Clopidogrel Influences Fracture Healing Under Ischemic Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Surgical Procedure
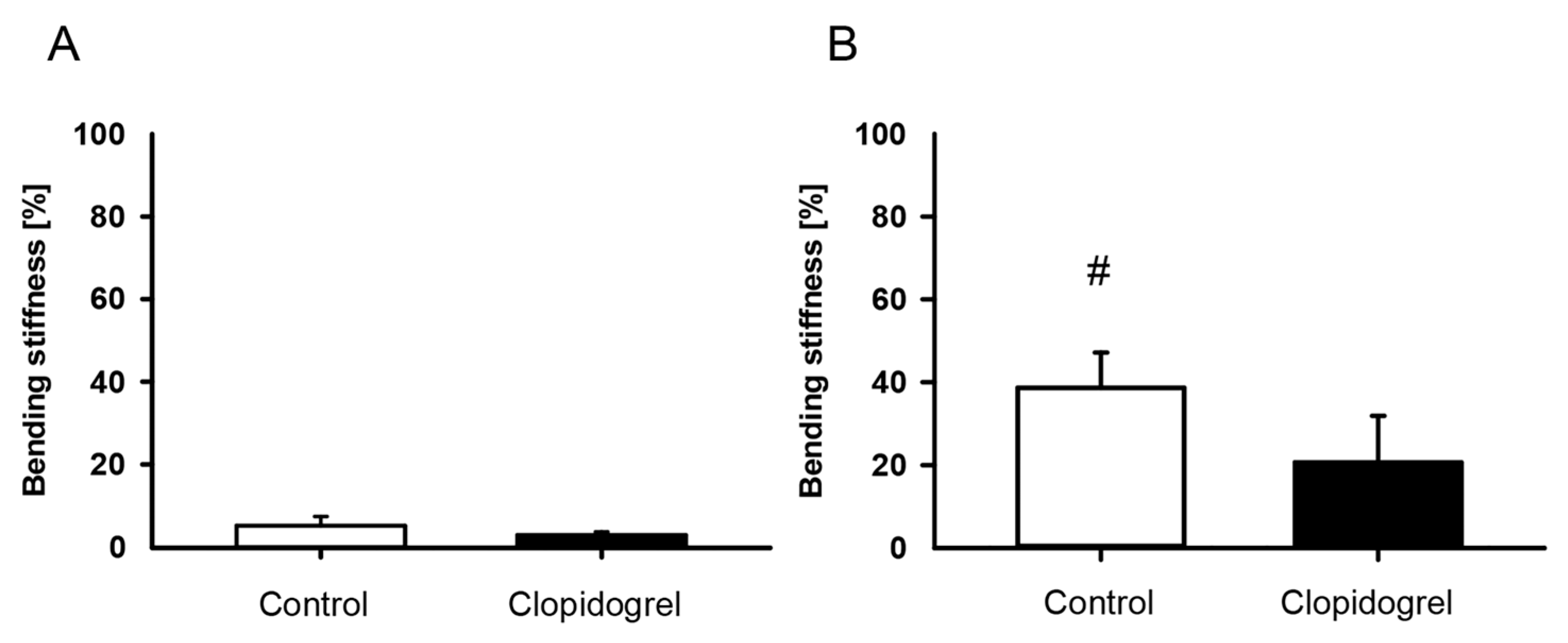
2.3. Biomechanical Analysis
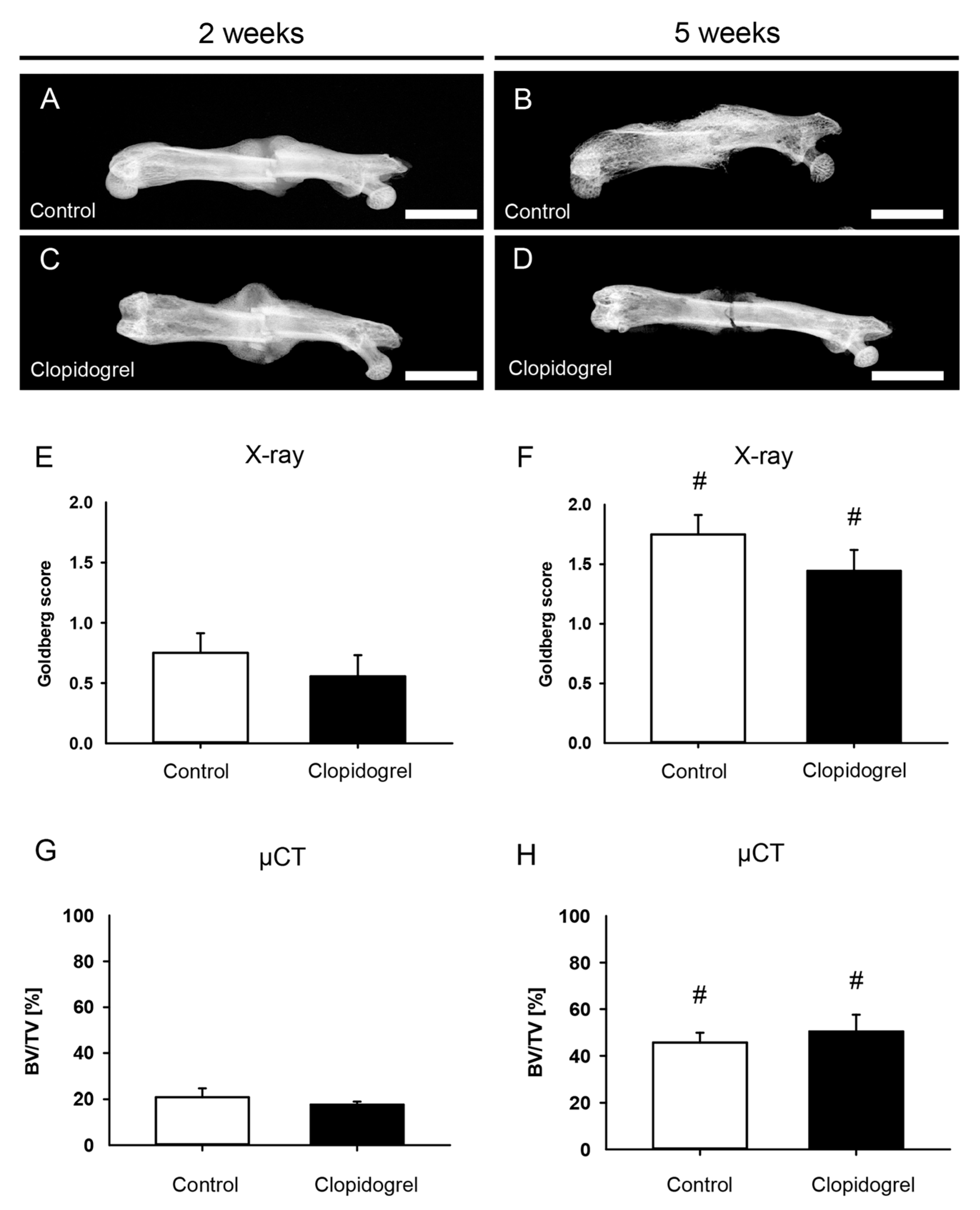
2.4. Radiological Analysis
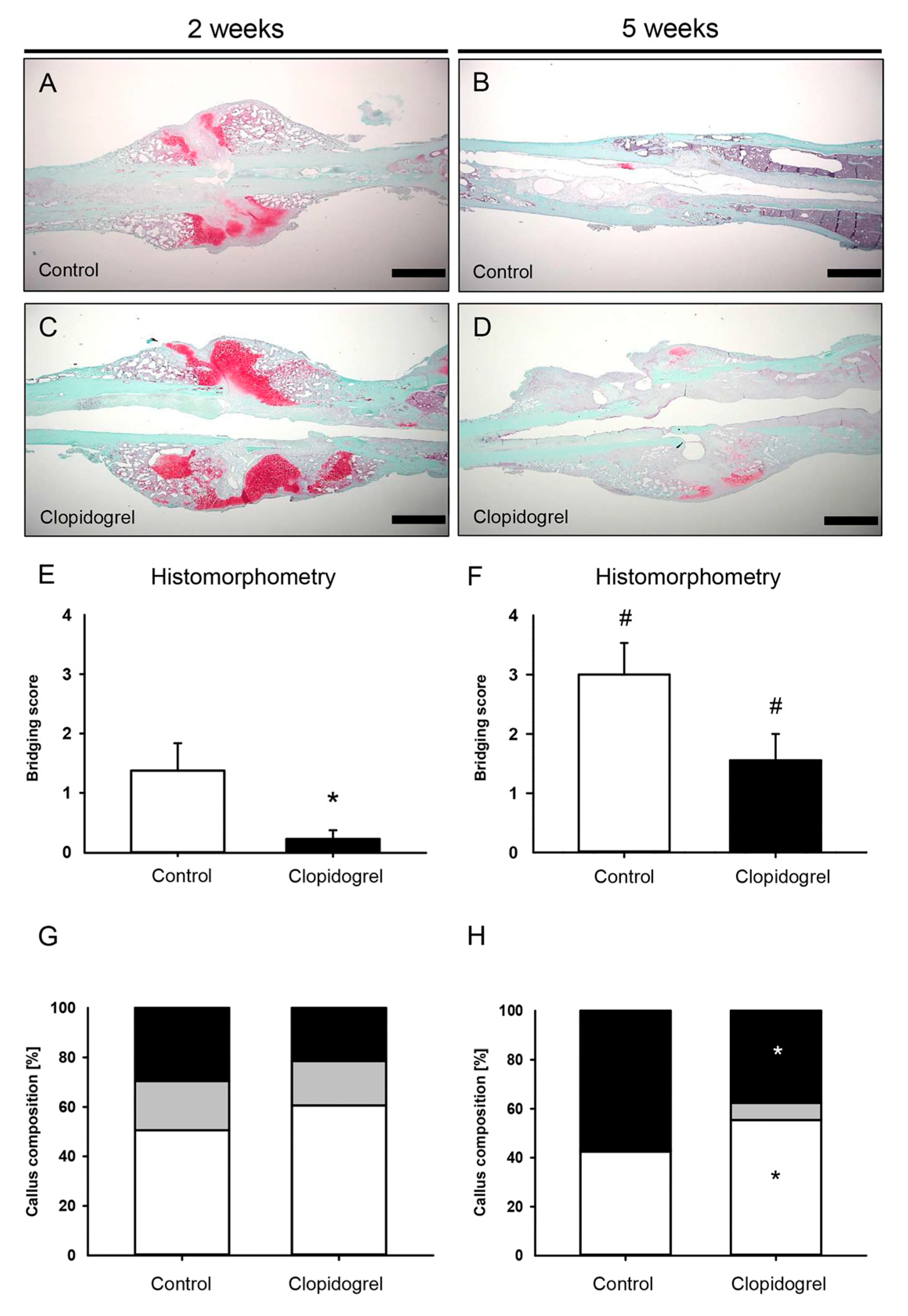
2.5. Histomorphometric Analysis
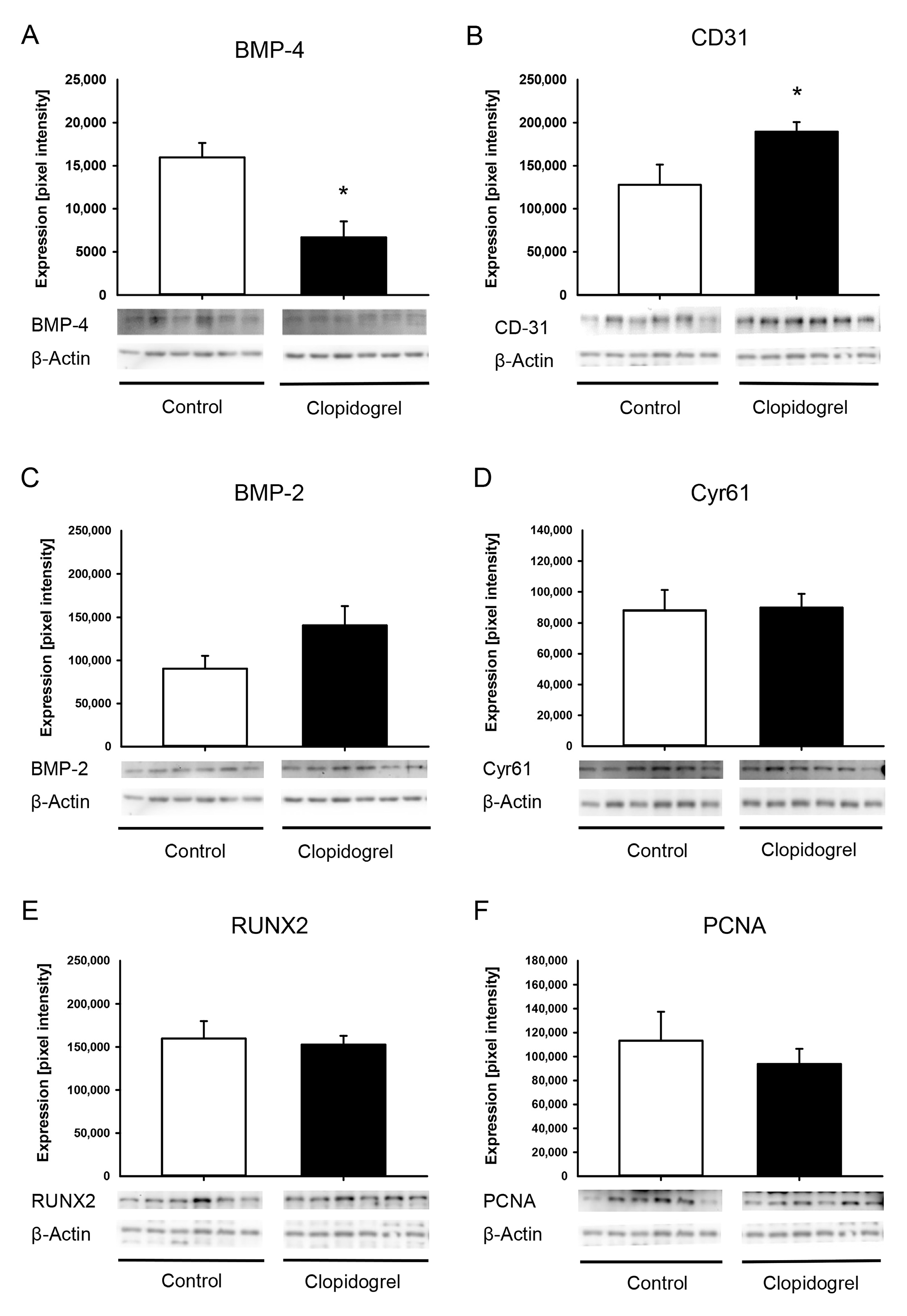
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
3.1. Biomechanical Analysis
3.2. Radiological Analysis
3.3. Histomorphometric Analysis
3.4. Western Blot Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMP | Bone Morphogenetic Protein |
| PAD | Peripheral Arterial Occlusive Disease |
| CLI | Critical Limb Ischemia |
| NIH | National Institutes of Health |
| µCT | Micro-Computed Tomography |
| BMD | Bone Mineral Density |
| CaHA | Calcium Hydroxyapatit |
| ROI | Region Of Interest |
| BV | Bone Volume |
| TV | Total Volume |
| TbN | Trabecular Number |
| TbSp | Trabecular Separation |
| TbTh | Trabecular Thickness |
| EDTA | Ethylenediaminetetraacetate |
| Cyr | Cystein-rich angiogenic inducer |
| RUNX | Runt related transcription factor |
| PCNA | Proliferating Cell Nuclear Antigen |
| SEM | Standard Error of the Mean |
References
- Mehta, S.R.; Yusuf, S.; Peters, R.J.; Bertrand, M.E.; Lewis, B.S.; Natarajan, M.K.; Malmberg, K.; Rupprecht, H.-J.; Zhao, F.; Chrolavicius, S.; et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: The PCI-CURE study. Lancet 2001, 358, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; Cannon, C.P.; Gibson, C.M.; López-Sendón, J.L.; Montalescot, G.; Theroux, P.; Claeys, M.J.; Cools, F.; Hill, K.A.; Skene, A.M.; et al. Addition of Clopidogrel to Aspirin and Fibrinolytic Therapy for Myocardial Infarction with ST-Segment Elevation. N. Engl. J. Med. 2005, 352, 1179–1189. [Google Scholar] [CrossRef]
- Yusuf, S.; Fox, K.A.A.; Tognoni, G.; Mehta, S.R. Effects of Clopidogrel in Addition to Aspirin in Patients with Acute Coronary Syndromes without ST-Segment Elevation. N. Engl. J. Med. 2001, 345, 494–502. [Google Scholar] [PubMed]
- Lambert, M.A.; Belch, J.J.F. Medical management of critical limb ischaemia: Where do we stand today? J. Intern. Med. 2013, 274, 295–307. [Google Scholar] [CrossRef]
- Kim, S.-W.; Kim, H.; Cho, H.-J.; Lee, J.-U.; Levit, R.; Yoon, Y. Human Peripheral Blood-Derived CD31+Cells Have Robust Angiogenic and Vasculogenic Properties and Are Effective for Treating Ischemic Vascular Disease. J. Am. Coll. Cardiol. 2010, 56, 593–607. [Google Scholar] [CrossRef]
- Lillis, T.; Veis, A.; Sakellaridis, N.; Tsirlis, A.; Dailiana, Z. Effect of clopidogrel in bone healing-experimental study in rabbits. World J. Orthop. 2019, 10, 434–445. [Google Scholar] [CrossRef]
- Schindeler, A.; McDonald, M.M.; Bokko, P.; Little, D.G. Bone remodeling during fracture repair: The cellular picture. Semin. Cell Dev. Biol. 2008, 19, 459–466. [Google Scholar] [CrossRef]
- Syberg, S.; Brandao-Burch, A.; Patel, J.J.; Hajjawi, M.; Arnett, T.R.; Schwarz, P.; Jorgensen, N.R.; Orriss, I.R. Clopidogrel (Plavix), a P2Y12 receptor antagonist, inhibits bone cell function in vitro and decreases trabecular bone in vivo. J. Bone Min. Res. 2012, 27, 2373–2386. [Google Scholar] [CrossRef]
- Mangiafico, R.A.; Russo, E.; Riccobene, S.; Pennisi, P.; Mangiafico, M.; D’Amico, F.; Fiore, C.E. Increased prevalence of peripheral arterial disease in osteoporotic postmenopausal women. J. Bone Min. Metab. 2006, 24, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Ungprasert, P.; Wijarnpreecha, K.; Thongprayoon, C.; Cheungpasitporn, W. Peripheral arterial disease and risk of hip fracture: A systematic review and meta-analysis of cohort studies. J. Postgrad. Med. 2018, 64, 220–225. [Google Scholar]
- Hald, S.M.; Möller, S.; García Rodríguez, L.A.; Al-Shahi Salman, R.; Sharma, M.; Christensen, H.; Hellfritzsch, M.; Pottegård, A.; Hallas, J.; Gaist, D. Trends in Incidence of Intracerebral Hemorrhage and Association with Antithrombotic Drug Use in Denmark 2005–2018. JAMA Netw. Open 2021, 4, e218380. [Google Scholar] [CrossRef]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Menger, M.M.; Stutz, J.; Ehnert, S.; Nussler, A.K.; Rollmann, M.F.; Herath, S.C.; Braun, B.J.; Pohlemann, T.; Menger, M.D.; Histing, T. Development of an ischemic fracture healing model in mice. Acta Orthop. 2022, 93, 466–471. [Google Scholar] [CrossRef]
- Holstein, J.H.; Matthys, R.; Histing, T.; Becker, S.C.; Fiedler, M.; Garcia, P.; Meier, C.; Pohlemann, T.; Menger, M.D. Development of a Stable Closed Femoral Fracture Model in Mice. J. Surg. Res. 2009, 153, 71–75. [Google Scholar] [CrossRef]
- Wong, P.C.; Crain, E.J.; Watson, C.A.; Jiang, X.; Hua, J.; Bostwick, J.S.; Ogletree, M.L.; Schumacher, W.A.; Rehfuss, R. Platelet aggregometry and receptor binding to predict the magnitude of antithrombotic and bleeding time effects of clopidogrel in rabbits. J. Cardiovasc. Pharmacol. 2007, 49, 316–324. [Google Scholar] [CrossRef]
- Orth, M.; Baudach, J.; Scheuer, C.; Osche, D.; Veith, N.T.; Braun, B.J.; Rollmann, M.F.; Herath, S.C.; Pohlemann, T.; Menger, M.D.; et al. Erythropoietin does not improve fracture healing in aged mice. Exp. Gerontol. 2019, 122, 1–9. [Google Scholar] [CrossRef]
- Orth, M.; Kruse, N.J.; Braun, B.J.; Scheuer, C.; Holstein, J.H.; Khalil, A.; Yu, X.; Murphy, W.L.; Pohlemann, T.; Laschke, M.W.; et al. BMP-2-coated mineral coated microparticles improve bone repair in atrophic non-unions. Eur. Cell Mater. 2017, 33, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, V.M.; Powell, A.; Shaffer, J.W.; Zika, J.; Bos, G.D.; Heiple, K.G. Bone grafting: Role of histocompatibility in transplantation. J. Orthop. Res. 1985, 3, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Gerstenfeld, L.C.; Wronski, T.J.; Hollinger, J.O.; Einhorn, T.A. Application of histomorphometric methods to the study of bone repair. J. Bone Miner. Res. 2005, 20, 1715–1722. [Google Scholar] [CrossRef]
- Savi, P.; Zachayus, J.L.; Delesque-Touchard, N.; Labouret, C.; Hervé, C.; Uzabiaga, M.F.; Pereillo, J.M.; Culouscou, J.M.; Bono, F.; Ferrara, P.; et al. The active metabolite of Clopidogrel disrupts P2Y12 receptor oligomers and partitions them out of lipid rafts. Proc. Natl. Acad. Sci. USA 2006, 103, 11069–11074. [Google Scholar] [CrossRef]
- Kumar, A.; Lutsey, P.L.; St Peter, W.L.; Schommer, J.C.; Van’t Hof, J.R.; Rajpurohit, A.; Farley, J.F. Prescription patterns of P2Y12 inhibitors following revascularization in the United States: 2013–2018. Clin. Transl. Sci. 2023, 16, 1886–1897. [Google Scholar] [CrossRef]
- Buckley, K.A.; Golding, S.L.; Rice, J.M.; Dillon, J.P.; Gallagher, J.A. Release and interconversion of P2 receptor agonists by human osteoblast-like cells. FASEB J. 2003, 17, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Floyd, D.H.; Hughes, A.; Xiang, J.; Schneider, J.G.; Uluckan, O.; Heller, E.; Deng, H.; Zou, W.; Craft, C.S.; et al. The ADP receptor P2RY12 regulates osteoclast function and pathologic bone remodeling. J. Clin. Investig. 2012, 122, 3579–3592. [Google Scholar] [CrossRef]
- Histing, T.; Stenger, D.; Scheuer, C.; Metzger, W.; Garcia, P.; Holstein, J.H.; Klein, M.; Pohlemann, T.; Menger, M.D. Pantoprazole, a proton pump inhibitor, delays fracture healing in mice. Calcif. Tissue Int. 2012, 90, 507–514. [Google Scholar] [CrossRef]
- Menger, M.M.; Merscher, B.; Scheuer, C.; Braun, B.J.; Herath, S.C.; Rollmann, M.F.; Stenger, D.; Spater, T.; Pohlemann, T.; Menger, M.D.; et al. Amlodipine accelerates bone healing in a stable closed femoral fracture model in mice. Eur. Cell Mater. 2021, 41, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cai, X.; Guo, J.; Li, X.; Li, W.; Liu, Y.; Qi, M. Cell communication and relevant signaling pathways in osteogenesis-angiogenesis coupling. Bone Res. 2025, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Kanczler, J.M.; Oreffo, R.O. Osteogenesis and angiogenesis: The potential for engineering bone. Eur. Cell Mater. 2008, 15, 100–114. [Google Scholar] [CrossRef]
- Rather, H.A.; Jhala, D.; Vasita, R. Dual functional approaches for osteogenesis coupled angiogenesis in bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109761. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Cai, F.; Liu, K.; Zhang, X.; Yusufu, A. Hypoxia During the Consolidation Phase of Distraction Osteogenesis Promotes Bone Regeneration. Front. Physiol. 2022, 13, 804469. [Google Scholar] [CrossRef]
- Park, S.; Sorenson, C.M.; Sheibani, N. PECAM-1 isoforms, eNOS and endoglin axis in regulation of angiogenesis. Clin. Sci. 2015, 129, 217–234. [Google Scholar] [CrossRef]
- Yang, M.; Li, C.; Xiao, Y.; Guo, Q.; Huang, Y.; Su, T.; Luo, X.; Jiang, T. Ophiopogonin D promotes bone regeneration by stimulating CD31 hi EMCN hi vessel formation. Cell Prolif. 2020, 53, e12784. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Jiang, W.; Phillips, F.M.; Haydon, R.C.; Peng, Y.; Zhou, L.; Luu, H.H.; An, N.; Breyer, B.; Vanichakarn, P.; et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J. Bone Joint Surg. Am. 2003, 85, 1544–1552. [Google Scholar] [CrossRef]
- Mandal, C.C.; Das, F.; Ganapathy, S.; Harris, S.E.; Choudhury, G.G.; Ghosh-Choudhury, N. Bone Morphogenetic Protein-2 (BMP-2) Activates NFATc1 Transcription Factor via an Autoregulatory Loop Involving Smad/Akt/Ca2+ Signaling. J. Biol. Chem. 2016, 291, 1148–1161. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, M.; Harris, S.E.; Mi, Z. Signal transduction and biological functions of bone morphogenetic proteins. Front. Biosci. 2004, 9, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-H.; Yang, R.-S.; Liou, H.-C.; Fu, W.-M. Enhancement of fibronectin synthesis and fibrillogenesis by BMP-4 in cultured rat osteoblast. J. Bone Min. Res. Off. J. Am. Soc. Bone Min. Res. 2003, 18, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Abe, E.; Yamamoto, M.; Taguchi, Y.; Lecka-Czernik, B.; O’Brien, C.A.; Economides, A.N.; Stahl, N.; Jilka, R.L.; Manolagas, S.C. Essential Requirement of BMPs-2/4 for Both Osteoblast and Osteoclast Formation in Murine Bone Marrow Cultures from Adult Mice: Antagonism by Noggin. J. Bone Min. Res. 2010, 15, 663–673. [Google Scholar] [CrossRef]
- Kozawa, O.; Hatakeyama, D.; Tokuda, H.; Oiso, Y.; Matsuno, H.; Uematsu, T. Sphingomyelinase amplifies BMP-4-induced osteocalcin synthesis in osteoblasts: Role of ceramide. Cell Signal. 2002, 14, 999–1004. [Google Scholar] [CrossRef]
- Orth, M.; Altmeyer, M.A.B.; Scheuer, C.; Braun, B.J.; Holstein, J.H.; Eglin, D.; D’Este, M.; Histing, T.; Laschke, M.W.; Pohlemann, T.; et al. Effects of locally applied adipose tissue-derived microvascular fragments by thermoresponsive hydrogel on bone healing. Acta Biomater. 2020, 77, 201–211. [Google Scholar] [CrossRef]
- Orth, M.; Fritz, T.; Stutz, J.; Scheuer, C.; Ganse, B.; Bullinger, Y.; Lee, J.S.; Murphy, W.L.; Laschke, M.W.; Menger, M.D.; et al. Local Application of Mineral-Coated Microparticles Loaded with VEGF and BMP-2 Induces the Healing of Murine Atrophic Non-Unions. Front. Bioeng. Biotechnol. 2020, 9, 809397. [Google Scholar] [CrossRef]
- Peng, H.; Usas, A.; Olshanski, A.; Ho, A.M.; Gearhart, B.; Cooper, G.M.; Huard, J. VEGF Improves, Whereas sFlt1 Inhibits, BMP2-Induced Bone Formation and Bone Healing Through Modulation of Angiogenesis. J. Bone Min. Res. 2005, 20, 2017–2027. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schreiber, S.; Stutz, J.; Keller, L.; Metzger, W.; Fritz, T.; Schönbeck, C.; Osche, D.; Örgel, M.; Menger, M.D.; Pohlemann, T.; et al. Clopidogrel Influences Fracture Healing Under Ischemic Conditions. Biomedicines 2025, 13, 2286. https://doi.org/10.3390/biomedicines13092286
Schreiber S, Stutz J, Keller L, Metzger W, Fritz T, Schönbeck C, Osche D, Örgel M, Menger MD, Pohlemann T, et al. Clopidogrel Influences Fracture Healing Under Ischemic Conditions. Biomedicines. 2025; 13(9):2286. https://doi.org/10.3390/biomedicines13092286
Chicago/Turabian StyleSchreiber, Sebastian, Janine Stutz, Lukas Keller, Wolfgang Metzger, Tobias Fritz, Christian Schönbeck, David Osche, Marcus Örgel, Michael D. Menger, Tim Pohlemann, and et al. 2025. "Clopidogrel Influences Fracture Healing Under Ischemic Conditions" Biomedicines 13, no. 9: 2286. https://doi.org/10.3390/biomedicines13092286
APA StyleSchreiber, S., Stutz, J., Keller, L., Metzger, W., Fritz, T., Schönbeck, C., Osche, D., Örgel, M., Menger, M. D., Pohlemann, T., Liodakis, E., Laschke, M. W., & Orth, M. (2025). Clopidogrel Influences Fracture Healing Under Ischemic Conditions. Biomedicines, 13(9), 2286. https://doi.org/10.3390/biomedicines13092286






