Clinical and dGEMRIC Evaluation of Microfragmented Adipose Tissue Versus Hyaluronic Acid in Inflammatory Phenotype of Knee Osteoarthritis: A Randomized Controlled Trial
Abstract
1. Introduction
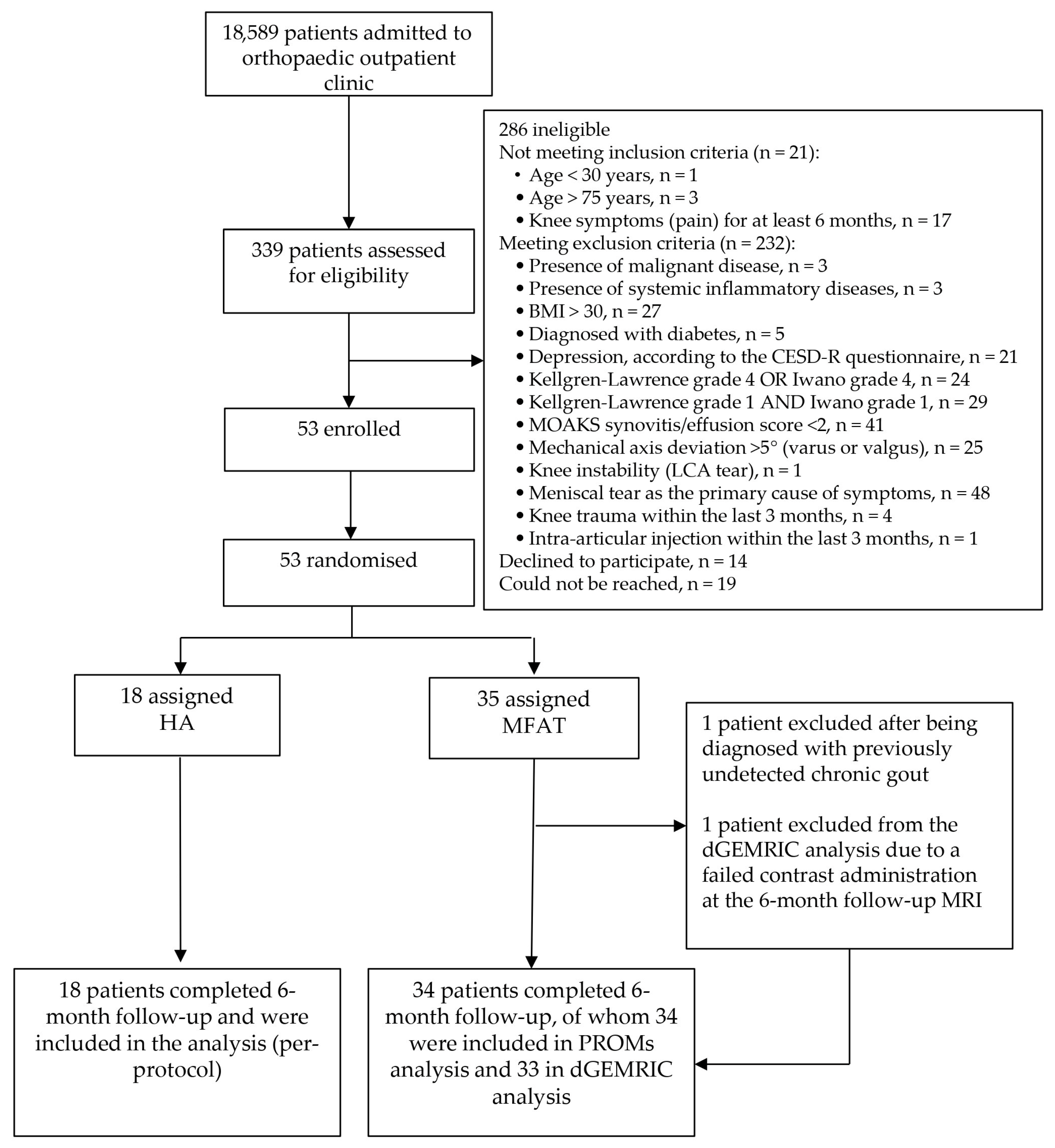
2. Materials and Methods
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
2.3. Randomization and Blinding
2.4. Interventions
2.4.1. Lipoaspiration and MFAT Preparation
2.4.2. Injection Procedure
2.5. Clinical Outcome Measures
2.5.1. Patient-Reported Outcome Measures (PROMs)
2.5.2. Minimal Clinically Important Difference (MCID) and Ceiling Effect Adjustments
2.6. Imaging Assessment
2.7. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Clinical Outcomes (PROMs)
3.2.1. KOOS
3.2.2. WOMAC
3.2.3. Visual Analog Scale (VAS)
3.2.4. Responder Analysis
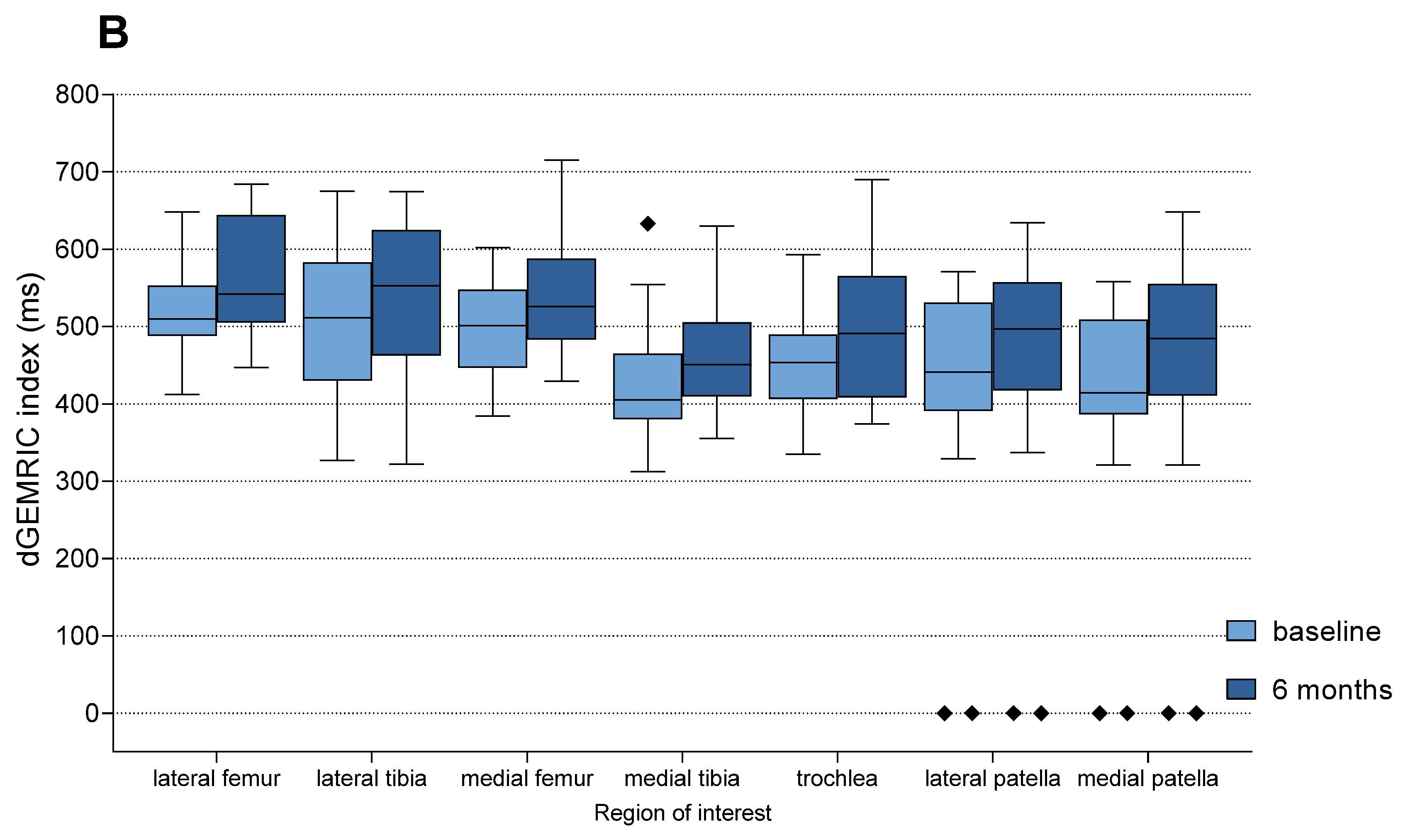
3.3. Imaging Outcomes (dGEMRIC)
3.4. Correlation Between Clinical and Imaging Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CESD-R | Center for Epidemiologic Studies Depression Scale Revised |
| dGEMRIC | Delayed Gadolinium-Enhanced Magnetic Resonance Imaging of Cartilage |
| GAG | Glycosaminoglycan |
| HA | Hyaluronic acid |
| IQR | Interquartile range |
| KOOS | Knee Injury and Osteoarthritis Outcome Score |
| LF | Lateral femur |
| LP | Lateral patella |
| LT | Lateral tibia |
| MCID | Minimal clinically important difference |
| MF | Medial femur |
| MFAT | Microfragmented adipose tissue |
| MOAKS | MRI Osteoarthritis Knee Score |
| MP | Medial patella |
| MRI | Magnetic resonance imaging |
| MSC | Mesenchymal stem cell |
| MT | Medial tibia |
| OA | Osteoarthritis |
| PPV | Positive predictive value |
| PROMs | Patient-reported outcome measures |
| RCT | Randomized controlled trial |
| ROI | Region of interest |
| SVF | Stromal vascular fraction |
| TR | Trochlea |
| VAS | Visual analogue scale |
| WOMAC | Western Ontario and McMaster Universities Osteoarthritis Index |
References
- Nelson, A.E. Osteoarthritis Year in Review 2017: Clinical. Osteoarthr. Cartil. 2018, 26, 319–325. [Google Scholar] [CrossRef]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A Disease of the Joint as an Organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Primorac, D.; Molnar, V.; Rod, E.; Jeleč, Ž.; Čukelj, F.; Matišić, V.; Vrdoljak, T.; Hudetz, D.; Hajsok, H.; Borić, I. Knee Osteoarthritis: A Review of Pathogenesis and State-of-the-Art Non-Operative Therapeutic Considerations. Genes 2020, 11, 854. [Google Scholar] [CrossRef]
- Allen, K.D.; Thoma, L.M.; Golightly, Y.M. Epidemiology of Osteoarthritis. Osteoarthr. Cartil. 2022, 30, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Liu, Q.; Yin, H.; Wang, K.; Diao, N.; Zhang, Y.; Lin, J.; Guo, A. Prevalence Trends of Site-Specific Osteoarthritis from 1990 to 2019: Findings from the Global Burden of Disease Study 2019. Arthritis Rheumatol. 2022, 74, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Krakowski, P.; Rejniak, A.; Sobczyk, J.; Karpiński, R. Cartilage Integrity: A Review of Mechanical and Frictional Properties and Repair Approaches in Osteoarthritis. Healthcare 2024, 12, 1648. [Google Scholar] [CrossRef]
- Yucesoy, B.; Charles, L.E.; Baker, B.; Burchfiel, C.M. Occupational and Genetic Risk Factors for Osteoarthritis: A Review. Work 2015, 50, 261–273. [Google Scholar] [CrossRef]
- Roemer, F.W.; Jarraya, M.; Collins, J.E.; Kwoh, C.K.; Hayashi, D.; Hunter, D.J.; Guermazi, A. Structural Phenotypes of Knee Osteoarthritis: Potential Clinical and Research Relevance. Skelet. Radiol. 2023, 52, 2021–2030. [Google Scholar] [CrossRef]
- Mahmoudian, A.; Lohmander, L.S.; Mobasheri, A.; Englund, M.; Luyten, F.P. Early-Stage Symptomatic Osteoarthritis of the Knee—Time for Action. Nat. Rev. Rheumatol. 2021, 17, 621–632. [Google Scholar] [CrossRef]
- Karpiński, R.; Prus, A.; Baj, J.; Radej, S.; Prządka, M.; Krakowski, P.; Jonak, K. Articular Cartilage: Structure, Biomechanics, and the Potential of Conventional and Advanced Diagnostics. Appl. Sci. 2025, 15, 6896. [Google Scholar] [CrossRef]
- Dell’Isola, A.; Allan, R.; Smith, S.L.; Marreiros, S.S.P.; Steultjens, M. Identification of Clinical Phenotypes in Knee Osteoarthritis: A Systematic Review of the Literature. BMC Musculoskelet. Disord. 2016, 17, 425. [Google Scholar] [CrossRef]
- Dell’Isola, A.; Steultjens, M. Classification of Patients with Knee Osteoarthritis in Clinical Phenotypes: Data from the Osteoarthritis Initiative. BMC Musculoskelet. Disord. 2018, 13, e0191045. [Google Scholar] [CrossRef]
- van der Esch, M.; Knoop, J.; van der Leeden, M.; Roorda, L.D.; Lems, W.F.; Knol, D.L.; Dekker, J. Clinical Phenotypes in Patients with Knee Osteoarthritis: A Study in the Amsterdam Osteoarthritis Cohort. Osteoarthr. Cartil. 2015, 23, 544–549. [Google Scholar] [CrossRef]
- Van Spil, W.E.; Kubassova, O.; Boesen, M.; Bay-Jensen, A.-C.; Mobasheri, A. Osteoarthritis Phenotypes and Novel Therapeutic Targets. Biochem. Pharmacol. 2019, 165, 41–48. [Google Scholar] [CrossRef]
- Hunter, D.J.; Guermazi, A.; Lo, G.H.; Grainger, A.J.; Conaghan, P.G.; Boudreau, R.M.; Roemer, F.W. Evolution of Semi-Quantitative Whole Joint Assessment of Knee OA: MOAKS (MRI Osteoarthritis Knee Score). Osteoarthr. Cartil. 2011, 19, 990–1002. [Google Scholar] [CrossRef] [PubMed]
- Sellam, J.; Berenbaum, F. The Role of Synovitis in Pathophysiology and Clinical Symptoms of Osteoarthritis. Nat. Rev. Rheumatol. 2010, 6, 625–635. [Google Scholar] [CrossRef]
- Molnar, V.; Pavelić, E.; Vrdoljak, K.; Čemerin, M.; Klarić, E.; Matišić, V.; Bjelica, R.; Brlek, P.; Kovačić, I.; Tremolada, C.; et al. Mesenchymal Stem Cell Mechanisms of Action and Clinical Effects in Osteoarthritis: A Narrative Review. Genes 2022, 13, 949. [Google Scholar] [CrossRef] [PubMed]
- Shoukrie, S.I.; Venugopal, S.; Dhanoa, R.K.; Selvaraj, R.; Selvamani, T.Y.; Zahra, A.; Malla, J.; Hamouda, R.K.; Hamid, P.F. Safety and Efficacy of Injecting Mesenchymal Stem Cells into a Human Knee Joint to Treat Osteoarthritis: A Systematic Review. Cureus 2022, 14, e24823. [Google Scholar] [CrossRef] [PubMed]
- Hudetz, D.; Borić, I.; Rod, E.; Jeleč, Ž.; Radić, A.; Vrdoljak, T.; Skelin, A.; Lauc, G.; Trbojević-Akmačić, I.; Plečko, M.; et al. The Effect of Intra-Articular Injection of Autologous Microfragmented Fat Tissue on Proteoglycan Synthesis in Patients with Knee Osteoarthritis. Genes 2017, 8, 270. [Google Scholar] [CrossRef]
- Hudetz, D.; Borić, I.; Rod, E.; Jeleč, Ž.; Kunovac, B.; Polašek, O.; Vrdoljak, T.; Plečko, M.; Skelin, A.; Polančec, D.; et al. Early Results of Intra-Articular Micro-Fragmented Lipoaspirate Treatment in Patients with Late Stages Knee Osteoarthritis: A Prospective Study. Croat. Med. J. 2019, 60, 227–236. [Google Scholar] [CrossRef]
- Borić, I.; Hudetz, D.; Rod, E.; Jeleč, Ž.; Vrdoljak, T.; Skelin, A.; Polašek, O.; Plečko, M.; Trbojević-Akmačić, I.; Lauc, G.; et al. A 24-Month Follow-Up Study of the Effect of Intra-Articular Injection of Autologous Microfragmented Fat Tissue on Proteoglycan Synthesis in Patients with Knee Osteoarthritis. Genes 2019, 10, 1051. [Google Scholar] [CrossRef]
- Altman, R.; Manjoo, A.; Fierlinger, A.; Niazi, F.; Nicholls, M. The Mechanism of Action for Hyaluronic Acid Treatment in the Osteoarthritic Knee: A Systematic Review. BMC Musculoskelet. Disord. 2015, 16, 321. [Google Scholar] [CrossRef]
- Roos, E.M.; Lohmander, L.S. The Knee Injury and Osteoarthritis Outcome Score (KOOS): From Joint Injury to Osteoarthritis. Health Qual. Life Outcomes 2003, 1, 64. [Google Scholar] [CrossRef] [PubMed]
- Emara, A.; El-Khouly, G.; Boettner, F. Diagnosis-Specific Thresholds of the Minimal Clinically Important Difference and Patient Acceptable Symptom State for KOOS After Total Knee Arthroplasty. J. Orthop. Surg. Res. 2024, 19, 35. [Google Scholar] [CrossRef] [PubMed]
- Boffa, A.; Andriolo, L.; Franceschini, M.; Di Martino, A.; Asunis, E.; Grassi, A.; Zaffagnini, S.; Filardo, G. Minimal Clinically Important Difference and Patient Acceptable Symptom State in Patients with Knee Osteoarthritis Treated with PRP Injection. Orthop. J. Sports Med. 2021, 9, 23259671211026242. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.D.C.; Perriman, D.M.; Fearon, A.M.; Couldrick, J.M.; Scarvell, J.M. Minimal Important Change and Difference for Knee Osteoarthritis Outcome Measurement Tools After Non-Surgical Interventions: A Systematic Review. BMJ Open 2023, 13, e063026. [Google Scholar] [CrossRef]
- Clement, N.D.; Bardgett, M.; Weir, D.; Holland, J.; Gerrand, C.; Deehan, D.J. What Is the Minimum Clinically Important Difference for the WOMAC Index After TKA? Clin. Orthop. Relat. Res. 2018, 476, 2005–2014. [Google Scholar] [CrossRef]
- Escobar, A.; Quintana, J.M.; Bilbao, A.; Aróstegui, I.; Lafuente, I.; Vidaurreta, I. Responsiveness and Clinically Important Differences for the WOMAC and SF-36 After Total Knee Replacement. Osteoarthr. Cartil. 2007, 15, 273–280. [Google Scholar] [CrossRef]
- Danoff, J.R.; Goel, R.; Sutton, R.; Maltenfort, M.G.; Austin, M.S. How Much Pain Is Significant? Defining the Minimal Clinically Important Difference for the Visual Analog Scale for Pain After Total Joint Arthroplasty. J. Arthroplast. 2018, 33, S71–S75.e2. [Google Scholar] [CrossRef]
- Tiderius, C.J.; Hawezi, Z.K.; Olsson, L.E.; Dahlberg, L.E. Pre-Contrast T1 and Cartilage Thickness as Confounding Factors in dGEMRIC When Evaluating Human Cartilage Adaptation to Physical Activity. BMC Med. Imaging 2020, 20, 1. [Google Scholar] [CrossRef]
- Baria, M.; Barker, T.; Durgam, S.; Pedroza, A.; Flanigan, D.; Jia, L.; Kaeding, C.; Magnussen, R. Microfragmented Adipose Tissue Is Equivalent to Platelet-Rich Plasma for Knee Osteoarthritis at 12 Months Posttreatment: A Randomized Controlled Trial. Orthop. J. Sports Med. 2024, 12, 23259671241233916. [Google Scholar] [CrossRef]
- Caplan, A.I.; Correa, D. The MSC: An Injury Drugstore. Cell Stem Cell 2011, 9, 11–15. [Google Scholar] [CrossRef]
- Hong, Z.; Chen, J.; Zhang, S.; Zhao, C.; Bi, M.; Chen, X.; Bi, Q. Intra-Articular Injection of Autologous Adipose-Derived Stromal Vascular Fraction for Knee Osteoarthritis: A Double-Blind Randomized Clinical Trial. Am. J. Sports Med. 2019, 47, 2820–2830. [Google Scholar] [CrossRef]
- Han, J.H.; Jung, M.; Chung, K.; Moon, H.S.; Jung, S.H.; Byun, J.; Kim, S.H. Intra-articular Stromal Vascular Fraction and Mesenchymal Stem Cell Injections Show Variable Efficacy and Higher Potential Complications Compared to Corticosteroid and Hyaluronic Acid in Treatment of Knee Osteoarthritis: A Meta-analysis of Randomized Controlled Trials. Arthroscopy 2025, 41, 3666–3680.e13. [Google Scholar] [CrossRef]
- Pas, H.I.; Winters, M.; Haisma, H.J.; Koenis, M.J.J.; Tol, J.L.; Moen, M.H. Stem cell injections in knee osteoarthritis: A systematic review of the literature. Br. J. Sports Med. 2017, 51, 1125–1133. [Google Scholar] [CrossRef]
- Tiderius, C.J.; Olsson, L.E.; Leander, P.; Ekberg, O.; Dahlberg, L. Delayed Gadolinium-Enhanced MRI of Cartilage (dGEMRIC) in Early Knee Osteoarthritis. Magn. Reson. Med. 2003, 49, 488–492. [Google Scholar] [CrossRef]
- Van Tiel, J.; Reijman, M.; Bos, P.K.; Hermans, J.; Van Buul, G.M.; Bron, E.E.; Klein, S.; Verhaar, J.A.N.; Krestin, G.P.; Bierma-Zeinstra, S.M.A.; et al. Delayed Gadolinium-Enhanced MRI of Cartilage (dGEMRIC) Shows No Change in Cartilage Structural Composition After Viscosupplementation in Patients with Early-Stage Knee Osteoarthritis. PLoS ONE 2013, 8, e79785. [Google Scholar] [CrossRef]
- Van Ginckel, A.; Baelde, N.; Almqvist, K.F.; Roosen, P.; McNair, P.; Witvrouw, E. Functional Adaptation of Knee Cartilage in Asymptomatic Female Novice Runners Compared to Sedentary Controls. A Longitudinal Analysis Using Delayed Gadolinium Enhanced Magnetic Resonance Imaging of Cartilage (dGEMRIC). Osteoarthr. Cartil. 2010, 18, 1564–1569. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Lu, V.; Khan, W. Adipose Tissue-Derived Mesenchymal Stem Cells as a Potential Restorative Treatment for Cartilage Defects: A PRISMA Review and Meta-Analysis. Pharmaceuticals 2021, 14, 1280. [Google Scholar] [CrossRef]
- Amann, E.; Wolff, P.; Breel, E.; Van Griensven, M.; Balmayor, E.R. Hyaluronic Acid Facilitates Chondrogenesis and Matrix Deposition of Human Adipose Derived Mesenchymal Stem Cells and Human Chondrocytes Co-Cultures. Acta Biomater. 2017, 52, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Felthaus, O.; Prantl, L. Adipose Tissue-Derived Therapies for Osteoarthritis: Multifaceted Mechanisms and Clinical Prospects. Cells 2025, 14, 669. [Google Scholar] [CrossRef] [PubMed]
- Glinkowski, W.; Śladowski, D.; Tomaszewski, W.; Pol-IAHA Study Group. Molecular Mechanisms and Therapeutic Role of Intra-Articular Hyaluronic Acid in Osteoarthritis: A Precision Medicine Perspective. J. Clin. Med. 2025, 14, 2547. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Jaramillo, D.; Millis, M.B.; Gray, M.L.; Burstein, D. Assessment of Early Osteoarthritis in Hip Dysplasia with Delayed Gadolinium-Enhanced Magnetic Resonance Imaging of Cartilage. J. Bone Jt. Surg. Am. 2003, 85, 1987–1992. [Google Scholar] [CrossRef]
- Bekkers, J.E.J.; Bartels, L.W.; Benink, R.J.; Tsuchida, A.I.; Vincken, K.L.; Dhert, W.J.A.; Creemers, L.B.; Saris, D.B.F. Delayed Gadolinium Enhanced MRI of Cartilage (dGEMRIC) Can Be Effectively Applied for Longitudinal Cohort Evaluation of Articular Cartilage Regeneration. Osteoarthr. Cartil. 2013, 21, 943–949. [Google Scholar] [CrossRef]
- Sethi, V.; Anand, C.; Della Pasqua, O. Clinical Assessment of Osteoarthritis Pain: Contemporary Scenario, Challenges, and Future Perspectives. Pain Ther. 2024, 13, 391–408. [Google Scholar] [CrossRef]
- Courties, A.; Kouki, I.; Soliman, N.; Mathieu, S.; Sellam, J. Osteoarthritis Year in Review 2024: Epidemiology and Therapy. Osteoarthr. Cartil. 2024, 32, 1397–1404. [Google Scholar] [CrossRef]
- Chin, S.; Collins, J.E. Analytic Challenges in Defining Structural Phenotypes in OA Clinical Trials: A Perspective. Osteoarthr. Imaging 2025, 100273. [Google Scholar] [CrossRef]
- Karsdal, M.A.; Rovati, L.C.; Tambiah, J.; Kubassova, O.; Ladel, C.; Berenbaum, F.; Bay-Jensen, A.-C.; Mclean, L.; Loeser, R.; Mobasheri, A.; et al. The Inflammatory Endotype in Osteoarthritis: Reflections from the 2024 OARSI Clinical Trials Symposium (CTS) with a Special Emphasis on Feasibility for Clinical Development. Osteoarthr. Cartil. Open 2025, 7, 100572. [Google Scholar] [CrossRef]
- Bannuru, R.R.; Schmid, C.H.; Kent, D.M.; Vaysbrot, E.E.; Wong, J.B.; McAlindon, T.E. Comparative Effectiveness of Pharmacologic Interventions for Knee Osteoarthritis: A Systematic Review and Network Meta-Analysis. Ann. Intern. Med. 2015, 162, 46–54. [Google Scholar] [CrossRef]





| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Age between 30 and 75 years | Presence of malignant, systemic inflammatory, or other systemic diseases that potentially cause knee pain or systemic inflammation |
| Kellgren–Lawrence OA grade 2–3 OR Iwano grade 2–3 for patellofemoral OA | BMI > 30 or diagnosed with diabetes |
| Knee symptoms (pain) for at least 6 months | ≥6 tender points distributed above and below the waist, bilaterally and axially |
| Mechanical axis deviation < 5° | Depression, according to the CESD-R questionnaire |
| Ability to comply with follow-up and study instructions | Kellgren–Lawrence grade 4 OR Iwano grade 4 |
| Signed informed consent | Kellgren–Lawrence grade 1 AND Iwano grade 1 |
| MOAKS synovitis/effusion score < 2 (i.e., no effusion on MRI) | |
| Post-traumatic knee OA | |
| History of surgical intervention on the affected knee | |
| Mechanical axis deviation > 5° (varus or valgus) | |
| Knee instability | |
| Meniscal or structural lesions as the primary cause of symptoms (e.g., bucket-handle or radial meniscal tears) | |
| Knee trauma within the last 3 months | |
| Intra-articular injection within the last 3 months (e.g., corticosteroids, HA, platelet-rich plasma, etc.) | |
| Other musculoskeletal conditions (e.g., Marfan syndrome, osteogenesis imperfecta) impairing clinical assessment | |
| Inability to abstain from NSAID use for 7 days before and during the follow-up | |
| Known allergies to lidocaine or adrenaline | |
| Coagulopathy, thrombocytopenia, or anticoagulation with PT < 0.70 | |
| Systemic immunosuppressive therapy | |
| Synovial chondromatosis or pigmented villonodular synovitis | |
| Active joint infection | |
| Pregnancy or intention to become pregnant during the study period | |
| History of chemotherapy or radiotherapy to the limbs or the adipose tissue harvesting site | |
| Psychiatric disorders impairing compliance | |
| Anticipated inability to attend follow-up assessments |
| MFAT (n = 35) | HA (n = 18) | p-Value | |
|---|---|---|---|
| Age (mean ± SD) | 53.9 ± 9.0 | 58.7 ± 9.5 | 0.104 |
| Sex (% female) | 74.3% | 77.8% | 0.780 |
| BMI (kg/m2, mean ± SD) | 26.6 ± 2.4 | 26.8 ± 2.5 | 0.851 |
| KOOS Subscore | Group | Mean | SD | Median (IQR) | p-Value |
|---|---|---|---|---|---|
| ΔKOOS Pain_6M | HA | 22.4 | 22.0 | 20.8 (34.0) | 0.623 |
| MFAT | 23.0 | 15.0 | 20.8 (28.5) | ||
| ΔKOOS Symptoms_6M | HA | 12.7 | 16.0 | 12.5 (18.8) | 0.008 |
| MFAT | 25.0 | 15.6 | 23.2 (15.2) | ||
| ΔKOOS ADL_6M | HA | 19.3 | 19.6 | 16.9 (34.9) | 0.307 |
| MFAT | 22.6 | 14.8 | 25.0 (23.2) | ||
| ΔKOOS Sport/Rec_6M | HA | 19.7 | 23.7 | 20.0 (35.0) | 0.122 |
| MFAT | 29.7 | 24.6 | 25.0 (42.5) | ||
| ΔKOOS QoL_6M | HA | 23.6 | 25.4 | 12.5 (46.9) | 0.323 |
| MFAT | 29.8 | 22.0 | 31.3 (37.5) |
| Region of Interest | MFAT | HA | p-Value (MFAT vs. HA) | ||
|---|---|---|---|---|---|
| Baseline dGEMRIC Mean (ms) | Mean % Change | Baseline dGEMRIC Mean (ms) | Mean % Change | ||
| Lateral femur | 499.9 | 11.3% | 519.7 | 7.7% | 0.174 |
| Lateral tibia | 487.4 | 11.5% | 507.1 | 7.5% | 0.211 |
| Medial femur | 489.1 | 9.2% | 500.7 | 8.7% | 0.737 |
| Medial tibia | 440.8 | 13.3% | 428.2 | 9.2% | 0.156 |
| Trochlea | 446.2 | 11.6% | 449.9 | 9.6% | 0.574 |
| Lateral patella | 452.6 | 10.7% | 464.4 | 8.2% | 0.358 |
| Medial patella | 441.0 | 10.1% | 454.8 | 7.8% | 0.343 |
| KOOS Pain Responder | KOOS Pain Non-Responder | |
|---|---|---|
| dGEMRIC responder | 23 | 0 |
| dGEMRIC non-responder | 14 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molnar, V.; Jeleč, Ž.; Rod, E.; Hudetz, D.; Brlek, P.; Borić, I.; Matišić, V.; Mešić, J.; Pavelić, E.S.; Vidović, D.; et al. Clinical and dGEMRIC Evaluation of Microfragmented Adipose Tissue Versus Hyaluronic Acid in Inflammatory Phenotype of Knee Osteoarthritis: A Randomized Controlled Trial. Biomedicines 2025, 13, 2301. https://doi.org/10.3390/biomedicines13092301
Molnar V, Jeleč Ž, Rod E, Hudetz D, Brlek P, Borić I, Matišić V, Mešić J, Pavelić ES, Vidović D, et al. Clinical and dGEMRIC Evaluation of Microfragmented Adipose Tissue Versus Hyaluronic Acid in Inflammatory Phenotype of Knee Osteoarthritis: A Randomized Controlled Trial. Biomedicines. 2025; 13(9):2301. https://doi.org/10.3390/biomedicines13092301
Chicago/Turabian StyleMolnar, Vilim, Željko Jeleč, Eduard Rod, Damir Hudetz, Petar Brlek, Igor Borić, Vid Matišić, Jana Mešić, Eduard Stjepan Pavelić, Dinko Vidović, and et al. 2025. "Clinical and dGEMRIC Evaluation of Microfragmented Adipose Tissue Versus Hyaluronic Acid in Inflammatory Phenotype of Knee Osteoarthritis: A Randomized Controlled Trial" Biomedicines 13, no. 9: 2301. https://doi.org/10.3390/biomedicines13092301
APA StyleMolnar, V., Jeleč, Ž., Rod, E., Hudetz, D., Brlek, P., Borić, I., Matišić, V., Mešić, J., Pavelić, E. S., Vidović, D., Blažević, D., Čukelj, F., Sabalić, S., Štivičić, J., Dujmović, T., Starešinić, M., Čemerin, M., Weinberger, D. G., Molnar, I., ... Primorac, D. (2025). Clinical and dGEMRIC Evaluation of Microfragmented Adipose Tissue Versus Hyaluronic Acid in Inflammatory Phenotype of Knee Osteoarthritis: A Randomized Controlled Trial. Biomedicines, 13(9), 2301. https://doi.org/10.3390/biomedicines13092301









