Nanomaterials—Tools, Technology and Methodology of Nanotechnology Based Biomedical Systems for Diagnostics and Therapy
Abstract
:Significance
1. Introduction

2. Definition of Terms Used in This Article
3. Applications of Nanotechnology to Life Sciences
3.1. Lab on a Chip
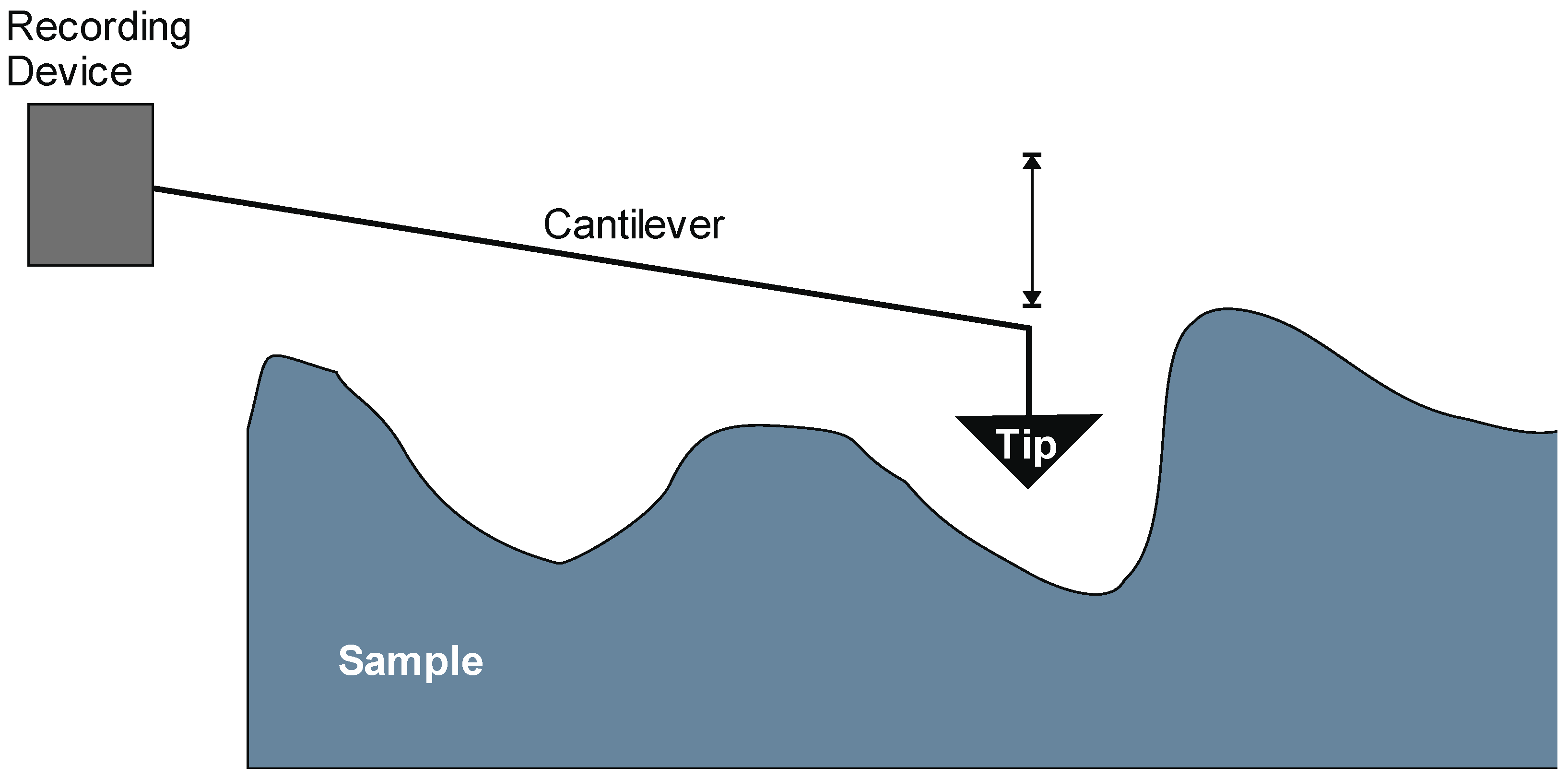
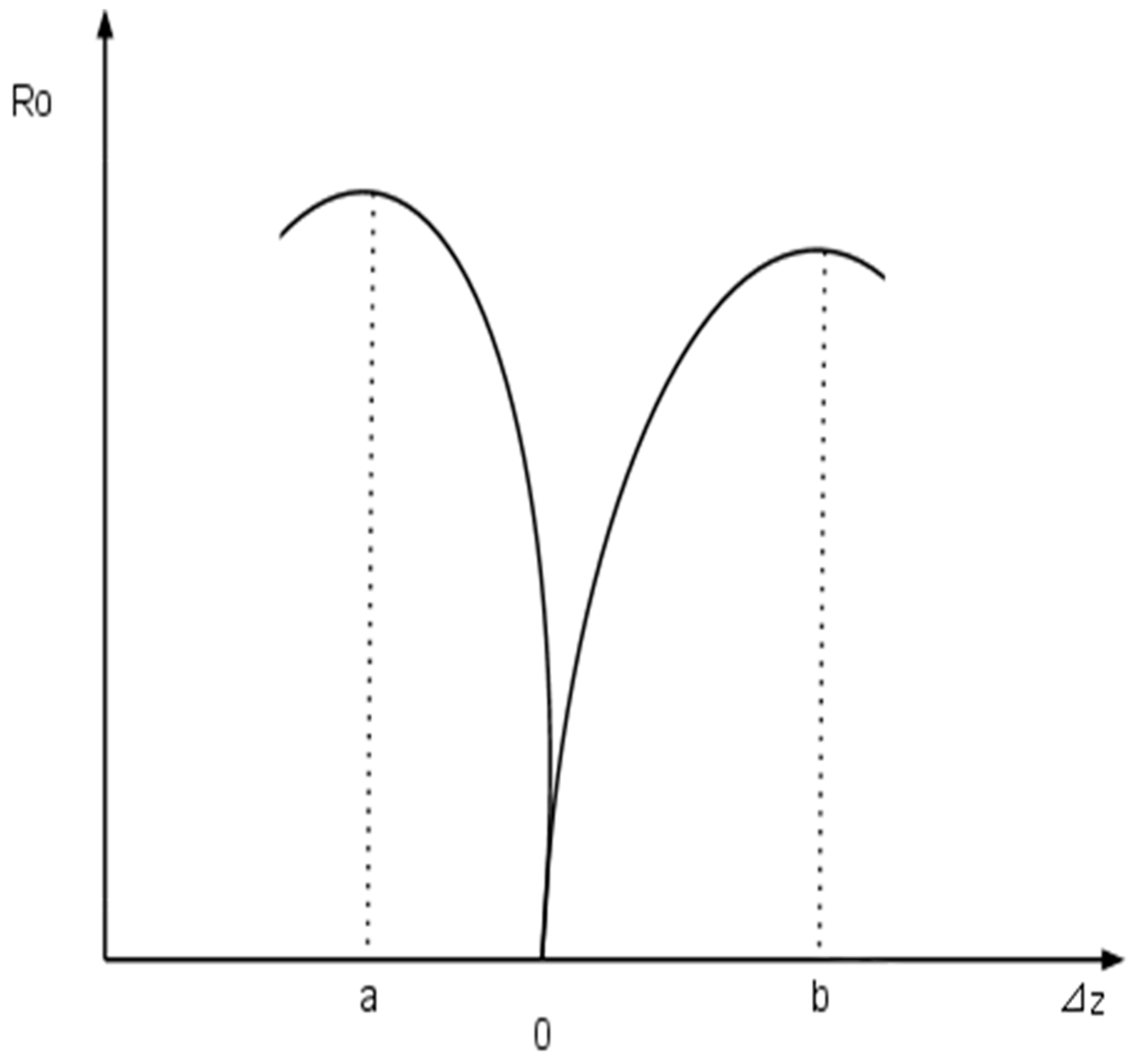
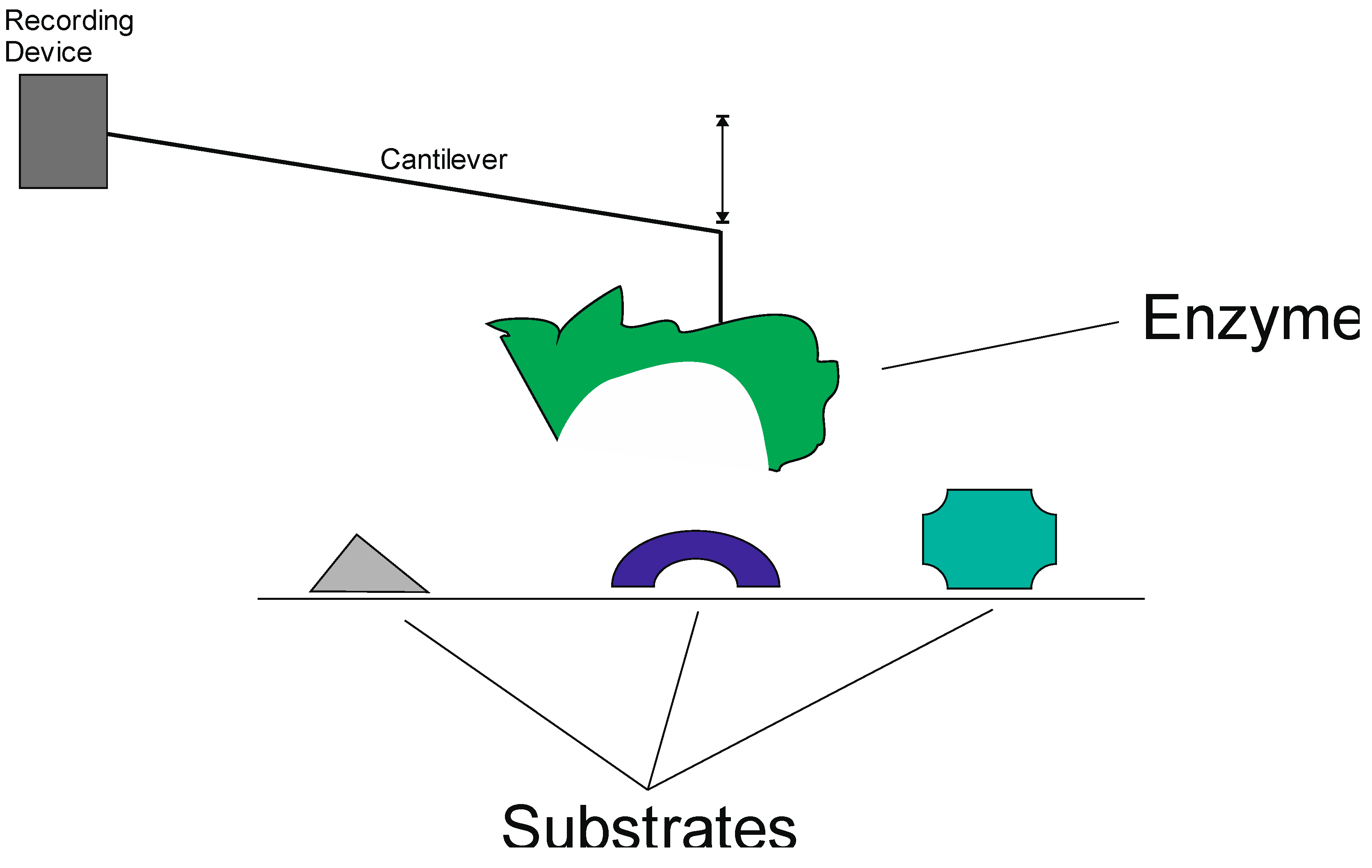
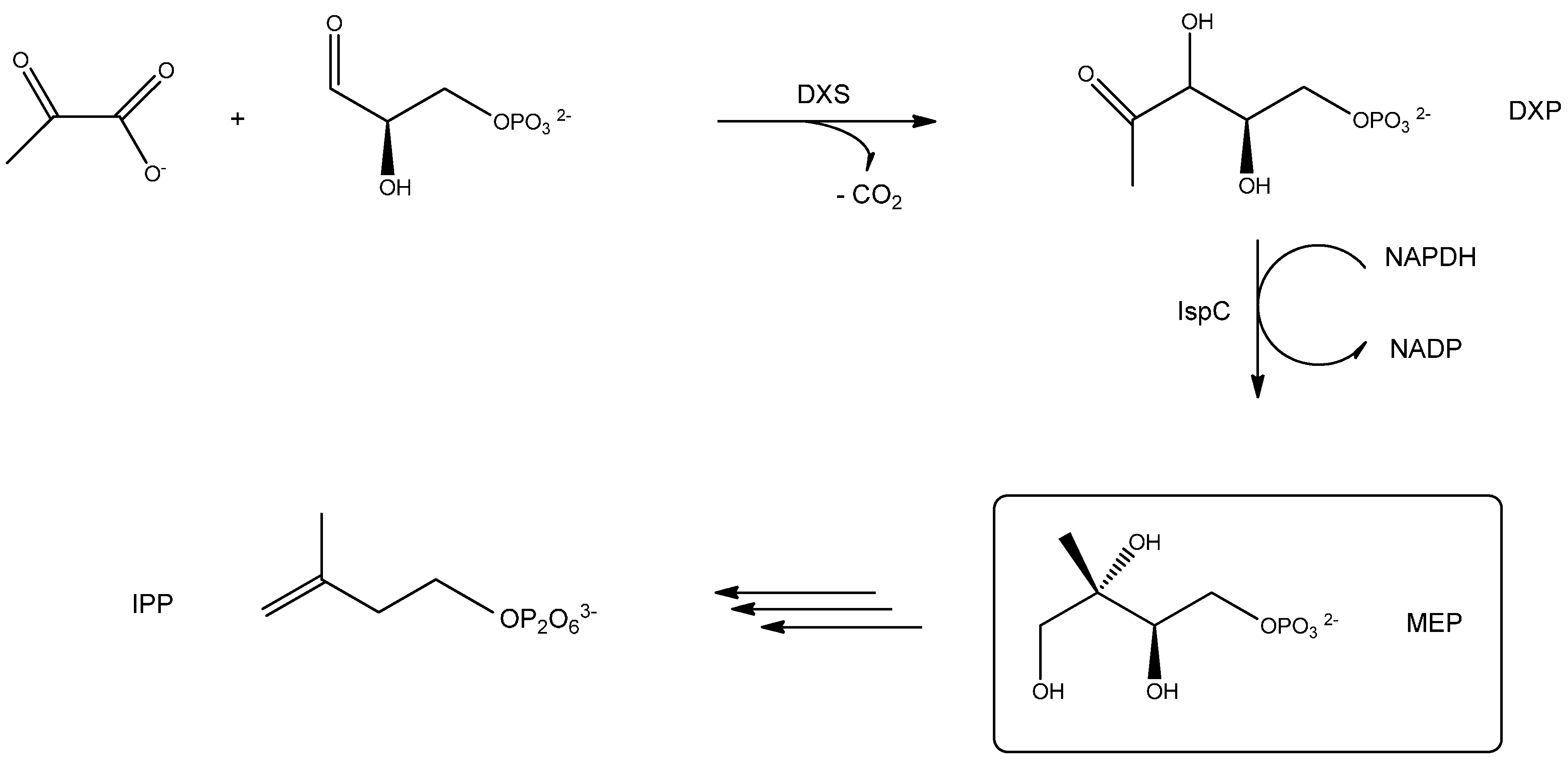
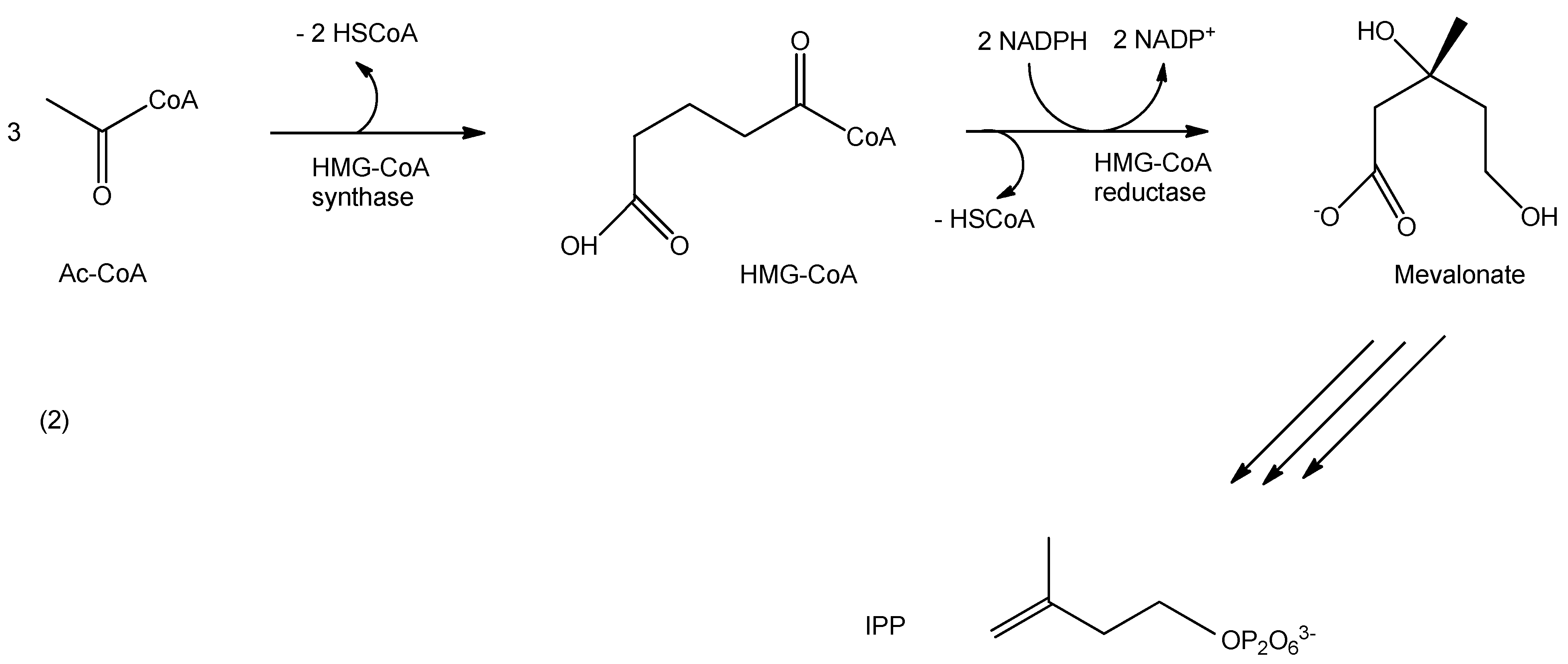
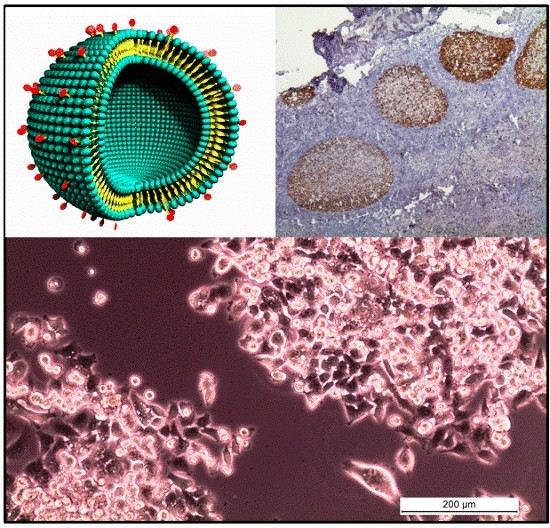
3.2. Chemical Noses and Tongues Based on Nano-Biomaterials
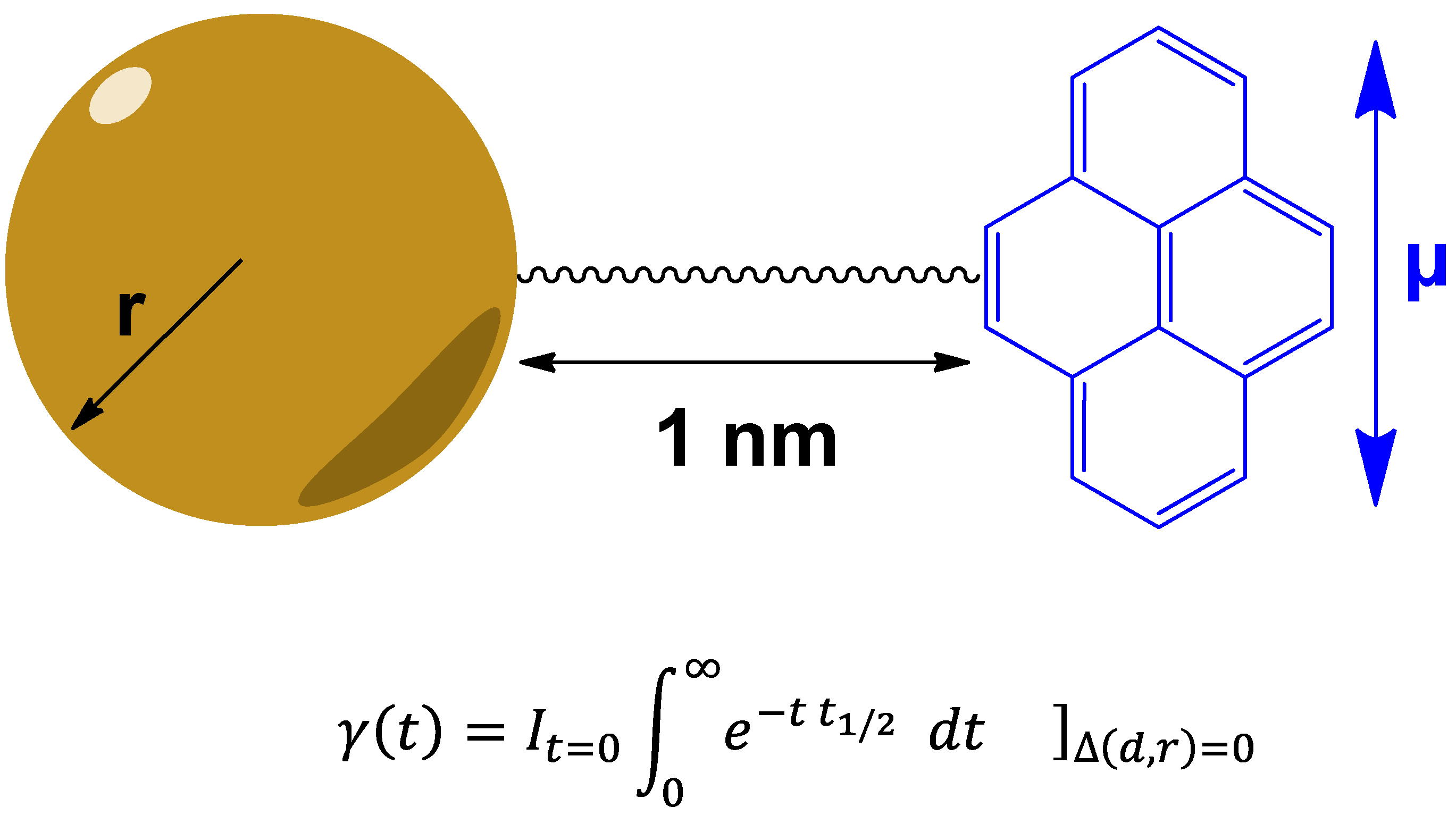
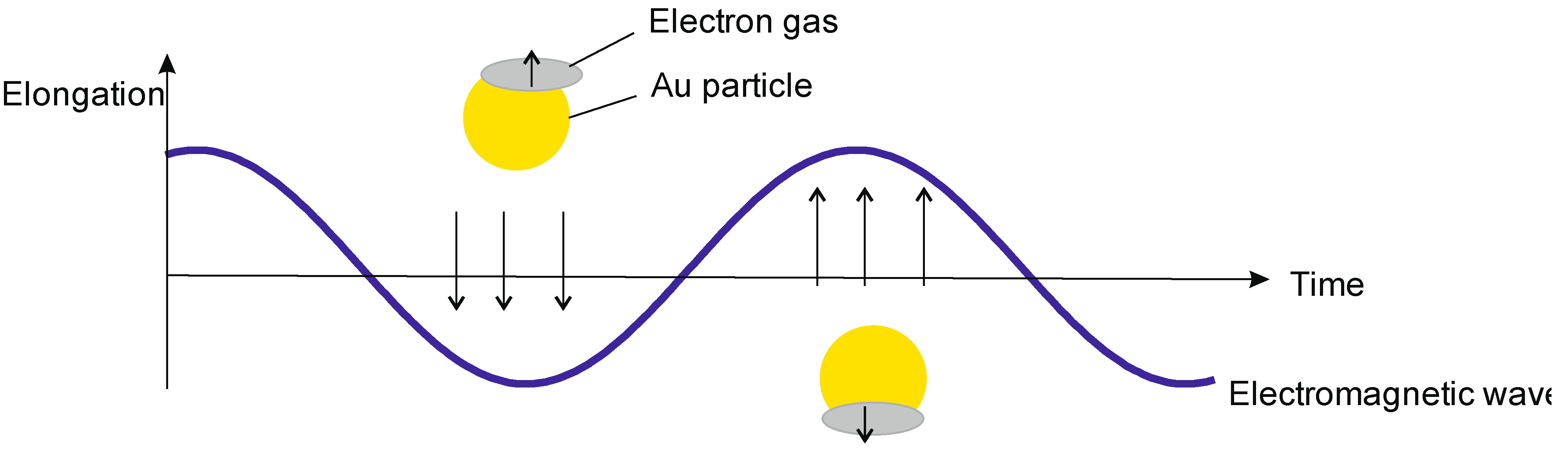
3.3. Theranostics Based on Quasi-Relativistic Effects
3.4. Cancer Nanotechnology
3.5. Chemically Functionalized Quasi-Atoms in Cancer Theranostics
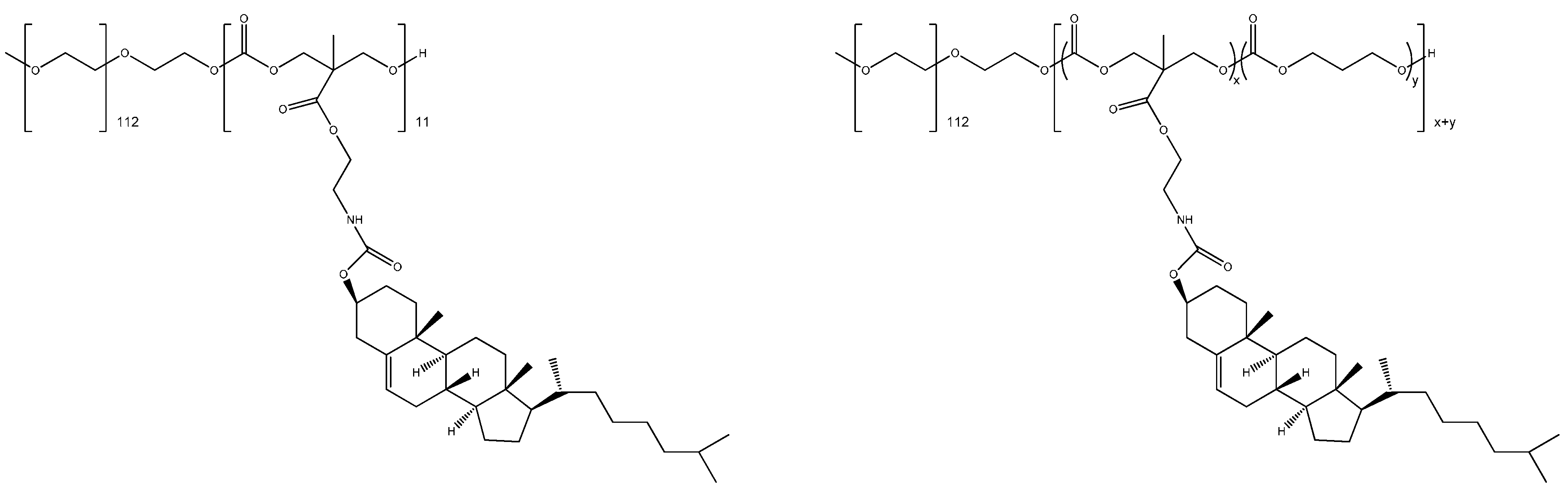
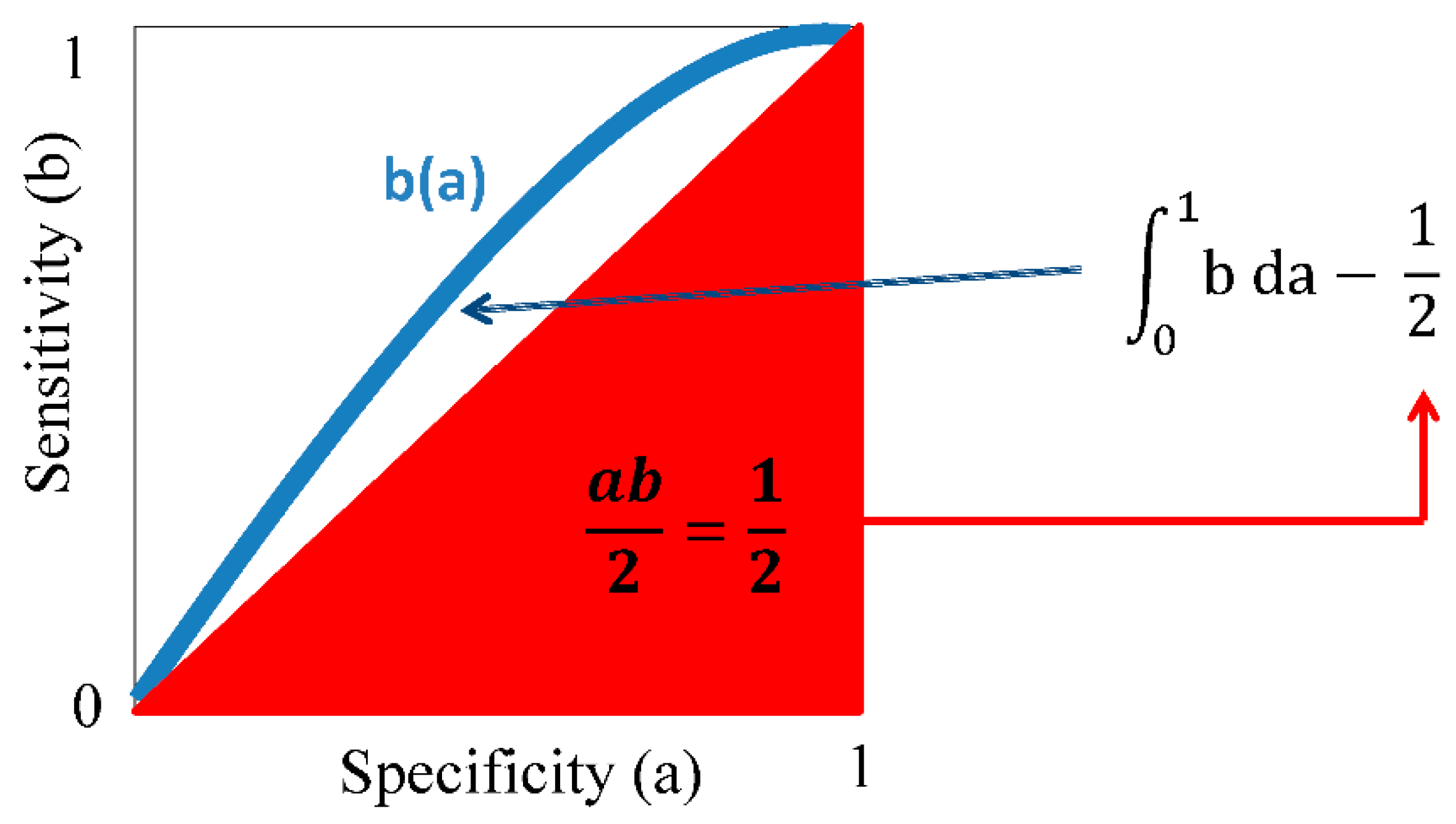
4. Comparing Theranostic Features Using a Better than a Random Guess Value
5. Perspective
Acknowledgements
Author Contributions
Conflicts of Interest
Other Remarks
Abbreviations
| Ac-CoA | acetyl-coenzyme A |
| AFM | atomic force microscopy |
| AUC | area under the receiver operating characteristic curve |
| brag | better than a random guess |
| α-EGFRII | anti-epidermal growth factor receptor type II antibody |
| DXP | 1-deoxy-d-xylulose-5-phosphate |
| DXS | 1-deoxy-d-xylulose-5-phosphate synthase |
| EGF | epidermal growth factor |
| EGFRII | epidermal growth factor receptor type II |
| GAP | glyceraldehyde 3-phosphate |
| HMG-CoA | hydroxy-3-methylglutaryl-coenzyme A |
| IPP | iso-pentenyl-diphosphate |
| IspC | 1-deoxy-d-xylulose-5-phosphate reducto-isomerase |
| MEP | 2-C-methyl-d-erythritol-4-phosphate |
| NADH | nicotinamide adenine dinucleotide |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| SFM | single-molecule force spectroscopy |
| TDP | thiamine di-phosphate |
References
- Jones, T.; Saba, N. Nanotechnology and drug delivery: An update in oncology. Pharmaceutics 2011, 3, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Mousa, S.A.; Bharali, D.J. Nanotechnology-Based detection and targeted therapy in cancer: Nano-Bio paradigms and applications. Cancers 2011, 3, 2888–2903. [Google Scholar] [CrossRef] [PubMed]
- Jo, D.H.; Lee, T.G.; Kim, J.H. Nanotechnology and nanotoxicology in retinopathy. Int. J. Mol. Sci. 2011, 12, 8288–8301. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.K.; Jang, E.; Lee, K.; Haam, S.; Huh, Y.M. Delivery of cancer therapeutics using nanotechnology. Pharmaceutics 2013, 5, 294–317. [Google Scholar] [CrossRef] [PubMed]
- Masotti, A.; Caporali, A. Preparation of magnetic carbon nanotubes (Mag-CNTs) for biomedical and biotechnological applications. Int. J. Mol. Sci. 2013, 14, 24619–24642. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Oh, B.K.; Choi, J.W. Development of a HIV-1 Virus Detection System Based on Nanotechnology. Sensors 2015, 15, 9915–9927. [Google Scholar] [CrossRef] [PubMed]
- Irshad, M.; Iqbal, N.; Mujahid, A.; Afzal, A.; Hussain, T.; Sharif, A.; Ahmad, E.; Athar, M.M. Molecularly Imprinted Nanomaterials for Sensor Applications. Nanomaterials 2013, 3, 615–637. [Google Scholar] [CrossRef]
- Perán, M.; García, M.A.; Lopez-Ruiz, E.; Jiménez, G.; Marchal, J.A. How Can Nanotechnology Help to Repair the Body? Advances in Cardiac, Skin, Bone, Cartilage and Nerve Tissue Regeneration. Materials 2013, 6, 1333–1359. [Google Scholar] [CrossRef]
- Kalantzi, O.-I.; Biskos, G. Methods for Assessing Basic Particle Properties and Cytotoxicity of Engineered Nanoparticles. Toxics 2014, 2, 79–91. [Google Scholar] [CrossRef]
- Wagner, V.; Dullaart, A.; Bock, A.K.; Zweck, A. The emerging nanomedicine landscape. Nat. Biotechnol. 2006, 24, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Sandhiya, S.; Dkhar, S.A.; Surendiran, A. Emerging trends of nanomedicine—An overview. Fundam. Clin. Pharmacol. 2009, 23, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Zafar, M.; Qasim, S.; Shahab, S.; Naseem, M.; AbuReqaiba, A. Advances in Nanotechnology for Restorative Dentistry. Materials 2015, 8, 717–731. [Google Scholar] [CrossRef]
- De la Zerda, A.; Gambhir, S.S. Drug delivery: Keeping tabs on nanocarriers. Nat. Nanotechnol. 2007, 2, 745–746. [Google Scholar] [CrossRef] [PubMed]
- Eaton, M. Nanomedicine: Industry-wise research. Nat. Mater. 2007, 6, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Ellis-Behnke, R.; Jonas, J.B. Redefining tissue engineering for nanomedicine in ophthalmology. Acta Ophthalmol. 2011, 89, e108–e114. [Google Scholar] [CrossRef] [PubMed]
- Zarbin, M.A.; Montemagno, C.; Leary, J.F.; Ritch, R. Nanomedicine in ophthalmology: The new frontier. Am. J. Ophthalmol. 2010, 150, 144–162. [Google Scholar] [CrossRef] [PubMed]
- Klenkler, B.J.; Griffith, M.; Becerril, C.; West-Mays, J.A.; Sheardown, H. EGF-grafted PDMS surfaces in artificial cornea applications. Biomaterials 2005, 26, 7286–7296. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, H.; Shimmura, S.; Kobayashi, H.; Taguchi, T.; Asano-Kato, N.; Uchino, Y.; Kato, M.; Shimazaki, J.; Tanaka, J.; Tsubota, K. Collagen-immobilized poly(vinyl alcohol) as an artificial cornea scaffold that supports a stratified corneal epithelium. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 76, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Bakhshandeh, H.; Soleimani, M.; Hosseini, S.S.; Hashemi, H.; Shabani, I.; Shafiee, A.; Nejad, A.H.; Erfan, M.; Dinarvand, R.; Atyabi, F. Poly (ε-caprolactone) nanofibrous ring surrounding a polyvinyl alcohol hydrogel for the development of a biocompatible two-part artificial cornea. Int. J. Nanomed. 2011, 6, 1509–1515. [Google Scholar]
- Werner, L.; Storsberg, J.; Mauger, O.; Brasse, K.; Gerl, R.; Müller, M.; Tetz, M. Unusual pattern of glistening formation on a 3-piece hydrophobic acrylic intraocular lens. J. Cataract Refract. Surg. 2008, 34, 1604–1609. [Google Scholar] [CrossRef] [PubMed]
- Cretich, M.; Daaboul, G.G.; Sola, L.; Ünlü, M.S.; Chiari, M. Digital detection of biomarkers assisted by nanoparticles: application to diagnostics. Trends Biotechnol. 2015, 33, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Steiner, T. The hydrogen bond in the solid state. Angew. Chem. Int. Ed. Engl. 2002, 41, 49–76. [Google Scholar] [CrossRef]
- Sbaizero, O.; DelFavero, G.; Martinelli, V.; Long, C.S.; Mestroni, L. Analysis of long- and short-range contribution to adhesion work in cardiac fibroblasts: an atomic force microscopy study. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 49, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, U.S.; Gardel, M.L. United we stand: Integrating the actin cytoskeleton and cell-matrix adhesions in cellular mechanotransduction. J. Cell Sci. 2012, 125, 3051–3060. [Google Scholar] [CrossRef] [PubMed]
- Taubenberger, A.V.; Hutmacher, D.W.; Muller, D.J. Single-Cell force spectroscopy, an emerging tool to quantify cell adhesion to biomaterials. Tissue Eng. Part. B Rev. 2014, 20, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Arnold, S.; Holler, S.; Fan, X. Nano-Structures for Optics and Photonics, Taking Microcavity Label-Free Single Molecule Detection Deep into the Protein Realm: Cancer Marker Detection at the Ultimate Sensitivity. In Nano-Structures for Optics and Photonics; Springer: Dodrecht, The Netherlands, 2015; pp. 309–322. [Google Scholar]
- Aspelmeyer, M.; Kippenberg, T.J.; Marquardt, F. Cavity optomechanics. Rev. Mod. Phys. 2014, 86, 1391–1452. [Google Scholar] [CrossRef]
- Sisquella, X.; de Pourcq, K.; Alguacil, J.; Robles, J.; Sanz, F.; Anselmetti, D.; Imperial, S.; Fernàndez-Busquets, X. A single-molecule force spectroscopy nanosensor for the identification of new antibiotics and antimalarials. FASEB J. 2010, 24, 4203–4217. [Google Scholar] [CrossRef] [PubMed]
- Rohmer, M. The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat. Prod. Rep. 1999, 16, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Hunter, W.N. The non-mevalonate pathway of isoprenoid precursor biosynthesis. J. Biol. Chem. 2007, 282, 21573–21577. [Google Scholar] [CrossRef] [PubMed]
- Bald, I.; Keller, A. Molecular processes studied at a single-molecule level using DNA origami nanostructures and atomic force microscopy. Molecules 2014, 19, 13803–13823. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Ng, T.B. In-vitro nanodiagnostic platform through nanoparticles and DNA-RNA nanotechnology. Appl. Microbiol. Biotechnol. 2015, 99, 3359–3374. [Google Scholar] [CrossRef] [PubMed]
- Tsien, R.Y.; Rink, T.J.; Poenie, M. Measurement of cytosolic free Ca2+ in individual small cells using fluorescence microscopy with dual excitation wavelengths. Cell Calcium 1985, 6, 145–157. [Google Scholar] [CrossRef]
- Owen, C.S.; Shuler, R.L. Spectral evidence for non-calcium interactions of intracellular Indo-1. Biochem. Biophys. Res. Commun. 1989, 163, 328–333. [Google Scholar] [CrossRef]
- Bancel, F.; Salmon, J.M.; Vigo, J.; Vo-Dinh, T.; Viallet, P. Investigation of noncalcium interactions of fura-2 by classical and synchronous fluorescence spectroscopy. Anal. Biochem. 1992, 204, 231–238. [Google Scholar] [CrossRef]
- Shimomura, O.; Johnson, F.H.; Saiga, Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J. Cell Comp. Physiol. 1962, 59, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Nakai, J.; Ohkura, M.; Imoto, K. A high signal-to-noise Ca2+ probe composed of a single green fluorescent protein. Nat. Biotechnol. 2001, 19, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Kneen, M.; Farinas, J.; Li, Y.; Verkman, A.S. Green fluorescent protein as a noninvasive intracellular pH indicator. Biophys. J. 1998, 74, 1591–1599. [Google Scholar] [CrossRef]
- Llopis, J.; McCaffery, J.M.; Miyawaki, A.; Farquhar, M.G.; Tsien, R.Y. Measurement of cytosolic, mitochondrial, and Golgi pH in single living cells with green fluorescent proteins. Proc. Natl. Acad. Sci. USA 1998, 95, 6803–6808. [Google Scholar] [CrossRef] [PubMed]
- Tsien, R.Y. The green fluorescent protein. Annu. Rev. Biochem. 1998, 67, 509–544. [Google Scholar] [CrossRef] [PubMed]
- You, C.C.; Miranda, O.R.; Gider, B.; Ghosh, P.S.; Kim, I.B.; Erdogan, B.; Krovi, S.A.; Bunz, U.H.; Rotello, V.M. Detection and identification of proteins using nanoparticle-fluorescent polymer “chemical nose” sensors. Nat. Nanotechnol. 2007, 2, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Dulkeith, E.; Morteani, A.C.; Niedereichholz, T.; Klar, T.A.; Feldmann, J.; Levi, S.A.; van Veggel, F.C.; Reinhoudt, D.N.; Möller, M.; Gittins, D.I. Fluorescence quenching of dye molecules near gold nanoparticles: Radiative and nonradiative effects. Phys. Rev. Lett. 2002, 89, 203002. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.D. Advances in electronic-nose technologies for the detection of volatile biomarker metabolites in the human breath. Metabolites 2015, 5, 140–163. [Google Scholar] [CrossRef] [PubMed]
- Homola, J.; Yee, S.S.; Gauglitz, G. Surface plasmon resonance sensors: review. Sens. Actuators B: Chem. 1999, 54, 3–15. [Google Scholar] [CrossRef]
- Yguerabide, J.; Yguerabide, E.E. Light-scattering submicroscopic particles as highly fluorescent analogs and their use as tracer labels in clinical and biological applications: I. Theory. Anal. Biochem. 1998, 262, 137–156. [Google Scholar] [CrossRef] [PubMed]
- Yguerabide, J.; Yguerabide, E.E. Light-Scattering submicroscopic particles as highly fluorescent analogs and their use as tracer labels in clinical and biological applications: II. Experimental Characterization. Anal. Biochem. 1998, 262, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Luk’yanchuk, B.; Zheludev, N.I.; Maier, S.A.; Halas, N.J.; Nordlander, P.; Giessen, H.; Chong, C.T. The Fano resonance in plasmonic nanostructures and metamaterials. Nat. Mater. 2010, 9, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Tok, R.U.; Ow-Yang, C.; Şendur, K. Unidirectional broadband radiation of honeycomb plasmonic antenna array with broken symmetry. Opt. Express 2011, 19, 22731–22742. [Google Scholar] [CrossRef] [PubMed]
- Dregely, D.; Taubert, R.; Dorfmüller, J.; Vogelgesang, R.; Kern, K.; Giessen, H. 3D optical Yagi-Uda nanoantenna array. Nat. Commun. 2011, 2, 267. [Google Scholar] [CrossRef] [PubMed]
- Lucas, L.J.; Tellez, C.; Castilho, M.L.; Lee, C.L.D.; Hupman, M.A.; Vieira, L.S.; Ferreira, I.; Raniero, L.; Hewitt, K.C. Development of a sensitive, stable and EGFR-specific molecular imaging agent for surface enhanced Raman spectroscopy. J. Raman Spectrosc. 2015, 46, 434–446. [Google Scholar] [CrossRef]
- Badawi, M.I.; Ahmed, M.M. Gold nanoparticles as high-resolution imaging contrast agents for early cancer diagnoses: Computational study. Int. J. Chem. Appl. Biol. Sci. 2014, 1, 12–17. [Google Scholar] [CrossRef]
- Rogalla, S.; Contag, C.H. Early cancer detection at the epithelial surface. Cancer J. 2015, 21, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, S.; Hollander, S.M.; Munasinghe, J.P.; Brinster, L.R.; Mercado-Matos, J.R.; Li, J.; Regales, L.; Pao, W.; Jänne, P.A.; Wong, K.K.; et al. Epidermal growth factor receptor as a novel molecular target for aggressive papillary tumors in the middle ear and temporal bone. Oncotarget 2015, 6, 11357–11368. [Google Scholar] [PubMed]
- Jackisch, C.; Scappaticci, F.A.; Heinzmann, D.; Bisordi, F.; Schreitmüller, T.; Minckwitz, G.V.; Cortés, J. Neoadjuvant breast cancer treatment as a sensitive setting for trastuzumab biosimilar development and extrapolation. Future Oncol. 2015, 11, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Clark, E.; Baselga, J. Treatment of HER2-positive metastatic breast cancer. N. Engl. J. Med. 2015, 372, 1964–1965. [Google Scholar] [CrossRef] [PubMed]
- Schönfeld, K.; Sahm, C.; Zhang, C.; Naundorf, S.; Brendel, C.; Odendahl, M.; Nowakowska, P.; Bönig, H.; Köhl, U.; Kloess, S.; et al. Selective inhibition of tumor growth by clonal NK cells expressing an ErbB2/HER2-specific chimeric antigen receptor. Mol. Ther. 2015, 23, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Hanna, W.M.; Rüschoff, J.; Bilous, M.; Coudry, R.A.; Dowsett, M.; Osamura, R.Y.; Penault-Llorca, F.; van de Vijver, M.; Viale, G. HER2 in situ hybridization in breast cancer: Clinical implications of polysomy 17 and genetic heterogeneity. Mod. Pathol. 2014, 27, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Pollock, N.I.; Grandis, J.R. HER2 as a therapeutic target in head and neck squamous cell carcinoma. Clin. Cancer Res. 2015, 21, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Yu, D. Molecular mechanisms of erbB2-mediated breast cancer chemoresistance. Adv. Exp. Med. Biol. 2007, 608, 119–129. [Google Scholar] [PubMed]
- Kim, S.S.; Rait, A.; Kim, E.; Pirollo, K.F.; Nishida, M.; Farkas, N.; Dagata, J.A.; Chang, E.H. A nanoparticle carrying the p53 gene targets tumors including cancer stem cells, sensitizes glioblastoma to chemotherapy and improves survival. ACS Nano 2014, 8, 5494–5514. [Google Scholar] [CrossRef] [PubMed]
- Moitra, K.L.; Lou, H.; Dean, M. Multidrug efflux pumps and cancer stem cells: Insights into multidrug resistance and therapeutic development. Clin. Pharmacol. Ther. 2011, 89, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Patra, H.K.; Ul Khaliq, N.; Romu, T.; Wiechec, E.; Borga, M.; Turner, A.P.; Tiwari, A. MRI-visual order-disorder micellar nanostructures for smart cancer theranostics. Adv. Healthc. Mater. 2014, 3, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi, A.; de Mayolo, E.A.; Patra, H.K.; Golabi, M.; Parlak, O.; Gunnarsson, R.; Campos, R.; Vishnu, R.; Elhag, S.; Subramanain, S.; et al. Keys and regulators of nanoscale theranostics. Adv. Mater. Lett. 2015, 6, 87–98. [Google Scholar]
- Patra, H.K.; Turner, A.P. The potential legacy of cancer nanotechnology: Cellular selection. Trends Biotechnol. 2014, 32, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Liao, L.C.; Lu, P.L.; Lo, C.L.; Tsai, H.C.; Huang, C.Y.; Wei, K.C.; Yen, T.C.; Hsiue, G.H. The accumulation of dual pH and temperature responsive micelles in tumors. Biomaterials 2012, 33, 4576–4588. [Google Scholar] [CrossRef] [PubMed]
- Schmaljohann, D. Thermo- and pH-responsive polymers in drug delivery. Adv. Drug Deliv Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Akiyama, Y.; Okano, T. Temperature-Responsive polymer modified surface for cell sheet engineering. Polymers 2012, 4, 1478–1498. [Google Scholar] [CrossRef]
- Tan, C.; Wang, Y.; Fan, W. Exploring polymeric micelles for improved delivery of anticancer agents: recent developments in preclinical studies. Pharmaceutics 2013, 5, 201–219. [Google Scholar] [CrossRef] [PubMed]
- Pacardo, D.B.; Ligler, F.S.; Gu, Z. Programmable nanomedicine: Synergistic and sequential drug delivery systems. Nanoscale 2015, 7, 3381–3391. [Google Scholar] [CrossRef] [PubMed]
- Schaffner, M.; England, G.; Kolle, M.; Aizenberg, J.; Vogel, N. Combining Bottom-Up Self-Assembly with Top-Down Microfabrication to Create Hierarchical Inverse Opals with High Structural Order. Small 2015. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zheng, Y.; Munro, C.J.; Ji, Y.; Braunschweig, A.B. Carbohydrate nanotechnology: hierarchical assembly using nature’s other information carrying biopolymers. Curr. Opin. Biotechnol. 2014, 34C, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Sknepnek, R.; Anderson, J.A.; Lamm, M.H.; Schmalian, J.; Travesset, A. Nanoparticle ordering via functionalized block copolymers in solution. ACS Nano. 2008, 2, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Lalitha, K.; Prasad, Y.S.; Maheswari, C.U.; Sridharan, V.; John, G.; Nagarajan, S. Stimuli responsive hydrogels derived from a renewable resource: Synthesis, self-assembly in water and application in drug delivery. J. Mater. Chem. B 2015. [Google Scholar] [CrossRef]
- Wei, T.; Chen, C.; Liu, J.; Liu, C.; Posocco, P.; Liu, X.; Cheng, Q.; Huo, S.; Liang, Z.; Fermeglia, M.; et al. Anticancer drug nanomicelles formed by self-assembling amphiphilic dendrimer to combat cancer drug resistance. Proc. Natl. Acad. Sci. USA 2015, 112, 2978–2983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arseneault, M.; Wafer, C.; Morin, J.F. Recent Advances in Click Chemistry Applied to Dendrimer Synthesis. Molecules 2015, 20, 9263–9294. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lin, C.; Li, J.; Qu, Y.; Ren, J. In vitro and in vivo gene transfection using biodegradable and low cytotoxic nanomicelles based on dendritic block copolymers. J. Mater. Chem. B 2015, 3, 688–699. [Google Scholar] [CrossRef]
- McDermott, S.P.; Eppert, K.; Lechman, E.R.; Doedens, M.; Dick, J.E. Comparison of human cord blood engraftment between immunocompromised mouse strains. Blood 2010, 116, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Szot, G.L.; Yadav, M.; Lang, J.; Kroon, E.; Kerr, J.; Kadoya, K.; Brandon, E.P.; Baetge, E.E.; Bour-Jordan, H.; Bluestone, J.A. Tolerance induction and reversal of diabetes in mice transplanted with human embryonic stem cell-derived pancreatic endoderm. Cell Stem Cell 2015, 16, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Twibanire, J.K.; Grindley, T.B. Polyester Dendrimers: Smart Carriers for Drug Delivery. Polymers 2014, 6, 179–213. [Google Scholar] [CrossRef]
- Wang, D.; Tu, C.; Su, Y.; Zhang, C.; Greiser, U.; Zhu, X.; Yan, D.; Wang, W. Supramolecularly engineered phospholipids constructed by nucleobase molecular recognition: Upgraded generation of phospholipids for drug delivery. Chem. Sci. 2015, 6, 3775–3787. [Google Scholar] [CrossRef]
- Lee, A.L.; Venkataraman, S.; Sirat, S.B.; Gao, S.; Hedrick, J.L.; Yang, Y.Y. The use of cholesterol-containing biodegradable block copolymers to exploit hydrophobic interactions for the delivery of anticancer drugs. Biomaterials 2012, 33, 1921–1928. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, W.; Ding, J.; Lu, S.; Wang, X.; Wang, C.; Chen, X. Cholesterol-Enhanced polylactide-based stereocomplex micelle for effective delivery of doxorubicin. Materials 2015, 8, 216–230. [Google Scholar] [CrossRef]
- Cao, L.; Shi, X.; Chang, H.; Zhang, Q.; He, Y. pH-Dependent recognition of apoptotic and necrotic cells by the human dendritic cell receptor DEC205. Proc. Natl. Acad. Sci. USA 2015, 112, 7237–7242. [Google Scholar] [CrossRef] [PubMed]
- Reshkin, S.J.; Greco, M.R.; Cardone, R.A. Role of pHi, and proton transporters in oncogene-driven neoplastic transformation. Philos. Trans. B 2014, 369. [Google Scholar] [CrossRef] [PubMed]
- Amith, S.R.; Wilkinson, J.M.; Baksh, S.; Fliegel, L. The Na+/H+ exchanger (NHE1) as a novel co-adjuvant target in paclitaxel therapy of triple-negative breast cancer cells. Oncotarget 2015, 6, 1262–1275. [Google Scholar] [PubMed]
- Parks, S.K.; Chiche, J.; Pouyssegur, J. pH control mechanisms of tumor survival and growth. J. Cell. Physiol. 2011, 226, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Plaks, V.; Kong, N.; Werb, Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell 2015, 16, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Cho, S.H.; Yuk, S.H. Polymeric microspheres composed of pH/temperature-sensitive polymer complex. Biomaterials 2001, 22, 2495–2499. [Google Scholar] [CrossRef]
- Böhm, I.; Kreth, S.K.; Ritter, H. Hyperbranched polyethylenimine bearing cyclodextrin moieties showing temperature and pH controlled dye release. Beilstein J. Org. Chem. 2011, 7, 1130–1134. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Meng, F.; Deng, C.; Klok, H.A.; Zhong, Z. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials 2013, 34, 3647–3657. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Chen, J.; Bi, Y.; Xu, X.; Zhou, H.; Gao, J.; Hu, Y.; Zhao, Y.; Chai, Z. Near-Infrared light remote-controlled intracellular anti-cancer drug delivery using thermo/pH sensitive nanovehicle. Acta Biomater. 2015, 17, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Lebrecht, A.; Boehm, D.; Schmidt, M.; Koelbl, H.; Schwirz, R.L.; Grus, F.H. Diagnosis of breast cancer by tear proteomic pattern. Cancer Genomics Proteomics 2009, 6, 177–182. [Google Scholar] [PubMed]
- Anderson, K.S.; Sibani, S.; Wallstrom, G.; Qiu, J.; Mendoza, E.A.; Raphael, J.; Hainsworth, E.; Montor, W.R.; Wong, J.; Park, J.G.; et al. Protein microarray signature of autoantibody biomarkers for the early detection of breast cancer. J. Proteome Res. 2011, 10, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.K.; Chen, L.; Ao, M.H.; Chiu, J.H.; Zhang, Z.; Zhang, H.; Chan, D.W. Serum fucosylated prostate-specific antigen (PSA) improves the differentiation of aggressive from non-aggressive prostate cancers. Theranostics 2015, 5, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Wang, Y.; Li, M.; Chen, P.; Zhou, Z.; Liu, Y.; Zhao, T.; Zhang, Z.; Wang, C.; Sun, X.; Qiu, P. Validation of the Memorial Sloan Kettering Cancer Center nomogram for predicting non-sentinel lymph node metastasis in sentinel lymph node-positive breast-cancer patients. Onco Targets Ther. 2015, 8, 487–493. [Google Scholar] [PubMed]
- Brenner, S. Nobel lecture. Nature’s gift to science. Biosci. Rep. 2003, 23, 225–237. [Google Scholar] [CrossRef] [PubMed]
- De La Iglesia, D.; Chiesa, S.; Kern, J.; Maojo, V.; Martin-Sanchez, F.; Potamias, G.; Moustakis, V.; Mitchell, J.A. Nanoinformatics: New challenges for biomedical informatics at the nano level. Stud. Health Technol. Inform. 2009, 150, 987–991. [Google Scholar] [PubMed]
- Maojo, V.; Fritts, M.; de la Iglesia, D.; Cachau, R.E.; Garcia-Remesal, M.; Mitchell, J.A.; Kulikowski, C. Nanoinformatics: A new area of research in nanomedicine. Int. J. Nanomed. 2012, 7, 3867–3890. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.G.; Klaessig, F.; Harper, S.L.; Fritts, M.; Hoover, M.D.; Gaheen, S.; Stokes, T.H.; Reznik-Zellen, R.; Freund, E.T.; Klemm, J.D.; et al. Informatics and standards for nanomedicine technology. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011, 3, 511–532. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, C.; Storsberg, J. Nanomaterials—Tools, Technology and Methodology of Nanotechnology Based Biomedical Systems for Diagnostics and Therapy. Biomedicines 2015, 3, 203-223. https://doi.org/10.3390/biomedicines3030203
Schmidt C, Storsberg J. Nanomaterials—Tools, Technology and Methodology of Nanotechnology Based Biomedical Systems for Diagnostics and Therapy. Biomedicines. 2015; 3(3):203-223. https://doi.org/10.3390/biomedicines3030203
Chicago/Turabian StyleSchmidt, Christian, and Joachim Storsberg. 2015. "Nanomaterials—Tools, Technology and Methodology of Nanotechnology Based Biomedical Systems for Diagnostics and Therapy" Biomedicines 3, no. 3: 203-223. https://doi.org/10.3390/biomedicines3030203
APA StyleSchmidt, C., & Storsberg, J. (2015). Nanomaterials—Tools, Technology and Methodology of Nanotechnology Based Biomedical Systems for Diagnostics and Therapy. Biomedicines, 3(3), 203-223. https://doi.org/10.3390/biomedicines3030203