Abstract
Acute respiratory distress syndrome (ARDS) is a life-threatening condition with high mortality. A central driver in its pathogenesis is alveolar epithelial cell (AEC) dysfunction, which leads to disruption of the epithelial barrier, impaired fluid clearance, and dysregulated inflammatory responses. This review summarizes the key mechanisms underlying AEC injury, including programmed cell death (apoptosis, pyroptosis, necroptosis, ferroptosis), oxidative stress, mitochondrial dysfunction, epigenetic reprogramming (DNA methylation, histone modifications), metabolic rewiring (succinate accumulation), and spatiotemporal heterogeneity revealed by single-cell sequencing and spatial transcriptomics. Multicellular crosstalk involving epithelial–immune–endothelial networks and the gut-lung axis further shapes disease progression. Building on these mechanistic foundations, we evaluate emerging AEC-targeted interventions such as pharmacologic agents (antioxidants, anti-inflammatories), biologics (mesenchymal stem cells and engineered exosomes), and gene-based approaches (adeno-associated virus and CRISPR-Cas9 systems delivered via smart nanocarriers). Complementary strategies include microbiome modulation through probiotics, short-chain fatty acids, or fecal microbiota transplantation, and biomarker-guided precision medicine (e.g., sRAGE, exosomal miRNAs) to enable promise individualized regimens. We also discuss translational hurdles, including nanotoxicity, mesenchymal stem cell (MSC) heterogeneity, and gene-editing safety, and highlight future opportunities involving AI-driven multi-omics, lung-on-chip platforms, and epithelium-centered regenerative therapies. By integrating mechanistic insights with innovative therapeutic strategies, this review aims to outline a roadmap toward epithelium-targeted, precision-guided therapies for ARDS.
1. Introduction
Acute respiratory distress syndrome (ARDS) represents a major challenge in critical care medicine, characterized by high morbidity, mortality, and healthcare burden. Its early features primarily include alveolar epithelial cell (AEC) injury, alveolar-capillary barrier dysfunction, and the activation of an acute inflammatory response [1,2]. These pathological changes lead to pulmonary edema, hypoxemia, and respiratory failure. Mechanisms such as apoptosis and autophagy play critical roles in ARDS progression, not only damaging the lungs but also promoting multi-organ dysfunction, which further increases mortality [3,4,5]. Even among survivors, long-term complications are common, including pulmonary fibrosis (PF), impaired lung function, chronic fatigue, muscle weakness and cognitive impairment. These sequelae greatly reduce patients’ quality of life and impose a substantial burden on families and healthcare systems [3,6]. A deeper understanding of ARDS pathogenesis and the development of targeted therapies are expected to improve clinical outcomes and enhance patients’ quality of life. Progress in this area may also alleviate the broader economic and psychosocial burdens.
AECs, as key components of the alveolar-capillary barrier, play a vital role in the pathophysiology of ARDS. The functions of the alveolar-capillary barrier include maintaining barrier integrity, regulating fluid balance, and participating in the immune response [3,7]. There are two primary types of AECs including alveolar type I epithelial cells (AT1 cells) and alveolar type II epithelial cells (AT2 cells). AT1 cells account for 95% of the alveolar surface area with a flat morphology that covers most of the alveolar surface. Their main function is to form a thin and wide barrier to facilitate gas exchange. In contrast, AT2 cells are cuboidal in shape, constitute about 5% of the alveolar surface area, and are interspersed among AT1 cells. AT2 cells perform multiple essential functions, including the secretion of pulmonary surfactant, regulation of fluid balance, and serving as progenitor cells for alveolar repair [8,9]. The advent of advanced technologies, such as single-cell RNA sequencing, spatial transcriptomics, and metabolomics, has significantly advanced our understanding of AEC heterogeneity, plasticity, and dynamic responses during ARDS. These technologies help elucidate the molecular mechanisms driving epithelial dysfunction and identify novel therapeutic targets.
In this review, we comprehensively examine the mechanistic underpinnings of AEC dysfunction in ARDS, including oxidative stress, programmed cell death, epigenetic reprogramming, and metabolic alterations. We further highlight the spatiotemporal dynamics of AECs and their crosstalk with immune and endothelial cells. Finally, we summarize emerging epithelial-targeted interventions, from pharmacologic agents and biologics to gene-based therapies, and discuss their translational potential and challenges. This review aims to bridge fundamental insights with innovative therapies to guide future precision medicine approaches in ARDS.
2. Alterations of Epithelial Cell Function During Ards
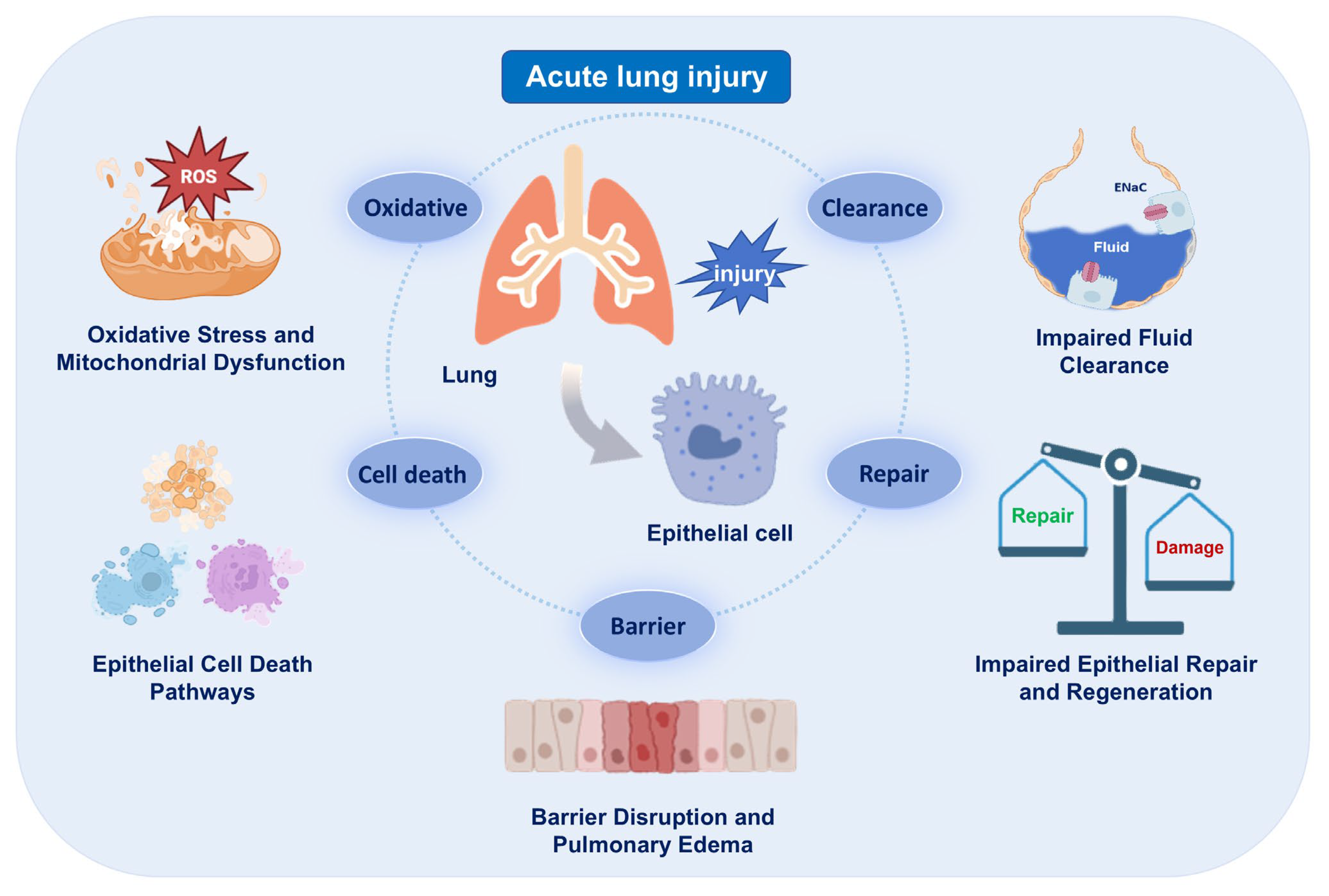
During the pathogenesis of ARDS, AECs serve as the main targets of injury. Their structural and functional disruption compromises the integrity of the alveolar–capillary barrier, leading to pulmonary edema, impaired gas exchange and the amplification of inflammatory cascades. Damaged AECs undergo various forms of programmed cell death, oxidative damage and mitochondrial dysfunction, and exhibit impaired fluid clearance and regenerative capacity. This chapter provides a systematic overview of key pathological processes involving AECs, highlighting the contribution of epithelial dysfunction to ARDS progression (Figure 1).
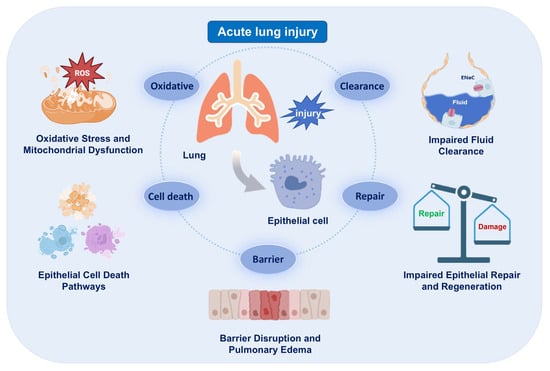
Figure 1.
Epithelial injury and its consequences in acute respiratory distress syndrome (ARDS). Schematic summarizing major epithelial phenotypes (cell death, mitochondrial dysfunction, impaired fluid clearance) and their impact on barrier integrity. The images illustrated in the figures were adapted from https://app.biorender.com.
2.1. Barrier Disruption and Pulmonary Edema
Epithelial barrier disruption in ARDS can lead to alveolar edema, inflammatory cell infiltration and impaired gas exchange [10,11]. During the acute phase, inflammatory cells such as neutrophils and macrophages accumulate and release proteases, which cleave junctional proteins (such as E-cadherin) and degrades the extracellular matrix (ECM) [12,13]. The release of mediators such as tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) triggers the rearrangement of actin cytoskeleton in AECs, further increasing permeability [14,15]. As a result, protein-rich fluid leaks into the alveolar space, resulting in pulmonary edema. ARDS also induces oxidative stress that contributes to lipid peroxidation and membrane damage in AECs [16,17]. The detailed mechanisms of oxidative injury and mitochondrial dysfunction are discussed in Section 2.3. In addition, evidence indicates that impaired alveolar fluid clearance further exacerbates pulmonary edema, and the detailed mechanisms of fluid clearance dysfunction are discussed in Section 2.4.
2.2. Cell Death Pathways
In ARDS, loss of AECs via programmed cell death not only compromises barrier integrity but also drives inflammation and edema. Apoptosis occurs through intrinsic and extrinsic pathways. Cellular stress (e.g., DNA damage) activates the intrinsic pathway via Bax/Bak-mediated mitochondrial outer membrane permeabilization (MOMP), releasing cytochrome c to form the APAF-1 apoptosome, which activates caspase-9 to cleave and activate executioner caspases-3/7. Death receptors (e.g., Fas/TNFR) initiate the extrinsic pathway by assembling the DISC complex with Fas-associated death domain protein (FADD) and procaspase-8, activating caspase-8. These pathways cause DNA fragmentation, cell shrinkage, and apoptotic body formation. In AECs, excessive apoptosis induces cell detachment, disrupting intercellular junctions and compromising barrier function in ARDS [18,19,20,21].
Pyroptosis is a programmed cell death pathway triggered by inflammasome assembly such as NLRP3. The classical pathway depends on caspase-1 activation, while the non-classical pathway is mediated by caspase-4/5 (human) or caspase-11 (mouse). Activated caspases cleave gasdermin D (GSDMD), and the released GSDMD-N-terminal domain oligomerization forms pores in the plasma membrane, leading to osmotic lysis of cells. This process also promotes the maturation and release of IL-1β and IL-18, thereby amplifying the inflammatory cascade [22,23,24].
Necroptosis of AECs is mediated by the RIPK1-RIPK3-MLKL signaling axis. When AECs are stimulated by pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs), RIPK3 activates and phosphorylates mixed lineage kinase domain-like protein (MLKL). Phosphorylated MLKL oligomerizes and translocates to the plasma membrane, forming permeable pores. This causes the loss of cell membrane integrity and release of intracellular contents, directly damaging the alveolar-capillary barrier and exacerbating ARDS pathology [25,26,27].
Additionally, ferroptosis is an iron-dependent form of cell death driven by lipid peroxidation and GPX4 inactivation. It contributes to epithelial damage under oxidative stress and overlaps with mitochondrial dysfunction in ARDS.
2.3. Oxidative Stress and Mitochondrial Dysfunction
In ARDS, mitochondrial dysfunction triggers pathological reactive oxygen species (ROS) overproduction in AECs, subverting antioxidant defenses [28]. The resulting oxidative storm impairs cellular function by disrupting membrane integrity via lipid peroxidation, inactivating critical enzymes via protein carbonylation, and inducing bioenergetic failure via mtDNA damage. These alterations synergize with ROS-mediated Nrf2 suppression and PINK1/Parkin mitophagy impairment to sustain self-amplifying oxidative stress [21,29,30]. ROS can also alter intracellular signaling proteins and transcription factors, impair normal cell metabolism and promote apoptosis [31,32]. Meanwhile, decreased activities of key antioxidant enzymes further reduce ROS clearance, aggravating oxidative damage [33]. Together, these mechanisms lead to loss of epithelial barrier integrity, trigger programmed cell death and critically drive ARDS progression. For the functional consequences of ROS on epithelial ion transport and alveolar fluid clearance, see Section 2.4.
2.4. Impaired Fluid Clearance
AECs maintain alveolar dryness for efficient gas exchange by regulating ion and water transport through epithelial sodium channels (ENaC), Na+/K+-ATPase and aquaporin 5 (AQP5). Impaired fluid clearance also delays epithelial repair and promotes fibroblast activation, thereby increasing the risk of PF [34,35]. In ARDS, a combination of factors leads to impaired fluid clearance in AECs. Pro-inflammatory factors can lead to the degradation of tight junctions, increased barrier permeability, and inhibition of ENaC expression and activity [36,37,38,39]. Excessive ROS produced by macrophages, neutrophils and damaged AECs inactivate ENaC and reduce Na+/K+-ATPase activity [40,41]. TNF-α inhibits AQP5 transcription through Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells (NF-κB), and High Mobility Group Box 1 (HMGB1) down-regulates AQP5 transcription through Toll-like receptor 4 (TLR4) [42,43].
In addition, AT2 cells possess the ability to proliferate and differentiate into AT1 cells, but this ability is inhibited in ARDS due to oxidative stress and inflammatory factors. This inhibition leads to reduced secretion of surface-active substances, including surfactant proteins A (SP-A) and surfactant proteins D (SP-D), leading to increased alveolar collapse and fluid retention [44,45], in line with the functional roles of AT2 cells as described in the Introduction (Section 1). Persistent obstacles to fluid clearance lead to increased fibroblast activation and collagen deposition, further damaging the alveolar structure [46].
2.5. Impaired Epithelial Repair and Regeneration
During ARDS, the balance between AEC damage and repair influences disease progression and prognosis. An imbalance in this process contributes directly to respiratory failure. Inflammatory factors, such as Transforming Growth Factor-Beta (TGF-β), and oxidative stress could inhibit the proliferation of AT2 cells [47,48]. The dysregulation of the Wnt/β-catenin pathway impairs the differentiation of AT2 cells into AT1 cells, thereby compromising their regenerative capacity [49]. Furthermore, the reduced secretion of SP-A and SP-D by AT2 cells promotes alveolar collapse and weakens immune defense [49]. Injury results in aberrant DNA methylation in the promoter region of repair-related genes, leading to their repressed transcription [50]. Furthermore, enhanced histone deacetylase (HDAC) activity silences pro-repair genes such as AQP5. Collectively, these disruptions to AEC repair mechanisms exacerbate the imbalance between cellular damage and repair, thereby driving disease progression [51,52].
3. Tools and Approaches Enabling New Insights
This section concisely overviews the key experimental and computational platforms discussed in this review. It focuses on single-cell transcriptomics, spatial omics, and single-cell and spatial metabolomics. These technologies establish the technical foundation for the mechanistic insights detailed in Section 4.
3.1. Single-Cell RNA Sequencing (ScRNA-Seq) for Resolving Cell States and Trajectories
scRNA-seq measures gene expression in individual cells. It is a powerful tool for uncovering cellular heterogeneity, rare transitional states like Krt8+ alveolar differentiation intermediates, and injury-related differentiation trajectories. Standard analytical workflows typically involve sequential steps including quality control, normalization, dimensionality reduction, clustering, and trajectory inference. Researchers should consider key limitations when interpreting scRNA-seq data, such as capture bias, transcript dropout, and the loss of spatial context [53,54].
3.2. Spatial Transcriptomics and Multiplexed Proteomics Recovering Spatial Context
Spatial methodologies bridge the gap left by scRNA-seq by providing crucial spatial context. Several techniques map molecular data directly onto tissue structures. These include in situ hybridization panels, untargeted spatial transcriptomics, and imaging mass cytometry, together offering a complementary perspective to single-cell data. These techniques help localize distinct cell states to specific anatomical niches and reveal spatial patterns in cytokine or metabolite distribution. Each technology involves a balance between resolution, molecular depth, and throughput. Integration with single-cell data from dissociated cells often produces the most comprehensive biological insights [55,56].
3.3. Single-Cell and Spatial Metabolomics with Multi-Omic Integration
Emerging approaches in single-cell metabolic inference and spatial metabolomics enable direct correlations between transcriptional states and metabolic functions. Examples include detecting succinate accumulation or inferring succinate dehydrogenase (SDH) complex dysfunction [57,58]. Multi-omic integration of transcriptomic, epigenomic, and metabolomic data enables more robust testing of mechanistic hypotheses. Current technical challenges include limited sensitivity in metabolite detection at cellular resolution and computational challenges in cross-modal data integration. To validate these findings, orthogonal approaches such as targeted biochemistry or perturbation experiments are essential [59,60].
Section 4 presents specific applications and key findings derived from these platforms, including work on succinate dehydrogenase A (SDHA)/succinate and alveolar differentiation intermediates. The reader is directed to the methodological introductions above for technical context.
4. Key Molecular Mechanisms and Cellular Crosstalk in ARDS
In ARDS, AECs are governed by an intricate interplay of intracellular signaling cascades, epigenetic reprogramming, and metabolic adaptations. Based on the single-cell and spatial multi-omics technologies introduced in Chapter 3, the following sections detail the mechanistic insights these tools have enabled (Table 1). In this chapter, we first dissect the activation dynamics and crosstalk among pro-inflammatory pathways, then investigate how epigenetic modifiers such as DNA methylation shifts, histone modifications and non-coding RNAs, reshape transcriptional landscapes. Subsequent sections explore metabolic rewiring to accelerated glycolysis and fatty acid oxidation (FAO), and finally analyze the bidirectional crosstalk between epithelium and immune cells that orchestrates tissue injury and repair processes.
4.1. Activation of Inflammatory Signaling Pathways
In ARDS, a complex network exists within AECs where multiple signaling pathways cross-regulate and amplify inflammatory responses, ultimately leading to cellular dysfunction and apoptosis.
First, the binding of TNF-α or IL-1β rapidly activates the IκB kinase (IKK) complex, enabling nuclear translocation of NF-κB and driving transcription of pro-inflammatory and pro-apoptotic genes such as TNF and IL-6 [61]. Second, the c-Jun N-terminal kinase (JNK), a classical mitogen-activated protein kinase (MAPK) family member, is persistently activated in the inflammatory milieu. Activated JNK directly phosphorylates Bcl-2 family proteins, triggering mitochondria-mediated apoptosis, while p38 MAPK contributes to lung injury by regulating inflammatory gene transcription and inducing cell cycle arrest [62]. Finally, IL-6 family cytokines engage their receptors to activate the signal-transducing GP130 subunit and Janus kinase (JAK) tyrosine kinases, leading to Signal Transducer and Activator of Transcription 3 (STAT3) phosphorylation, dimerization, and nuclear accumulation. STAT3 then enhances pro-inflammatory gene transcription, amplifying the inflammatory cascade [63,64]. Notably, NF-κB, MAPK, and JAK/STAT3 pathways engage in cross-regulation. For example, NF-κB can up-regulate JAK/STAT3 components, while STAT3 modulates NF-κB activity, collectively driving ARDS initiation and progression [65,66].
Overall, these synergistic pro-inflammatory signaling pathways establish an efficiently amplifiable and tightly regulated inflammatory network, representing a pivotal molecular mechanism in ARDS pathogenesis.
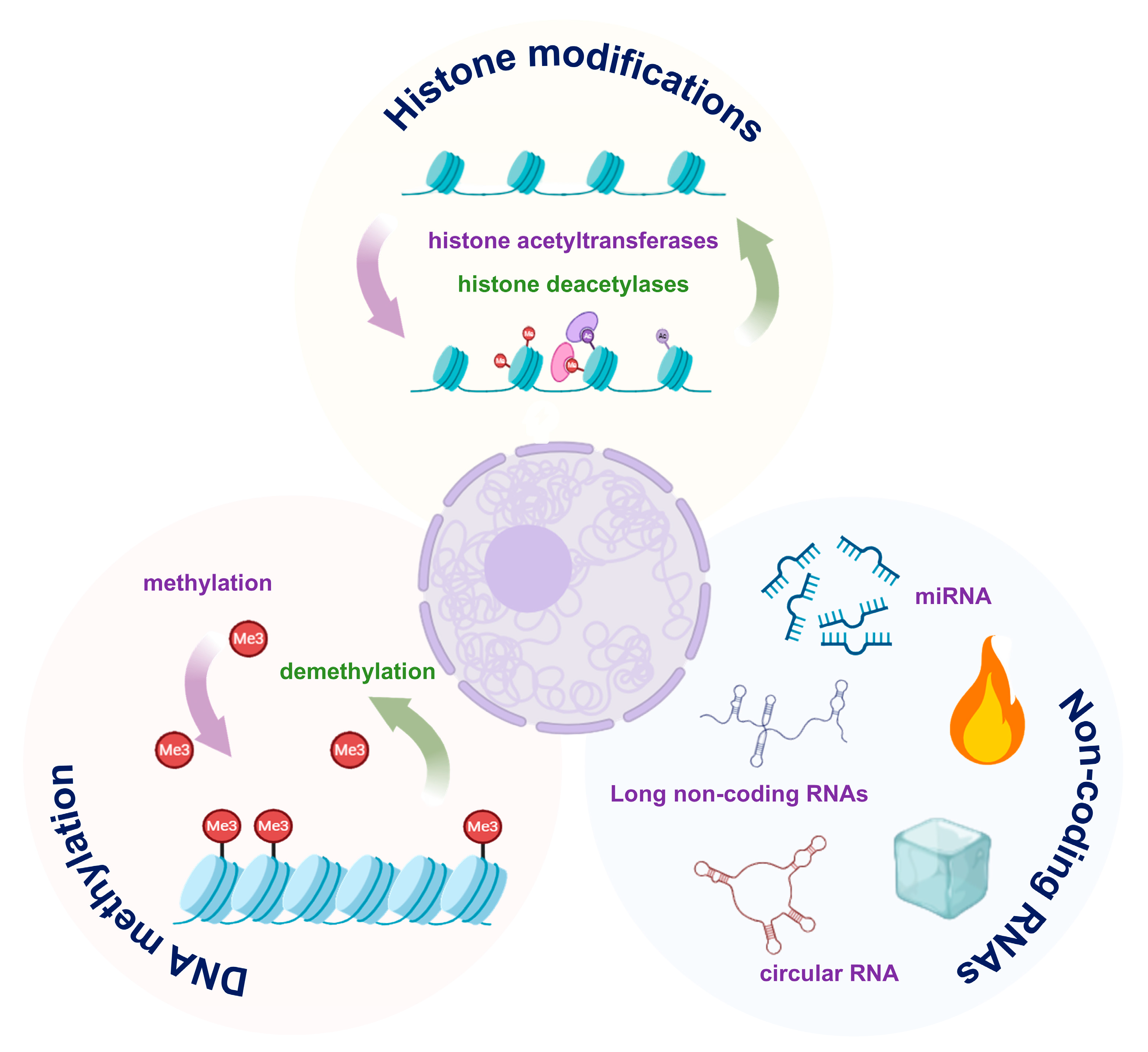
4.2. Epigenetic Regulation
In ARDS, epigenetic regulation (Figure 2), including DNA methylation, histone modifications, and non-coding RNAs, emerges as a key mechanism driving AEC injury, by modulating gene expression without altering the DNA sequence [67,68].
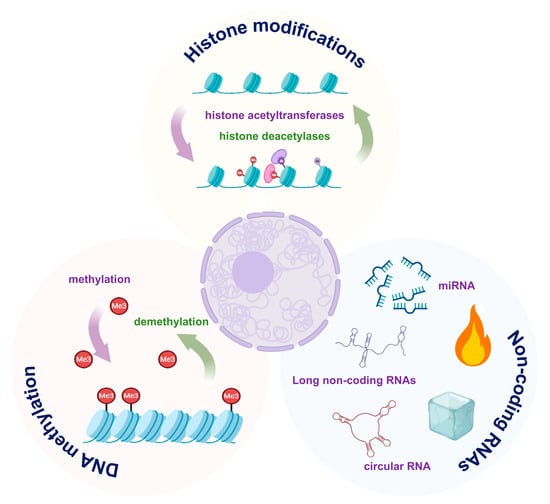
Figure 2.
Major epigenetic mechanisms in alveolar epithelial cell (AEC) dysfunction. DNA methylation, histone modifications and non-coding RNAs modulate pro-inflammatory and pro-repair gene programs. The images illustrated in the figures were adapted from https://app.biorender.com.

Table 1.
Summary of Key Molecular Mechanisms in AEC Dysfunction during ARDS.
Table 1.
Summary of Key Molecular Mechanisms in AEC Dysfunction during ARDS.
| Mechanism | Affected Aec Process | Key Molecular Players/Pathway | References |
|---|---|---|---|
| Pro-inflammatory signaling | Cytokine production, apoptosis, barrier dysfunction | NF-κB, JNK/p38 MAPK, JAK/STAT3, TNF-α, IL-1β, IL-6 | [52,61,62,63,64,65] |
| Programmed cell death | Cell death, release of DAMPs, propagation of inflammation | Caspase-8, MLKL (Necroptosis), GSDMD (Pyroptosis) | [66,67] |
| Epigenetic reprogramming | Transcriptional silencing of repair genes, sustained inflammatory gene expression | DNA methylation (AQP5), HATs/H3K27ac, HDACs, H3K4me3, H3K27me3, miR-155, miR146a | [69,70,71] |
| Metabolic reprogramming | Energy production, succinate signaling, HIF-1α activation | Impaired FAO, SDHA dysfunction, Succinate accumulation, Glycolytic switch, HIF-1α | [72,73,74,75] |
| Epithelial–immune–endothelial crosstalk | Immune cell recruitment, barrier integrity, fibrotic remodeling | CCL2, CXCL1, CXCL8, VEGF, HMGB1, VE-cadherin | [75,76,77,78,79,80,81,82,83] |
| Gut-Lung Axis | Barrier function, inflammasome activation, systemic inflammation | SCFAs/Butyrate (protective), TMAO (detrimental), NLRP3 | [84,85,86,87,88,89,90,91] |
AEC, alveolar epithelial cell; ARDS, acute respiratory distress syndrome; AQP5, aquaporin-5; CCL2, C-C motif chemokine ligand 2; CXCL1, C-X-C motif chemokine ligand 1; DAMPs, damage-associated molecular patterns; FAO, fatty acid oxidation; GSDMD, gasdermin D; HATs, histone acetyltransferases; HDACs, histone deacetylases; HIF-1α, hypoxia-inducible factor 1-alpha; HMGB1, high mobility group box 1; IL, interleukin; JAK/STAT3, Janus kinase/signal transducer and activator of transcription 3; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; MLKL, mixed lineage kinase domain-like; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NLRP3, NLR family pyrin domain containing 3; SCFAs, short-chain fatty acids; SDHA, succinate dehydrogenase complex flavoprotein subunit A; TMAO, trimethylamine N-oxide; TNF-α, tumor necrosis factor-alpha; VE-cadherin, vascular endothelial cadherin; VEGF, vascular endothelial growth factor.
4.2.1. DNA Methylation
During the progression of ARDS, a portion of the DNA undergoes methylation, resulting in the silencing of repair genes and the activation of pro-inflammatory pathways. Stress-induced hypermethylation of key repair factors has been shown to suppress AT2 cell proliferation and their differentiation into AT1 cells.
Conversely, hypomethylation of inflammatory genes maintains cytokine production and barrier disruption [69,70]. AQP5 promoter methylation was observed to result in reduced expression of water channel proteins and impaired fluid clearance [71]. Further studies revealed that promoter demethylation of pro-inflammatory genes enhances their transcriptional activity, thereby amplifying the inflammatory response [72].
4.2.2. Histone Modifications
Histone modifications, such as acetylation and methylation, are critical epigenetic regulators of chromatin remodeling and gene expression. They play a key role in the pathogenesis of ARDS. In response to inflammatory stimuli, the recruitment of histone acetyltransferases (HATs) promotes H3K27 acetylation (H3K27ac) at promoters of pro-inflammatory genes. This open chromatin state facilitates NF-κB-driven transcription of cytokines, thereby exacerbating epithelial injury [73]. Conversely, activation of histone deacetylases (HDACs) removes acetyl groups from the promoters of anti-inflammatory and reparative genes (e.g., IL-10, SP-A), leading to their transcriptional silencing.
Beyond acetylation, alterations in histone methylation also contribute to dysregulated inflammation and impaired repair. For instance, persistent inflammatory responses have been linked to aberrant histone methylation marks, including the activating H3K4me3 and the repressive H3K27me3, which are enriched at the promoters of pro-inflammatory genes [74,75]. Moreover, the deposition of the repressive mark H3K27me3 at promoters of epithelial repair genes effectively inhibits AT2 cell proliferation. Notably, inhibition of the H3K27 methyltransferase was shown to reverse this repression, restore reparative gene expression, and promote alveolar regeneration [76,77].
4.2.3. Non-Coding RNAs
Non-coding RNAs regulate inflammation and cell fate. Pro-inflammatory miRNAs, such as miR-155, amplify inflammatory responses by inhibiting SOCS1 expression and enhancing JAK-STAT pathway activity [78,79]. In contrast, anti-inflammatory miRNAs, such as miR-146a, have been found to target TRAF6/IRAK1 and negatively regulate NF-κB signaling [80]. Long non-coding RNAs (lncRNAs) also affect AEC function through interactions with chromatin [81].
4.3. Metabolic Reprogramming
Based on the single-cell and spatial metabolomics tools introduced in Chapter 3, the following summarizes specific findings on epithelial metabolic rewiring in ARDS. Impairment of FAO in AECs has been shown to exacerbate ALI. Conversely, AECs may ameliorate lung inflammation by modulating mitochondrial FAO [82,83]. Recent single-cell transcriptomic analyses in murine acute lung injury (ALI) models have revealed that dysfunction of SDHA leads to intracellular succinate accumulation and Hypoxia-Inducible Factor 1 Alpha Subunit (HIF-1α) stabilization, driving a metabolic switch of AT2 cells toward glycolysis [84,85]. In a mechanical ventilation induced ALI model, AT2-specific deletion of SDHA (sdhaloxp/loxp SPC-CreER mice) recapitulated succinate accumulation and HIF-1α activation, and improved barrier integrity with reduced epithelial injury [84]. Accumulated succinate not only alters epithelial metabolism but also acts as a paracrine signal, and its SUCNR1-mediated immunoregulatory effects are discussed in Section 5.4.
Complementing these single-cell insights, spatial metabolomics techniques are beginning to map succinate gradients and other small molecule distributions in injured lung tissue. Although comprehensive imaging studies in classic ALI models such as lipopolysaccharide (LPS) or bleomycin (BLM) remain limited, the existing imaging methods have demonstrated the feasibility of locating small molecules under inflammatory conditions. Collectively, these findings underscore that epithelial metabolic reprogramming not only reflects injury severity but actively shapes the immune microenvironment. Integrating spatial metabolomics with single-cell multi-omics will be essential for dissecting the dynamic metabolite-cell interactions that drive ALI progression and for identifying novel therapeutic targets.
4.4. Epithelial Heterogeneity and Plasticity
Following the metabolic changes summarized above, scRNA-seq and spatial transcriptomics provide the cellular resolution needed to define transitional states and repair trajectories. Emerging technologies such as single-cell RNA sequencing (scRNA-seq) and spatial transcriptomics have transformed our understanding of ARDS by shifting from bulk “population” analyses to integrated high-resolution spatiotemporal maps of epithelial diversity and dynamics. They provide real-time insights into both the emergence of distinct AEC subpopulations during injury and repair, and the impact of their spatial context and interactions with neighboring cells and the ECM on disease progression.
Following lung injury, scRNA-seq studies have shown that airway and alveolar progenitors do not fully differentiate into mature AT1 cells but instead converge on a Krt8+ “alveolar differentiation intermediate” (ADI) transitional state. Spatial transcriptomics further localizes these ADI cells predominantly at the alveolar-bronchiolar junction, colocalizing with fibroblasts and suggesting their involvement in early fibrotic processes [81,86]. In animal models, activation of Yap/Taz signaling drives expansion of Krt8+ cells and accelerates alveolar repair, whereas epithelial-specific deletion of Yap and Taz blocks AT2 differentiation, resulting in persistent collagen deposition, neutrophilic inflammation and impaired lung function [86,87]. Moreover, single-cell trajectory analyses reveal that failed repair correlates with upregulation of pro-fibrotic gene programs and dysregulation of Wnt/β-catenin signaling, suggesting Wnt/β-catenin imbalance as a critical barrier to effective regeneration [86,88,89].
Together, these findings not only deepen mechanistic insights into ARDS pathogenesis but also highlight Yap/Taz and Wnt/β-catenin pathways as promising targets for precision interventions.
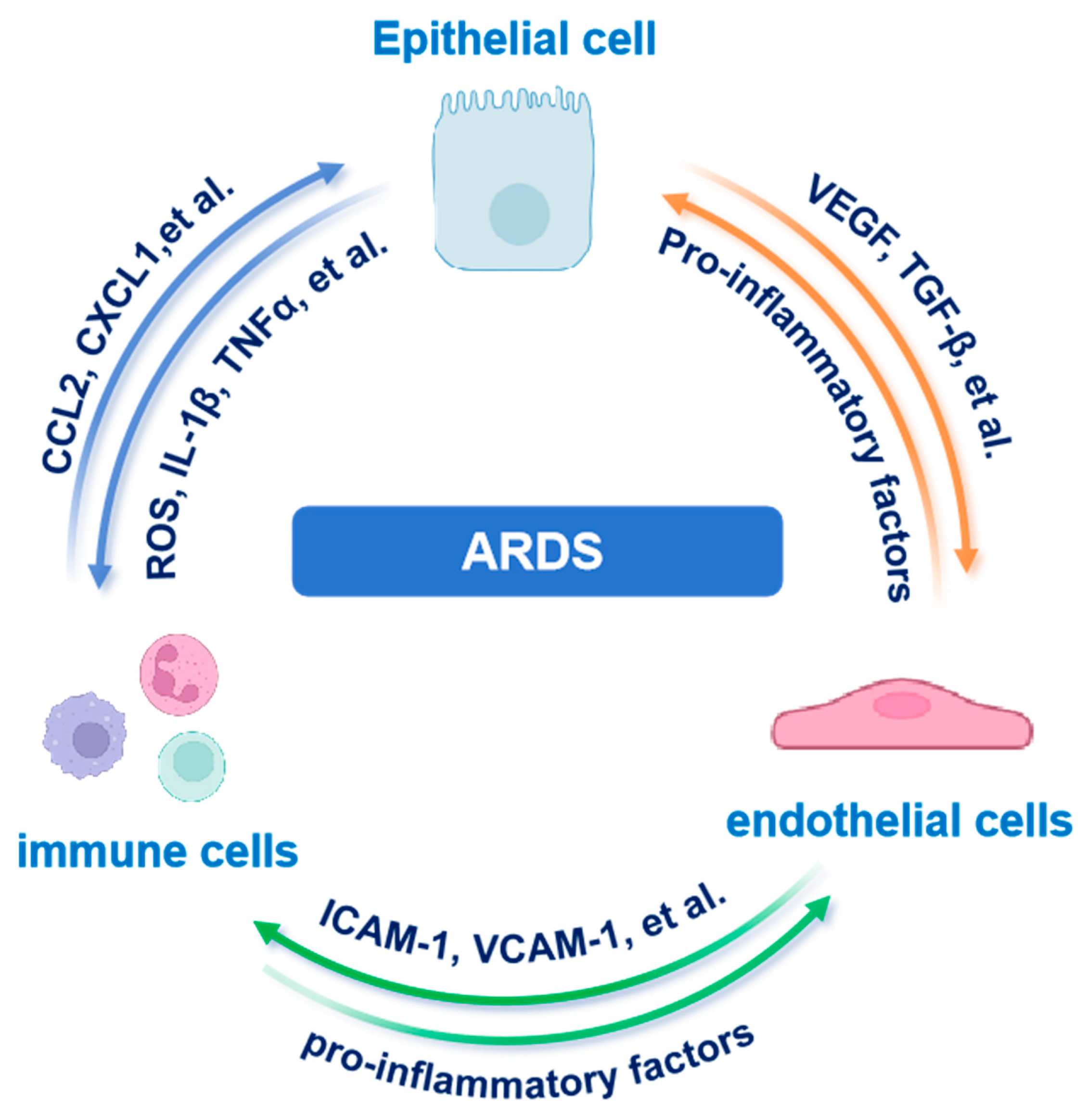
4.5. Epithelial–Immune–Endothelial Crosstalk
Building on the epithelial heterogeneity and metabolic rewiring discussed above, we next examine how AECs communicate with immune and endothelial cells to orchestrate ARDS pathogenesis. In ARDS, AECs, immune cells and endothelial cells (ECs) form a dynamic multicellular network that governs both inflammation and barrier repair. Recent scRNA-seq and spatial transcriptomics studies have uncovered previously unrecognized heterogeneity among AT2 cells, identifying subpopulations with high expression of pro-inflammatory cytokines and chemokines such as CCL2, CXCL1 and CXCL8. These AT2 subsets actively orchestrate immune cell recruitment and fuel local inflammation [11,87].
Moreover, spatial omics coupled with high parameter imaging has precisely mapped cytokine rich epithelial micro niches (“hotspots”) in situ. For instance, GeoMx Digital Spatial Profiling revealed that AECs exhibit upregulation of cytokines and matrix-remodeling genes, coinciding with dense CD68+macrophage infiltration in SARS-CoV-2–associated ARDS [90]. Inflammatory alveolar macrophage-derived microvesicles have been implicated in damaging AECs, thereby promoting pulmonary edema and exacerbating lung injury [91]. Imaging mass cytometry further revealed spatial colocalization of AECs with both CD15+CD11b+ polymorphonuclear neutrophils and CD68+ macrophages, frequently near ECs. Spatial network analyses then revealed that these ECs actively participate in recruiting and activating local immune cells. Together, these findings underscore a critical epithelial–immune–endothelial crosstalk driving early inflammatory and fibrotic remodeling in ARDS [92,93].
Beyond epithelial-immune interactions, ECs play a central role in modulating vascular permeability and immune cell extravasation. Injured AECs release key mediators such as Vascular Endothelial Growth Factor (VEGF), and DAMPs such as HMGB1. These molecules disrupt endothelial junctional proteins such as Vascular Endothelial Cadherin (VE-cadherin), increasing capillary leak. Conversely, activated ECs secrete IL-6 and granulocyte-macrophage colony-stimulating factor (GM-CSF), amplifying leukocyte recruitment and perpetuating epithelial injury [94,95,96].
Collectively, these bidirectional signals establish a self-perpetuating inflammatory loop (Figure 3). Epithelial-derived chemokines initiate immune cell recruitment and activation. Immune-derived mediators, including cytokines and ROS, subsequently disrupt endothelial integrity, potentiating immune infiltration and epithelial injury. Spatially resolved studies define multicellular hubs (AECs, immune cells, ECs) as sites where triadic crosstalk drives fibrotic remodeling and barrier dysfunction in ARDS. Moreover, transplantation of human embryonic stem cell-derived AT2 cells has been shown to alleviate ARDS in mouse models, further supporting the important role of AECs in lung homeostasis and injury resolution [97].
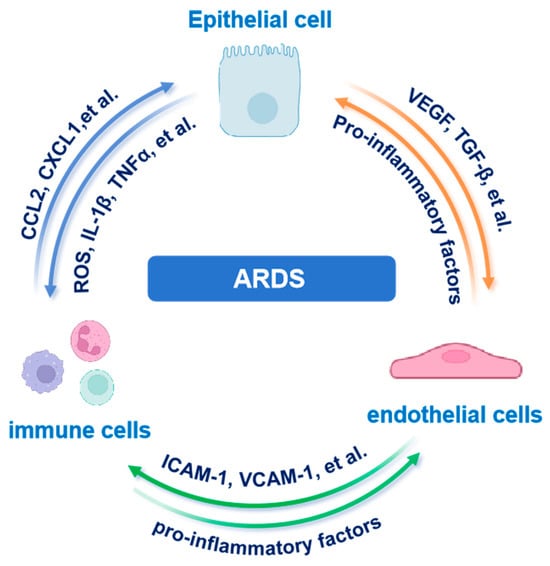
Figure 3.
Tripartite epithelial–immune–endothelial crosstalk in ARDS. AEC-derived chemokines recruit immune cells that amplify endothelial leak and further epithelial injury. Abbreviations: AEC, alveolar epithelial cell; ARDS, acute respiratory distress syndrome; CCL2, C-C motif chemokine ligand 2; CXCL1, C-X-C motif chemokine ligand 1; ICAM-1, intercellular adhesion molecule 1; IL-1β, interleukin-1 beta; TGF-β, transforming growth factor beta; TNFα, tumor necrosis factor alpha; VCAM-1, vascular cell adhesion molecule 1; VEGF, vascular endothelial growth factor. The images illustrated in the figures were adapted from https://app.biorender.com.
4.6. Modulation of Alveolar Epithelium by the Gut-Lung Axis
Beyond local cell–cell interactions, systemic modulators including the gut-lung axis can influence epithelial resilience and repair. The gut-lung axis plays a critical role in maintaining pulmonary homeostasis and alveolar epithelial integrity during ARDS. Dysbiosis of the gut microbiota and increased intestinal permeability facilitate the translocation of microbial products and viable bacteria into the systemic circulation. This process amplifies systemic inflammation, exacerbates AEC injury, and disrupts barrier function [98,99]. Preclinical studies have shown that depletion of beneficial taxa, such as Akkermansia muciniphila, worsens alveolar damage, whereas probiotic supplementation restores epithelial integrity, highlighting the distal regulatory role of the gut microbiota on lung epithelium [100,101].
Moreover, microbiota-derived metabolites directly influence AEC function. Short-chain fatty acids (SCFAs), particularly butyrate, enhance mitochondrial activity and upregulate tight junction proteins such as ZO-1, thereby mitigating alveolar edema. In contrast, dysbiosis-associated metabolites, including trimethylamine N-oxide (TMAO), promote NLRP3 inflammasome activation and pyroptosis in AECs [101,102,103]. These findings underscore the therapeutic potential of microbiome-targeted interventions. Strategies such as fecal microbiota transplantation (FMT), SCFA analogs, or engineered probiotics have shown promise in preserving alveolar-capillary barrier function and improving outcomes in preclinical ALI models [104,105].
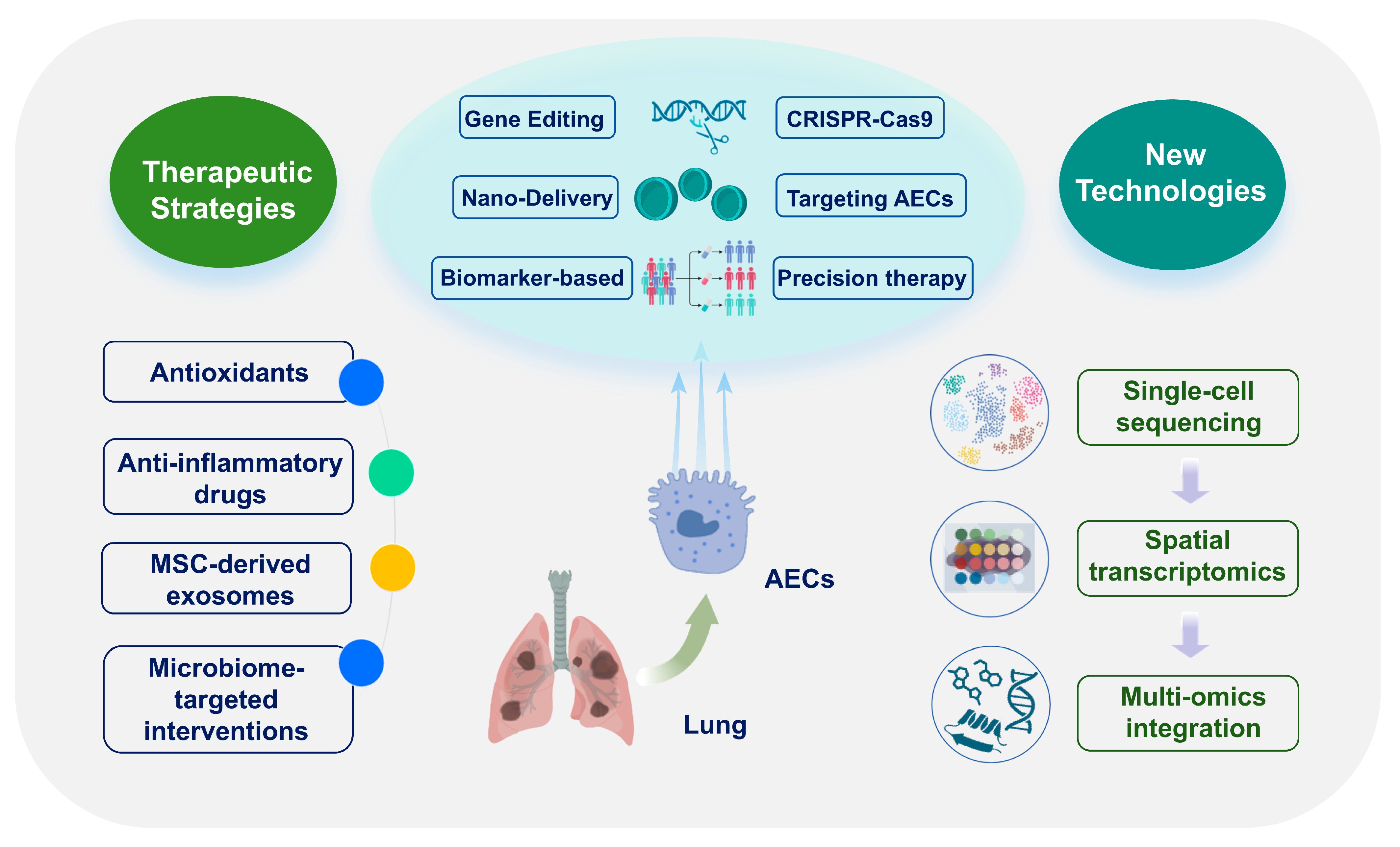
5. Therapeutic Strategies Targeting Epithelial Cells
AEC dysfunction is a key driver of alveolar-capillary barrier failure and immune dysregulation in ARDS and represents a critical target for therapeutic intervention. This chapter synthesizes mechanistically targeted strategies to preserve AEC function, including antioxidant and anti-inflammatory agents, cell and gene therapies, advanced delivery systems, microbiome modulation, and biomarker-guided precision medicine (Figure 4 and Table 2).
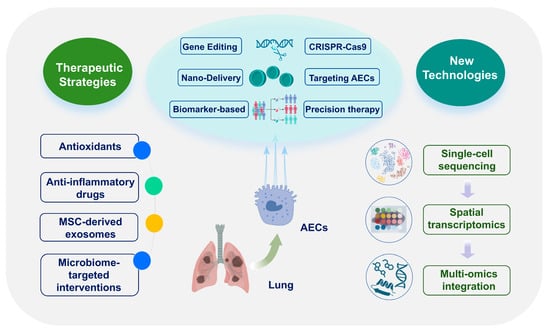
Figure 4.
Epithelial-targeted therapeutic strategies. Overview of antioxidants, biologics, cell/gene therapies, nanodelivery, microbiome interventions and biomarker-guided precision approaches. Abbreviations: AEC, alveolar epithelial cell; MSC, mesenchymal stem cell. The images illustrated in the figures were adapted from https://app.biorender.com.

Table 2.
Summary of Novel Epithelial-Targeted Therapeutic Strategies for ARDS.
5.1. Antioxidant and Anti-Inflammatory Therapies
5.1.1. Antioxidants
Oxidative stress is a key factor in AEC damage during ARDS, and antioxidant therapy has been shown to alleviate this damage. N-acetylcysteine (NAC), a precursor of glutathione, has been shown to effectively reduce oxidative stress and maintain the integrity of AEC membranes. In the trial registered as NCT04374461 and in several small randomized or open-label studies, NAC was associated with reductions in oxidative-stress biomarkers and, in some reports, modest improvements in oxygenation. However, these small studies did not consistently demonstrate benefit on hard clinical endpoints such as mortality or ventilator-free days. Overall, the data indicate a biological effect of NAC but highlight the need for larger, biomarker-guided trials to determine whether these antioxidant effects translate into clear clinical benefit [106,107]. Similarly, melatonin has shown protective effects in ALI models by activating the nuclear factor erythroid 2-related factor 2 signaling pathway and enhancing the expression of key antioxidant enzymes, thereby reducing alveolar edema and inflammatory responses [108,109]. These findings support the therapeutic potential of antioxidants in limiting epithelial damage mediated by oxidative stress in ALI.
5.1.2. Anti-Inflammatory Drugs
Glucocorticoids remain the cornerstone of anti-inflammatory therapy for ARDS, and they work mainly by inhibiting the NF-κB signaling pathway, thereby reducing the production of key cytokines such as IL-6 and TNF-α [110,111]. However, prolonged glucocorticoid exposure can compromise epithelial repair and immune surveillance, which underscores the need to carefully balance anti-inflammatory benefits against potential delays in lung recovery.
Another strategy targets specific cytokine axes. The IL-1 receptor antagonist anakinra blocks the IL-1β signaling pathway, preventing epithelial pyroptosis and amplification of downstream inflammation. In the randomized trial registered as NCT04339712 in patients with COVID-19–associated ARDS, anakinra was generally well tolerated and was associated with reductions in inflammatory biomarkers and, in some analyses, reduced ventilator dependence; however, results have been inconsistent across studies, suggesting that benefit may be restricted to biomarker-defined subgroups and that prospective, stratified trials are needed [112,113].
Together, these approaches illustrate two complementary modes of anti-inflammatory action including broad-spectrum inhibition by glucocorticoids and targeted cytokine blockade, both of which have differential effects on epithelial integrity and repair.
5.2. Cell and Gene Therapies
Cell and gene therapies have emerged as promising approaches to restore AEC function and attenuate lung injury in ARDS. Among them, mesenchymal stem cells (MSCs) have been extensively studied due to their immunomodulatory, anti-apoptotic and regenerative properties. Preclinical studies have demonstrated that both systemic and intratracheal administration of bone marrow or adipose-derived MSCs can reduce alveolar permeability, enhance AEC survival, and promote epithelial repair [114,115,116]. These effects are largely mediated by paracrine signaling, including the release of growth factors, cytokines and extracellular vesicles that influence AEC behavior [117,118].
Gene-based interventions provide an additional layer of targeted control over epithelial pathophysiology. Adeno-associated virus (AAV) vectors have been used to deliver genes that encode anti-inflammatory or barrier-protective molecules directly to AECs, thereby improving alveolar stability [119,120]. Furthermore, CRISPR-Cas9 genome editing offers a powerful platform for correcting gene dysfunctions or modulating key signaling pathways involved in epithelial injury and regeneration [121,122]. However, considerable challenges persist, mirroring the limitations of the pharmacologic approaches noted in Section 5.1. Efficient delivery of therapies remains a major challenge. Transplanted cells often fail to engraft in damaged lung tissue, and viral or non-viral vectors show inconsistent transduction rates. Immunogenicity introduces further risks, including the immune-mediated rejection of allogeneic cells and adverse reactions to gene-editing tools. Safety issues also remain, particularly uncertainties around long-term consequences and off-target effects. Future work should focus on developing integrated strategies, such as biomaterial-assisted delivery, nanocarrier systems, and immune-modulatory regimens, to improve the efficacy and safety of advanced cell and gene therapies.
5.3. Exosomes and Nanodelivery Systems
As nature’s inherent nanocarriers, exosomes have attracted significant interest for the treatment of ARDS. This stems from their intrinsic biocompatibility and ability to transport bioactive cargo across the alveolar barrier [123,124]. MSC-derived exosomes demonstrate substantial therapeutic efficacy in diverse ALI models [125]. These exosomes, whether loaded with anti-inflammatory miRNAs (such as miR-146a-5p) or engineered to display lung-homing peptides, function by reducing pro-inflammatory cytokines (including TNF-α and IL-1β), enhancing IL-10 secretion, and restoring alveolar barrier integrity [125,126,127].
In parallel, synthetic nano-delivery systems have achieved notable advances. Lipid nanoparticles (LNPs) and polymeric platforms enable precision delivery of therapeutic genes and drugs. Neutrophil membrane-coated poly (lactic-co-glycolic acid) (PLGA) nanoparticles carrying TLR4 siRNA selectively target inflammatory macrophages to suppress NF-κB activation and mitigate LPS-induced lung injury [128]. Mannosylated LNPs delivering miR-146a improve survival in hemorrhagic-shock-induced ARDS. And 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP)-modified LNPs encapsulating sPD-L1 mRNA induce lung-restricted immunosuppression in ARDS models [129].
Future research will focus on “intelligent” responsive nanomaterials, which can release therapeutic drugs when the level of reactive oxygen species or proteinase activity increases, thereby achieving precise temporal control of pulmonary inflammation. The synergistic combination of biomimetic exosomes and programmable synthetic nanocarriers provides a transformative approach for individualized treatment of ARDS, but it needs to overcome the obstacle of scaling up production and prove its efficacy in advanced disease models.
5.4. Precision Medicine and Biomarkers
In recent years, multi-omics technologies such as scRNA-seq, metabolomics and proteomics have revealed the dynamic changes of biomarkers in blood and bronchoalveolar lavage fluid (BALF). These advances have improved our understanding of ARDS and provided important insights for clinical classification, prognosis, and targeted therapy [130]. The scRNA-seq identified ADIs, which arise during the transformation of AT2 to AT1 cells after lung injury and are associated with epithelial repair. These transitional cells are enriched in fibrotic lungs and exhibit pro-inflammatory and stress-responsive features [86]. Accumulated succinate in injured epithelium not only affects epithelial metabolism but also acts on macrophages through SUCNR1, thereby promoting pro-inflammatory responses, as discussed in Section 4.3 [131]. Soluble receptor for advanced glycation end products (sRAGE) is a biomarker of AT1 cell injury, with elevated levels correlating with disease severity in ARDS [132]. Furthermore, exosomal miRNAs (including miR-146a) can serve as potential biomarkers for the intensity of inflammatory response and may help to identify patients who will benefit from anti-inflammatory interventions [126,133]. Finally, scRNA-seq has further revealed epithelial heterogeneity, identified profibrotic AT2 subsets within patient lungs, and facilitated the selection of targeted agents, such as TGF-β inhibitors, for individualized treatment [134,135].
5.5. Microbiome Targeted Interventions
ARDS-associated gut–lung axis dysregulation increases intestinal permeability, facilitating microbial products to enter the circulation and worsen alveolar epithelial inflammation. Targeting the microbiome shows preclinical promise. In animal models, specific probiotics such as Lactobacillus rhamnosus GG and Bifidobacterium breve modulate CD4+ regulatory T cell (Treg) and T helper 17 cell (Th17) balance, reduce pulmonary inflammation and improve epithelial barrier function [98,136,137]. Short-chain fatty acids (SCFAs), especially butyrate, appear to activate the epithelial free fatty acid receptors GPR41 (also known as FFAR3) and GPR43 (FFAR2), resulting in inhibition of inflammation and facilitating mucus layer repair in these models [138,139]. Fecal microbiota transplantation (FMT) similarly reduces inflammation and mortality in animals. However, its clinical translation encounters challenges in standardization and safety [140,141]. These microbiome interventions show protection in animal ALI models, and high-quality clinical trials are needed to validate their therapeutic potential.
Future research needs to utilize sophisticated tools like humanized mouse models and lung-on-a-chip technology to carefully evaluate probiotic strains and microbial metabolites. The key is finding those that can both stably establish themselves in the gut and actively promote epithelial repair. At the same time, we must develop better ways to deliver these agents directly to the airways and establish standardized methods to consistently measure their effectiveness and safety. These efforts will help move these promising approaches from the lab to the clinic faster.
6. Challenges and Future Perspectives
6.1. Translational Challenges
The clinical translation of ARDS therapies faces multifaceted technical hurdles. Nano-delivery systems are challenged by low lung-targeting efficiency and systemic toxicity, though surface modifications (such as lung-specific ligands) and nebulized formulations offer promising solutions [142,143,144]. Gene editing precision remains compromised by off-target effects, necessitating high-fidelity Cas9 variants (such as eSpCas9) and AI-optimized guide RNA design to enhance specificity [145,146,147,148]. Concurrently, MSC heterogeneity, driven by donor variability and culture conditions, undermines therapeutic consistency, mandating GMP-compliant cell banking and standardized protocols [149,150,151]. Personalized approaches are hindered by inadequate biomarkers. Integrating multi-omics signatures (such as sRAGE, IL-6, Ang-2, miRNAs) with real-time biosensors and machine learning is critical for dynamic subtyping and treatment optimization [152,153,154].
Safety and ethical complexities further impede translational progress. Cationic lipid-based nanocarriers risk pulmonary inflammation, prompting a shift toward biodegradable polymers or exosomes with improved biocompatibility [155,156,157]. Gene editing vectors (such as AAVs) face immunogenicity barriers, driving exploration of engineered variants and non-viral platforms (such as PiggyBac, mRNA-Cas9) to enable repeated dosing [158,159]. Crucially, CRISPR therapies raise unresolved ethical concerns, including germline contamination and carcinogenic risks, demanding strict somatic-cell editing limits, IRB oversight per Helsinki principles, and long-term registries spanning over 10 years to monitor delayed toxicity [160,161,162,163,164,165,166]. Human-lung-chip platforms provide vital preclinical tools for dose safety validation [167].
6.2. Emerging Technologies Driving Mechanistic Insights
6.2.1. Single-Cell and Spatial Omics
The scRNA-seq in bleomycin-injured murine lungs has revealed a population of transitional Krt8+ alveolar progenitors. These analyses suggest the Hippo-Yap/Taz signaling axis as a therapeutically targetable pathway. Pharmacologic inhibition of Yap/Taz suppresses Krt8+ cell proliferation, while genetic ablation of Yap1 severely compromises alveolar regeneration. These complementary approaches indicate that modulating Hippo-Yap/Taz activity may enhance epithelial repair [86,168]. Further leveraging single-cell trajectory analysis, researchers observed an IL-1β–HIF1α–mediated glycolytic shift that traps AT2 cells in a damage-associated transient progenitor (DATP) state. Importantly, interruption of IL-1β or HIF1α signaling reverses this differentiation arrest and promotes AT1 maturation. This highlights cytokine blockade (IL-1β) and metabolic reprogramming (HIF1α inhibition) as promising combination therapy for epithelial regeneration [169].
Translating these insights to human pathology, spatial transcriptomic profiling of fibrotic lungs identified localized “fibrotic hotspots”. These niches are characterized by aberrant activation of Notch and TGF-β pathways within KRT17+ epithelial clusters, co-localized with myofibroblast accumulation and stress-response signatures [170]. This spatial resolution directly informs precision therapeutic strategies, such as aerosolized delivery of TGF-β inhibitors or Notch modulators, designed to selectively disrupt pro-fibrotic signaling within these microenvironments [134,171,172]. Consequently, the integration of single-cell dynamics with spatial architecture offers a powerful framework to identify context-dependent therapeutic targets and advance mechanism-based interventions for ARDS and PF.
6.2.2. Mechanobiology and Microfluidic “Lung-on-Chip” Platforms
Studies in mechanobiology utilizing tunable substrates and cyclic stretch have demonstrated that elevated ECM stiffness and mechanical strain promote Yap/Taz nuclear translocation in AECs. This mechanotransduction pathway critically regulates epithelial barrier integrity and potentiates pro-fibrotic responses [173,174]. To model these dynamics, microfluidic lung-on-chip platforms have been developed. These devices incorporate a stretchable, ECM-coated membrane that mimics the alveolar-capillary interface, subject to controlled fluidic shear stress and cyclic mechanical deformation. This setup enables real-time quantification of key parameters including vascular permeability, transepithelial electrical resistance (TEER), and cell–matrix adhesion kinetics [175,176].
Notably, advanced iterations integrate adjacent microchambers for fibroblast or immune cell co-culture, coupled with embedded biosensors. This design facilitates high-resolution spatiotemporal analysis of mechanobiological crosstalk within a human-relevant microenvironment [177,178]. Collectively, lung-on-chip systems offer a physiologically mimetic platform for deconvoluting epithelial-matrix mechanosignaling and accelerating the discovery of mechano-targeted therapeutics.
6.2.3. AI-Driven Multi-Omics Integration
AI and graph-based computational models are transforming multi-omics integration to decode cell–cell communication networks, significantly accelerating target identification. Techniques spanning random forest-based feature selection to graph neural networks infer key regulatory hubs and pathways obscured in single-omic analyses. For example, integrative metabolomic-proteomic profiling of ALI models identified succinate-SUCNR1 signaling as a critical immunometabolic driver. Subsequent AI validation prioritized this axis for therapeutic targeting [59,131,179]. By reconstructing disease-relevant networks, these approaches not only pinpoint druggable targets but also predict synergistic drug combinations, accelerating precision medicine design.
6.3. Toward Epithelium Centered Precision ARDS Therapy
Current mechanistic insights into AEC injury and repair underpin an epithelium-centered precision paradigm for ARDS. This approach begins with patient stratification through epithelial endotyping. Integrated multi-omics signatures and spatial biomarkers, such as alveolar damage indicators, sRAGE, and exosomal miRNA profiles, are utilized to classify patients into hyper-inflammatory, metabolically dysregulated or fibrotic-prone subgroups [175,180]. Endotype classification provides a mechanistic framework for therapeutic selection. Hyper-inflammatory states may be targeted by IL-1β or NF-κB pathway inhibitors. For metabolically dysregulated endotypes, SUCNR1 antagonists or HIF1α modulators represent rational candidates. In fibrotic-prone cases, localized TGF-β/Notch or YAP/TAZ pathway modulation is proposed to inhibit fibroblast activation. These targeted agents are delivered via lung-optimized carriers such as aerosolized nanoparticles or engineered exosomes to enhance epithelial specificity while minimizing systemic exposure. This strategy aligns with evolving precision medicine paradigms but requires further validation in ARDS-specific contexts.
Therapeutic efficacy is dynamically monitored using bedside biosensors, such as electrochemical assays for BALF biomarkers and exhaled miRNA panels, enabling dose adjustments based on biological feedback. Patient-derived lung organoids and lung-on-chip platforms support preclinical drug sensitivity testing, while AI-driven decision systems integrate clinical and multi-omics data to guide regimen optimization. Collectively, this framework shifts ARDS management toward precision regenerative medicine through synchronized endotype targeting, adaptive delivery, and biosensor-guided monitoring.
7. Conclusions
ARDS is characterized by AEC dysfunction, which underlies barrier disruption, impaired fluid clearance, and dysregulated inflammatory signaling [19]. This pathophysiological cascade is driven by multiple mechanisms, including NF-κB activation, ROS-mediated oxidative stress, and epigenetic modifications. Recent applications of spatial transcriptomics and scRNA-seq reveal profound epithelial heterogeneity in ARDS. Transitional progenitor cells accumulate while AT2-to-AT1 differentiation is impaired, and distinct AEC subtypes actively remodel local immune microenvironments. These insights advance mechanistic understanding and highlight novel therapeutic targets.
Conventional therapies such as antioxidants, anti-inflammatory agents, and growth factors can provide transient protection of epithelial integrity and promote regeneration. However, inconsistent therapeutic efficacy and off-target toxicity frequently limit their clinical application [19,114]. Precision medicine strategies aim to overcome these limitations through biomarker-guided patient stratification, CRISPR-Cas9 genome editing, and nanoparticle-based drug delivery [181]. Single cell endotyping identifies individuals with impaired regenerative capacity. For such patients, combination therapy employing keratinocyte growth factor (KGF) and MSCs may enhance epithelial repair efficacy. LNP-encapsulated siRNA mediates localized anti-inflammatory effects within damaged alveolar areas.
The clinical translation of individualized strategies requires overcoming critical barriers. First, integrated analysis of multi-omics datasets is required to identify actionable therapeutic targets [182]. Second, interoperable analytical workflows enable cohesive collaboration integrating molecular biology, bioengineering, computational, and clinical practice [61,182]. Third, ensuring CRISPR-Cas9 safety requires high-fidelity nucleases and rigorous genomic screening to minimize off-target effects. Fourth, thorough preclinical and clinical assessment of nanocarriers under Good Manufacturing Practice (GMP) must evaluate biodistribution patterns, toxicity profiles, and cost-effective manufacturing scalability [182,183]. Finally, widespread adoption of spatial and single-cell technologies requires standardized protocols, AI-assisted tissue processing, and multicenter registries for longitudinal tracking of biomarkers, genomic features, and clinical outcomes.
A multilevel translational strategy integrates high-resolution spatial omics empowered by multiplexed in situ hybridization and AI modeling. This approach further combines advanced human-relevant models such as lung-on-chip systems, epithelial-immune co-cultures and patient-derived organoids. This integrated approach accelerates validation of nanoparticle biodistribution and gene-editing precision [184,185]. Concurrently, de-identified multicenter biomarker registries will guide adaptive clinical trial designs and real-world evidence synthesis [186,187]. By coupling targeted delivery with molecular mapping, this framework shifts ARDS management from symptomatic support toward reestablishing epithelial-immune homeostasis. Such targeted interventions may improve survival, functional recovery, and long-term quality of life.
Author Contributions
Writing—original draft preparation, J.W.; writing-literature collection, J.W.; writing-figure preparation, J.W.; writing—review and editing, J.W.; conceptualization and supervision, J.W. and J.C.; funding acquisition, J.W. and J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 82373547) and supported by Jiangsu Province Science and Technology Plan Project ‘Provincial Frontier Technology R&D Program’ (BF2024054).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
No new data were created.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ALI | acute lung injury |
| ARDS | acute respiratory distress syndrome |
| AEC | alveolar epithelial cell |
| PF | pulmonary fibrosis |
| AT1 cells | alveolar type I epithelial cells |
| AT2 cells | alveolar type II epithelial cells |
| ECM | extracellular matrix |
| TNF-α | tumor necrosis factor-α |
| IL-1β | interleukin-1β |
| MOMP | mitochondrial outer membrane permeabilization |
| FADD | Fas-associated death domain protein |
| GSDMD | gasdermin D |
| PAMPs | pathogen-associated molecular patterns |
| DAMPs | damage-associated molecular patterns |
| MLKL | mixed lineage kinase domain-like protein |
| ROS | reactive oxygen species |
| ENaC | epithelial sodium channels |
| AQP5 | aquaporin 5 |
| HMGB1 | High Mobility Group Box 1 |
| TLR4 | Toll-like receptor 4 |
| JNK | the c-Jun N-terminal kinase |
| MAPK | mitogen-activated protein kinase |
| JAK | Janus kinase |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| HATs | histone acetyltransferases |
| lncRNAs | Long non-coding RNAs |
| SDHA | succinate dehydrogenase A |
| CXCL1 | Chemokine Ligand 1 |
| LPS | lipopolysaccharide |
| BLM | bleomycin |
| scRNA-seq | single-cell RNA sequencing |
| VEGF | Vascular Endothelial Growth Factor |
| VE-cadherin | Vascular Endothelial Cadherin |
| GM-CSF | granulocyte-macrophage colony-stimulating factor |
| SCFAs | Short-chain fatty acids |
| TMAO | trimethylamine N-oxide |
| FMT | fecal microbiota transplantation |
| NAC | N-acetylcysteine |
| AAV | Adeno-associated virus |
| LNPs | Lipid nanoparticles |
| PLGA | poly(lactic-co-glycolic acid) |
| BALF | bronchoalveolar lavage fluid |
| DATP | damage-associated transient progenitor |
| TEER | transepithelial electrical resistance |
References
- Swenson, K.E.; Swenson, E.R. Pathophysiology of Acute Respiratory Distress Syndrome and COVID-19 Lung Injury. Crit. Care Clin. 2021, 37, 749–776. [Google Scholar] [CrossRef]
- Rohrich, S.; Hofmanninger, J.; Negrin, L.; Langs, G.; Prosch, H. Radiomics score predicts acute respiratory distress syndrome based on the initial CT scan after trauma. Eur. Radiol. 2021, 31, 5443–5453. [Google Scholar] [CrossRef]
- Bos, L.D.J.; Ware, L.B. Acute Respiratory Distress Syndrome 2022 1 Acute respiratory distress syndrome: Causes, pathophysiology, and phenotypes. Lancet 2022, 400, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, N.; Martinon, D.; Moreira, D.; Crother, T.; Arditi, M.; Shimada, K. Autophagy Protects Against Developing Increased Lung Permeability and Hypoxemia by Down Regulating Inflammasome Activity and IL-1β in LPS Plus Mechanical Ventilation-Induced Acute Lung Injury. Front. Immunol. 2020, 11, 207. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Qin, Q.; Lu, J. Pathophysiological mechanisms of ARDS: A narrative review from molecular to organ-level perspectives. Respir. Res. 2025, 26, 54. [Google Scholar] [CrossRef] [PubMed]
- Herridge, M.; Moss, M.; Hough, C.; Hopkins, R.; Rice, T.; Bienvenu, O.; Azoulay, E. Recovery and outcomes after the acute respiratory distress syndrome (ARDS) in patients and their family caregivers. Intensive Care Med. 2016, 42, 725–738. [Google Scholar] [CrossRef]
- Xie, R.; Tan, D.; Liu, B.; Xiao, G.; Gong, F.; Zhang, Q.; Qi, L.; Zheng, S.; Yuan, Y.; Yang, Z.; et al. Acute respiratory distress syndrome (ARDS): From mechanistic insights to therapeutic strategies. MedComm 2025, 6, e70074. [Google Scholar] [CrossRef]
- Yang, J.; Pan, X.; Wang, L.; Yu, G. Alveolar cells under mechanical stressed niche: Critical contributors to pulmonary fibrosis. Mol. Med. 2020, 26, 95. [Google Scholar] [CrossRef]
- Kass-Gergi, S.; Vaughan, A. Alveolar Repair after Viral Injury: A Tale of Two Cell Types. Am. J. Respir. Cell Mol. Biol. 2022, 67, 273–274. [Google Scholar] [CrossRef]
- Meng, L.; Wang, M.; Gao, Y.; Chen, L.; Wang, K.; Gao, W.; Liu, Q. Dopamine D1 receptor agonist alleviates acute lung injury via modulating inflammatory responses in macrophages and barrier function in airway epithelial cells. Free Radic. Biol. Med. 2023, 202, 2–16. [Google Scholar] [CrossRef]
- Li, X.; Jamal, M.; Guo, P.; Jin, Z.; Zheng, F.; Song, X.; Zhan, J.; Wu, H. Irisin alleviates pulmonary epithelial barrier dysfunction in sepsis-induced acute lung injury via activation of AMPK/SIRT1 pathways. Biomed. Pharmacother. 2019, 118, 109363. [Google Scholar] [CrossRef]
- Scozzi, D.; Liao, F.; Krupnick, A.S.; Kreisel, D.; Gelman, A.E. The role of neutrophil extracellular traps in acute lung injury. Front. Immunol. 2022, 13, 953195. [Google Scholar] [CrossRef]
- Bhattacharya, J.; Westphalen, K. Macrophage-epithelial interactions in pulmonary alveoli. Semin. Immunopathol. 2016, 38, 461–469. [Google Scholar] [CrossRef]
- Huang, Q.; Le, Y.; Li, S.; Bian, Y. Signaling pathways and potential therapeutic targets in acute respiratory distress syndrome (ARDS). Respir. Res. 2024, 25, 30. [Google Scholar] [CrossRef]
- Zhai, R.; Ma Bonda, W.L.; Leclaire, C.; Saint-Beat, C.; Theilliere, C.; Belville, C.; Coupet, R.; Blondonnet, R.; Bouvier, D.; Blanchon, L.; et al. Effects of sevoflurane on lung epithelial permeability in experimental models of acute respiratory distress syndrome. J. Transl. Med. 2023, 21, 397. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Cong, Z.; Yang, C.; Zhu, X. Inhibition of LPS-induced Nox2 activation by VAS2870 protects alveolar epithelial cells through eliminating ROS and restoring tight junctions. Biochem. Biophys. Res. Commun. 2020, 524, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, S.; Xiang, D.; Ju, L.; Shen, D.; Wang, X.; Wang, Y. Friend or Foe? The Roles of Antioxidants in Acute Lung Injury. Antioxidants 2021, 10, 1956. [Google Scholar] [CrossRef]
- Li, Y.; Cao, Y.; Xiao, J.; Shang, J.; Tan, Q.; Ping, F.; Huang, W.; Wu, F.; Zhang, H.; Zhang, X. Inhibitor of apoptosis-stimulating protein of p53 inhibits ferroptosis and alleviates intestinal ischemia/reperfusion-induced acute lung injury. Cell Death Differ. 2020, 27, 2635–2650. [Google Scholar] [CrossRef]
- Li, N.; Liu, B.; Xiong, R.; Li, G.; Wang, B.; Geng, Q. HDAC3 deficiency protects against acute lung injury by maintaining epithelial barrier integrity through preserving mitochondrial quality control. Redox Biol. 2023, 63, 102746. [Google Scholar] [CrossRef]
- Larson-Casey, J.L.; He, C.; Carter, A.B. Mitochondrial quality control in pulmonary fibrosis. Redox Biol. 2020, 33, 101426. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, X.; Wu, Q.; Zhu, J.; Que, Y.; Wang, Y.; Ding, Y.; Yang, Y.; Jin, J.; Zhang, X.; et al. BCAP31 Alleviates Lipopolysaccharide-Mediated Acute Lung Injury via Induction of PINK1/Parkin in Alveolar Epithelial Type II Cell. Research 2024, 7, 0498. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, C.; Song, Y. Molecular mechanisms and roles of pyroptosis in acute lung injury. Chin. Med. J. 2022, 135, 2417–2426. [Google Scholar] [CrossRef]
- Liu, J.; Song, K.; Lin, B.; Chen, Z.; Liu, Y.; Qiu, X.; He, Q.; Zuo, Z.; Yao, X.; Huang, X.; et al. The suppression of HSPA8 attenuates NLRP3 ubiquitination through SKP2 to promote pyroptosis in sepsis-induced lung injury. Cell Biosci. 2024, 14, 56. [Google Scholar] [CrossRef]
- Wang, J.; Li, L.-L.; Zhao, Z.-A.; Niu, C.-Y.; Zhao, Z.-G. NLRP3 Inflammasome-mediated pyroptosis in acute lung injury: Roles of main lung cell types and therapeutic perspectives. Int. Immunopharmacol. 2025, 154, 114560. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.E.; Price, D.R.; Ryter, S.W.; Choi, A.M.K. Necroptosis: A crucial pathogenic mediator of human disease. JCI Insight 2019, 4, e128834. [Google Scholar] [CrossRef] [PubMed]
- Khanpour, M.A.; Moradiani, F.; Parsanasab, G.-M.; Karimzadeh, R. Dimethyl fumarate inhibits necroptosis and alleviates systemic inflammatory response syndrome by blocking the RIPK1-RIPK3-MLKL axis. Opt. Laser Technol. 2023, 162, 109236. [Google Scholar] [CrossRef]
- Morgan, M.J.; Kim, Y.-S. Roles of RIPK3 in necroptosis, cell signaling, and disease. Exp. Mol. Med. 2022, 54, 1695–1704. [Google Scholar] [CrossRef]
- Hou, L.; Zhang, J.; Liu, Y.; Fang, H.; Liao, L.; Wang, Z.; Yuan, J.; Wang, X.; Sun, J.; Tang, B.; et al. MitoQ alleviates LPS-mediated acute lung injury through regulating Nrf2/Drp1 pathway. Free Radic. Biol. Med. 2021, 165, 219–228. [Google Scholar] [CrossRef]
- Gumeni, S.; Papanagnou, E.-D.; Manola, M.S.; Trougakos, I.P. Nrf2 activation induces mitophagy and reverses Parkin/Pink1 knock down-mediated neuronal and muscle degeneration phenotypes. Cell Death Dis. 2021, 12, 671. [Google Scholar] [CrossRef]
- Song, C.; Zhang, A.; Zhang, M.; Song, Y.; Huangfu, H.; Jin, S.; Sun, Y.; Zhang, C.; Shi, D.; Wang, J.; et al. Nrf2/PINK1-mediated mitophagy induction alleviates sodium fluoride-induced hepatic injury by improving mitochondrial function, oxidative stress, and inflammation. Ecotoxicol. Environ. Saf. 2023, 252, 114646. [Google Scholar] [CrossRef]
- Lennicke, C.; Cocheme, H.M. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell 2021, 81, 3691–3707. [Google Scholar] [CrossRef]
- Li, P.-C.; Wang, B.-R.; Li, C.-C.; Lu, X.; Qian, W.-S.; Li, Y.-J.; Jin, F.-G.; Mu, D.-G. Seawater inhalation induces acute lung injury via ROS generation and the endoplasmic reticulum stress pathway. Int. J. Mol. Med. 2018, 41, 2505–2516. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, X.; Kang, Y.; Cai, M.; Yan, J.; Zang, C.; Gao, Y.; Qi, Y. Scutellarein Suppresses the Production of ROS and Inflammatory Mediators of LPS-Activated Bronchial Epithelial Cells and Attenuates Acute Lung Injury in Mice. Antioxidants 2024, 13, 710. [Google Scholar] [CrossRef] [PubMed]
- Gupte, V.V.; Ramasamy, S.K.; Reddy, R.; Lee, J.; Weinreb, P.H.; Violette, S.M.; Guenther, A.; Warburton, D.; Driscoll, B.; Minoo, P. Overexpression of fibroblast growth factor-10 during both inflammatory and fibrotic phases attenuates bleomycin-induced pulmonary fibrosis in mice. Am. J. Respir. Crit. Care Med. 2009, 180, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yan, S.-F.; Hao, Y.; Jin, S.-W. Specialized Pro-resolving Mediators Regulate Alveolar Fluid Clearance during Acute Respiratory Distress Syndrome. Chin. Med. J. 2018, 131, 982–989. [Google Scholar] [CrossRef]
- Bai, Y.; Ma, C.; Jing, X.; Wu, J.; Yao, S.; Tian, L.; Dong, X.; An, Z.; Ren, W. Resveratrol alleviates blast lung injury by modulating the epithelial sodium channel (ENaC) via the PI3K/AKT pathway. Int. Immunopharmacol. 2025, 147, 113995. [Google Scholar] [CrossRef]
- Peters, D.M.; Vadasz, I.; Wujak, L.; Wygrecka, M.; Olschewski, A.; Becker, C.; Herold, S.; Papp, R.; Mayer, K.; Rummel, S.; et al. TGF-β directs trafficking of the epithelial sodium channel ENaC which has implications for ion and fluid transport in acute lung injury. Proc. Natl. Acad. Sci. USA 2014, 111, E374–E383. [Google Scholar] [CrossRef]
- Wynne, B.M.; Zou, L.; Linck, V.; Hoover, R.S.; Ma, H.-P.; Eaton, D.C. Regulation of Lung epithelial Sodium Channels by Cytokines and Chemokines. Front. Immunol. 2017, 8, 766. [Google Scholar] [CrossRef]
- Herrero, R.; Prados, L.; Ferruelo, A.; Puig, F.; Pandolfi, R.; Guillamat-Prats, R.; Moreno, L.; Matute-Bello, G.; Artigas, A.; Esteban, A.; et al. Fas activation alters tight junction proteins in acute lung injury. Thorax 2019, 74, 69–82. [Google Scholar] [CrossRef]
- Xu, H.-R.; Yang, Q.; Xiang, S.-Y.; Zhang, P.-H.; Ye, Y.; Chen, Y.; Xu, K.-W.; Ren, X.-Y.; Mei, H.-X.; Shen, C.-X.; et al. Rosuvastatin Enhances Alveolar Fluid Clearance in Lipopolysaccharide-Induced Acute Lung Injury by Activating the Expression of Sodium Channel and Na,K-ATPase via the PI3K/AKT/Nedd4-2 Pathway. J. Inflamm. Res. 2021, 14, 1537–1549. [Google Scholar] [CrossRef]
- Wang, H.; Zentner, M.; Deng, H.; Kim, K.; Wu, R.; Yang, P.; Ann, D. Oxidative stress disrupts glucocorticoid hormone-dependent transcription of the amiloride-sensitive epithelial sodium channel α-subunit in lung epithelial cells through ERK-dependent and thioredoxin-sensitive pathways. J. Biol. Chem. 2000, 275, 8600–8609. [Google Scholar] [CrossRef]
- Towne, J.; Krane, C.; Bachurski, C.; Menon, A. Tumor necrosis factor-α inhibits aquaporin 5 expression in mouse lung epithelial cells. J. Biol. Chem. 2001, 276, 18657–18664. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, C.; Parisis, D.; Chivasso, C.; Hajiabbas, M.; Soyfoo, M.S.; Delporte, C. Aquaporin-5 Dynamic Regulation. Int. J. Mol. Sci. 2023, 24, 1889. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.J.; Sun, L.; Luo, Z.C.; Zhang, Y.; Wang, X.Z.; Liao, Y.Z.; Tong, X.; Shan, J.J. Potential Therapeutic Applications of Pulmonary Surfactant Lipids in the Host Defence Against Respiratory Viral Infections. Front. Immunol. 2021, 12, 730022. [Google Scholar] [CrossRef] [PubMed]
- Dushianthan, A.; Grocott, M.; Murugan, G.; Wilkinson, T.; Postle, A. Pulmonary Surfactant in Adult ARDS: Current Perspectives and Future Directions. Diagnostics 2023, 13, 2964. [Google Scholar] [CrossRef]
- Quesnel, C.; Nardelli, L.; Piednoir, P.; Leçon, V.; Marchal-Somme, J.; Lasocki, S.; Bouadma, L.; Philip, I.; Soler, P.; Crestani, B.; et al. Alveolar fibroblasts in acute lung injury: Biological behaviour and clinical relevance. Eur. Respir. J. 2010, 35, 1312–1321. [Google Scholar] [CrossRef]
- Zhang, F.; Nielsen, L.; Lucas, J.; Mason, R. Transforming growth factor-β antagonizes alveolar type II cell proliferation induced by keratinocyte growth factor. Am. J. Respir. Cell Mol. Biol. 2004, 31, 679–686. [Google Scholar] [CrossRef]
- Eldredge, L. Preventable ATII Proliferation after Hyperoxia: The “Tempo” of Folate Metabolism in the Neonatal Lung. Am. J. Respir. Cell Mol. Biol. 2022, 66, 353–355. [Google Scholar] [CrossRef]
- Abdelwahab, E.M.M.; Rapp, J.; Feller, D.; Csongei, V.; Pal, S.; Bartis, D.; Thickett, D.R.; Pongracz, J.E. Wnt signaling regulates trans-differentiation of stem cell like type 2 alveolar epithelial cells to type 1 epithelial cells. Respir. Res. 2019, 20, 204. [Google Scholar] [CrossRef]
- Bartczak, K.; Bialas, A.J.; Kotecki, M.J.; Gorski, P.; Piotrowski, W.J. More than a Genetic Code: Epigenetics of Lung Fibrosis. Mol. Diagn. Ther. 2020, 24, 665–681. [Google Scholar] [CrossRef]
- Flodby, P.; Li, C.; Liu, Y.; Wang, H.; Rieger, M.E.; Minoo, P.; Crandall, E.D.; Ann, D.K.; Borok, Z.; Zhou, B. Cell-specific expression of aquaporin-5 (AQP5) in alveolar epithelium is directed by GATA6/Sp1 via histone acetylation. Sci. Rep. 2017, 7, 3473. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.; Geng, B.; Jiang, W.; Hu, Y.; Hu, Z.; Hao, B.; Li, N.; Geng, Q. Histone deacetylase 3 deletion in alveolar type 2 epithelial cells prevents bleomycin-induced pulmonary fibrosis. Clin. Epigenet. 2023, 15, 182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Wang, D.; Peng, M.; Tang, L.; Ouyang, J.W.; Xiong, F.; Guo, C.; Tang, Y.Y.; Zhou, Y.J.; Liao, Q.J.; et al. Single-cell RNA sequencing in cancer research. J. Exp. Clin. Cancer Res. 2021, 40, 81. [Google Scholar] [CrossRef] [PubMed]
- Jovic, D.; Liang, X.; Zeng, H.; Lin, L.; Xu, F.P.; Luo, Y.L. Single-cell RNA sequencing technologies and applications: A brief overview. Clin. Transl. Med. 2022, 12, e694. [Google Scholar] [CrossRef]
- Rao, A.; Barkley, D.; França, G.S.; Yanai, I. Exploring tissue architecture using spatial transcriptomics. Nature 2021, 596, 211–220. [Google Scholar] [CrossRef]
- Williams, C.G.; Lee, H.J.; Asatsuma, T.; Vento-Tormo, R.; Haque, A. An introduction to spatial transcriptomics for biomedical research. Genome Med. 2022, 14, 68. [Google Scholar] [CrossRef]
- Hu, T.; Allam, M.; Cai, S.; Henderson, W.; Yueh, B.; Garipcan, A.; Ievlev, A.V.; Afkarian, M.; Beyaz, S.; Coskun, A.F. Single-cell spatial metabolomics with cell-type specific protein profiling for tissue systems biology. Nat. Commun. 2023, 14, 8260. [Google Scholar] [CrossRef]
- Rabelink, T.J.; Wang, G.Q.; Heijs, B.; Kostidis, S.; Mahfouz, A.; Rietjens, R.; Bijkerk, R.; Koudijs, A.; van der Pluijm, L.; Van den Berg, C.W.; et al. Analyzing Cell Type-Specific Dynamics of Metabolism in Kidney Repair. J. Am. Soc. Nephrol. 2022, 33, 13–14. [Google Scholar] [CrossRef]
- Vandereyken, K.; Sifrim, A.; Thienpont, B.; Voet, T. Methods and applications for single-cell and spatial multi-omics. Nat. Rev. Genet. 2023, 24, 494–515. [Google Scholar] [CrossRef]
- Zheng, Y.T.; Liu, Y.Q.; Yang, J.C.; Dong, L.H.; Zhang, R.; Tian, S.; Yu, Y.; Ren, L.Y.; Hou, W.W.; Zhu, F.; et al. Multi-omics data integration using ratio-based quantitative profiling with Quartet reference materials. Nat. Biotechnol. 2024, 42, 1133–1149. [Google Scholar] [CrossRef]
- Millar, M.W.; Fazal, F.; Rahman, A. Therapeutic Targeting of NF-κB in Acute Lung Injury: A Double-Edged Sword. Cells 2022, 11, 3317. [Google Scholar] [CrossRef]
- Bo, L.; Li, Y.; Liu, W.; Jin, F.; Li, C. Selective inhibition of JNK mitochondrial location is protective against seawater inhalation-induced ALI/ARDS. Mol. Med. Reports 2021, 24, 515. [Google Scholar] [CrossRef]
- Cao, L.; Song, H.; Zhou, S.; Lan, K.; Lv, K.; Huang, M. The STAT3 inhibitor B9 alleviates lipopolysaccharide-induced acute lung injury through its anti-inflammatory effects. Int. Immunopharmacol. 2024, 135, 112221. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Lou, S.; Zheng, F.; Gao, H.; Wang, N.; Tian, S.; Huang, G.; Zhao, H. Hydnocarpin D attenuates lipopolysaccharide-induced acute lung injury via MAPK/NF-κB and Keap1/Nrf2/HO−1 pathway. Phytomedicine 2022, 101, 154143. [Google Scholar] [CrossRef] [PubMed]
- Paris, A.J.; Hayer, K.E.; Oved, J.H.; Avgousti, D.C.; Toulmin, S.A.; Zepp, J.A.; Zacharias, W.J.; Katzen, J.B.; Basil, M.C.; Kremp, M.M.; et al. STAT3-BDNF-TrkB signalling promotes alveolar epithelial regeneration after lung injury. Nat. Cell Biol. 2020, 22, 1197–1210. [Google Scholar] [CrossRef]
- Cao, F.; Chen, G.; Xu, Y.; Wang, X.; Tang, X.; Zhang, W.; Song, X.; Yang, X.; Zeng, W.; Xie, J. METTL14 contributes to acute lung injury by stabilizing NLRP3 expression in an IGF2BP2-dependent manner. Cell Death Dis. 2024, 15, 43. [Google Scholar] [CrossRef]
- Wu, D.; Shi, Y.; Zhang, H.; Miao, C. Epigenetic mechanisms of Immune remodeling in sepsis: Targeting histone modification. Cell Death Dis. 2023, 14, 112. [Google Scholar] [CrossRef]
- Ren, L.; Chang, Y.-F.; Jiang, S.-H.; Li, X.-H.; Cheng, H.-P. DNA methylation modification in Idiopathic pulmonary fibrosis. Front. Cell Dev. Biol. 2024, 12, 1416325. [Google Scholar] [CrossRef]
- Zuttion, M.S.S.R.; Moore, S.K.L.; Chen, P.; Beppu, A.K.; Hook, J.L. New Insights into the Alveolar Epithelium as a Driver of Acute Respiratory Distress Syndrome. Biomolecules 2022, 12, 1273. [Google Scholar] [CrossRef]
- Rump, K.; Spellenberg, T.; von Busch, A.; Wolf, A.; Ziehe, D.; Thon, P.; Rahmel, T.; Adamzik, M.; Koos, B.; Unterberg, M. AQP5-1364A/C Polymorphism Affects AQP5 Promoter Methylation. Int. J. Mol. Sci. 2022, 23, 11813. [Google Scholar] [CrossRef]
- Lian, B.; Kawasaki, T.; Kano, N.; Ori, D.; Ikegawa, M.; Isotani, A.; Kawai, T. Regulation of Il6 expression by single CpG methylation in downstream of Il6 transcription initiation site. iScience 2022, 25, 104118. [Google Scholar] [CrossRef] [PubMed]
- Afthab, M.; Hambo, S.; Kim, H.; Alhamad, A.; Harb, H. Particulate matter-induced epigenetic modifications and lung complications. Eur. Respir. Rev. 2024, 33, 240129. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Liu, B.; Fu, T.; Liu, Y.; Li, B.; Li, N.; Geng, Q. The Role of Histone Deacetylases in Acute Lung Injury-Friend or Foe. Int. J. Mol. Sci. 2023, 24, 7876. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhang, Y.; Li, J.; Dang, Y.; Hu, D. Regulation of histone H3K27 methylation in inflammation and cancer. Mol. Biomed. 2025, 6, 14. [Google Scholar] [CrossRef]
- Dai, Y.; Chen, J.; Duan, Q. Epigenetic mechanism of EZH2-mediated histone methylation modification in regulating ferroptosis of alveolar epithelial cells in sepsis-induced acute lung injury. Drug Dev. Res. 2024, 85, e22263. [Google Scholar] [CrossRef]
- Seasock, M.J.; Shafiquzzaman, M.; Ruiz-Echartea, M.E.; Kanchi, R.S.; Tran, B.T.; Simon, L.M.; Meyer, M.D.; Erice, P.A.; Lotlikar, S.L.; Wenlock, S.C.; et al. Let-7 restrains an epigenetic circuit in AT2 cells to prevent fibrogenic intermediates in pulmonary fibrosis. Nat. Commun. 2025, 16, 4353. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, Y.; Zhang, L.; Zhao, H. MicroRNA-155 Participates in Smoke-Inhalation-Induced Acute Lung Injury through Inhibition of SOCS-1. Molecules 2020, 25, 1022. [Google Scholar] [CrossRef]
- Hawez, A.; Taha, D.; Algaber, A.; Madhi, R.; Rahman, M.; Thorlacius, H. MiR-155 regulates neutrophil extracellular trap formation and lung injury in abdominal sepsis. J. Leukoc. Biol. 2022, 111, 391–400. [Google Scholar] [CrossRef]
- Vaughn, A.E.; Lehmann, T.; Sul, C.; Wallbank, A.M.; Lyttle, B.D.; Bardill, J.; Burns, N.; Apte, A.; Nozik, E.S.; Smith, B.; et al. CNP-miR146a Decreases Inflammation in Murine Acute Infectious Lung Injury. Pharmaceutics 2023, 15, 2210. [Google Scholar] [CrossRef]
- Sucre, J.M.S.; Bock, F.; Negretti, N.M.; Benjamin, J.T.; Gulleman, P.M.; Dong, X.; Ferguson, K.T.; Jetter, C.S.; Han, W.; Liu, Y.; et al. Alveolar repair following LPS-induced injury requires cell-ECM interactions. JCI Insight 2023, 8, e167211. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Xie, N.; Banerjee, S.; Ge, J.; Guo, S.; Liu, G. Impairment of Fatty Acid Oxidation in Alveolar Epithelial Cells Mediates Acute Lung Injury. Am. J. Respir. Cell Mol. Biol. 2019, 60, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.-P.; Cheng, C.-N.; Chen, Y.-J.; Hsu, C.-L.; Huang, Y.-L.; Hsieh, M.-S.; Kuo, H.-C.; Lin, Y.-T.; Juan, Y.-H.; Nakahira, K.; et al. Alveolar epithelial cells mitigate neutrophilic inflammation in lung injury through regulating mitochondrial fatty acid oxidation. Nat. Commun. 2024, 15, 7241. [Google Scholar] [CrossRef] [PubMed]
- Vohwinkel, C.U.; Coit, E.J.; Burns, N.; Elajaili, H.; Hernandez-Saavedra, D.; Yuan, X.; Eckle, T.; Nozik, E.; Tuder, R.M.; Eltzschig, H.K. Targeting alveolar-specific succinate dehydrogenase A attenuates pulmonary inflammation during acute lung injury. FASEB J. 2021, 35, e21468. [Google Scholar] [CrossRef]
- Huang, H.; Li, G.; He, Y.; Chen, J.; Yan, J.; Zhang, Q.; Li, L.; Cai, X. Cellular succinate metabolism and signaling in inflammation: Implications for therapeutic intervention. Front. Immunol. 2024, 15, 1404441. [Google Scholar] [CrossRef]
- Strunz, M.; Simon, L.M.; Ansari, M.; Kathiriya, J.J.; Angelidis, I.; Mayr, C.H.; Tsidiridis, G.; Lange, M.; Mattner, L.F.; Yee, M.; et al. Alveolar regeneration through a Krt8+transitional stem cell state that persists in human lung fibrosis. Nat. Commun. 2020, 11, 3559. [Google Scholar] [CrossRef]
- Digiovanni, G.T.; Han, W.; Sherrill, T.P.; Taylor, C.J.; Nichols, D.S.; Geis, N.M.; Singha, U.K.; Calvi, C.L.; McCall, A.S.; Dixon, M.M.; et al. Epithelial Yap/Taz are required for functional alveolar regeneration following acute lung injury. JCI Insight 2023, 8, e173374. [Google Scholar] [CrossRef]
- Dudchenko, O.; Ordovas-Montanes, J.; Bingle, C.D. Respiratory epithelial cell types, states and fates in the era of single-cell RNA-sequencing. Biochem. J. 2023, 480, 921–939. [Google Scholar] [CrossRef]
- Nabhan, A.N.; Webster, J.D.; Adams, J.J.; Blazer, L.; Everrett, C.; Eidenschenk, C.; Arlantico, A.; Fleming, I.; Brightbill, H.D.; Wolters, P.J.; et al. Targeted alveolar regeneration with Frizzled-specific agonists. Cell 2023, 186, 2995–3012.e15. [Google Scholar] [CrossRef]
- Margaroli, C.; Benson, P.; Sharma, N.S.; Madison, M.C.; Robison, S.W.; Arora, N.; Ton, K.; Liang, Y.; Zhang, L.; Patel, R.P.; et al. Spatial mapping of SARS-CoV-2 and H1N1 lung injury identifies differential transcriptional signatures. Cell Rep. Med. 2021, 2, 100242. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, J.; Qin, C.; Liang, Y.; Chen, S.; Hei, F. Inflammatory alveolar macrophage-derived microvesicles damage lung epithelial cells and induce lung injury. Immunol. Lett. 2022, 241, 23–34. [Google Scholar] [CrossRef]
- Rendeiro, A.F.; Ravichandran, H.; Bram, Y.; Chandar, V.; Kim, J.; Meydan, C.; Park, J.; Foox, J.; Hether, T.; Warren, S.; et al. The spatial landscape of lung pathology during COVID-19 progression. Nature 2021, 593, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Franzen, L.; Lindvall, M.O.; Huhn, M.; Ptasinski, V.; Setyo, L.; Keith, B.P.; Collin, A.; Oag, S.; Volckaert, T.; Borde, A.; et al. Mapping spatially resolved transcriptomes in human and mouse pulmonary fibrosis. Nat. Genet. 2024, 56, 1725–1736. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Yin, J.; Zheng, Z.; Li, L.; Feng, X. Endothelial cell dynamics in sepsis-induced acute lung injury and acute respiratory distress syndrome: Pathogenesis and therapeutic implications. Cell Commun. Signal. 2024, 22, 241. [Google Scholar] [CrossRef]
- Price, D.R.; Garcia, J.G.N. A Razor’s Edge: Vascular Responses to Acute Inflammatory Lung Injury/Acute Respiratory Distress Syndrome. Annu. Rev. Physiol. 2024, 86, 505–529. [Google Scholar] [CrossRef] [PubMed]
- Korde, A.; Haslip, M.; Pednekar, P.; Khan, A.; Chioccioli, M.; Mehta, S.; Lopez-Giraldez, F.; Bermejo, S.; Rojas, M.; Dela Cruz, C.; et al. MicroRNA-1 protects the endothelium in acute lung injury. JCI Insight 2023, 8, e164816. [Google Scholar] [CrossRef]
- Wang, D.; Morales, J.E.; Calame, D.G.; Alcorn, J.L.; Wetsel, R.A. Transplantation of Human Embryonic Stem Cell-Derived Alveolar Epithelial Type II Cells Abrogates Acute Lung Injury in Mice. Mol. Ther. 2010, 18, 625–634. [Google Scholar] [CrossRef]
- Ziaka, M.; Exadaktylos, A. Gut-derived immune cells and the gut-lung axis in ARDS. Crit. Care 2024, 28, 220. [Google Scholar] [CrossRef]
- Dang, A.T.; Marsland, B.J. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol. 2019, 12, 843–850. [Google Scholar] [CrossRef]
- Han, B.; Chao, K.; Wang, D.; Sun, Y.; Ding, X.; Zhang, X.; Liu, S.; Du, J.; Luo, Y.; Wang, H.; et al. A purified membrane protein from Akkermansia muciniphila blunted the sepsis-induced acute lung injury by modulation of gut microbiota in rats. Int. Immunopharmacol. 2023, 121, 110432. [Google Scholar] [CrossRef]
- Shen, J.; Wang, S.; Xia, H.; Han, S.; Wang, Q.; Wu, Z.; Zhuge, A.; Li, S.; Chen, H.; Lv, L.; et al. Akkermansia muciniphila attenuated lipopolysaccharide-induced acute lung injury by modulating the gut microbiota and SCFAs in mice. Food Funct. 2023, 14, 10401–10417. [Google Scholar] [CrossRef]
- Wu, K.; Yuan, Y.; Yu, H.; Dai, X.; Wang, S.; Sun, Z.; Wang, F.; Fei, H.; Lin, Q.; Jiang, H.; et al. The gut microbial metabolite trimethylamine N-oxide aggravates GVHD by inducing M1 macrophage polarization in mice. Blood 2020, 136, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, Y.; Jin, S.; Lv, J.; Li, M.; Feng, N. The gut microbiota derived metabolite trimethylamine N-oxide: Its important role in cancer and other diseases. Biomed. Pharmacother. 2024, 177, 117031. [Google Scholar] [CrossRef] [PubMed]
- Gai, X.; Wang, H.; Li, Y.; Zhao, H.; He, C.; Wang, Z.; Zhao, H. Fecal Microbiota Transplantation Protects the Intestinal Mucosal Barrier by Reconstructing the Gut Microbiota in a Murine Model of Sepsis. Front. Cell. Infect. Microbiol. 2021, 11, 736204. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhou, Y.; Xu, J.; Zhang, Y.; Wang, H.; Zhao, C.; Huang, H.; Yang, J.; Huang, C.; Li, Y.; et al. Short-chain fatty acid-producing bacterial strains attenuate experimental ulcerative colitis by promoting M2 macrophage polarization via JAK/STAT3/FOXO3 axis inactivation. J. Transl. Med. 2024, 22, 369. [Google Scholar] [CrossRef]
- Faghfouri, A.H.; Zarezadeh, M.; Tavakoli-Rouzbehani, O.M.; Radkhah, N.; Faghfuri, E.; Kord-Varkaneh, H.; Tan, S.C.; Ostadrahimi, A. The effects of N-acetylcysteine on inflammatory and oxidative stress biomarkers: A systematic review and meta-analysis of controlled clinical trials. Eur. J. Pharmacol. 2020, 884, 173368. [Google Scholar] [CrossRef]
- Raghu, G.; Berk, M.; Campochiaro, P.A.; Jaeschke, H.; Marenzi, G.; Richeldi, L.; Wen, F.Q.; Nicoletti, F.; Calverley, P.M.A. The Multifaceted Therapeutic Role of N-Acetylcysteine (NAC) in Disorders Characterized by Oxidative Stress. Curr. Neuropharmacol. 2021, 19, 1202–1224. [Google Scholar] [CrossRef]
- Kang, J.-Y.; Xu, M.-M.; Sun, Y.; Ding, Z.-X.; Wei, Y.-Y.; Zhang, D.-W.; Wang, Y.-G.; Shen, J.-L.; Wu, H.-M.; Fei, G.-H. Melatonin attenuates LPS-induced pyroptosis in acute lung injury by inhibiting NLRP3-GSDMD pathway via activating Nrf2/HO-1 signaling axis. Int. Immunopharmacol. 2022, 109, 108782. [Google Scholar] [CrossRef]
- Atasever, A.; Tekin, S.; Bolat, I.; Bolat, M.; Dag, Y.; Cinar, B.; Sengul, E.; Yildirim, S.; Warda, M.; Celebi, F. The effects of melatonin on oxidative stress, inflammation, apoptosis and Nrf2/HO-1 in acrylamide-induced lung injury in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025. [Google Scholar] [CrossRef]
- Al-Harbi, N.O.; Imam, F.; Al-Harbi, M.M.; Ansari, M.A.; Zoheir, K.M.A.; Korashy, H.M.; Sayed-Ahmed, M.M.; Attia, S.M.; Shabanah, O.A.; Ahmad, S.F. Dexamethasone Attenuates LPS-induced Acute Lung Injury through Inhibition of NF-κB, COX-2, and Pro-inflammatory Mediators. Immunol. Investig. 2016, 45, 349–369. [Google Scholar] [CrossRef]
- Terzi, F.; Demirci, B.; Cinar, I.; Alhilal, M.; Erol, H.S. Effects of tocilizumab and dexamethasone on the downregulation of proinflammatory cytokines and upregulation of antioxidants in the lungs in oleic acid-induced ARDS. Respir. Res. 2022, 23, 249. [Google Scholar] [CrossRef]
- Cavalli, G.; De Luca, G.; Campochiaro, C.; Della-Torre, E.; Ripa, M.; Canetti, D.; Oltolini, C.; Castiglioni, B.; Din, C.T.; Boffini, N.; et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: A retrospective cohort study. Lancet Rheumatol. 2020, 2, E325–E331. [Google Scholar] [CrossRef]
- Kyriazopoulou, E.; Poulakou, G.; Milionis, H.; Metallidis, S.; Adamis, G.; Tsiakos, K.; Fragkou, A.; Rapti, A.; Damoulari, C.; Fantoni, M.; et al. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: A double-blind, randomized controlled phase 3 trial. Nat. Med. 2021, 27, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Lv, Y.; Xuan, R.; Han, P.; Xu, H.; Ma, X. Human placental mesenchymal stem cells transplantation repairs the alveolar epithelial barrier to alleviate lipopolysaccharides-induced acute lung injury. Biochem. Pharmacol. 2024, 229, 116547. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Cui, Y.; Xin, S.; Fu, Y.; Ding, Y.; Hao, L.; Nie, H. Mesenchymal stem cell conditioned medium alleviates acute lung injury through KGF-mediated regulation of epithelial sodium channels. Biomed. Pharmacother. 2023, 169, 115896. [Google Scholar] [CrossRef]
- Liu, P.; Yang, S.; Shao, X.; Li, C.; Wang, Z.; Dai, H.; Wang, C. Mesenchymal Stem Cells-Derived Exosomes Alleviate Acute Lung Injury By Inhibiting Alveolar Macrophage Pyroptosis. Stem Cells Transl. Med. 2024, 13, 371–386. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yang, J.; Fang, J.; Zhou, Y.; Candi, E.; Wang, J.; Hua, D.; Shao, C.; Shi, Y. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct. Target. Ther. 2022, 7, 92. [Google Scholar] [CrossRef]
- McLaughlin, C.; Datta, P.; Singh, Y.P.; Lo, A.; Horchler, S.; Elcheva, I.A.; Ozbolat, I.T.; Ravnic, D.J.; Koduru, S.V. Mesenchymal Stem Cell-Derived Extracellular Vesicles for Therapeutic Use and in Bioengineering Applications. Cells 2022, 11, 3366. [Google Scholar] [CrossRef]
- Chan, Y.K.; Wang, S.K.; Chu, C.J.; Copland, D.A.; Letizia, A.J.; Verdera, H.C.; Chiang, J.J.; Sethi, M.; Wang, M.K.; Neidermyer, W.J., Jr.; et al. Engineering adeno-associated viral vectors to evade innate immune and inflammatory responses. Sci. Transl. Med. 2021, 13, eabd3438. [Google Scholar] [CrossRef]
- Li, R.; Zeng, J.; Ren, T. Expression of DEL-1 in alveolar epithelial cells prevents lipopolysaccharide-induced inflammation, oxidative stress, and eosinophil recruitment in acute lung injury. Int. Immunopharmacol. 2022, 110, 108961. [Google Scholar] [CrossRef]
- Chen, K.; Han, H.; Zhao, S.; Xu, B.; Yin, B.; Lawanprasert, A.; Trinidad, M.; Burgstone, B.W.; Murthy, N.; Doudna, J.A. Lung and liver editing by lipid nanoparticle delivery of a stable CRISPR-Cas9 ribonucleoprotein. Nat. Biotechnol. 2024, 43, 1445–1457. [Google Scholar] [CrossRef] [PubMed]
- Alapati, D.; Zacharias, W.J.; Hartman, H.A.; Rossidis, A.C.; Stratigis, J.D.; Ahn, N.J.; Coons, B.; Zhou, S.; Li, H.; Singh, K.; et al. In utero gene editing for monogenic lung disease. Sci. Transl. Med. 2019, 11, eaav8375. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Zhang, C.; Lv, N.; Liang, Z.; Ma, T.; Cheng, H.; Xia, Y.; Shi, L. AdMSC-derived exosomes alleviate acute lung injury via transferring mitochondrial component to improve homeostasis of alveolar macrophages. Theranostics 2022, 12, 2928–2947. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xiao, K.; Xie, L. Advances in the use of exosomes for the treatment of ALI/ARDS. Front. Immunol. 2022, 13, 971189. [Google Scholar] [CrossRef]
- Jiang, K.; Yang, J.; Guo, S.; Zhao, G.; Wu, H.; Deng, G. Peripheral Circulating Exosome-Mediated Delivery of miR-155 as a Novel Mechanism for Acute Lung Inflammation. Mol. Ther. 2019, 27, 1758–1771. [Google Scholar] [CrossRef]
- Pei, Z.; Cen, J.; Zhang, X.; Gong, C.; Sun, M.; Meng, W.; Mao, G.; Wan, J.; Hu, B.; He, X.; et al. MiR-146a-5p delivered by hucMSC extracellular vesicles modulates the inflammatory response to sulfur mustard-induced acute lung injury. Stem Cell. Res. Ther. 2023, 14, 149. [Google Scholar] [CrossRef]
- Kim, G.; Lee, Y.; Ha, J.; Han, S.; Lee, M. Engineering exosomes for pulmonary delivery of peptides and drugs to inflammatory lung cells by inhalation. J. Control. Release 2021, 330, 684–695. [Google Scholar] [CrossRef]
- Cao, L.; Du, M.; Cai, M.; Feng, Y.; Miao, J.; Sun, J.; Song, J.; Du, B. Neutrophil membrane-coated nanoparticles for targeted delivery of toll-like receptor 4 siRNA ameliorate LPS-induced acute lung injury. Int. J. Pharm. 2025, 668, 124960. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, Y.; Wang, J.; Su, J.; Zhou, D.; Yu, X.; Xu, Y.; Yang, W. Application of Lung-Targeted Lipid Nanoparticle- delivered mRNA of soluble PD-L1 via SORT Technology in Acute Respiratory Distress Syndrome. Theranostics 2023, 13, 4974–4992. [Google Scholar] [CrossRef]
- Zheng, Z.; Qiao, X.; Yin, J.; Kong, J.; Han, W.; Qin, J.; Meng, F.; Tian, G.; Feng, X. Advancements in omics technologies: Molecular mechanisms of acute lung injury and acute respiratory distress syndrome (Review). Int. J. Mol. Med. 2025, 55, 38. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Yan, Z.-Z.; Luo, S.-D.; Hu, J.-J.; Wu, M.; Zhao, J.; Liu, W.-F.; Li, C.; Liu, K.-X. Gut microbiota-derived succinate aggravates acute lung injury after intestinal ischaemia/reperfusion in mice. Eur. Respir. J. 2023, 61, 2200840. [Google Scholar] [CrossRef]
- Jabaudon, M.; Blondonnet, R.; Roszyk, L.; Bouvier, D.; Audard, J.; Clairefond, G.; Fournier, M.; Marceau, G.; Dechelotte, P.; Pereira, B.; et al. Soluble Receptor for Advanced Glycation End-Products Predicts Impaired Alveolar Fluid Clearance in Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2015, 192, 191–199. [Google Scholar] [CrossRef]
- Song, Y.; Dou, H.; Li, X.; Zhao, X.; Li, Y.; Liu, D.; Ji, J.; Liu, F.; Ding, L.; Ni, Y.; et al. Exosomal miR-146a Contributes to the Enhanced Therapeutic Efficacy of Interleukin-1-Primed Mesenchymal Stem Cells Against Sepsis. Stem Cells 2017, 35, 1208–1221. [Google Scholar] [CrossRef]
- Habermann, A.C.; Gutierrez, A.J.; Bui, L.T.; Yahn, S.L.; Winters, N.I.; Calvi, C.L.; Peter, L.; Chung, M.-I.; Taylor, C.J.; Jetter, C.; et al. Single-cell RNA sequencing reveals profibrotic roles of distinct epithelial and mesenchymal lineages in pulmonary fibrosis. Sci. Adv. 2020, 6, eaba1972. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, Y.; Katsura, H.; Fujimura, T.; Ogata, A.; Baba, S.; Yamaoka, A.; Kihara, M.; Abe, T.; Nishimura, O.; Kadota, M.; et al. Autocrine TGF-β-positive feedback in profibrotic AT2-lineage cells plays a crucial role in non-inflammatory lung fibrogenesis. Nat. Commun. 2023, 14, 4956. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Wang, S.; Huang, Y.; Wu, Z.; Han, S.; Xia, H.; Chen, H.; Li, L. Lactobacillus reuteri Ameliorates Lipopolysaccharide-Induced Acute Lung Injury by Modulating the Gut Microbiota in Mice. Nutrients 2023, 15, 4256. [Google Scholar] [CrossRef]
- Li, F.; Wang, Z.; Cao, Y.; Pei, B.; Luo, X.; Liu, J.; Ge, P.; Luo, Y.; Ma, S.; Chen, H. Intestinal Mucosal Immune Barrier: A Powerful Firewall Against Severe Acute PancreatitisAssociated Acute Lung Injury via the Gut-Lung Axis. J. Inflamm. Res. 2024, 17, 2173–2193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Jian, Y.-P.; Zhang, Y.-N.; Li, Y.; Gu, L.-T.; Sun, H.-H.; Liu, M.-D.; Zhou, H.-L.; Wang, Y.-S.; Xu, Z.-X. Short-chain fatty acids in diseases. Cell Commun. Signal. 2023, 21, 212. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, M.T.; Han, J.H. GPR41 and GPR43: From development to metabolic regulation. Biomed. Pharmacother. 2024, 175, 116735. [Google Scholar] [CrossRef]
- Hua, F.; Cui, E.; Lv, L.; Wang, B.; Li, L.; Lu, H.; Chen, N.; Chen, W. Fecal microbiota transplantation from HUC-MSC-treated mice alleviates acute lung injury in mice through anti-inflammation and gut microbiota modulation. Front. Microbiol. 2023, 14, 1243102. [Google Scholar] [CrossRef]
- Xiong, Y.; Brinkman, C.C.; Hittle, L.E.; Fricke, W.F.; Mongodin, E.F.; Bromberg, J.S. Gut Microbiota Determines Graft Survival Outcome Through Regulation of Anti-Inflammatory and Pro-Inflammatory Responses. Transplantation 2018, 102, S333. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, J.; Woods, P.S.; Chesarino, N.M.; Liu, C.; Lee, L.J.; Nana-Sinkam, S.P.; Davis, I.C. Selective targeting of alveolar type II respiratory epithelial cells by anti-surfactant protein-C antibody-conjugated lipoplexes. J. Control. Release 2015, 203, 140–149. [Google Scholar] [CrossRef]
- Wan, Q.; Zhang, X.; Zhou, D.; Xie, R.; Cai, Y.; Zhang, K.; Sun, X. Inhaled nano-based therapeutics for pulmonary fibrosis: Recent advances and future prospects. J. Nanobiotechnol. 2023, 21, 215. [Google Scholar] [CrossRef]
- Huang, K.; Liu, Y.; Miao, H.; Li, Y.; Fan, Q.; Zhang, H.; Zuo, C.; Zhu, J.; Zheng, Q.; Deng, C.; et al. Nebulized Lipid Nanoparticles Based on Degradable Ionizable Glycerolipid for Potent Pulmonary mRNA Delivery. ACS Nano 2025, 19, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Dagdas, Y.S.; Kleinstiver, B.P.; Welch, M.M.; Sousa, A.A.; Harrington, L.B.; Sternberg, S.H.; Joung, K.J.; Yildiz, A.; Doudna, J.A. Enhanced Proofreading Governs CRISPR-Cas9 Targeting Accuracy. Biophys. J. 2018, 114, 194A. [Google Scholar] [CrossRef]
- Zhang, L.; He, W.; Fu, R.; Xu, H. Guide-specific loss of efficiency and off-target reduction with Cas9 variants. bioRxiv 2023. [Google Scholar] [CrossRef]
- Tyumentseva, M.; Tyumentsev, A.; Akimkin, V. CRISPR/Cas9 Landscape: Current State and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 16077. [Google Scholar] [CrossRef]
- Chuai, G.; Ma, H.; Yan, J.; Chen, M.; Hong, N.; Xue, D.; Zhou, C.; Zhu, C.; Chen, K.; Duan, B.; et al. DeepCRISPR: Optimized CRISPR guide RNA design by deep learning. Genome Biol. 2018, 19, 80. [Google Scholar] [CrossRef]
- Dunn, C.M.; Kameishi, S.; Grainger, D.W.; Okano, T. Strategies to address mesenchymal stem/stromal cell heterogeneity in immunomodulatory profiles to improve cell-based therapies. Acta Biomater. 2021, 133, 114–125. [Google Scholar] [CrossRef]
- Chen, T.; Ye, B.; Tan, J.; Yang, H.; He, F.; Khalil, R. CD146+Mesenchymal stem cells treatment improves vascularization, muscle contraction and VEGF expression, and reduces apoptosis in rat ischemic hind limb. Biochem. Pharmacol. 2021, 190, 114530. [Google Scholar] [CrossRef]
- Terheyden-Keighley, D.; Huehne, M.; Berger, T.; Hiller, B.; Martins, S.; Gamerschlag, A.; Sabour, D.; Meffert, A.; Kislat, A.; Slotta, C.; et al. GMP-compliant iPS cell lines show widespread plasticity in a new set of differentiation workflows for cell replacement and cancer immunotherapy. Stem Cells Transl. Med. 2024, 13, 898–911. [Google Scholar] [CrossRef]
- Olivier, M.; Asmis, R.; Hawkins, G.A.; Howard, T.D.; Cox, L.A. The Need for Multi-Omics Biomarker Signatures in Precision Medicine. Int. J. Mol. Sci. 2019, 20, 4781. [Google Scholar] [CrossRef]
- Nicora, G.; Vitali, F.; Dagliati, A.; Geifman, N.; Bellazzi, R. Integrated Multi-Omics Analyses in Oncology: A Review of Machine Learning Methods and Tools. Front. Oncol. 2020, 10, 1030. [Google Scholar] [CrossRef]
- Reel, P.S.; Reel, S.; Pearson, E.; Trucco, E.; Jefferson, E. Using machine learning approaches for multi-omics data analysis: A review. Biotechnol. Adv. 2021, 49, 107739. [Google Scholar] [CrossRef]
- Wei, X.; Shao, B.; He, Z.; Ye, T.; Luo, M.; Sang, Y.; Liang, X.; Wang, W.; Luo, S.; Yang, S.; et al. Cationic nanocarriers induce cell necrosis through impairment of Na+/K+-ATPase and cause subsequent inflammatory response. Cell Res. 2015, 25, 237–253. [Google Scholar] [CrossRef]
- Tenchov, R.; Sasso, J.M.; Wang, X.; Liaw, W.-S.; Chen, C.-A.; Zhou, Q.A. Exosomes-Nature’s Lipid Nanoparticles, a Rising Star in Drug Delivery and Diagnostics. ACS Nano 2022, 16, 17802–17846. [Google Scholar] [CrossRef]
- Zhang, M.; Zang, X.; Wang, M.; Li, Z.; Qiao, M.; Hu, H.; Chen, D. Exosome-based nanocarriers as bio-inspired and versatile vehicles for drug delivery: Recent advances and challenges. J. Mater. Chem. B 2019, 7, 2421–2433. [Google Scholar] [CrossRef]
- Moreno, A.M.; Palmer, N.; Aleman, F.; Chen, G.; Pla, A.; Jiang, N.; Chew, W.L.; Law, M.; Mali, P. Immune-orthogonal orthologues of AAV capsids and of Cas9 circumvent the immune response to the administration of gene therapy. Nat. Biomed. Eng. 2019, 3, 842. [Google Scholar] [CrossRef]
- Shirley, J.L.; de Jong, Y.P.; Terhorst, C.; Herzog, R.W. Immune Responses to Viral Gene Therapy Vectors. Mol. Ther. 2020, 28, 709–722. [Google Scholar] [CrossRef]
- Zuo, E.; Sun, Y.; Wei, W.; Yuan, T.; Ying, W.; Sun, H.; Yuan, L.; Steinmetz, L.M.; Li, Y.; Yang, H. Cytosine base editor generates substantial off-target single-nucleotide variants in mouse embryos. Science 2019, 364, 289–292. [Google Scholar] [CrossRef]
- Akhavanfard, S.; Padmanabhan, R.; Yehia, L.; Cheng, F.; Eng, C. Comprehensive germline genomic profiles of children, adolescents and young adults with solid tumors. Nat. Commun. 2020, 11, 2206. [Google Scholar] [CrossRef]
- Schleidgen, S.; Dederer, H.-G.; Sgodda, S.; Cravcisin, S.; Lueneburg, L.; Cantz, T.; Heinemann, T. Human germline editing in the era of CRISPR-Cas: Risk and uncertainty, inter-generational responsibility, therapeutic legitimacy. BMC Med. Ethics 2020, 21, 87. [Google Scholar] [CrossRef]
- Saha, K.; Sontheimer, E.J.; Brooks, P.J.; Dwinell, M.R.; Gersbach, C.A.; Liu, D.R.; Murray, S.A.; Tsai, S.Q.; Wilson, R.C.; Anderson, D.G.; et al. The NIH Somatic Cell Genome Editing program. Nature 2021, 592, 195–204. [Google Scholar] [CrossRef]
- Irfan, M.; Majeed, H.; Iftikhar, T.; Ravi, P.K. A review on molecular scissoring with CRISPR/Cas9 genome editing technology. Toxicol. Res. 2024, 13, tfae105. [Google Scholar] [CrossRef]
- Kumar, A.D.; Atallah-Yunes, S.A.; Rajeeve, S.; Abdelhak, A.; Hashmi, H.; Corraes, A.; Htut, M.; Rodriguez-Otero, P.; Parrondo, R.D.; Chhabra, S.; et al. Delayed Neurotoxicity after CAR-T in Multiple Myeloma: Results from a Global IMWG Registry. Blood 2024, 144, 4758–4760. [Google Scholar] [CrossRef]
- Yang, Y.; Li, L.; Tian, J.; Ma, L.; Wu, Y.; Luo, Q.; Luo, Y. Delayed immune-related adverse events profile associated with immune checkpoint inhibitors: A real-world analysis. Front. Pharmacol. 2024, 15, 1453429. [Google Scholar] [CrossRef]
- Bovard, D.; Sandoz, A.; Luettich, K.; Frentzel, S.; Iskandar, A.; Marescotti, D.; Trivedi, K.; Guedj, E.; Dutertre, Q.; Peitsch, M.C.; et al. A lung/liver-on-a-chip platform for acute and chronic toxicity studies. Lab Chip 2018, 18, 3814–3829. [Google Scholar] [CrossRef]
- Warren, R.; Lyu, H.; Klinkhammer, K.; De Langhe, S.P. Hippo signaling impairs alveolar epithelial regeneration in pulmonary fibrosis. Elife 2023, 12, e85092. [Google Scholar] [CrossRef]
- Choi, J.; Park, J.-E.; Tsagkogeorga, G.; Yanagita, M.; Koo, B.-K.; Han, N.; Lee, J.-H. Inflammatory Signals Induce AT2 Cell-Derived Damage-Associated Transient Progenitors that Mediate Alveolar Regeneration. Cell Stem Cell 2020, 27, 366–382. [Google Scholar] [CrossRef]
- Jin, C.; Chen, Y.; Wang, Y.; Li, J.; Liang, J.; Zheng, S.; Zhang, L.; Li, Q.; Wang, Y.; Ling, F.; et al. Single-cell RNA sequencing reveals special basal cells and fibroblasts in idiopathic pulmonary fibrosis. Sci. Rep. 2024, 14, 15778. [Google Scholar] [CrossRef]
- Mayr, C.H.; Santacruz, D.; Jarosch, S.; Bleck, M.; Dalton, J.; McNabola, A.; Lempp, C.; Neubert, L.; Rath, B.; Kamp, J.C.; et al. Spatial transcriptomic characterization of pathologic niches in IPF. Sci. Adv. 2024, 10, eadl5473. [Google Scholar] [CrossRef]
- Blumhagen, R.Z.; Kurche, J.S.; Cool, C.D.; Walts, A.D.; Heinz, D.; Fingerlin, T.E.; Yang, I.V.; Schwartz, D.A. Spatially distinct molecular patterns of gene expression in idiopathic pulmonary fibrosis. Respir. Res. 2023, 24, 287. [Google Scholar] [CrossRef]
- Tiskratok, W.; Chuinsiri, N.; Limraksasin, P.; Kyawsoewin, M.; Jitprasertwong, P. Extracellular Matrix Stiffness: Mechanotransduction and Mechanobiological Response-Driven Strategies for Biomedical Applications Targeting Fibroblast Inflammation. Polymers 2025, 17, 822. [Google Scholar] [CrossRef]
- Panciera, T.; Azzolin, L.; Cordenonsi, M.; Piccolo, S. Mechanobiology of YAP and TAZ in physiology and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 758–770. [Google Scholar] [CrossRef]
- Chen, W.; Zhu, Y.; Liu, R.; Kong, B.; Xia, N.; Zhao, Y.; Sun, L. Screening Therapeutic Effects of MSC-EVs to Acute Lung Injury Model on A Chip. Adv. Healthc. Mater. 2024, 13, 2303123. [Google Scholar] [CrossRef]
- Wong, J.F.; Simmons, C.A. Microfluidic assay for the on-chip electrochemical measurement of cell monolayer permeability. Lab Chip 2019, 19, 1060–1070. [Google Scholar] [CrossRef]
- Yousafzai, M.S.; Hammer, J.A. Using Biosensors to Study Organoids, Spheroids and Organs-on-a-Chip: A Mechanobiology Perspective. Biosensors 2023, 13, 905. [Google Scholar] [CrossRef]
- Ding, S.; Zhang, H.; Wang, X. Microfluidic-Chip-Integrated Biosensors for Lung Disease Models. Biosensors 2021, 11, 456. [Google Scholar] [CrossRef]
- Liu, T.; Salguero, P.; Petek, M.; Martinez-Mira, C.; Balzano-Nogueira, L.; Ramsak, Z.; McIntyre, L.; Gruden, K.; Tarazona, S.; Conesa, A. PaintOmics 4: New tools for the integrative analysis of multi-omics datasets supported by multiple pathway databases. Nucleic Acids Res. 2022, 50, W551–W559. [Google Scholar] [CrossRef]
- Yaqub, N.; Wayne, G.; Birchall, M.; Song, W. Recent advances in human respiratory epithelium models for drug discovery. Biotechnol. Adv. 2022, 54, 107832. [Google Scholar] [CrossRef]
- Zhuang, C.; Kang, M.; Lee, M. Delivery systems of therapeutic nucleic acids for the treatment of acute lung injury/acute respiratory distress syndrome. J. Control. Release 2023, 360, 1–14. [Google Scholar] [CrossRef]
- Lin, P.; Gao, R.; Fang, Z.; Yang, W.; Tang, Z.; Wang, Q.; Wu, Y.; Fang, J.; Yu, W. Precise nanodrug delivery systems with cell-specific targeting for ALI/ARDS treatment. Int. J. Pharm. 2023, 644, 123321. [Google Scholar] [CrossRef]
- Gu, Z.; Sun, M.; Liu, J.; Huang, Q.; Wang, Y.; Liao, J.; Shu, T.; Tao, M.; Mao, G.; Pei, Z.; et al. Endothelium-Derived Engineered Extracellular Vesicles Protect the Pulmonary Endothelial Barrier in Acute Lung Injury. Adv. Sci. 2024, 11, 2306156. [Google Scholar] [CrossRef] [PubMed]
- Tiroille, V.; Krug, A.; Bokobza, E.; Kahi, M.; Bulcaen, M.; Ensinck, M.M.; Geurts, M.H.; Hendriks, D.; Vermeulen, F.; Larbret, F.; et al. Nanoblades allow high-level genome editing in murine and human organoids. Mol. Ther.-Nucleic Acids 2023, 33, P57–P74. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.V.T.; Ye, S.; Gkouzioti, V.; van Wolferen, M.E.; Yengej, F.Y.; Melkert, D.; Siti, S.; de Jong, B.; Besseling, P.J.; Spee, B.; et al. A human kidney and liver organoid-based multi-organ-on-a-chip model to study the therapeutic effects and biodistribution of mesenchymal stromal cell-derived extracellular vesicles. J. Extracell. Vesicles 2022, 11, 12280. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Gately, L.; Lok, S.W.; Tran, B.; Lee, M.; Wong, R.; Markman, B.; Dunn, K.; Wong, V.; Loft, M.; et al. Leveraging Comprehensive Cancer Registry Data to Enable a Broad Range of Research, Audit and Patient Support Activities. Cancers 2022, 14, 4131. [Google Scholar] [CrossRef]
- Prang, K.-H.; Karanatsios, B.; Verbunt, E.; Wong, H.-L.; Yeung, J.; Kelaher, M.; Gibbs, P. Clinical registries data quality attributes to support registry-based randomised controlled trials: A scoping review. Contemp. Clin. Trials 2022, 119, 106843. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).