Three-Dimensional Cartilage Tissue Engineering Using Placenta-Derived Extra-Embryonic Mesenchymal Stem Cells: From Isolation to Differentiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Culture of Human Placenta-Derived MSCs
2.2. MSC Isolation Protocols
2.2.1. Placental Dissection and Preprocessing
2.2.2. Trypsin with Collagenase Protocol
2.2.3. Trypsin-Only Protocol
2.3. Subculture and Expansion of MSCs
2.4. Characterization of MSC
2.5. Two-Dimensional and Three-Dimensional Chondrogenic Differentiation of Mesenchymal Stem Cells
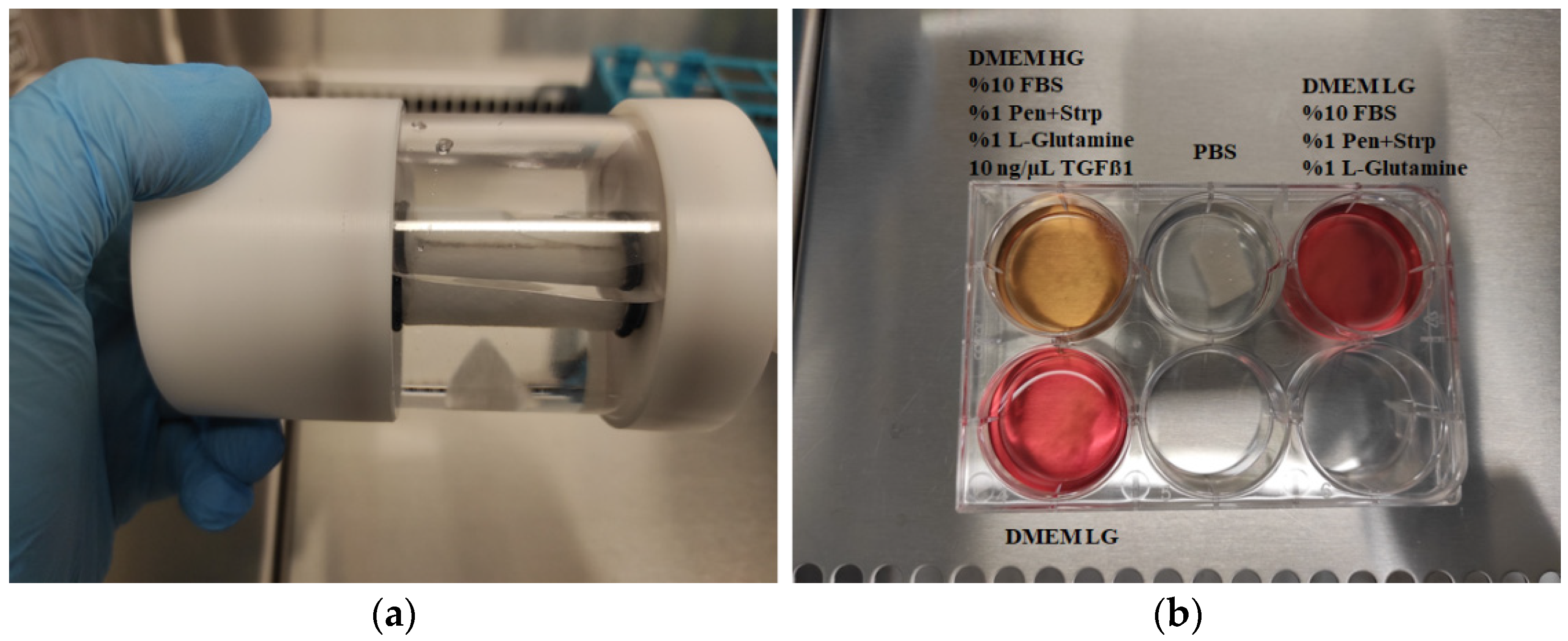
2.5.1. Two-Dimensional Culture
2.5.2. Three-Dimensional Culture
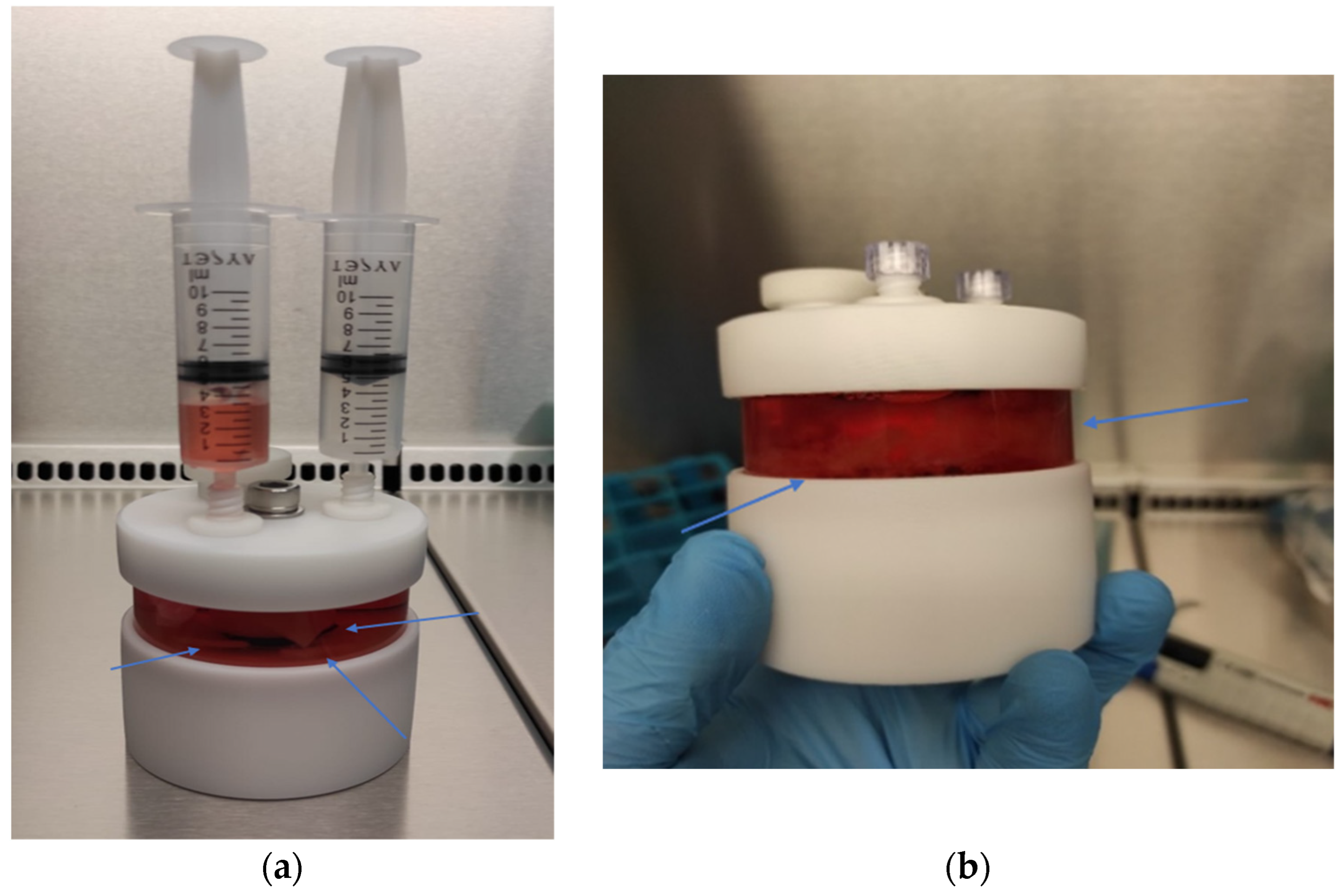
2.6. Three-Dimensional Chondrocyte Cultures Using PLGA Scaffolds
2.6.1. Preparation of PLGA Sheets
2.6.2. Static 3D Cell Culture of Chondrocytes
2.6.3. Dynamic 3D Cell Culture of Chondrocytes
2.6.4. Three-Dimensional Cell Culture for PLGA Degradation
3. Results
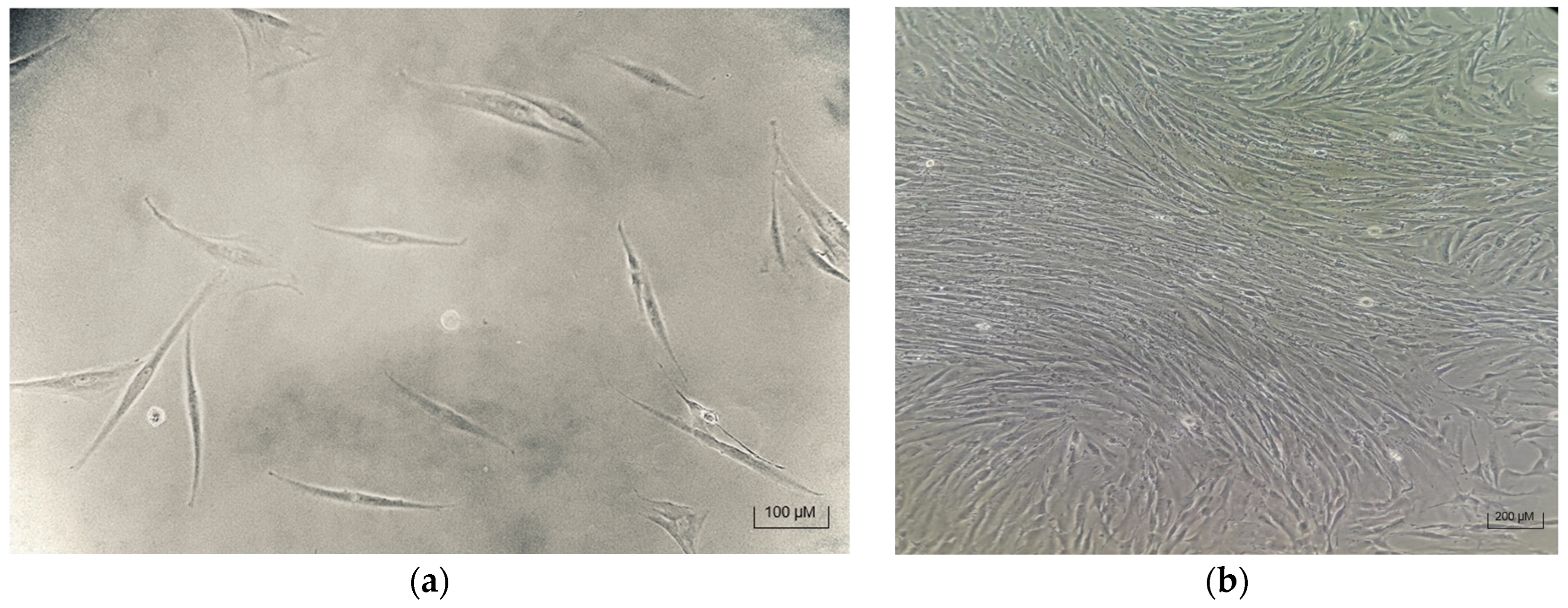
3.1. Successful MSC Isolation and Attachment
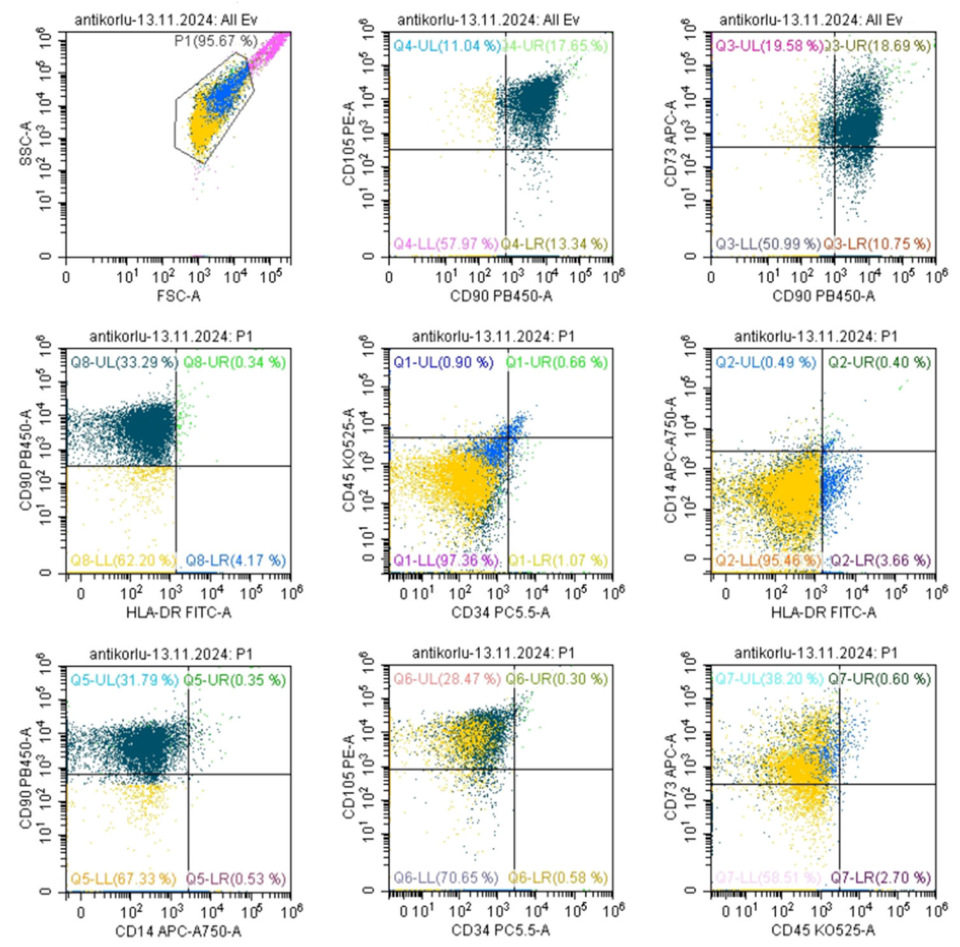
3.2. MSC Characterization—Marker Expression Analysis
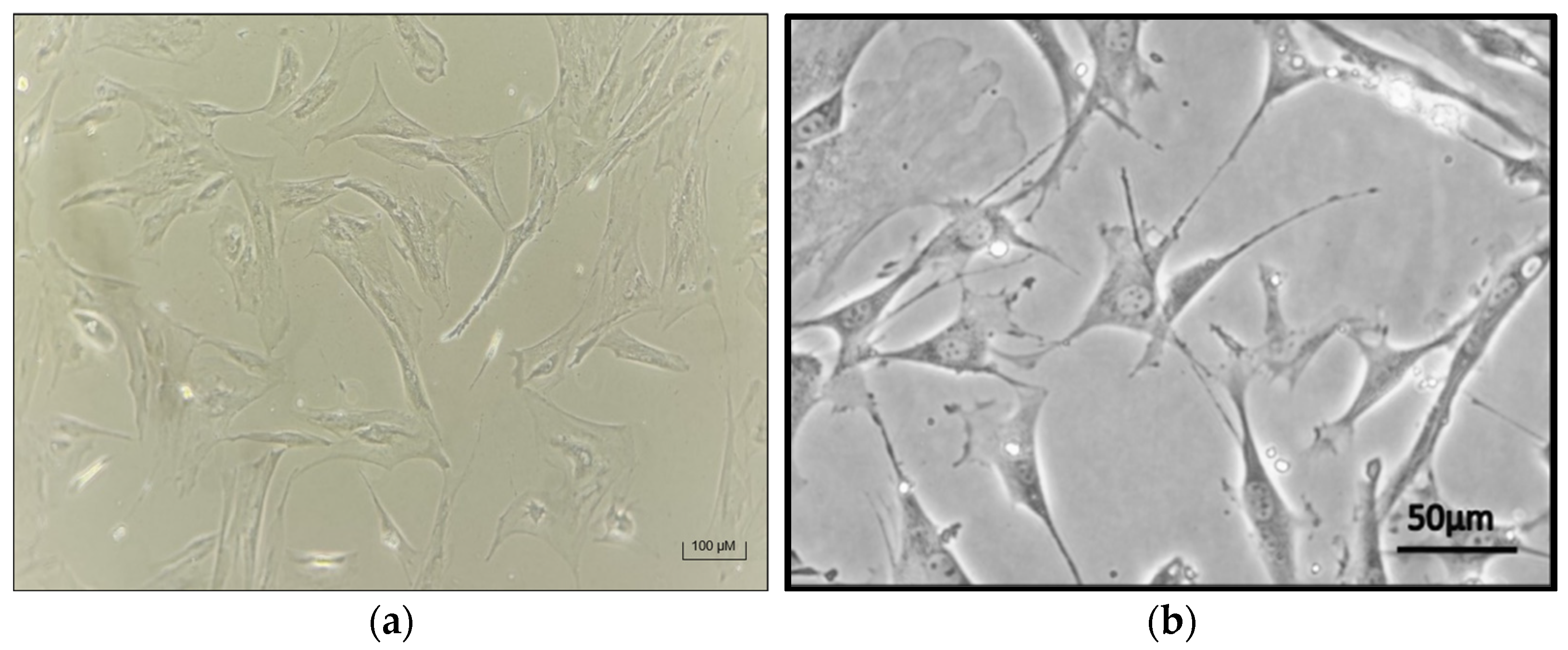
3.3. Successful Chondrocyte Differentiation in 2D and 3D Cultures
3.4. PLGA Biopolymer Degradation Timeline
3.5. RCCS-Mediated PLGA Biopolymer Degradation
3.6. Assessing ECM Formation in Chondrogenic Tissue
3.7. Biopolymer Degradation in 3D Cell Cultures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MSC | Mesenchymal Stem Cell |
| 2D | 2-Dimensional |
| 3D | 3-Dimensional |
| PGA | Poly(glycolic acid) |
| PLA | Poly(lactic acid) |
| PLGA | Poly(lactic-co-glycolic acid) |
| PCL | Polycaprolactone |
| RCCS | Rotary Cell Culture Systems |
| ECM | Extracellular Matrix |
| STLV | Slow-Turning Lateral Vessel |
| PBS | Phosphate-Buffered Saline |
| FBS | Fetal Bovine Serum |
| H&E | Hematoxylin and Eosin |
| TGF-β1 | Transforming Growth Factor beta-1 |
| GAG | Glycosaminoglycan |
| RWV | Rotating wall vessel |
References
- Huang, J.; Liu, Q.; Xia, J.; Chen, X.; Xiong, J.; Yang, L.; Liang, Y. Modification of mesenchymal stem cells for cartilage-targeted therapy. J. Transl. Med. 2022, 20, 515. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.M.; Pham, P.T.; Bach, T.Q.; Ngo, A.T.L.; Nguyen, Q.T.; Phan, T.T.K.; Nguyen, G.H.; Le, P.T.T.; Hoang, V.T.; Forsyth, N.R.; et al. Stem cell-based therapy for human diseases. Signal Transduct. Target. Ther. 2022, 7, 272. [Google Scholar] [CrossRef] [PubMed]
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-derived stem cells for regenerative medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef]
- Gnecchi, M.; Melo, L.G. Bone marrow-derived mesenchymal stem cells: Isolation, expansion, characterization, viral transduction, and production of conditioned medium. Methods Mol. Biol. 2009, 482, 281–294. [Google Scholar] [CrossRef]
- Jiang, Y.; Cai, Y.; Zhang, W.; Yin, Z.; Hu, C.; Tong, T.; Lu, P.; Zhang, S.; Neculai, D.; Tuan, R.S.; et al. Human Cartilage-Derived Progenitor Cells from Committed Chondrocytes for Efficient Cartilage Repair and Regeneration. Stem Cells Transl. Med. 2016, 5, 733–744. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- González, P.L.; Carvajal, C.; Cuenca, J.; Alcayaga-Miranda, F.; Figueroa, F.E.; Bartolucci, J.; Salazar-Aravena, L.; Khoury, M. Chorion Mesenchymal Stem Cells Show Superior Differentiation, Immunosuppressive, and Angiogenic Potentials in Comparison with Haploidentical Maternal Placental Cells. Stem Cells Transl. Med. 2015, 4, 1109–1121. [Google Scholar] [CrossRef]
- Perry, B.C.; Zhou, D.; Wu, X.; Yang, F.C.; Byers, M.A.; Chu, T.M.; Hockema, J.J.; Woods, E.J.; Goebel, W.S. Collection, cryopreservation, and characterization of human dental pulp-derived mesenchymal stem cells for banking and clinical use. Tissue Eng. Part C Methods 2008, 14, 149–156. [Google Scholar] [CrossRef]
- Barlow, S.; Brooke, G.; Chatterjee, K.; Price, G.; Pelekanos, R.; Rossetti, T.; Doody, M.; Venter, D.; Pain, S.; Gilshenan, K.; et al. Comparison of human placenta- and bone marrow-derived multipotent mesenchymal stem cells. Stem Cells Dev. 2008, 17, 1095–1107. [Google Scholar] [CrossRef]
- Biswas, A.; Rajasekaran, R.; Saha, B.; Dixit, K.; Vaidya, P.V.; Ojha, A.K.; Dhara, S. Human placenta/umbilical cord derivatives in regenerative medicine—Prospects and challenges. Biomater. Sci. 2023, 11, 4789–4821. [Google Scholar] [CrossRef]
- Choi, Y.S.; Park, Y.B.; Ha, C.W.; Kim, J.A.; Heo, J.C.; Han, W.J.; Oh, S.Y.; Choi, S.J. Different characteristics of mesenchymal stem cells isolated from different layers of full term placenta. PLoS ONE 2017, 12, e0172642. [Google Scholar] [CrossRef]
- Nahian, A.; Sapra, A. Histology, Chondrocytes. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Carballo, C.B.; Nakagawa, Y.; Sekiya, I.; Rodeo, S.A. Basic Science of Articular Cartilage. Clin. Sports Med. 2017, 36, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Brody, L.T. Knee osteoarthritis: Clinical connections to articular cartilage structure and function. Phys. Ther. Sport 2015, 16, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Mancini, I.A.D.; Vindas Bolaños, R.A.; Brommer, H.; Castilho, M.; Ribeiro, A.; van Loon, J.; Mensinga, A.; van Rijen, M.H.P.; Malda, J.; van Weeren, R. Fixation of Hydrogel Constructs for Cartilage Repair in the Equine Model: A Challenging Issue. Tissue Eng. Part C Methods 2017, 23, 804–814. [Google Scholar] [CrossRef]
- Morouço, P.; Fernandes, C.; Santos-Rocha, R. Osteoarthritis, Exercise, and Tissue Engineering: A Stimulating Triad for Health Professionals. J. Aging Res. 2019, 2019, 1935806. [Google Scholar] [CrossRef]
- Hiemer, B.; Krogull, M.; Zander, K.; Gruttner, C.; Bergschmidt, P.; Tischer, Y.; Wree, A.; Bader, R.; Pasold, J. Chondrogenic differentiation of human chondrocytes and stem cells in different cell culture systems using IGF-1-coupled particles. J. Tissue Sci. Eng. 2017, 8, 1000203. [Google Scholar]
- Carriel, V.; Geuna, S.; Alaminos, M. Ex Vivo and In Vivo Stem Cells-Based Tissue Engineering Strategies for Their Use in Regenerative Medicine. Stem Cells Int. 2018, 2018, 7143930. [Google Scholar] [CrossRef]
- Klangjorhor, J.; Phitak, T.; Pruksakorn, D.; Pothacharoen, P.; Kongtawelert, P. Comparison of growth factor adsorbed scaffold and conventional scaffold with growth factor supplemented media for primary human articular chondrocyte 3D culture. BMC Biotechnol. 2014, 14, 108. [Google Scholar] [CrossRef]
- Darling, E.M.; Athanasiou, K.A. Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J. Orthop. Res. 2005, 23, 425–432. [Google Scholar] [CrossRef]
- van der Kraan, P.M. Differential Role of Transforming Growth Factor-beta in an Osteoarthritic or a Healthy Joint. J. Bone Metab. 2018, 25, 65–72. [Google Scholar] [CrossRef]
- Pfeifer, C.G.; Karl, A.; Kerschbaum, M.; Berner, A.; Lang, S.; Schupfner, R.; Koch, M.; Angele, P.; Nerlich, M.; Mueller, M.B. TGF-β Signalling is Suppressed under Pro-Hypertrophic Conditions in MSC Chondrogenesis Due to TGF-β Receptor Downregulation. Int. J. Stem Cells 2019, 12, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Diao, H.; Wang, J.; Shen, C.; Xia, S.; Guo, T.; Dong, L.; Zhang, C.; Chen, J.; Zhao, J.; Zhang, J. Improved cartilage regeneration utilizing mesenchymal stem cells in TGF-β1 gene-activated scaffolds. Tissue Eng. Part A 2009, 15, 2687–2698. [Google Scholar] [CrossRef] [PubMed]
- MacBarb, R.F.; Makris, E.A.; Hu, J.C.; Athanasiou, K.A. A chondroitinase-ABC and TGF-β1 treatment regimen for enhancing the mechanical properties of tissue-engineered fibrocartilage. Acta Biomater. 2013, 9, 4626–4634. [Google Scholar] [CrossRef] [PubMed]
- Tangyuenyong, S.; Kongdang, P.; Sirikaew, N.; Ongchai, S. First study on the effect of transforming growth factor beta 1 and insulin-like growth factor 1 on the chondrogenesis of elephant articular chondrocytes in a scaffold-based 3D culture model. Vet. World 2022, 15, 1869–1879. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, H.; Dong, W.; Bai, J.; Gao, B.; Xia, D.; Feng, B.; Chen, M.; He, X.; Yin, M.; et al. Tissue-engineered trachea from a 3D-printed scaffold enhances whole-segment tracheal repair. Sci. Rep. 2017, 7, 5246. [Google Scholar] [CrossRef]
- Jiang, T.; Kai, D.; Liu, S.; Huang, X.; Heng, S.; Zhao, J.; Chan, B.Q.Y.; Loh, X.J.; Zhu, Y.; Mao, C.; et al. Mechanically cartilage-mimicking poly(PCL-PTHF urethane)/collagen nanofibers induce chondrogenesis by blocking NF-kappa B signaling pathway. Biomaterials 2018, 178, 281–292. [Google Scholar] [CrossRef]
- Maadani, A.M.; Salahinejad, E. Performance comparison of PLA- and PLGA-coated porous bioceramic scaffolds: Mechanical, biodegradability, bioactivity, delivery and biocompatibility assessments. J. Control. Release 2022, 351, 1–7. [Google Scholar] [CrossRef]
- Corrò, C.; Novellasdemunt, L.; Li, V.S. A brief history of organoids. Am. J. Physiol. Cell Physiol. 2020, 319, C151–C165. [Google Scholar] [CrossRef]
- Sittinger, M. Tissue engineering: Artificial tissue replacement containing vital components. Laryngorhinootologie 1995, 74, 695–699. [Google Scholar] [CrossRef]
- Bacakova, L.; Zarubova, J.; Travnickova, M.; Musilkova, J.; Pajorova, J.; Slepicka, P.; Kasalkova, N.S.; Svorcik, V.; Kolska, Z.; Motarjemi, H. Stem cells: Their source, potency and use in regenerative therapies with focus on adipose-derived stem cells–A review. Biotechnol. Adv. 2018, 36, 1111–1126. [Google Scholar] [CrossRef]
- Kim, J.; Koo, B.-K.; Knoblich, J.A. Human organoids: Model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 2020, 21, 571–584. [Google Scholar] [CrossRef]
- Kim, M.B.; Hwangbo, S.; Jang, S.; Jo, Y.K. Bioengineered Co-culture of organoids to recapitulate host-microbe interactions. Mater. Today Bio 2022, 16, 100345. [Google Scholar] [CrossRef]
- Bisgin, A.; Mujde, C. Bioreactor-Based Tissue Models as an Alternative Approach in Cancer Research. In Handbook of Animal Models and Its Uses in Cancer Research; Pathak, S., Banerjee, A., Bisgin, A., Eds.; Springer Nature: Singapore, 2022; pp. 1–16. [Google Scholar]
- Duval, K.; Grover, H.; Han, L.H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef]
- Erickson, L.E. Bioreactors for commodity products. In Reference Module in Life Sciences: Comprehensive Biotechnology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 3, pp. 683–689. [Google Scholar] [CrossRef]
- Vandermies, M.; Fickers, P. Bioreactor-scale strategies for the production of recombinant protein in the yeast Yarrowia lipolytica. Microorganisms 2019, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Licata, J.P.; Schwab, K.H.; Har-El, Y.E.; Gerstenhaber, J.A.; Lelkes, P.I. Bioreactor Technologies for Enhanced Organoid Culture. Int. J. Mol. Sci. 2023, 24, 11427. [Google Scholar] [CrossRef] [PubMed]
- Steimberg, N.; Angiero, F.; Farronato, D.; Berenzi, A.; Cossellu, G.; Ottonello, A.; Kaigler, D.; Mazzoleni, G. Advanced 3D Models Cultured to Investigate Mesenchymal Stromal Cells of the Human Dental Follicle. Tissue Eng. Part C Methods 2018, 24, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, P.; Torres, M.J.; Arancibia, R.; Aulestia, F.; Vergara, M.; Carrión, F.; Osses, N.; Altamirano, C. Dynamic Culture of Mesenchymal Stromal/Stem Cell Spheroids and Secretion of Paracrine Factors. Front. Bioeng. Biotechnol. 2022, 10, 916229. [Google Scholar] [CrossRef]
- Jacobs, I.N.; Redden, R.A.; Goldberg, R.; Hast, M.; Salowe, R.; Mauck, R.L.; Doolin, E.J. Pediatric laryngotracheal reconstruction with tissue-engineered cartilage in a rabbit model. Laryngoscope 2016, 126 (Suppl. S1), S5–S21. [Google Scholar] [CrossRef]
- Szychlinska, M.A.; Castrogiovanni, P.; Nsir, H.; Di Rosa, M.; Guglielmino, C.; Parenti, R.; Calabrese, G.; Pricoco, E.; Salvatorelli, L.; Magro, G.; et al. Engineered cartilage regeneration from adipose tissue derived-mesenchymal stem cells: A morphomolecular study on osteoblast, chondrocyte and apoptosis evaluation. Exp. Cell Res. 2017, 357, 222–235. [Google Scholar] [CrossRef]
- Oliveira, M.S.; Barreto-Filho, J.B. Placental-derived stem cells: Culture, differentiation and challenges. World J. Stem Cells 2015, 7, 769–775. [Google Scholar] [CrossRef]
- Ciucă, D.; Soritau, O.; Susman, S.; Pop, V.; Mihu, C. Isolation and characterization of chorionic mesenchyal stem cells from the placenta. Rom. J. Morphol. Embryol. 2011, 52, 803–808. [Google Scholar]
- Samsonraj, R.M.; Raghunath, M.; Nurcombe, V.; Hui, J.H.; van Wijnen, A.J.; Cool, S.M. Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem Cells Transl. Med. 2017, 6, 2173–2185. [Google Scholar] [CrossRef]
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, A.G. Mesenchymal Stem Cell Migration and Tissue Repair. Cells 2019, 8, 784. [Google Scholar] [CrossRef] [PubMed]
- Araújo, A.B.; Furlan, J.M.; Salton, G.D.; Schmalfuss, T.; Röhsig, L.M.; Silla, L.M.R.; Passos, E.P.; Paz, A.H. Isolation of human mesenchymal stem cells from amnion, chorion, placental decidua and umbilical cord: Comparison of four enzymatic protocols. Biotechnol. Lett. 2018, 40, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Kwon, A.; Kim, Y.; Kim, M.; Kim, J.; Choi, H.; Jekarl, D.W.; Lee, S.; Kim, J.M.; Shin, J.C.; Park, I.Y. Tissue-specific Differentiation Potency of Mesenchymal Stromal Cells from Perinatal Tissues. Sci. Rep. 2016, 6, 23544. [Google Scholar] [CrossRef]
- Pelekanos, R.A.; Sardesai, V.S.; Futrega, K.; Lott, W.B.; Kuhn, M.; Doran, M.R. Isolation and Expansion of Mesenchymal Stem/Stromal Cells Derived from Human Placenta Tissue. J. Vis. Exp. JoVE 2016, 112, e54204. [Google Scholar] [CrossRef]
- Ventura Ferreira, M.S.; Bienert, M.; Müller, K.; Rath, B.; Goecke, T.; Opländer, C.; Braunschweig, T.; Mela, P.; Brümmendorf, T.H.; Beier, F.; et al. Comprehensive characterization of chorionic villi-derived mesenchymal stromal cells from human placenta. Stem Cell Res. Ther. 2018, 9, 28. [Google Scholar] [CrossRef]
- Kyo, S.; Takakura, M.; Tanaka, M.; Kanaya, T.; Sagawa, T.; Kohama, T.; Ishikawa, H.; Nakano, T.; Shimoya, K.; Inoue, M. Expression of telomerase activity in human chorion. Biochem. Biophys. Res. Commun. 1997, 241, 498–503. [Google Scholar] [CrossRef]
- ATCC. Available online: https://www.atcc.org/products/crl-2846#product-references (accessed on 31 July 2023).
- STEMCELL Technologies. Available online: https://www.stemcell.com/products/mesencult-acf-chondrogenic-differentiation-medium.html (accessed on 25 April 2021).
- Jagur-Grodzinski, J. Biomedical application of functional polymers. React. Funct. Polym. 1999, 39, 99–138. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640. [Google Scholar] [CrossRef]
- Athanasiou, K.A.; Niederauer, G.G.; Agrawal, C.M. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/ polyglycolic acid copolymers. Biomaterials 1996, 17, 93–102. [Google Scholar] [CrossRef]
- Xia, P.; Wang, X.; Qu, Y.; Lin, Q.; Cheng, K.; Gao, M.; Ren, S.; Zhang, T.; Li, X. TGF-β1-induced chondrogenesis of bone marrow mesenchymal stem cells is promoted by low-intensity pulsed ultrasound through the integrin-mTOR signaling pathway. Stem Cell Res. Ther. 2017, 8, 281. [Google Scholar] [CrossRef] [PubMed]
- Ebisawa, K.; Hata, K.; Okada, K.; Kimata, K.; Ueda, M.; Torii, S.; Watanabe, H. Ultrasound enhances transforming growth factor beta-mediated chondrocyte differentiation of human mesenchymal stem cells. Tissue Eng. 2004, 10, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Almalki, S.G.; Agrawal, D.K. Key transcription factors in the differentiation of mesenchymal stem cells. Differ. Res. Biol. Divers. 2016, 92, 41–51. [Google Scholar] [CrossRef]
- Rodrigues, C.A.; Fernandes, T.G.; Diogo, M.M.; da Silva, C.L.; Cabral, J.M. Stem cell cultivation in bioreactors. Biotechnol. Adv. 2011, 29, 815–829. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mujde, C.; Bisgin, A. Three-Dimensional Cartilage Tissue Engineering Using Placenta-Derived Extra-Embryonic Mesenchymal Stem Cells: From Isolation to Differentiation. Biomedicines 2025, 13, 2291. https://doi.org/10.3390/biomedicines13092291
Mujde C, Bisgin A. Three-Dimensional Cartilage Tissue Engineering Using Placenta-Derived Extra-Embryonic Mesenchymal Stem Cells: From Isolation to Differentiation. Biomedicines. 2025; 13(9):2291. https://doi.org/10.3390/biomedicines13092291
Chicago/Turabian StyleMujde, Cem, and Atil Bisgin. 2025. "Three-Dimensional Cartilage Tissue Engineering Using Placenta-Derived Extra-Embryonic Mesenchymal Stem Cells: From Isolation to Differentiation" Biomedicines 13, no. 9: 2291. https://doi.org/10.3390/biomedicines13092291
APA StyleMujde, C., & Bisgin, A. (2025). Three-Dimensional Cartilage Tissue Engineering Using Placenta-Derived Extra-Embryonic Mesenchymal Stem Cells: From Isolation to Differentiation. Biomedicines, 13(9), 2291. https://doi.org/10.3390/biomedicines13092291





