.; Houston, S.A.; et al. Interleukin-22 binding protein (IL-22BP) is constitutively expressed by a subset of conventional dendritic cells and is strongly induced by retinoic acid. Mucosal Immunol. 2014, 7, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Kobold, S.; Völk, S.; Clauditz, T.; Küpper, N.J.; Minner, S.; Tufman, A.; Düwell, P.; Lindner, M.; Koch, I.; Heidegger, S.; et al. Interleukin-22 is frequently expressed in small- and large-cell lung cancer and promotes growth in chemotherapy-resistant cancer cells. J. Thorac. Oncol. 2013, 8, 1032–1042. [Google Scholar] [CrossRef] [PubMed]
- Huber, S.; Gagliani, N.; Zenewicz, L.A.; Huber, F.J.; Bosurgi, L.; Hu, B.; Hedl, M.; Zhang, W.; O’Connor, W.; Murphy, A.J.; et al. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature 2012, 491, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Kirchberger, S.; Royston, D.J.; Boulard, O.; Thornton, E.; Franchini, F.; Szabady, R.L.; Harrison, O.; Powrie, F. Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. J. Exp. Med. 2013, 210, 917–931. [Google Scholar] [CrossRef] [PubMed]
- Von Burg, N.; Chappaz, S.; Baerenwaldt, A.; Horvath, E.; Bose Dasgupta, S.; Ashok, D.; Pieters, J.; Tacchini-Cottier, F.; Rolink, A.; Acha-Orbea, H.; et al. Activated group 3 innate lymphoid cells promote T-cell-mediated immune responses. Proc. Natl. Acad. Sci. USA 2014, 111, 12835–12840. [Google Scholar] [CrossRef] [PubMed]
- Hepworth, M.R.; Fung, T.C.; Masur, S.H.; Kelsen, J.R.; McConnell, F.M.; Dubrot, J.; Withers, D.R.; Hugues, S.; Farrar, M.A.; Reith, W.; et al. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria-specific CD4+ T cells. Science 2015, 348, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Benchetrit, F.; Ciree, A.; Vives, V.; Warnier, G.; Gey, A.; Sautès-Fridman, C.; Fossiez, F.; Haicheur, N.; Fridman, W.H.; Tartour, E.; et al. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood 2002, 99, 2114–2121. [Google Scholar] [CrossRef] [PubMed]
- Kryczek, I.; Wei, S.; Szeliga, W.; Vatan, L.; Zou, W. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood 2009, 114, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Weber, G.F.; Gaertner, F.C.; Erl, W.; Janssen, K.-P.; Blechert, B.; Holzmann, B.; Weighardt, H.; Essler, M. IL-22-mediated tumor growth reduction correlates with inhibition of ERK1/2 and AKT phosphorylation and induction of cell cycle arrest in the G2-M phase. J. Immunol. 2006, 177, 8266–8272. [Google Scholar] [CrossRef] [PubMed]
- Carrega, P.; Loiacono, F.; Di Carlo, E.; Scaramuccia, A.; Mora, M.; Conte, R.; Benelli, R.; Spaggiari, G.M.; Cantoni, C.; Campana, S.; et al. NCR+ILC3 concentrate in human lung cancer and associate with intratumoral lymphoid structures. Nat. Commun. 2015, 6, 8280. [Google Scholar] [CrossRef] [PubMed]
- Eisenring, M.; Berg, J.; Kristiansen, G.; Saller, E.; Becher, B. IL-12 initiates tumor rejection via lymphoid tissue-inducer cells bearing the natural cytotoxicity receptor NKp46. Nat. Immunol. 2010, 11, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Moskalenko, M.; Pan, M.; Fu, Y.; de Moll, E.H.; Hashimoto, D.; Mortha, A.; Leboeuf, M.; Jayaraman, P.; Bernardo, S.; Sikora, A.G.; et al. Requirement for innate immunity and CD90+ NK1.1− lymphocytes to treat established melanoma with chemo-immunotherapy. Cancer Immunol. Res. 2015, 3, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Sonnenberg, G.F.; Monticelli, L.A.; Elloso, M.M.; Fouser, L.A.; Artis, D. CD4+ lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity 2011, 34, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Shields, J.D.; Kourtis, I.C.; Tomei, A.A.; Roberts, J.M.; Swartz, M.A. Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21. Science 2010, 328, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 1991, 9, 271–296. [Google Scholar] [CrossRef] [PubMed]
- Van Beek, J.J.P.; Wimmers, F.; Hato, S.V.; de Vries, I.J.M.; Sköld, A.E. Dendritic cell cross talk with innate and innate-like effector cells in antitumor immunity: Implications for DC vaccination. Crit. Rev. Immunol. 2014, 34, 517–536. [Google Scholar] [CrossRef] [PubMed]
- Powell, N.; Walker, A.W.; Stolarczyk, E.; Canavan, J.B.; Gökmen, M.R.; Marks, E.; Jackson, I.; Hashim, A.; Curtis, M.A.; Jenner, R.G.; et al. The transcription factor T-bet regulates intestinal inflammation mediated by interleukin-7 receptor+ innate lymphoid cells. Immunity 2012, 37, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Kinnebrew, M.A.; Buffie, C.G.; Diehl, G.E.; Zenewicz, L.A.; Leiner, I.; Hohl, T.M.; Flavell, R.A.; Littman, D.R.; Pamer, E.G. Interleukin 23 Production by Intestinal CD103+CD11b+ Dendritic Cells in Response to Bacterial Flagellin Enhances Mucosal Innate Immune Defense. Immunity 2012, 36, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Hazenberg, M.D.; Spits, H. Human innate lymphoid cells. Blood 2014, 124, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A. IL-2: The first effective immunotherapy for human cancer. J. Immunol. 2014, 192, 5451–5458. [Google Scholar] [CrossRef] [PubMed]
- Galvin, K.C.; Dyck, L.; Marshall, N.A.; Stefanska, A.M.; Walsh, K.P.; Moran, B.; Higgins, S.C.; Dungan, L.S.; Mills, K.H.G. Blocking retinoic acid receptor-α enhances the efficacy of a dendritic cell vaccine against tumours by suppressing the induction of regulatory T cells. Cancer Immunol. Immunother. 2013, 62, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Benson, D.M.; Bakan, C.E.; Mishra, A.; Hofmeister, C.C.; Efebera, Y.; Becknell, B.; Baiocchi, R.A.; Zhang, J.; Yu, J.; Smith, M.K.; et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: A therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood 2010, 116, 2286–2294. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).