The Diagnostic Role of Novel Echocardiography Indices and Arterial Stiffness in Diabetic Cardiomyopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Resting Echocardiography
2.3. Diastolic Stress Echocardiography (DSTE)
2.4. Global Longitudinal Strain (GLS) and Myocardial Work (MW)
2.5. Cardio-Ankle Vascular Index (CAVI) Measurement
2.6. Blood Measurements
2.7. Statistical Analysis
3. Results
3.1. Study Population
3.2. Echocardiographic Findings
3.3. Diastolic Stress Echocardiography
3.4. Follow-Up Results
3.5. Biomarkers
3.6. Correlations
4. Discussion
4.1. Overall Findings
4.2. Echocardiographic Indices (GLS, Myocardial Work, and LAVI)
4.3. Arterial Stiffness
4.4. Biomarkers
4.5. Diastolic Stress Echocardiography
4.6. Follow-Up and Prognostic Implications
4.7. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BNP | B-type natriuretic peptide |
| BP | Blood pressure |
| BMI | Body mass index |
| CAVI | Cardio-Ankle Vascular Index |
| DBCM | Diabetic cardiomyopathy |
| DSTE | Diastolic stress echocardiography |
| GCW | Global constructive work |
| GLS | Global longitudinal strain |
| GWE | Global work efficiency |
| GWI | Global myocardial work index |
| GWW | Global wasted work |
| HF | Heart failure |
| HFpEF | HF with preserved ejection fraction |
| HFrEF | HF with reduced ejection fraction |
| HFA-PEFF | Heart Failure Association Pre-test Probability of HFpEF |
| HsTn | High-sensitivity troponin |
| IQR | Interquartile range |
| LA | Left atrium |
| LAVI | Left atrium volume index |
| LV | Left ventricle |
| LVEF | Left ventricular ejection fraction |
| LVFP | Left ventricular filling pressures |
| LVMI | left ventricular mass index |
| MW | Myocardial work |
| PWV | Pulse wave velocity |
| RV | Right ventricle |
| STA | Speckle tracking analysis |
| SD | Standard deviation |
| TAPSE | Tricuspid annular plane systolic excursion |
| TRVmax | Tricuspid regurgitation velocity maximum |
References
- Glovaci, D.; Fan, W.; Wong, N.D. Epidemiology of Diabetes Mellitus and Cardiovascular Disease. Curr. Cardiol. Rep. 2019, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Kim, J. Diabetic Cardiomyopathy: Where We Are and Where We Are Going. Korean J. Intern. Med. 2017, 32, 404–421. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, T.; Pafundi, P.C.; Galiero, R.; Albanese, G.; Di Martino, A.; Caturano, A.; Vetrano, E.; Rinaldi, L.; Sasso, F.C. The Diabetic Cardiomyopathy: The Contributing Pathophysiological Mechanisms. Front. Med. 2021, 8, 695792. [Google Scholar] [CrossRef]
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ. Res. 2018, 122, 624–638. [Google Scholar] [CrossRef]
- Lorenzo-Almorós, A.; Tuñón, J.; Orejas, M.; Cortés, M.; Egido, J.; Lorenzo, Ó. Diagnostic Approaches for Diabetic Cardiomyopathy. Cardiovasc. Diabetol. 2017, 16, 28. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Aboyans, V.; Blacher, J.; Brodmann, M.; Brutsaert, D.L.; Chirinos, J.A.; De Carlo, M.; Delgado, V.; Lancellotti, P.; Lekakis, J.; et al. The Role of Ventricular-Arterial Coupling in Cardiac Disease and Heart Failure: Assessment, Clinical Implications and Therapeutic Interventions. A Consensus Document of the ESC Working Group on Aorta & Peripheral Vascular Diseases, EACVI, and HFA. Eur. J. Heart Fail. 2019, 21, 402–424. [Google Scholar] [CrossRef] [PubMed]
- Pieske, B.; Tschöpe, C.; de Boer, R.A.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.P.; et al. How to Diagnose Heart Failure with Preserved Ejection Fraction: The HFA-PEFF Diagnostic Algorithm: A Consensus Recommendation from the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2019, 40, 3297–3317. [Google Scholar] [CrossRef]
- Nishi, T.; Kobayashi, Y.; Christle, J.W.; Cauwenberghs, N.; Boralkar, K.; Moneghetti, K.; Amsallem, M.; Hedman, K.; Contrepois, K.; Myers, J.; et al. Incremental Value of Diastolic Stress Test in Identifying Subclinical Heart Failure in Patients with Diabetes Mellitus. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Iwakura, K. Heart Failure in Patients with Type 2 Diabetes Mellitus: Assessment with Echocardiography and Effects of Antihyperglycemic Treatments. J. Echocardiogr. 2019, 17, 177–186. [Google Scholar] [CrossRef]
- Liao, L.; Shi, B.; Ding, Z.; Chen, L.; Dong, F.; Li, J.; Zhong, Y.; Xu, J. Echocardiographic Study of Myocardial Work in Patients with Type 2 Diabetes Mellitus. BMC Cardiovasc. Disord. 2022, 22, 59. [Google Scholar] [CrossRef]
- Prenner, S.B.; Chirinos, J.A. Arterial Stiffness in Diabetes Mellitus. Atherosclerosis 2015, 238, 370–379. [Google Scholar] [CrossRef]
- Kim, H.L.; Jo, S.H. Arterial Stiffness and Heart Failure with Preserved Ejection Fraction. J. Korean Med. Sci. 2024, 39, e195. [Google Scholar] [CrossRef]
- Chirinos, J.A.; Segers, P.; Hughes, T.; Townsend, R. Large-Artery Stiffness in Health and Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 74, 1237–1263. [Google Scholar] [CrossRef] [PubMed]
- Namba, T.; Masaki, N.; Takase, B.; Adachi, T. Arterial Stiffness Assessed by Cardio-Ankle Vascular Index. Int. J. Mol. Sci. 2019, 20, 3664. [Google Scholar] [CrossRef] [PubMed]
- Limpijankit, T.; Vathesatogkit, P.; Matchariyakul, D.; Yingchoncharoen, T.; Siriyotha, S.; Thakkinstian, A.; Sritara, P. Cardio-Ankle Vascular Index as a Predictor of Major Adverse Cardiovascular Events in Metabolic Syndrome Patients. Clin. Cardiol. 2021, 44, 1628–1635. [Google Scholar] [CrossRef] [PubMed]
- Kumric, M.; Ticinovic Kurir, T.; Borovac, J.A.; Bozic, J. Role of Novel Biomarkers in Diabetic Cardiomyopathy. World J. Diabetes 2021, 12, 685–705. [Google Scholar] [CrossRef]
- Ianoș, R.D.; Cozma, A.; Lucaciu, R.L.; Hangan, A.C.; Negrean, V.; Mercea, D.C.; Ciulei, G.; Pop, C.; Procopciuc, L.M. Role of Circulating Biomarkers in Diabetic Cardiomyopathy. Biomedicines 2024, 12, 2153. [Google Scholar] [CrossRef]
- Mahmood, A.; Dhall, E.; Primus, C.P.; Gallagher, A.; Zakeri, R.; Mohammed, S.F.; Chahal, A.A.; Ricci, F.; Aung, N.; Mohammed, K.Y. HFpEF Management: A Systematic Review of Clinical Practice Guidelines and Recommendations. Eur. Heart J. Qual. Care Clin. Outcomes 2024, 10, 571–589. [Google Scholar] [CrossRef]
- World Medical Association. Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects; WMA: Fortaleza, Brazil, 2013. [Google Scholar]
- Huang, D.; Cui, C.; Zheng, Q.; Li, Y.; Liu, Y.; Hu, Y.; Wang, Y.; Liu, R.; Liu, L. Quantitative Analysis of Myocardial Work by Non-Invasive Left Ventricular Pressure-Strain Loop in Patients with Type 2 Diabetes Mellitus. Front. Cardiovasc. Med. 2021, 8, 733339. [Google Scholar] [CrossRef]
- Cao, W.; Deng, Y.; Lv, L.; Liu, X.; Luo, A.; Yin, L.; Li, Z. Assessment of Left Ventricular Function in Patients with Type 2 Diabetes Mellitus by Non-Invasive Myocardial Work. Front. Endocrinol. 2023, 14, 1241307. [Google Scholar] [CrossRef]
- Li, G.A.; Huang, J.; Sheng, X.; Fan, L. Assessment of Subclinical LV Myocardial Dysfunction in Type 2 Diabetes Mellitus Patients with or without Hypertension by Myocardial Work. Diabetol. Metab. Syndr. 2023, 15, 180. [Google Scholar] [CrossRef]
- Zaborska, B.; Sikora-Frąc, M.; Smarż, K.; Pilichowska-Paszkiet, E.; Budaj, A.; Sitkiewicz, D.; Sygitowicz, G. The Role of Galectin-3 in Heart Failure: Diagnostic, Prognostic and Therapeutic Potential. Int. J. Mol. Sci. 2023, 24, 13111. [Google Scholar] [CrossRef]
- Dong, R.; Zhang, M.; Hu, Q.; Zheng, S.; Soh, A.; Zheng, Y.; Yuan, H. Galectin-3 as a Novel Biomarker for Disease Diagnosis and a Target for Therapy. Int. J. Mol. Med. 2018, 41, 599–614. [Google Scholar] [CrossRef]
- Tavolinejad, H.; Erten, O.; Maynard, H.; Chirinos, J.A. Prognostic Value of Cardio-Ankle Vascular Index for Cardiovascular and Kidney Outcomes: Systematic Review and Meta-Analysis. JACC Adv. 2024, 3, 101019. [Google Scholar] [CrossRef] [PubMed]
- Osawa, K.; Nakanishi, R.; Miyoshi, T.; Rahmani, S.; Ceponiene, I.; Nezarat, N.; Kanisawa, M.; Qi, H.; Jayawardena, E.; Kim, N.; et al. Correlation of Arterial Stiffness with Left Atrial Volume Index and Left Ventricular Mass Index in Young Adults: Evaluation by Coronary Computed Tomography Angiography. Heart Lung Circ. 2019, 28, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Lee, B.W.; Kim, H.M.; Shin, J.Y.; Kang, E.S.; Cha, B.S.; Lee, E.J.; Lim, S.-K.; Lee, H.C. Associations between Cardio-Ankle Vascular Index and Microvascular Complications in Type 2 Diabetes Mellitus Patients. J. Atheroscler. Thromb. 2011, 18, 328–336. [Google Scholar] [CrossRef]
- Huo, J.L.; Feng, Q.; Pan, S.; Fu, W.-J.; Liu, Z.; Liu, Z. Diabetic Cardiomyopathy: Early Diagnostic Biomarkers, Pathogenetic Mechanisms, and Therapeutic Interventions. Cell Death Discov. 2023, 9, 256. [Google Scholar] [CrossRef]
- Landolfo, M.; Spannella, F.; Giulietti, F.; Ortensi, B.; Stella, L.; Carlucci, M.A.; Galeazzi, R.; Turchi, F.; Luconi, M.P.; Zampa, R.; et al. Detecting Heart Stress Using NT-proBNP in Patients with Type 2 Diabetes Mellitus and Hypertension or High-Normal Blood Pressure: A Cross-Sectional Multicentric Study. Cardiovasc. Diabetol. 2024, 23, 391. [Google Scholar] [CrossRef] [PubMed]
- Gouda, P.; Liu, Y.; Butler, J.; Del Prato, S.; Ibrahim, N.E.; Lam, C.S.P.; Marwick, T.; Rosenstock, J.; Tang, W.; Zannad, F.; et al. Relationship between NT-proBNP, Echocardiographic Abnormalities and Functional Status in Patients with Subclinical Diabetic Cardiomyopathy. Cardiovasc. Diabetol. 2024, 23, 378. [Google Scholar] [CrossRef] [PubMed]
- Kadoglou, N.P.E.; Bouwmeester, S.; de Lepper, A.G.W.; de Kleijn, M.C.; Herold, I.H.F.; Bouwman, A.R.A.; Korakianitis, I.; Simmers, T.; Bracke, F.A.L.E.; Houthuizen, P. The Prognostic Role of Global Longitudinal Strain and NT-proBNPin Heart Failure Patients Receiving Cardiac Resynchronization Therapy. J. Pers. Med. 2024, 14, 188. [Google Scholar] [CrossRef]
- Myhre, P.L.; O’Meara, E.; Claggett, B.L.; de Denus, S.; Jarolim, P.; Anand, I.S.; Beldhuis, I.E.; Fleg, J.L.; Lewis, E.; Pitt, B.; et al. Cardiac Troponin I and Risk of Cardiac Events in Patients with HFpEF. Circ. Heart Fail. 2018, 11, e005312. [Google Scholar] [CrossRef]
- Ianoș, R.D.; Iancu, M.; Pop, C.; Lucaciu, R.L.; Hangan, A.C.; Rahaian, R.; Cozma, A.; Negrean, V.; Mercea, D.; Procopciuc, L.M. Predictive Value of NT-proBNP, FGF21, Galectin-3 and Copeptin in Advanced HF in Patients with Preserved and Mildly Reduced EF and Type 2 Diabetes Mellitus. Medicina 2024, 60, 1841. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.R.; Cheng, C.H.; Liu, J.C.; Chen, H.Y.; Chen, J.J.; Cheng, T.H. Understanding Galectin-3’s Role in Diastolic Dysfunction: A Contemporary Perspective. Life 2024, 14, 906. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Yu, C.; Zhang, Z.; Cong, J. Timosaponin AIII Alleviates Type 2 Diabetes-Induced Cardiomyopathy by Targeting Galectin-3 (LGALS3). Exp. Cell Res. 2025, 450, 114665. [Google Scholar] [CrossRef]
- Flores-Ramírez, R.; Azpiri-López, J.R.; González-González, J.G.; Ordaz-Farías, A.; González-Carrillo, L.E.; Carrizales-Sepúlveda, E.F.; Vera-Pineda, R. Global Longitudinal Strain as a Biomarker in Diabetic Cardiomyopathy: A Comparative Study with Galectin-3 in Patients with Preserved EF. Arch. Cardiol. Mex. 2017, 87, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Echouffo-Tcheugui, J.B.; Zhang, S.; Florido, R.; Pankow, J.S.; Michos, E.D.; Goldberg, R.B.; Nambi, V.; Gerstenblith, G.; Post, W.S.; Blumenthal, R.S.; et al. Galectin-3, Metabolic Risk, and Incident Heart Failure: The ARIC Study. J. Am. Heart Assoc. 2024, 13, e031607. [Google Scholar] [CrossRef]
- Schmitt, V.H.; Prochaska, J.H.; Föll, A.S.; Schulz, A.; Keller, K.; Hahad, O.; Koeck, T.; Tröbs, S.-O.; Rapp, S.; Beutel, M.; et al. Galectin-3 for Prediction of Cardiac Function Compared to NT-proBNP in Individuals with Prediabetes and Type 2 Diabetes Mellitus. Sci. Rep. 2021, 11, 19012. [Google Scholar] [CrossRef]
- Patel, D.A.; Lavie, C.J.; Milani, R.V.; Ventura, H.O. Left Atrial Volume Index Predictive of Mortality Independent of LV Geometry in a Large Clinical Cohort with Preserved EF. Mayo Clin. Proc. 2011, 86, 730–737. [Google Scholar] [CrossRef]
- Kadoglou, N.P.E.; Mouzarou, A.; Hadjigeorgiou, N.; Korakianitis, I.; Myrianthefs, M.M. Challenges in Echocardiography for the Diagnosis and Prognosis of Non-Ischemic Hypertensive Heart Disease. J. Clin. Med. 2024, 13, 2708. [Google Scholar] [CrossRef]
- Alseenmy, A.A.; Attia, W.M.; Khalaf, H.A.S.; Amin, F.R. Assessment of Left Ventricular Diastolic Reserve in Diabetic Patients by Stress Echocardiography. Al-Azhar Int. Med. J. 2023, 4, 1721. [Google Scholar] [CrossRef]
- Kadoglou, N.P.E.; Papadopoulos, C.H.; Krommydas, A. Prognostic Value of Exercise-Induced Pulmonary Hypertension in Asymptomatic Patients with Primary Mitral Regurgitation. J. Cardiol. 2022, 79, 306–310. [Google Scholar] [CrossRef] [PubMed]
| Variables | DBCM Group (n = 37) | No-DBCM Group (n = 88) | Non-Diabetic Controls (n = 42) | p-Value |
|---|---|---|---|---|
| Age (years) | 62 ± 6 | 60 ± 8 | 63 ± 5 | 0.115 |
| Men (n) | 22 (59.5%) | 48 (54.5%) | 25 (59.5%) | 0.981 |
| Hypertension (n) | 25 (69.44%) | 47 (53.40%) | 25 (59.5%) | 0.300 |
| Dyslipidemia (n) | 27 (75%) | 61 (72.61%) | 29 (69%) | 0.787 |
| Smoking (n) | 10 (27.77%) | 20 (23.80%) | 11 (26.19%) | 0.645 |
| BMI (kg/m2) | 29.86 ± 9.17 | 29.88 ± 9.15 | 28.56 ± 4.55 | 0.088 |
| SBP (mmHg) | 148 ± 17 | 143 ± 13 | 138 ± 7 | 0.102 |
| DBP (mmHg) | 88 ± 7 | 89 ± 8 | 85 ± 8 | 0.702 |
| RCAVI (m/s) | 8.45 ± 1.14 | 7.78 ± 1.21 | 6.86 ± 1.07 | 0.026 a,b,c |
| LCAVI (m/s) | 8.56 ± 1.21 | 7.86 ± 1.24 | 6.90 ± 1.04 | 0.022 a,b,c |
| CAVI (m/s) | 8.51 ± 1.17 | 7.82 ± 1.22 | 6.88 ± 1.05 | <0.001 a,b,c |
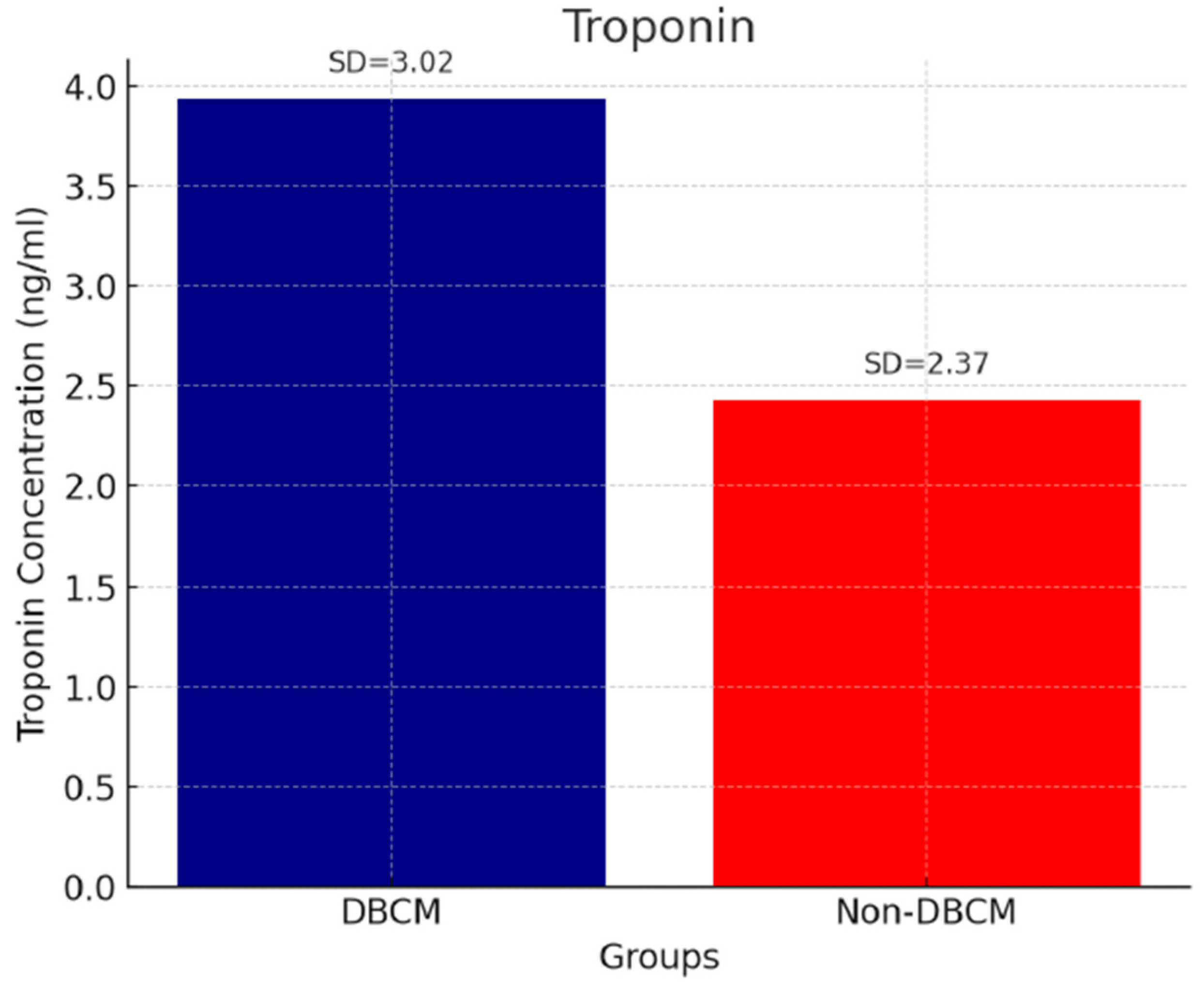
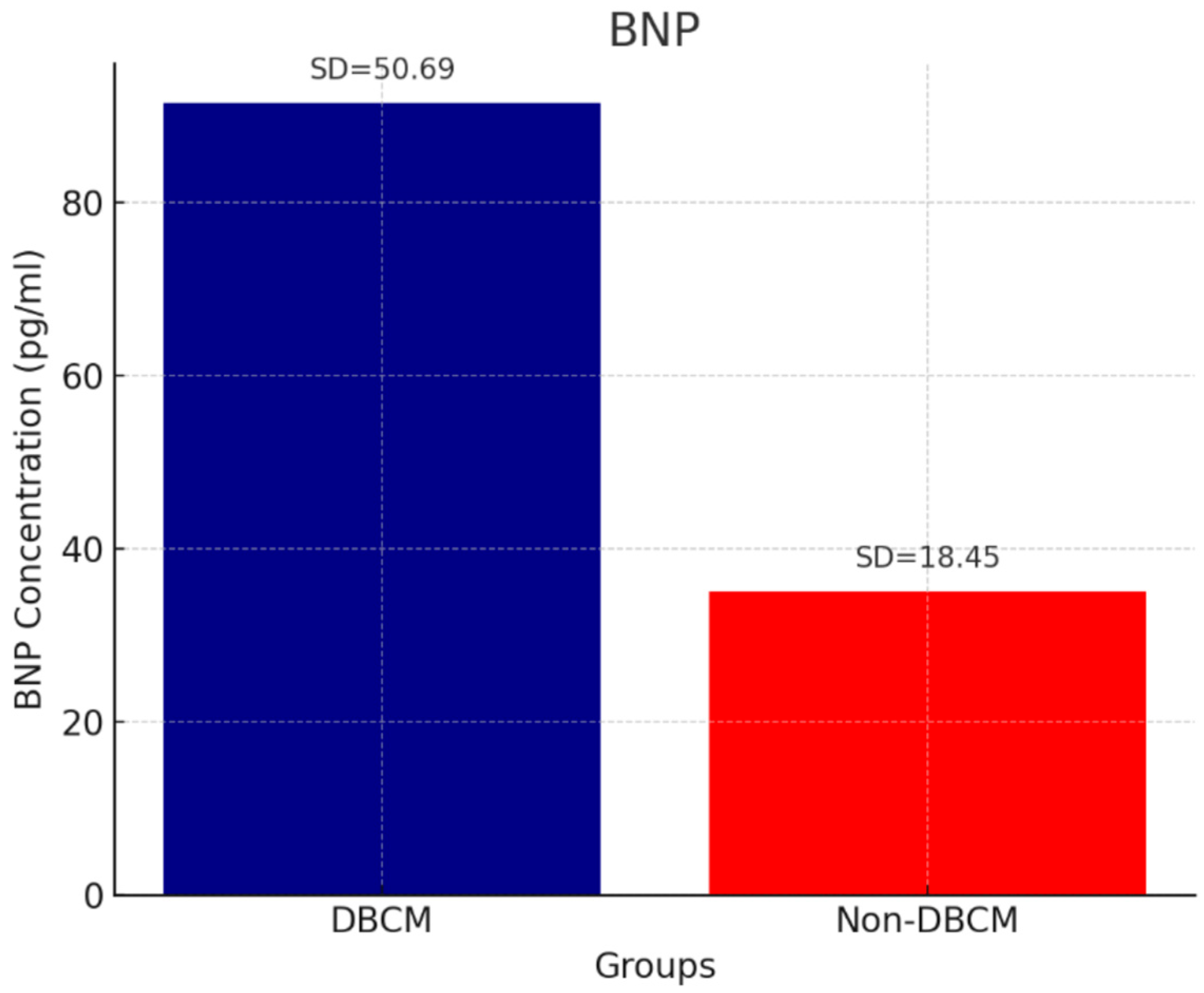
| BNP (pg/mL) | 91.48 ± 50.69 | 35.10 ± 18.45 | 29.79 ± 11.63 | <0.001 a,b |
| Troponin (ng/mL) | 3.94 ± 1.02 | 2.43 ± 1.01 | 2.07 ± 1.37 | <0.001 a,b |
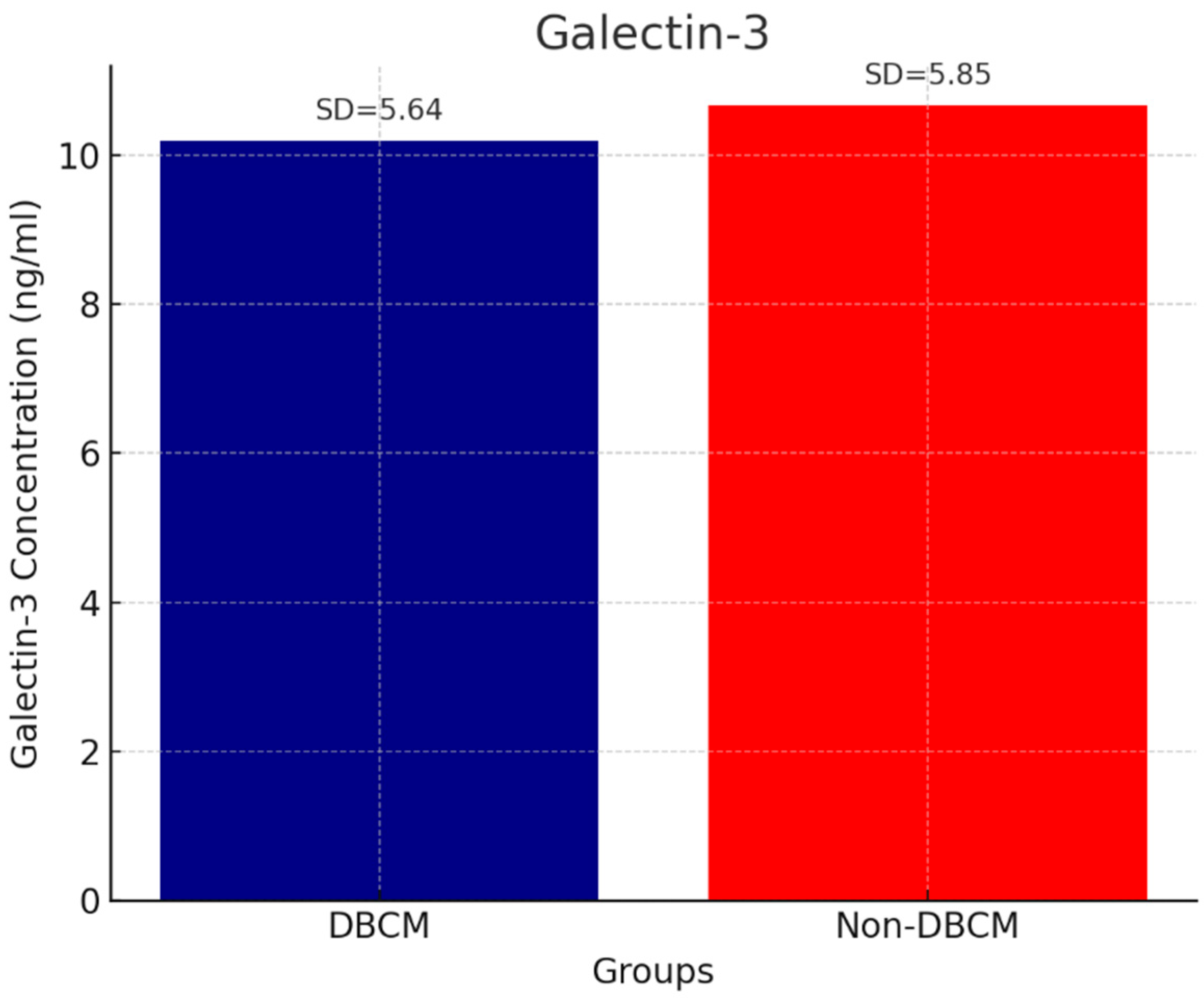
| Galectin |
| Parameters | DBCM Group (n = 37) | No-DBCM Group (n = 88) | Non-Diabetic Controls (n = 42) | p-Value |
|---|---|---|---|---|
| LVEF (%) | 63 ± 5 | 63 ± 5 | 63 ± 7 | 0.968 |
| E/A | 0.931 ± 0.217 | 0.931 ± 0.298 | 0.975 ± 0.298 | 0.799 |
| E/e′ | 8.64 ± 2.31 | 7.59 ± 1.91 | 7.01 ± 1.1 | 0.020 a,b |
| TAPSE (cm) | 2.47 ± 0.422 | 2.31 ± 0.39 | 2.68 ± 0.532 | 0.069 |
| TRVmax (m/s) | 2.21 ± 0.31 | 2.05 ± 0.32 | 2.11 ± 0.44 | 0.274 |
| RV S’ (m/s) | 0.14 ± 0.016 | 0.14 ± 0.029 | 0.15 ± 0.015 | 0.830 |
| LAVI (mL/m2) | 39.71 ± 8.27 | 33.46 ± 9.50 | 31.11 ± 5.60 | 0.012 |
| SV (mL) | 60.30 ± 19.05 | 71.77 ± 19.84 | 75.13 ± 16.46 | 0.039 |
| GLS (%) | −18.87 ± 2.65 | −18.73 ± 1.50 | −19.22 ± 1.12 | 0.790 |
| GWI (mmHg%) | 2197.79 ± 544.30 | 2042.11 ± 497.11 | 2055.89 ± 600.84 | 0.200 |
| GWE (%) | 95.66 ± 2.74 | 95.47 ± 3.02 | 95.91 ± 3.05 | 0.810 |
| GWW (%) | 80.58 ± 36.32 | 91.68 ± 44.20 | 90.11 ± 44.20 | 0.573 |
| GCW (mmHg%) | 2267.26 ± 333.21 | 2232.73 ± 361.14 | 2309.95 ± 580.23 | 0.714 |
| E/A exercise | 1.05 ± 0.17 | 1.17 ± 0.39 | 1.21 ± 0.25 | 0.075 |
| E/e′ exercise | 10.82 ± 3.59 | 8.50 ± 2.35 | 7.02 ± 1.77 | <0.001 a,b,c |
| TRVmax exercise (m/s) | 3.09 ± 0.5 | 2.35 ± 0.35 | 2.31 ± 0.44 | <0.001 a,b |
| DTRVmax (m/s) | 0.88 ± 0.2 | 0.3 ± 0.11 | 0.3 ± 0.12 | <0.001 a,b |
| HFA-PEFF score: 2–4 at rest (n) | 9 | 75 | - | - |
| HFA-PEFF score: 0–1 at rest (n) | 0 | 13 | 42 | - |
| Added points to final HFA-PEFF score from DSTE (n) | 0 points:11 1 points:23 | - | - | - |
| Parameters | DBCM Group (n = 36) | No-DBCM Group (n = 86) | p-Value |
|---|---|---|---|
| LVEF (%) | 64 ± 6 | 64 ± 5 | 0.919 |
| E/A | 0.95 ± 0.27 | 0.96 ± 0.32 | 0.852 |
| E/e′ | 8.48 ± 2.57 | 7.78 ± 2.99 | 0.081 |
| TAPSE (cm) | 2.31 ± 0.51 | 2.40 ± 0.42 | 0.382 |
| TRVmax (m/s) | 2.3 ± 0.3 | 2.1 ± 0.3 | 0.368 |
| RV S’ (m/s) | 0.13 ± 0.02 | 0.14 ± 0.03 | 0.397 |
| LAVI (mL/m2) | 37.98 ± 8.04 | 33.11 ± 8.12 | 0.049 |
| GLS (%) | −18.38 ± 2.11 | −18.88 ± 2.66% | 0.398 |
| GWI (mmHg%) | 2087.82 ± 456.20 | 2101.07 ± 589.12 | 0.511 |
| GWE (%) | 95.13 ± 3.25 | 95.89 ± 3.88 | 0.789 |
| GWW (%) | 89.85 ± 47.21 | 85.24 ± 41.25 | 0.676 |
| GWC (mmHg%) | 2221.25 ± 391.15 | 2318 ± 398.41 | 0.487 |
| E/A exercise | 1.11 ± 0.13 | 1.09 ± 0.21 | 0.354 |
| E/e′ exercise | 10.55 ± 4.03 | 8.10 ± 3.56 | <0.001 |
| TRVmax exercise (m/s) | 2.95 ± 0.37 | 2.31 ± 0.20 | <0.001 |
| HFA-PEFF score: 0–1 at rest (n) | 0 | 12 | <0.001 |
| HFA-PEFF score: 2–4 at rest (n) | 7 | 65 | <0.001 |
| Added points to final HFA-PEFF score from DSTE (n) | 0 points: 12 1 points: 21 2–3 points: 3 | 0 points: 84 1 points: 2 2–3 points: 0 | - |
| TRVmax exercise ≥ 2.9 m/s (n) | 29 | 7 | - |
| Medications | DBCM Group (n = 36) | No-DBCM Group (n = 86) | ||
|---|---|---|---|---|
| Baseline | End | Baseline | End | |
| ACEIs/ARBs | 21 | 28 | 44 | 51 |
| CCBs | 13 | 12 | 24 | 26 |
| Diuretics | 5 | 14 | 2 | 2 |
| Statins | 27 | 28 | 61 | 63 |
| SGLT-2 inhibitors | 4 | 33 | 6 | 8 |
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| E/e′ | 1.40 (0.1.18–1.91) | 0.030 | 1.15 (1.02–1.58) | 0.673 |
| CAVI | 2.11 (1.51–2.75) | 0.007 | 1.91 (1.69–2.42) | 0.042 |
| Troponin | 1.25 (1.01–2.58) | 0.040 | 1.05 (1.01–2.21) | 0.532 |
| BNP | 7.55 (4.28–11.78) | <0.001 | 5.45 (3.81–9.02) | 0.006 |
| DSTE-TRVmax | 10.41 (12.07–20.88) | <0.001 | 8.56 (6.62–11.63) | <0.001 |
| DSTE-E/e′ ratio | 1.60 (0.95–2.25) | 0.048 | 1.15 (1.03–1.45) | 0.342 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khattab, E.; Sokratous, S.; Kyriakou, M.; Parpas, G.; Korakianitis, I.; Papakyriakopoulou, P.; Kadoglou, N.P.E. The Diagnostic Role of Novel Echocardiography Indices and Arterial Stiffness in Diabetic Cardiomyopathy. Biomedicines 2025, 13, 2317. https://doi.org/10.3390/biomedicines13092317
Khattab E, Sokratous S, Kyriakou M, Parpas G, Korakianitis I, Papakyriakopoulou P, Kadoglou NPE. The Diagnostic Role of Novel Echocardiography Indices and Arterial Stiffness in Diabetic Cardiomyopathy. Biomedicines. 2025; 13(9):2317. https://doi.org/10.3390/biomedicines13092317
Chicago/Turabian StyleKhattab, Elina, Stefanos Sokratous, Michaela Kyriakou, Georgios Parpas, Ioannis Korakianitis, Paraskevi Papakyriakopoulou, and Nikolaos P. E. Kadoglou. 2025. "The Diagnostic Role of Novel Echocardiography Indices and Arterial Stiffness in Diabetic Cardiomyopathy" Biomedicines 13, no. 9: 2317. https://doi.org/10.3390/biomedicines13092317
APA StyleKhattab, E., Sokratous, S., Kyriakou, M., Parpas, G., Korakianitis, I., Papakyriakopoulou, P., & Kadoglou, N. P. E. (2025). The Diagnostic Role of Novel Echocardiography Indices and Arterial Stiffness in Diabetic Cardiomyopathy. Biomedicines, 13(9), 2317. https://doi.org/10.3390/biomedicines13092317








