A Potential Role of Adropin in Inflammatory Rheumatic Diseases—What Do We Know So Far?
Abstract
1. Introduction
2. Methods
3. Mechanisms of Adropin Action
3.1. Metabolic Mechanisms
3.2. Immune Mechanisms
3.3. Vascular Mechanisms
3.4. Adropin’s Interactions with Molecular and Inflammatory Pathways in ARDs
4. Clinical Evidence in Autoimmune Rheumatic Diseases
5. Therapeutic Potential and Translational Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKT | protein kinase B |
| ARDs | autoimmune rheumatic diseases |
| BD | Behçet’s disease |
| BMI | body mass index |
| ERK1/2 | extracellular signal-regulated kinase 1/2 |
| ECs | endothelial cells |
| Enho | energy homeostasis-associated |
| IL | interleukin |
| IR | insulin resistance |
| KD | Kawasaki disease |
| LXRα | liver X receptor alpha |
| MPO-ANCA | myeloperoxidase anti-neutrophil cytoplasmic antibodies |
| NF-κB | nuclear factor kappa B |
| NO | nitric oxide |
| OA | osteoarthritis |
| PPAR-γ | peroxisome proliferator activated receptor-γ |
| RA | rheumatoid arthritis |
| SLE | systemic lupus erythematosus |
| SSc | systemic sclerosis |
| TNF-α | tumor necrosis factor α |
| Tregs | regulatory T cells |
| VEGFR2 | vascular endothelial growth factor receptor 2 |
| eNOS | endothelial nitric oxide synthase |
| mRNA | messenger ribonucleic acid |
| pSjS | primary Sjögren’s Syndrome |
References
- Kumar, K.G.; Trevaskis, J.L.; Lam, D.D.; Sutton, G.M.; Koza, R.A.; Chouljenko, V.N.; Kousoulas, K.G.; Rogers, P.M.; Kesterson, R.A.; Thearle, M.; et al. Identification of Adropin as a Secreted Factor Linking Dietary Macronutrient Intake with Energy Homeostasis and Lipid Metabolism. Cell Metab. 2008, 8, 468–481. [Google Scholar] [CrossRef]
- Aydin, S.; Kuloglu, T.; Aydin, S.; Eren, M.N.; Yilmaz, M.; Kalayci, M.; Sahin, I.; Kocaman, N.; Citil, C.; Kendir, Y. Expression of adropin in rat brain, cerebellum, kidneys, heart, liver, and pancreas in streptozotocin-induced diabetes. Mol. Cell. Biochem. 2013, 380, 73–81. [Google Scholar] [CrossRef]
- Gao, S.; McMillan, R.P.; Zhu, Q.; Lopaschuk, G.D.; Hulver, M.W.; Butler, A.A. Therapeutic effects of adropin on glucose tolerance and substrate utilization in diet-induced obese mice with insulin resistance. Mol. Metab. 2015, 4, 310–324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, Q.; Huang, Z.; Jiang, Q. Adropin inhibited tilapia hepatic glucose output and triglyceride accumulation via AMPK activation. J. Endocrinol. 2020, 246, 109–122. [Google Scholar] [CrossRef]
- Jasaszwili, M.; Billert, M.; Strowski, M.Z.; Nowak, K.W.; Skrzypski, M. Adropin as A Fat-Burning Hormone with Multiple Functions—Review of a Decade of Research. Molecules 2020, 25, 549. [Google Scholar] [CrossRef]
- Butler, A.A.; Tam, C.S.; Stanhope, K.L.; Wolfe, B.M.; Ali, M.R.; O’KEeffe, M.; St-Onge, M.-P.; Ravussin, E.; Havel, P.J. Low Circulating Adropin Concentrations with Obesity and Aging Correlate with Risk Factors for Metabolic Disease and Increase after Gastric Bypass Surgery in Humans. J. Clin. Endocrinol. Metab. 2012, 97, 3783–3791. [Google Scholar] [CrossRef]
- Yosaee, S.; Khodadost, M.; Esteghamati, A.; Speakman, J.R.; Shidfar, F.; Nazari, M.N.; Bitarafan, V.; Djafarian, K. Metabolic Syndrome Patients Have Lower Levels of Adropin When Compared With Healthy Overweight/Obese and Lean Subjects. Am. J. Men’s Health 2016, 11, 426–434. [Google Scholar] [CrossRef]
- Zang, H.; Jiang, F.; Cheng, X.; Xu, H.; Hu, X. Serum adropin levels are decreased in Chinese type 2 diabetic patients and negatively correlated with body mass index. Endocr. J. 2018, 65, 685–691. [Google Scholar] [CrossRef]
- Ghoshal, S.; Stevens, J.R.; Billon, C.; Girardet, C.; Sitaula, S.; Leon, A.S.; Rao, D.; Skinner, J.S.; Rankinen, T.; Bouchard, C.; et al. Adropin: An endocrine link between the biological clock and cholesterol homeostasis. Mol. Metab. 2018, 8, 51–64. [Google Scholar] [CrossRef]
- Jasaszwili, M.; Pruszyńska-Oszmałek, E.; Wojciechowicz, T.; Strowski, M.Z.; Nowak, K.W.; Skrzypski, M. Adropin Slightly Modulates Lipolysis, Lipogenesis and Expression of Adipokines but Not Glucose Uptake in Rodent Adipocytes. Genes 2021, 12, 914. [Google Scholar] [CrossRef] [PubMed]
- Mushala, B.A.S.; Scott, I. Adropin: A hepatokine modulator of vascular function and cardiac fuel metabolism. Am. J. Physiol. Circ. Physiol. 2020, 320, H238–H244. [Google Scholar] [CrossRef]
- Wong, C.-M.; Wang, Y.; Lee, J.T.H.; Huang, Z.; Wu, D.; Xu, A.; Lam, K.S.L. Adropin Is a Brain Membrane-bound Protein Regulating Physical Activity via the NB-3/Notch Signaling Pathway in Mice. J. Biol. Chem. 2014, 289, 25976–25986. [Google Scholar] [CrossRef] [PubMed]
- Stein, L.M.; Yosten, G.L.C.; Samson, W.K. Adropin acts in brain to inhibit water drinking: Potential interaction with the orphan G protein-coupled receptor, GPR19. Am. J. Physiol. Integr. Comp. Physiol. 2016, 310, R476–R480. [Google Scholar] [CrossRef]
- Lovren, F.; Pan, Y.; Quan, A.; Singh, K.K.; Shukla, P.C.; Gupta, M.; Al-Omran, M.; Teoh, H.; Verma, S. Adropin Is a Novel Regulator of Endothelial Function. Circulation 2010, 122, S185–S192. [Google Scholar] [CrossRef] [PubMed]
- Rajala, M.W.; Scherer, P.E. Minireview: The Adipocyte—At the Crossroads of Energy Homeostasis, Inflammation, and Atherosclerosis. Endocrinology 2003, 144, 3765–3773. [Google Scholar] [CrossRef]
- Ali, I.I.; D’souza, C.; Singh, J.; Adeghate, E. Adropin’s Role in Energy Homeostasis and Metabolic Disorders. Int. J. Mol. Sci. 2022, 23, 8318. [Google Scholar] [CrossRef]
- Akcılar, R.; Koçak, F.E.; Şimşek, H.; Akcılar, A.; Bayat, Z.; Ece, E.; Kökdaşgil, H. The effect of adropin on lipid and glucose metabolism in rats with hyperlipidemia. Iran. J. Basic Med. Sci. 2016, 19, 245–251. [Google Scholar]
- Zhang, S.; Chen, Q.; Lin, X.; Chen, M.; Liu, Q. A Review of Adropin as the Medium of Dialogue between Energy Regulation and Immune Regulation. Oxidative Med. Cell. Longev. 2020, 2020, 3947806. [Google Scholar] [CrossRef]
- Feuerer, M.; Herrero, L.; Cipolletta, D.; Naaz, A.; Wong, J.; Nayer, A.; Lee, J.; Goldfine, A.B.; Benoist, C.; Shoelson, S.; et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 2009, 15, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Brnić, D.; Martinovic, D.; Zivkovic, P.M.; Tokic, D.; Tadin Hadjina, I.; Rusic, D.; Vilovic, M.; Supe-Domic, D.; Tonkic, A.; Bozic, J. Serum adropin levels are reduced in patients with inflammatory bowel diseases. Sci. Rep. 2020, 10, 9264. [Google Scholar] [CrossRef]
- Yolbas, S.; Kara, M.; Yilmaz, M.; Aydin, S.; Koca, S.S. Serum adropin level and ENHO gene expression in systemic sclerosis. Clin. Rheumatol. 2016, 35, 1535–1540. [Google Scholar] [CrossRef]
- Yolbas, S.; Kara, M.; Kalayci, M.; Yildirim, A.; Gundogdu, B.; Aydin, S.; Koca, S.S. ENHO gene expression and serum adropin level in rheumatoid arthritis and systemic lupus erythematosus. Adv. Clin. Exp. Med. 2018, 27, 1637–1641. [Google Scholar] [CrossRef]
- Gundogdu, G.; Gundogdu, K. A novel biomarker in patients with knee osteoarthritis: Adropin. Clin. Rheumatol. 2018, 37, 2179–2186. [Google Scholar] [CrossRef] [PubMed]
- Danolić, M.J.; Perković, D.; Petrić, M.; Barišić, I.; Gugo, K.; Božić, J. Adropin Serum Levels in Patients with Primary Sjögren’s Syndrome. Biomolecules 2021, 11, 1296. [Google Scholar] [CrossRef] [PubMed]
- Simac, P.; Perkovic, D.; Bozic, I.; Bilopavlovic, N.; Martinovic, D.; Bozic, J. Serum Adropin Levels in Patients with Rheumatoid Arthritis. Life 2022, 12, 169. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Van den Bossche, J.; O’Neill, L.A.; Menon, D. Macrophage Immunometabolism: Where Are We (Going)? Trends Immunol. 2017, 38, 395–406. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef]
- Sato, K.; Yamashita, T.; Shirai, R.; Shibata, K.; Okano, T.; Yamaguchi, M.; Mori, Y.; Hirano, T.; Watanabe, T. Adropin Contributes to Anti-Atherosclerosis by Suppressing Monocyte-Endothelial Cell Adhesion and Smooth Muscle Cell Proliferation. Int. J. Mol. Sci. 2018, 19, 1293. [Google Scholar] [CrossRef]
- Chen, S.; Zeng, K.; Liu, Q.-C.; Guo, Z.; Zhang, S.; Chen, X.-R.; Lin, J.-H.; Wen, J.-P.; Zhao, C.-F.; Lin, X.-H.; et al. Adropin deficiency worsens HFD-induced metabolic defects. Cell Death Dis. 2017, 8, e3008. [Google Scholar] [CrossRef]
- Butler, A.A.; Havel, P.J. Adropin and insulin resistance: Integration of endocrine, circadian, and stress signals regulating glucose metabolism. Obesity 2021, 29, 1799–1801. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ding, N.; Chen, C.; Gu, S.; Liu, J.; Wang, Y.; Lin, L.; Zheng, Y.; Li, Y. Adropin: A key player in immune cell homeostasis and regulation of inflammation in several diseases. Front. Immunol. 2025, 16, 1482308. [Google Scholar] [CrossRef]
- Chen, X.; Xue, H.; Fang, W.; Chen, K.; Chen, S.; Yang, W.; Shen, T.; Chen, X.; Zhang, P.; Ling, W. Adropin protects against liver injury in nonalcoholic steatohepatitis via the Nrf2 mediated antioxidant capacity. Redox Biol. 2019, 21, 101068. [Google Scholar] [CrossRef]
- Mamontova, A.; SégUret-Macé, S.; Esposito, B.; Chaniale, C.; Bouly, M.; Delhaye-Bouchaud, N.; Luc, G.; Staels, B.; Duverger, N.; Mariani, J.; et al. Severe Atherosclerosis and Hypoalphalipoproteinemia in the Staggerer Mouse, a Mutant of the Nuclear Receptor RORα. Circulation 1998, 98, 2738–2743. [Google Scholar] [CrossRef]
- Kopmels, B.; Mariani, J.; Delhaye-Bouchaud, N.; Audibert, F.; Fradelizi, D.; Wollman, E.E. Evidence for a Hyperexcitability State of Staggerer Mutant Mice Macrophages. J. Neurochem. 1992, 58, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Delerive, P.; Monte, D.; Dubois, G.; Trottein, F.; Fruchart-Najib, J.; Mariani, J.; Fruchart, J.-C.; Staels, B. The orphan nuclear receptor RORα is a negative regulator of the inflammatory response. EMBO Rep. 2001, 2, 42–48. [Google Scholar] [CrossRef]
- Takayanagi, H.; Iizuka, H.; Juji, T.; Nakagawa, T.; Yamamoto, A.; Miyazaki, T.; Koshihara, Y.; Oda, H.; Nakamura, K.; Tanaka, S. Involvement of receptor activator of nuclear factor κB ligand/osteoclast differentiation factor in osteoclastogenesis from synoviocytes in rheumatoid arthritis. Arthritis Rheum. 2000, 43, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Zielonka, J.; Sikora, A.; Joseph, J.; Kalyanaraman, B. Peroxynitrite Is the Major Species Formed from Different Flux Ratios of Co-generated Nitric Oxide and Superoxide. J. Biol. Chem. 2010, 285, 14210–14216. [Google Scholar] [CrossRef] [PubMed]
- Bozic, J.; Borovac, J.A.; Galic, T.; Kurir, T.T.; Supe-Domic, D.; Dogas, Z. Adropin and Inflammation Biomarker Levels in Male Patients With Obstructive Sleep Apnea: A Link With Glucose Metabolism and Sleep Parameters. J. Clin. Sleep Med. 2018, 14, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Gregersen, P.K.; I Amos, C.; Lee, A.T.; Lu, Y.; Remmers, E.F.; Kastner, D.L.; Seldin, M.F.; A Criswell, L.; Plenge, R.M.; Holers, V.M.; et al. REL, encoding a member of the NF-κB family of transcription factors, is a newly defined risk locus for rheumatoid arthritis. Nat. Genet. 2009, 41, 820–823. [Google Scholar] [CrossRef]
- Marcu, K.B.; Otero, M.; Olivotto, E.; Borzi, R.M.; Goldring, M.B. NF-κB Signaling: Multiple Angles to Target OA. Curr. Drug Targets 2010, 11, 599–613. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Dickel, N.; Györfi, A.-H.; SafakTümerdem, B.; Li, Y.-N.; Rigau, A.R.; Liang, C.; Hong, X.; Shen, L.; Matei, A.-E.; et al. Attenuation of fibroblast activation and fibrosis by adropin in systemic sclerosis. Sci. Transl. Med. 2024, 16, eadd6570. [Google Scholar] [CrossRef]
- Meyers, A.K.; Zhu, X. The NLRP3 Inflammasome: Metabolic Regulation and Contribution to Inflammaging. Cells 2020, 9, 1808. [Google Scholar] [CrossRef]
- Wu, L.; Fang, J.; Chen, L.; Zhao, Z.; Luo, Y.; Lin, C.; Fan, L. Low serum adropin is associated with coronary atherosclerosis in type 2 diabetic and non-diabetic patients. Clin. Chem. Lab. Med. 2014, 52, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-Y.; Zhao, P.; Wu, M.-C.; Liu, J.; Yin, W. Serum adropin levels are decreased in patients with acute myocardial infarction. Regul. Pept. 2014, 190–191, 46–49. [Google Scholar] [CrossRef]
- Gu, X.; Li, H.; Zhu, X.; Gu, H.; Chen, J.; Wang, L.; Harding, P.; Xu, W. Inverse Correlation Between Plasma Adropin and ET-1 Levels in Essential Hypertension. Medicine 2015, 94, e1712. [Google Scholar] [CrossRef]
- Boric-Skaro, D.; Mizdrak, M.; Luketin, M.; Martinovic, D.; Tokic, D.; Vilovic, M.; Supe-Domic, D.; Kurir, T.T.; Bozic, J. Serum Adropin Levels in Patients on Hemodialysis. Life 2021, 11, 337. [Google Scholar] [CrossRef]
- Gao, F.; Fang, J.; Chen, F.; Wang, C.; Chen, S.; Zhang, S.; Lv, X.; Zhang, J.; He, Q.; Weng, S.; et al. Enho Mutations Causing Low Adropin: A Possible Pathomechanism of MPO-ANCA Associated Lung Injury. EBioMedicine 2016, 9, 324–335. [Google Scholar] [CrossRef]
- Korkmaz, S.; Özgün, G.S. Serum adropin levels in psoriasis vulgaris and its relation with metabolic parameters. Turk. J. Med. Sci. 2019, 49, 110–115. [Google Scholar] [CrossRef]
- Yang, M.; Pei, Q.; Zhang, J.; Weng, H.; Jing, F.; Yi, Q. Association between adropin and coronary artery lesions in children with Kawasaki disease. Eur. J. Pediatr. 2021, 180, 2253–2259. [Google Scholar] [CrossRef] [PubMed]
- Kurz, B.; Lemke, A.K.; Fay, J.; Pufe, T.; Grodzinsky, A.J.; Schünke, M. Pathomechanisms of cartilage destruction by mechanical injury. Ann. Anat.-Anat. Anz. 2005, 187, 473–485. [Google Scholar] [CrossRef]
- Roman-Blas, J.; Jimenez, S. NF-κB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthr. Cartil. 2006, 14, 839–848. [Google Scholar] [CrossRef]
- Li, J.-X.; Cummins, C.L. Fresh insights into glucocorticoid-induced diabetes mellitus and new therapeutic directions. Nat. Rev. Endocrinol. 2022, 18, 540–557. [Google Scholar] [CrossRef] [PubMed]
- Peckett, A.J.; Wright, D.C.; Riddell, M.C. The effects of glucocorticoids on adipose tissue lipid metabolism. Metabolism 2011, 60, 1500–1510. [Google Scholar] [CrossRef]
- Infante, M.; Padilla, N.; Alejandro, R.; Caprio, M.; Della-Morte, D.; Fabbri, A.; Ricordi, C. Diabetes-Modifying Antirheumatic Drugs: The Roles of DMARDs as Glucose-Lowering Agents. Medicina 2022, 58, 571. [Google Scholar] [CrossRef]
- Barry, S.; Sheng, E.; Baker, J.F. Metabolic Consequences of Rheumatoid Arthritis. Arthritis Care Res. 2025. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Clegg, D.J.; Hevener, A.L. The Role of Estrogens in Control of Energy Balance and Glucose Homeostasis. Endocr. Rev. 2013, 34, 309–338. [Google Scholar] [CrossRef]
- Varlamov, O.; Bethea, C.L.; Roberts, C.T., Jr. Sex-Specific Differences in Lipid and Glucose Metabolism. Front. Endocrinol. 2015, 5, 241. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, S.K.; Bermas, B.; Costenbader, K.H. Sexual disparities in the incidence and course of SLE and RA. Clin. Immunol. 2013, 149, 211–218. [Google Scholar] [CrossRef]
- De Angelis, R.; Giuggioli, D.; Bajocchi, G.; Dagna, L.; Zanframundo, G.; Foti, R.; Cacciapaglia, F.; Cuomo, G.; Ariani, A.; Rosato, E.; et al. Sex-related Differences in Systemic Sclerosis: A Multicenter Cross-sectional Study From the National Registry of the Italian Society for Rheumatology. J. Rheumatol. 2021, 49, 176–185. [Google Scholar] [CrossRef]
- Zhou, J.-J.; Ma, J.-D.; Mo, Y.-Q.; Zheng, D.-H.; Chen, L.-F.; Wei, X.-N.; Dai, L. Down-regulating peroxisome proliferator-activated receptor-gamma coactivator-1beta alleviates the proinflammatory effect of rheumatoid arthritis fibroblast-like synoviocytes through inhibiting extracellular signal-regulated kinase, p38 and nuclear factor-kappaB activation. Arthritis Res. Ther. 2014, 16, 472. [Google Scholar] [CrossRef]
- Laragione, T.; Gulko, P.S. Liver X Receptor Regulates Rheumatoid Arthritis Fibroblast-like Synoviocyte Invasiveness, Matrix Metalloproteinase 2 Activation, Interleukin-6 and CXCL10. Mol. Med. 2012, 18, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Kasper, D.L.; Fauci, A.S.; Hauser, S.L.; Longo, D.L.; Jameson, J.L.; Loscalzo, J. (Eds.) Rheumatologic Disorders. In Harrison’s Principles of Internal Medicine, 20th ed.; McGraw-Hill: New York, NY, USA, 2018; pp. 2433–2445. [Google Scholar]
- Lee, H.M.; Kim, H.J.; Won, K.-J.; Choi, W.S.; Park, S.H.; Song, H.; Park, P.-J.; Park, T.-K.; Lee, C.-K.; Kim, B. Soluble Form of Vascular Cell Adhesion Molecule 1 Induces Migration and Proliferation of Vascular Smooth Muscle Cells. J. Vasc. Res. 2008, 45, 259–268. [Google Scholar] [CrossRef]
- Verma, S.; Buchanan, M.R.; Anderson, T.J. Endothelial Function Testing as a Biomarker of Vascular Disease. Circulation 2003, 108, 2054–2059. [Google Scholar] [CrossRef]
- Wanchu, A.; Khullar, M.; Sud, A.; Bambery, P. Elevated Nitric Oxide Production in Patients with Primary Sjögren’s Syndrome. Clin. Rheumatol. 2000, 19, 360–364. [Google Scholar] [CrossRef]
- Bartoloni, E.; Baldini, C.; Schillaci, G.; Quartuccio, L.; Priori, R.; Carubbi, F.; Bini, V.; Alunno, A.; Bombardieri, S.; De Vita, S.; et al. Cardiovascular disease risk burden in primary Sjögren’s syndrome: Results of a population-based multicentre cohort study. J. Intern. Med. 2015, 278, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Vaudo, G.; Bocci, E.B.; Shoenfeld, Y.; Schillaci, G.; Wu, R.; Del Papa, N.; Vitali, C.; Monache, F.D.; Marchesi, S.; Mannarino, E.; et al. Precocious intima-media thickening in patients with primary Sjögren’s syndrome. Arthritis Rheum. 2005, 52, 3890–3897. [Google Scholar] [CrossRef] [PubMed]
- Neumann, E.; Khawaja, K.; Müller-Ladner, U. G protein-coupled receptors in rheumatology. Nat. Rev. Rheumatol. 2014, 10, 429–436. [Google Scholar] [CrossRef]
- Denton, C.; Mblack, C. Scleroderma—Clinical and pathological advances. Best Pract. Res. Clin. Rheumatol. 2004, 18, 271–290. [Google Scholar] [CrossRef]
- Summers, G.D.; Metsios, G.S.; Stavropoulos-Kalinoglou, A.; Kitas, G.D. Rheumatoid cachexia and cardiovascular disease. Nat. Rev. Rheumatol. 2010, 6, 445–451. [Google Scholar] [CrossRef]
- Wei, J.; Zhu, H.; Komura, K.; Lord, G.; Tomcik, M.; Wang, W.; Doniparthi, S.; Tamaki, Z.; Hinchcliff, M.; Distler, J.H.W.; et al. A synthetic PPAR-γ agonist triterpenoid ameliorates experimental fibrosis: PPAR-γ-independent suppression of fibrotic responses. Ann. Rheum. Dis. 2014, 73, 446–454. [Google Scholar] [CrossRef]
- Rohrbach, A.S.; Hemmers, S.; Arandjelovic, S.; Corr, M.; A Mowen, K. PAD4 is not essential for disease in the K/BxN murine autoantibody-mediated model of arthritis. Arthritis Res. Ther. 2012, 14, R104. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, E.E.; Flier, J.S. Adipose Tissue as an Endocrine Organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef]
- Liang, W.; Qi, Y.; Yi, H.; Mao, C.; Meng, Q.; Wang, H.; Zheng, C. The Roles of Adipose Tissue Macrophages in Human Disease. Front. Immunol. 2022, 13, 908749. [Google Scholar] [CrossRef] [PubMed]
- Guymer, E.K.; Maruff, P.; Littlejohn, G.O. Clinical characteristics of 150 consecutive fibromyalgia patients attending an Australian public hospital clinic. Int. J. Rheum. Dis. 2012, 15, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Zhong, Z.; Shen, H.-W.; Wu, H.-P.; Xiang, P.; Hu, B. Intermedin as a prognostic factor for major adverse cardiovascular events in patients with ST-segment elevation acute myocardial infarction. Peptides 2014, 58, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Van den Hoek, J.; Boshuizen, H.C.; Roorda, L.D.; Tijhuis, G.J.; Nurmohamed, M.T.; Van den Bos, G.A.M.; Dekker, J. Mortality in patients with rheumatoid arthritis: A 15-year prospective cohort study. Rheumatol. Int. 2017, 37, 487–493. [Google Scholar] [CrossRef]
- Harford, K.A.; Reynolds, C.M.; McGillicuddy, F.C.; Roche, H.M. Fats, inflammation and insulin resistance: Insights to the role of macrophage and T-cell accumulation in adipose tissue. Proc. Nutr. Soc. 2011, 70, 408–417. [Google Scholar] [CrossRef]
- Berezina, T.A.; Berezin, O.O.; Hoppe, U.C.; Lichtenauer, M.; Berezin, A.E. Low Levels of Adropin Predict Adverse Clinical Outcomes in Outpatients with Newly Diagnosed Prediabetes after Acute Myocardial Infarction. Biomedicines 2024, 12, 1857. [Google Scholar] [CrossRef]
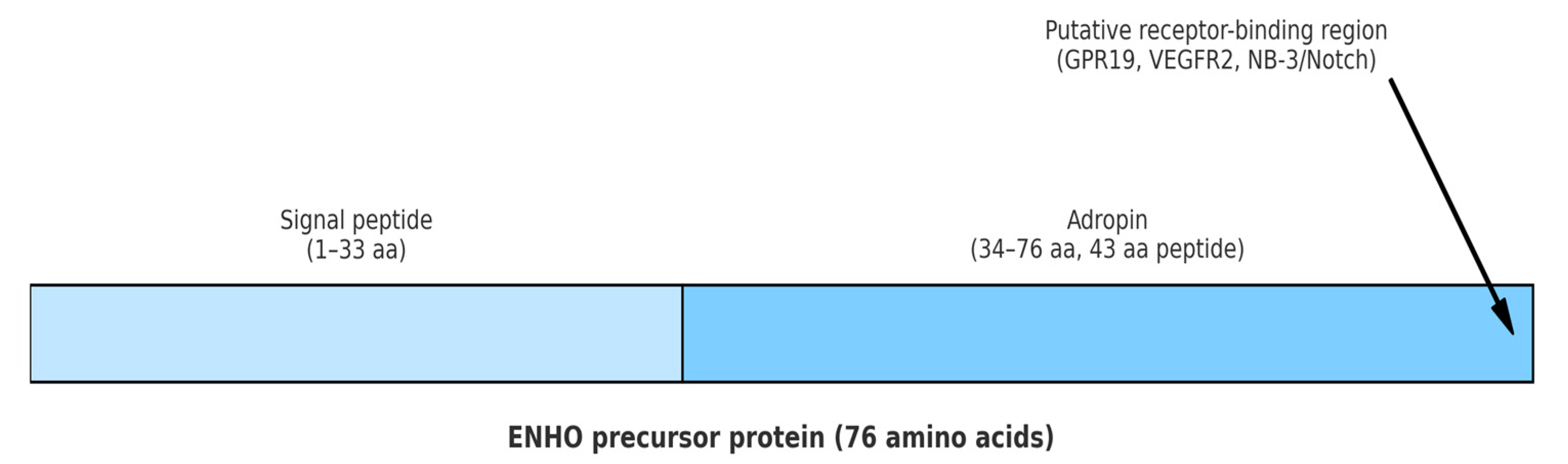
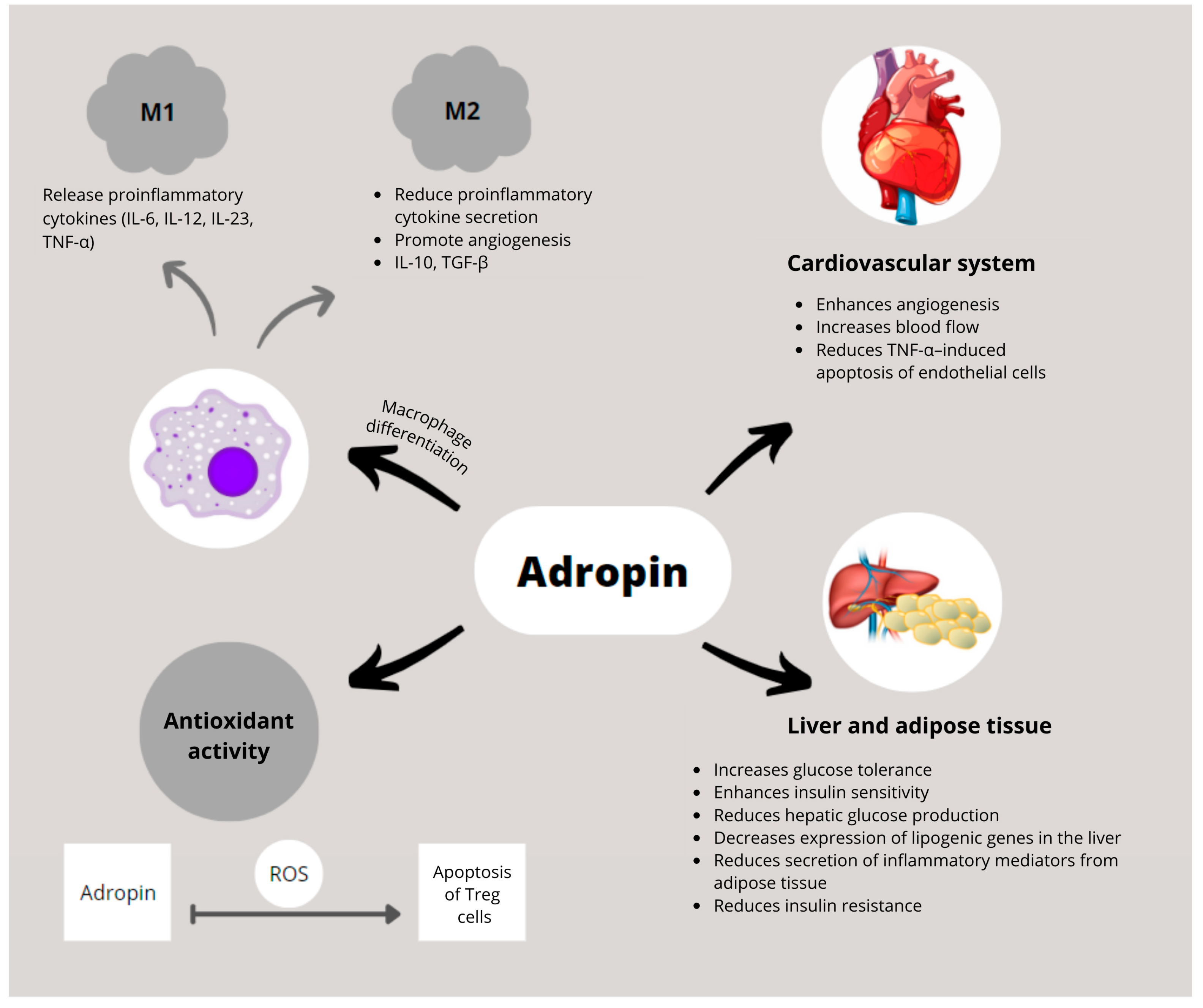
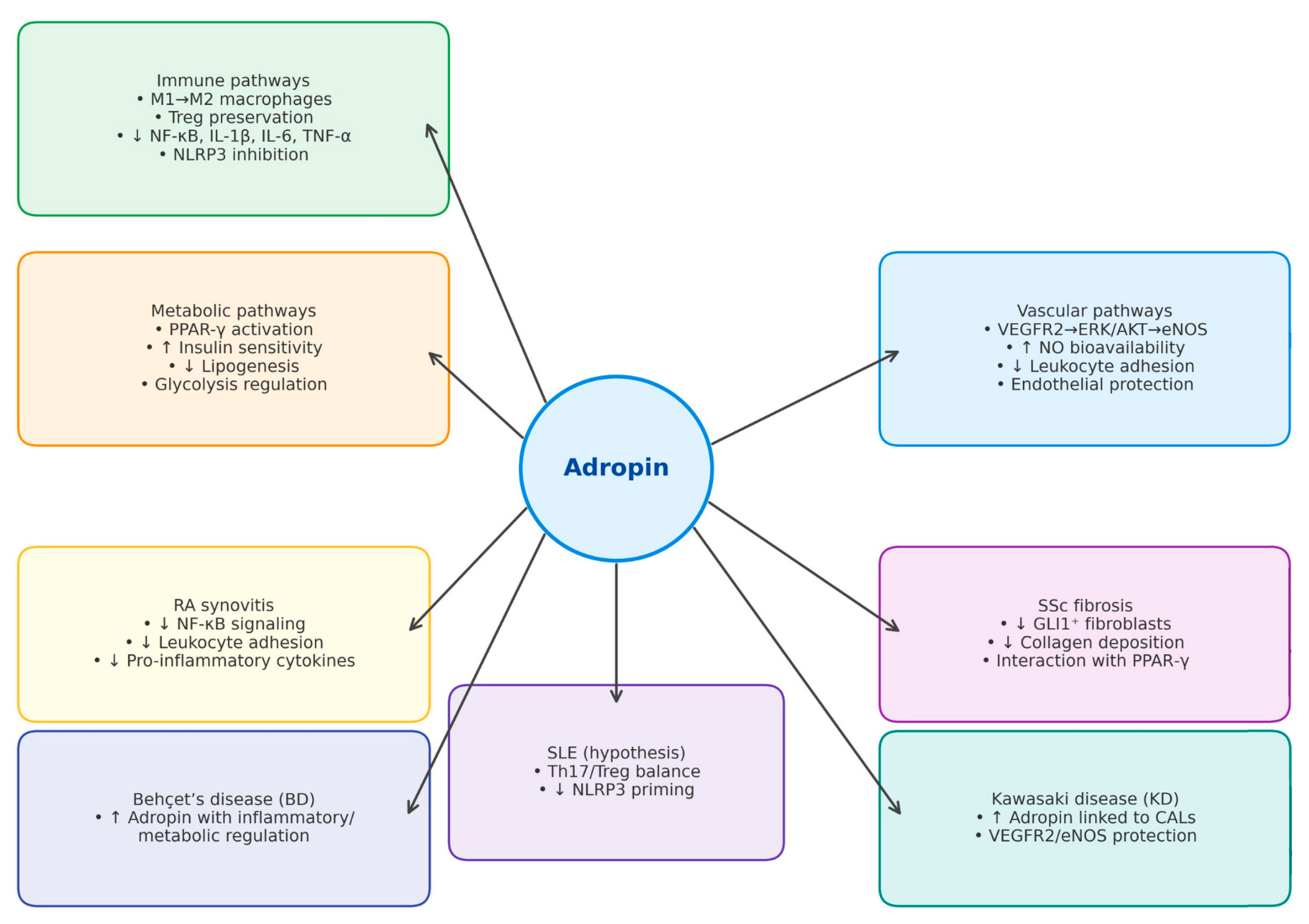
| Disease | References | Main Findings | Key Confounders |
| Rheumatoid Arthritis (RA) | [22,25] | Decreased serum adropin compared to controls; inverse correlation with TNF-α and glucose metabolism; inconsistent Enho expression findings | BMI and IR; disease duration; age; treatment status |
| Systemic Lupus Erythematosus (SLE) | [22] | No significant change in serum adropin compared to controls | Small sample size; heterogeneous cohorts; treatment effects not stratified |
| Osteoarthritis (OA) | [23] | Lower adropin correlated with greater OA severity, TNF-α, WBC, and NLR; lowest levels in patients with BMI ≥ 30 | Obesity; inflammatory burden; comorbid metabolic syndrome |
| Psoriasis (with MetS) | [50] | Decreased adropin in psoriasis; lowest in patients with MetS; associated with metabolic alterations | BMI; presence of metabolic syndrome |
| Primary Sjögren’s Syndrome (pSjS) | [24] | Increased adropin; positively correlated with HDL and anti-SSA/Ro52; negatively with SSDDI | Female predominance; autoantibody status; endothelial risk factors |
| Systemic Sclerosis (SSc) | [21,43] | Increased adropin; associated with fibrosis modulation via GLI1 and PPAR-γ; potential antifibrotic role | Leptin and adipokine imbalance; impaired PPAR-γ; disease duration; therapy |
| Behçet’s Disease (BD) | [21] | Increased adropin; potentially linked to inflammatory/metabolic dysregulation | Inflammatory burden; vascular involvement |
| Kawasaki Disease (KD) | [51] | Increased adropin in CAL + KD patients; correlated with CRP and procalcitonin | Pediatric cohort; acute inflammation; coronary artery lesions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simac, P.; Petric, M.; Jankovic Danolic, M.; Perković, D. A Potential Role of Adropin in Inflammatory Rheumatic Diseases—What Do We Know So Far? Biomedicines 2025, 13, 2300. https://doi.org/10.3390/biomedicines13092300
Simac P, Petric M, Jankovic Danolic M, Perković D. A Potential Role of Adropin in Inflammatory Rheumatic Diseases—What Do We Know So Far? Biomedicines. 2025; 13(9):2300. https://doi.org/10.3390/biomedicines13092300
Chicago/Turabian StyleSimac, Petra, Marin Petric, Marijana Jankovic Danolic, and Dijana Perković. 2025. "A Potential Role of Adropin in Inflammatory Rheumatic Diseases—What Do We Know So Far?" Biomedicines 13, no. 9: 2300. https://doi.org/10.3390/biomedicines13092300
APA StyleSimac, P., Petric, M., Jankovic Danolic, M., & Perković, D. (2025). A Potential Role of Adropin in Inflammatory Rheumatic Diseases—What Do We Know So Far? Biomedicines, 13(9), 2300. https://doi.org/10.3390/biomedicines13092300








