Necrotizing Enterocolitis: A Comprehensive Review on Toll-like Receptor 4-Mediated Pathophysiology, Clinical, and Therapeutic Insights
Abstract
1. Introduction
1.1. Clinical Aspects of NEC
1.2. Clinical Treatment Strategies
1.3. Feeding Practices
2. Pathogenesis and Mechanistic Insights
2.1. Experimental Models to Study the Pathogenesis of NEC
2.2. Compromised Gut Epithelium Triggering NEC Pathogenesis
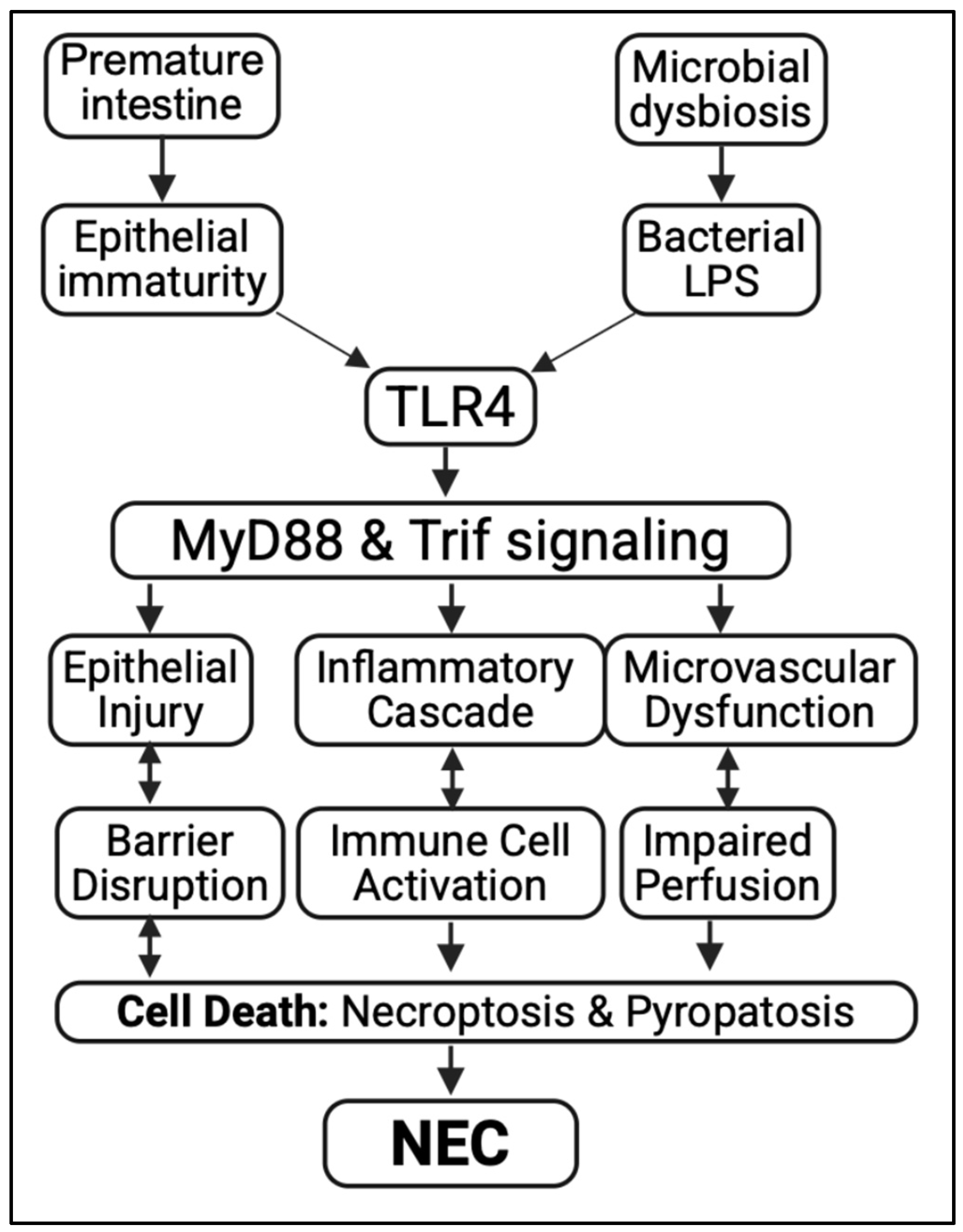
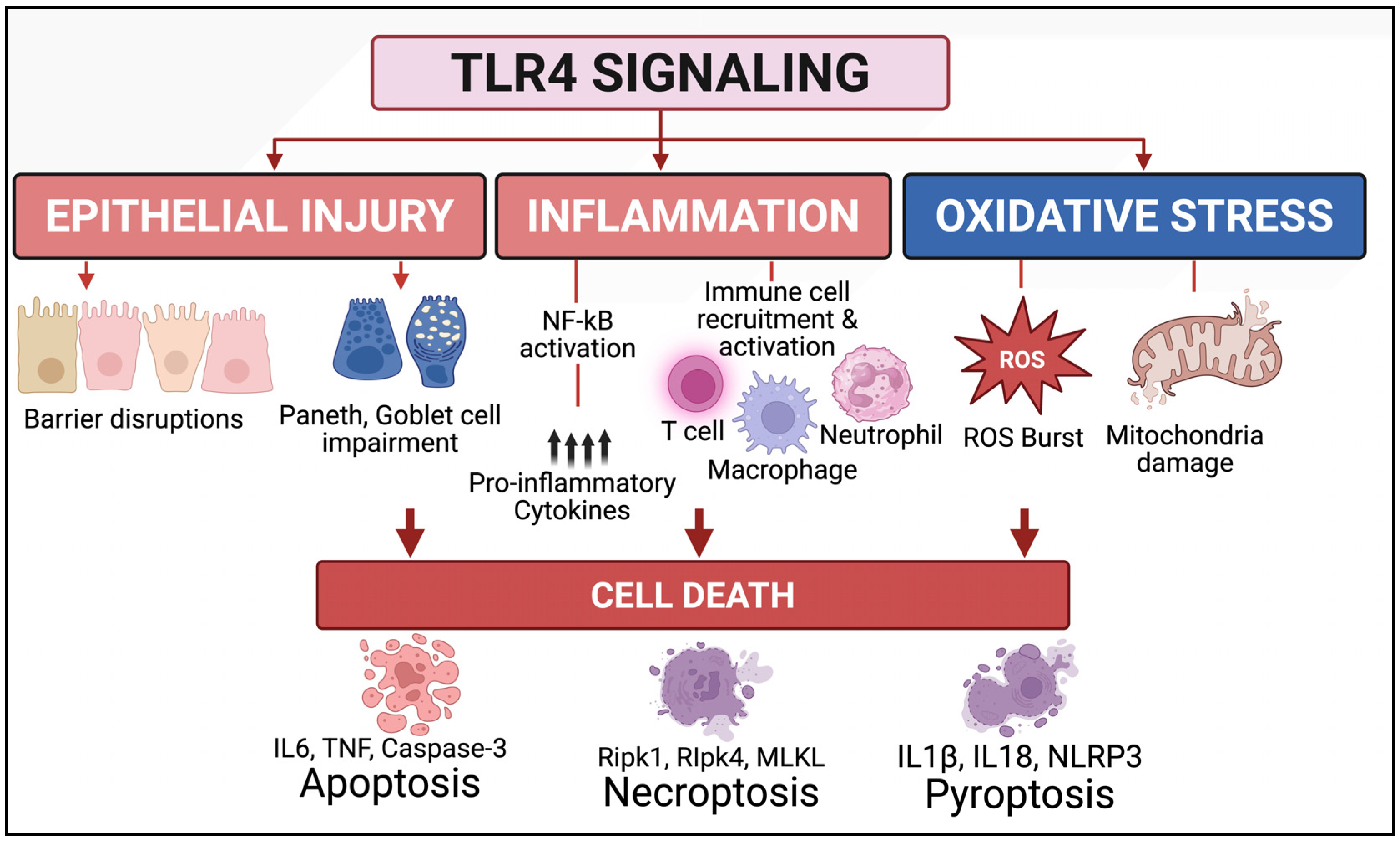
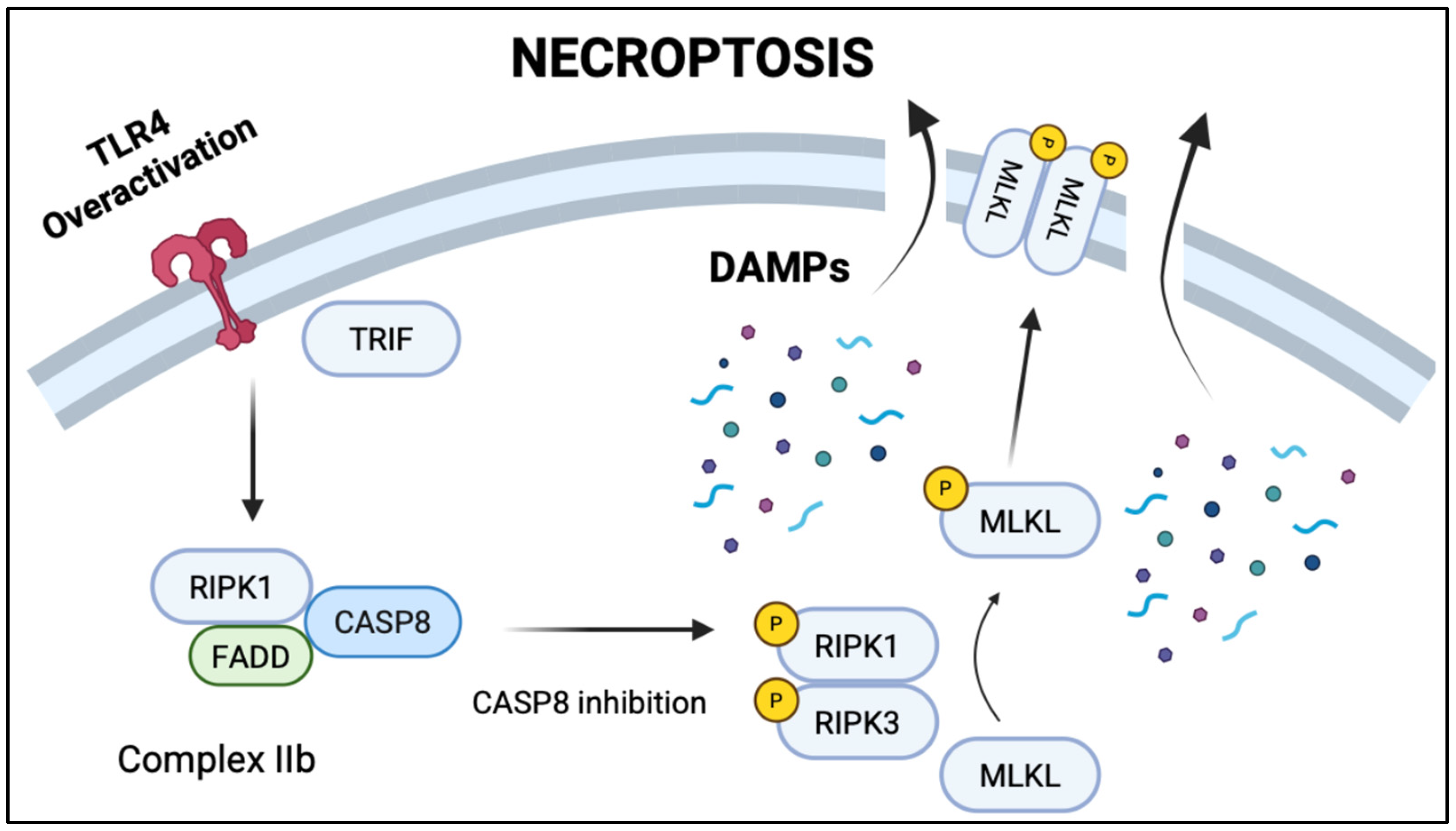
2.3. Toll-like Receptor 4 (TLR4) as a Key Driver of NEC Pathogenesis
2.4. Compromised Immune Landscape in NEC Pathogenesis
2.5. Compromised Enteric Nervous System in NEC Pathogenesis
2.6. Translational Advances: NEC Prevention and Treatment
3. Future Directions and Conclusions
4. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alsaied, A.; Islam, N.; Thalib, L. Global incidence of Necrotizing Enterocolitis: A systematic review and Meta-analysis. BMC Pediatr. 2020, 20, 344. [Google Scholar] [CrossRef]
- Cuna, A.; Sampath, V.; Khashu, M. Racial Disparities in Necrotizing Enterocolitis. Front. Pediatr. 2021, 9, 633088. [Google Scholar] [CrossRef]
- Dimmitt, R.A.; Meier, A.H.; Skarsgard, E.D.; Halamek, L.P.; Smith, B.M.; Moss, R.L. Salvage laparotomy for failure of peritoneal drainage in necrotizing enterocolitis in infants with extremely low birth weight. J. Pediatr. Surg. 2000, 35, 856–859. [Google Scholar] [CrossRef]
- Lai, K.C.; Lorch, S.A. Healthcare Costs of Major Morbidities Associated with Prematurity in US Children’s Hospitals. J. Pediatr. 2023, 256, 53–62.e4. [Google Scholar] [CrossRef] [PubMed]
- Bazacliu, C.; Neu, J. Necrotizing Enterocolitis: Long Term Complications. Curr. Pediatr. Rev. 2019, 15, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Sodhi, C.P.; Yamaguchi, Y.; Lu, P.; Ladd, M.R.; Werts, A.; Fulton, W.B.; Wang, S.; Prindle, T., Jr.; Hackam, D.J. Toll Like Receptor 4 Mediated Lymphocyte Imbalance Induces Nec-Induced Lung Injury. Shock 2019, 52, 215–223. [Google Scholar] [CrossRef]
- Nino, D.F.; Zhou, Q.; Yamaguchi, Y.; Martin, L.Y.; Wang, S.; Fulton, W.B.; Jia, H.; Lu, P.; Prindle, T., Jr.; Zhang, F.; et al. Cognitive impairments induced by necrotizing enterocolitis can be prevented by inhibiting microglial activation in mouse brain. Sci. Transl. Med. 2018, 10, eaan0237. [Google Scholar] [CrossRef]
- Zhou, Q.; Nino, D.F.; Yamaguchi, Y.; Wang, S.; Fulton, W.B.; Jia, H.; Lu, P.; Prindle, T., Jr.; Pamies, D.; Morris, M.; et al. Necrotizing enterocolitis induces T lymphocyte-mediated injury in the developing mammalian brain. Sci. Transl. Med. 2021, 13, eaay6621. [Google Scholar] [CrossRef]
- Young, K.; Kaiser, K.N.; Holler, E.; Markel, T.A. Addressing Health Inequities: Understanding the Relationship Between Social Determinants of Health and Necrotizing Enterocolitis. J. Pediatr. Surg. 2025, 60, 162176. [Google Scholar] [CrossRef]
- Kennedy, K.A.; Tyson, J.E.; Chamnanvanikij, S. Early versus delayed initiation of progressive enteral feedings for parenterally fed low birth weight or preterm infants. Cochrane Database Syst. Rev. 2000, CD001970. [Google Scholar] [CrossRef]
- Pace, E.; Yanowitz, T.D.; Waltz, P.; Morowitz, M.J. Antibiotic therapy and necrotizing enterocolitis. Semin. Pediatr. Surg. 2023, 32, 151308. [Google Scholar] [CrossRef]
- Hong, C.R.; Han, S.M.; Jaksic, T. Surgical considerations for neonates with necrotizing enterocolitis. Semin. Fetal Neonatal Med. 2018, 23, 420–425. [Google Scholar] [CrossRef]
- Sodhi, C.P. Portal venous gas value in predicting surgical necrotizing enterocolitis. Pediatr. Res. 2025, 97, 1451–1452. [Google Scholar] [CrossRef]
- Kallis, M.P.; Roberts, B.; Aronowitz, D.; Shi, Y.; Lipskar, A.M.; Amodio, J.B.; Aggarwal, A.; Sathya, C. Utilizing ultrasound in suspected necrotizing enterocolitis with equivocal radiographic findings. BMC Pediatr. 2023, 23, 134. [Google Scholar] [CrossRef]
- Kovler, M.L.; Sodhi, C.P.; Hackam, D.J. Precision-based modeling approaches for necrotizing enterocolitis. Dis. Model. Mech. 2020, 13, dmm044388. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.C.; Fletcher, J.G.; Nagpal, P.; Froemming, A.T.; Khandelwal, A. Mesenteric ischemia: What the radiologist needs to know. Cardiovasc. Diagn. Ther. 2019, 9 (Suppl. S1), S74–S87. [Google Scholar] [CrossRef] [PubMed]
- Sokou, R.; Mantzios, P.; Palioura, A.E.; Tsantes, A.G.; Lianou, A.; Piovani, D.; Tsante, K.A.; Lampropoulou, K.; Iacovidou, N.; Bonovas, S. Diagnostic and Prognostic Value of Hematological Parameters in Necrotizing Enterocolitis: A Systematic Review. J. Clin. Med. 2025, 14, 2530. [Google Scholar] [CrossRef]
- Sodhi, C.P.; Ahmad, R.; Jia, H.; Fulton, W.B.; Lopez, C.; Gonzalez Salazar, A.J.; Ishiyama, A.; Sampah, M.; Steinway, S.; Wang, S.; et al. The administration of amnion-derived multipotent cell secretome ST266 protects against necrotizing enterocolitis in mice and piglets. Am. J. Physiol. Gastrointest. Liver Physiol. 2022, 323, G265–G282. [Google Scholar] [CrossRef] [PubMed]
- Neal, M.D.; Jia, H.; Eyer, B.; Good, M.; Guerriero, C.J.; Sodhi, C.P.; Afrazi, A.; Prindle, T., Jr.; Ma, C.; Branca, M.; et al. Discovery and validation of a new class of small molecule Toll-like receptor 4 (TLR4) inhibitors. PLoS ONE 2013, 8, e65779. [Google Scholar] [CrossRef]
- Neal, M.D.; Leaphart, C.; Levy, R.; Prince, J.; Billiar, T.R.; Watkins, S.; Li, J.; Cetin, S.; Ford, H.; Schreiber, A.; et al. Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J. Immunol. 2006, 176, 3070–3079. [Google Scholar] [CrossRef]
- Egan, C.E.; Sodhi, C.P.; Good, M.; Lin, J.; Jia, H.; Yamaguchi, Y.; Lu, P.; Ma, C.; Branca, M.F.; Weyandt, S.; et al. Toll-like receptor 4-mediated lymphocyte influx induces neonatal necrotizing enterocolitis. J. Clin. Investig. 2016, 126, 495–508. [Google Scholar] [CrossRef]
- Olson, J.K.; Navarro, J.B.; Allen, J.M.; McCulloh, C.J.; Mashburn-Warren, L.; Wang, Y.; Varaljay, V.A.; Bailey, M.T.; Goodman, S.D.; Besner, G.E. An enhanced Lactobacillus reuteri biofilm formulation that increases protection against experimental necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G408–G419. [Google Scholar] [CrossRef]
- Klerk, D.H.; Moore, H.; Scheese, D.J.; Tragesser, C.; Raouf, Z.; Duess, J.W.; Tsuboi, K.; Sampah, M.E.; Lopez, C.M.; Williams-McLeod, S.; et al. Multi-strain probiotic administration decreases necrotizing enterocolitis severity and alters the epigenetic profile in mice. Pediatr. Res. 2024. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Bowles, W.H.D.; Gloster, T.M. Sialidase and Sialyltransferase Inhibitors: Targeting Pathogenicity and Disease. Front. Mol. Biosci. 2021, 8, 705133. [Google Scholar] [CrossRef]
- Good, M.; Sodhi, C.P.; Egan, C.E.; Afrazi, A.; Jia, H.; Yamaguchi, Y.; Lu, P.; Branca, M.F.; Ma, C.; Prindle, T., Jr.; et al. Breast milk protects against the development of necrotizing enterocolitis through inhibition of Toll-like receptor 4 in the intestinal epithelium via activation of the epidermal growth factor receptor. Mucosal Immunol. 2015, 8, 1166–1179. [Google Scholar] [CrossRef] [PubMed]
- Good, M.; Sodhi, C.P.; Yamaguchi, Y.; Jia, H.; Lu, P.; Fulton, W.B.; Martin, L.Y.; Prindle, T.; Nino, D.F.; Zhou, Q.; et al. The human milk oligosaccharide 2’-fucosyllactose attenuates the severity of experimental necrotising enterocolitis by enhancing mesenteric perfusion in the neonatal intestine. Br. J. Nutr. 2016, 116, 1175–1187. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, C.P.; Fulton, W.B.; Good, M.; Vurma, M.; Das, T.; Lai, C.S.; Jia, H.; Yamaguchi, Y.; Lu, P.; Prindle, T.; et al. Fat composition in infant formula contributes to the severity of necrotising enterocolitis. Br. J. Nutr. 2018, 120, 665–680. [Google Scholar] [CrossRef]
- Silano, M.; Milani, G.P.; Fattore, G.; Agostoni, C. Donor human milk and risk of surgical necrotizing enterocolitis: A meta-analysis. Clin. Nutr. 2019, 38, 1061–1066. [Google Scholar] [CrossRef]
- Quigley, M.; Embleton, N.D.; McGuire, W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst. Rev. 2019, 7, CD002971. [Google Scholar] [CrossRef]
- Jia, H.; Sodhi, C.P.; Yamaguchi, Y.; Lu, P.; Martin, L.Y.; Good, M.; Zhou, Q.; Sung, J.; Fulton, W.B.; Nino, D.F.; et al. Pulmonary Epithelial TLR4 Activation Leads to Lung Injury in Neonatal Necrotizing Enterocolitis. J. Immunol. 2016, 197, 859–871. [Google Scholar] [CrossRef]
- Robi, Y.G.; Sitote, T.M. Neonatal Disease Prediction Using Machine Learning Techniques. J. Healthc. Eng. 2023, 2023, 3567194. [Google Scholar] [CrossRef] [PubMed]
- Meeus, M.; Beirnaert, C.; Mahieu, L.; Laukens, K.; Meysman, P.; Mulder, A.; Van Laere, D. Clinical Decision Support for Improved Neonatal Care: The Development of a Machine Learning Model for the Prediction of Late-onset Sepsis and Necrotizing Enterocolitis. J. Pediatr. 2024, 266, 113869. [Google Scholar] [CrossRef] [PubMed]
- Han, S.M.; Hong, C.R.; Knell, J.; Edwards, E.M.; Morrow, K.A.; Soll, R.F.; Modi, B.P.; Horbar, J.D.; Jaksic, T. Trends in incidence and outcomes of necrotizing enterocolitis over the last 12 years: A multicenter cohort analysis. J. Pediatr. Surg. 2020, 55, 998–1001. [Google Scholar] [CrossRef] [PubMed]
- Embleton, N.D.; Jennifer Moltu, S.; Lapillonne, A.; van den Akker, C.H.P.; Carnielli, V.; Fusch, C.; Gerasimidis, K.; van Goudoever, J.B.; Haiden, N.; Iacobelli, S.; et al. Enteral Nutrition in Preterm Infants (2022): A Position Paper From the ESPGHAN Committee on Nutrition and Invited Experts. J. Pediatr. Gastroenterol. Nutr. 2023, 76, 248–268. [Google Scholar] [CrossRef]
- Jammeh, M.L.; Adibe, O.O.; Tracy, E.T.; Rice, H.E.; Clark, R.H.; Smith, P.B.; Greenberg, R.G. Racial/ethnic differences in necrotizing enterocolitis incidence and outcomes in premature very low birth weight infants. J. Perinatol. 2018, 38, 1386–1390. [Google Scholar] [CrossRef]
- Sheikh, J.; Allotey, J.; Kew, T.; Fernandez-Felix, B.M.; Zamora, J.; Khalil, A.; Thangaratinam, S.; Network, I.C. Effects of race and ethnicity on perinatal outcomes in high-income and upper-middle-income countries: An individual participant data meta-analysis of 2 198 655 pregnancies. Lancet 2022, 400, 2049–2062. [Google Scholar] [CrossRef]
- Nasiri, K.; Moodie, E.E.M.; Abenhaim, H.A. To What Extent Is the Association Between Race/Ethnicity and Fetal Growth Restriction Explained by Adequacy of Prenatal Care? A Mediation Analysis of a Retrospectively Selected Cohort. Am. J. Epidemiol. 2020, 189, 1360–1368. [Google Scholar] [CrossRef]
- Buck Louis, G.M.; Grewal, J.; Albert, P.S.; Sciscione, A.; Wing, D.A.; Grobman, W.A.; Newman, R.B.; Wapner, R.; D’Alton, M.E.; Skupski, D.; et al. Racial/ethnic standards for fetal growth: The NICHD Fetal Growth Studies. Am. J. Obstet. Gynecol. 2015, 213, 449.e1–449.e41. [Google Scholar] [CrossRef]
- Arechvo, A.; Voicu, D.; Gil, M.M.; Syngelaki, A.; Akolekar, R.; Nicolaides, K.H. Maternal race and pre-eclampsia: Cohort study and systematic review with meta-analysis. BJOG 2022, 129, 2082–2093. [Google Scholar] [CrossRef]
- Boakye, E.; Kwapong, Y.A.; Obisesan, O.; Ogunwole, S.M.; Hays, A.G.; Nasir, K.; Blumenthal, R.S.; Douglas, P.S.; Blaha, M.J.; Hong, X.; et al. Nativity-Related Disparities in Preeclampsia and Cardiovascular Disease Risk Among a Racially Diverse Cohort of US Women. JAMA Netw. Open 2021, 4, e2139564. [Google Scholar] [CrossRef]
- Eggemoen, A.R.; Falk, R.S.; Knutsen, K.V.; Lagerlov, P.; Sletner, L.; Birkeland, K.I.; Jenum, A.K. Vitamin D deficiency and supplementation in pregnancy in a multiethnic population-based cohort. BMC Pregnancy Childbirth 2016, 16, 7. [Google Scholar] [CrossRef] [PubMed]
- Pappas, A.; Kendrick, D.E.; Shankaran, S.; Stoll, B.J.; Bell, E.F.; Laptook, A.R.; Walsh, M.C.; Das, A.; Hale, E.C.; Newman, N.S.; et al. Chorioamnionitis and early childhood outcomes among extremely low-gestational-age neonates. JAMA Pediatr. 2014, 168, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Burris, H.H.; Baccarelli, A.A.; Wright, R.O.; Wright, R.J. Epigenetics: Linking social and environmental exposures to preterm birth. Pediatr. Res. 2016, 79, 136–140. [Google Scholar] [CrossRef]
- Martin, C.L.; Ghastine, L.; Lodge, E.K.; Dhingra, R.; Ward-Caviness, C.K. Understanding Health Inequalities Through the Lens of Social Epigenetics. Annu. Rev. Public. Health 2022, 43, 235–254. [Google Scholar] [CrossRef] [PubMed]
- Battersby, C.; Longford, N.; Mandalia, S.; Costeloe, K.; Modi, N.; UK Neonatal Collaborative Necrotising Enterocolitis (UKNC-NEC) Study Group. Incidence and enteral feed antecedents of severe neonatal necrotising enterocolitis across neonatal networks in England, 2012–2013: A whole-population surveillance study. Lancet Gastroenterol. Hepatol. 2017, 2, 43–51. [Google Scholar] [CrossRef]
- Fitzgibbons, S.C.; Ching, Y.; Yu, D.; Carpenter, J.; Kenny, M.; Weldon, C.; Lillehei, C.; Valim, C.; Horbar, J.D.; Jaksic, T. Mortality of necrotizing enterocolitis expressed by birth weight categories. J. Pediatr. Surg. 2009, 44, 1072–1075; discussion 1075–1076. [Google Scholar] [CrossRef]
- Neu, J.; Singh, R.; Demetrian, M.; Flores-Torres, J.; Hudak, M.; Zupancic, J.A.; Kronstrom, A.; Rastad, J.; Stromberg, S.; Thuresson, M.; et al. Clinical Characteristics of Necrotizing Enterocolitis Diagnosed by Independent Adjudication of Abdominal Radiographs, Laparotomy, or Autopsy in Preterm Infants in the “Connection Trial”. Am. J. Perinatol. 2025, 42, 268–280. [Google Scholar] [CrossRef]
- Pijpers, A.G.H.; Imren, C.; van Varsseveld, O.C.; Schattenkerk, L.D.E.; Keyzer-Dekker, C.M.G.; Hulscher, J.B.F.; Kooi, E.M.W.; van den Akker, C.H.P.; van Schuppen, J.; Taal, H.R.; et al. Risk Factors for 30-day Mortality in Patients with Surgically Treated Necrotizing Enterocolitis: A Multicenter Retrospective Cohort Study. Eur. J. Pediatr. Surg. 2025, 35, 332–340. [Google Scholar] [CrossRef]
- Indrio, F.; Neu, J.; Pettoello-Mantovani, M.; Marchese, F.; Martini, S.; Salatto, A.; Aceti, A. Development of the Gastrointestinal Tract in Newborns as a Challenge for an Appropriate Nutrition: A Narrative Review. Nutrients 2022, 14, 1405. [Google Scholar] [CrossRef]
- Blencowe, H.; Cousens, S.; Oestergaard, M.Z.; Chou, D.; Moller, A.B.; Narwal, R.; Adler, A.; Vera Garcia, C.; Rohde, S.; Say, L.; et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: A systematic analysis and implications. Lancet 2012, 379, 2162–2172. [Google Scholar] [CrossRef]
- Battersby, C.; Santhalingam, T.; Costeloe, K.; Modi, N. Incidence of neonatal necrotising enterocolitis in high-income countries: A systematic review. Arch. Dis. Child. Fetal Neonatal Ed. 2018, 103, F182–F189. [Google Scholar] [CrossRef] [PubMed]
- Qattea, I.; Farghaly, M.A.A.; Kattea, M.O.; Abdula, N.; Mohamed, M.A.; Aly, H. Survival of infants born at periviable gestation: The US national database. Lancet Reg. Health Am. 2022, 14, 100330. [Google Scholar] [CrossRef] [PubMed]
- Manogura, A.C.; Turan, O.; Kush, M.L.; Berg, C.; Bhide, A.; Turan, S.; Moyano, D.; Bower, S.; Nicolaides, K.H.; Galan, H.L.; et al. Predictors of necrotizing enterocolitis in preterm growth-restricted neonates. Am. J. Obstet. Gynecol. 2008, 198, 638.e1–638.e5. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.M.; Kandefer, S.; Walsh, M.C.; Bell, E.F.; Carlo, W.A.; Laptook, A.R.; Sanchez, P.J.; Shankaran, S.; Van Meurs, K.P.; Ball, M.B.; et al. Causes and timing of death in extremely premature infants from 2000 through 2011. N. Engl. J. Med. 2015, 372, 331–340. [Google Scholar] [CrossRef]
- Llanos, A.R.; Moss, M.E.; Pinzon, M.C.; Dye, T.; Sinkin, R.A.; Kendig, J.W. Epidemiology of neonatal necrotising enterocolitis: A population-based study. Paediatr. Perinat. Epidemiol. 2002, 16, 342–349. [Google Scholar] [CrossRef]
- Esposito, F.; Mamone, R.; Di Serafino, M.; Mercogliano, C.; Vitale, V.; Vallone, G.; Oresta, P. Diagnostic imaging features of necrotizing enterocolitis: A narrative review. Quant. Imaging Med. Surg. 2017, 7, 336–344. [Google Scholar] [CrossRef]
- Kim, J.H. Role of Abdominal US in Diagnosis of NEC. Clin. Perinatol. 2019, 46, 119–127. [Google Scholar] [CrossRef]
- Alexander, K.M.; Chan, S.S.; Opfer, E.; Cuna, A.; Fraser, J.D.; Sharif, S.; Khashu, M. Implementation of bowel ultrasound practice for the diagnosis and management of necrotising enterocolitis. Arch. Dis. Child. Fetal Neonatal Ed. 2021, 106, 96–103. [Google Scholar] [CrossRef]
- Bell, M.J.; Ternberg, J.L.; Feigin, R.D.; Keating, J.P.; Marshall, R.; Barton, L.; Brotherton, T. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 1978, 187, 1–7. [Google Scholar] [CrossRef]
- Challis, P.; Larsson, L.; Stoltz Sjostrom, E.; Serenius, F.; Domellof, M.; Elfvin, A. Validation of the diagnosis of necrotising enterocolitis in a Swedish population-based observational study. Acta Paediatr. 2019, 108, 835–841. [Google Scholar] [CrossRef]
- Vermont-Oxford-Database. Vermont Oxford Network Manual of Operations: Part 2 Data Definitions and Infant Data Forms, 2018. Manual of Operations: Part 2 Data Definitions & Infant Data Forms. Available online: www.vtoxford.org (accessed on 10 July 2025).
- Patel, R.M.; Ferguson, J.; McElroy, S.J.; Khashu, M.; Caplan, M.S. Defining necrotizing enterocolitis: Current difficulties and future opportunities. Pediatr. Res. 2020, 88 (Suppl. S1), 10–15. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. CDC/NHSN Surveillance Definitions for Specific Types of Infections. 2025. Available online: https://www.cdc.gov/nhsn/PDFs/pscManual/17pscNosInfDef_current.pdf (accessed on 10 July 2025).
- Battersby, C.; Longford, N.; Costeloe, K.; Modi, N.; UK Neonatal Collaborative Necrotising Enterocolitis Study Group. Development of a Gestational Age-Specific Case Definition for Neonatal Necrotizing Enterocolitis. JAMA Pediatr. 2017, 171, 256–263. [Google Scholar] [CrossRef]
- van der Heide, M.; Hulscher, J.B.F.; Bos, A.F.; Kooi, E.M.W. Near-infrared spectroscopy as a diagnostic tool for necrotizing enterocolitis in preterm infants. Pediatr. Res. 2021, 90, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Wahidi, L.S.; Sherman, J.; Miller, M.M.; Zaghouani, H.; Sherman, M.P. Early Persistent Blood Eosinophilia in Necrotizing Enterocolitis Is a Predictor of Late Complications. Neonatology 2015, 108, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Gill, E.M.; Jung, K.; Qvist, N.; Ellebaek, M.B. Antibiotics in the medical and surgical treatment of necrotizing enterocolitis. A systematic review. BMC Pediatr. 2022, 22, 66. [Google Scholar] [CrossRef]
- Moss, R.L.; Dimmitt, R.A.; Barnhart, D.C.; Sylvester, K.G.; Brown, R.L.; Powell, D.M.; Islam, S.; Langer, J.C.; Sato, T.T.; Brandt, M.L.; et al. Laparotomy versus peritoneal drainage for necrotizing enterocolitis and perforation. N. Engl. J. Med. 2006, 354, 2225–2234. [Google Scholar] [CrossRef]
- Shen, Y.; Lin, Y.; Fang, Y.F.; Wu, D.M.; He, Y.B. Efficacy of peritoneal drainage in very-low-birth-weight neonates with Bell’s stage II necrotizing enterocolitis: A single-center retrospective study. World J. Gastrointest. Surg. 2023, 15, 1416–1422. [Google Scholar] [CrossRef]
- Rath, C.; Samnakay, N.; Deshpande, G.; Sutyak, K.M.; Basani, L.; Simmer, K.; Fiander, M.; Rao, S.C. Peritoneal drainage versus laparotomy as initial treatment for surgical necrotising enterocolitis or spontaneous intestinal perforation in preterm very low birth weight infants. Cochrane Database Syst. Rev. 2025, 6, CD006182. [Google Scholar] [CrossRef]
- Goldfarb, M.; Choi, P.M.; Gollin, G. Primary Anastomosis Versus Stoma for Surgical Necrotizing Enterocolitis in US Children’s Hospitals. J. Surg. Res. 2024, 295, 296–301. [Google Scholar] [CrossRef]
- Ganji, N.; Kalish, B.; Offringa, M.; Li, B.; Anderson, J.; Baruchel, S.; Blakely, M.; De Coppi, P.; Eaton, S.; Gauda, E.; et al. Translating regenerative medicine therapies in neonatal necrotizing enterocolitis. Pediatr. Res. 2024, 96, 1609–1615. [Google Scholar] [CrossRef]
- Weeks, C.L.; Marino, L.V.; Johnson, M.J. A systematic review of the definitions and prevalence of feeding intolerance in preterm infants. Clin. Nutr. 2021, 40, 5576–5586. [Google Scholar] [CrossRef]
- Hadi, V.; Amiri Khosroshahi, R.; Imani, H.; Jahangirfard, B.; Majari, K.; Kiany, F.; Hadi, S. Impact of early versus delayed enteral nutrition on ICU outcomes: A comparative study on mortality, ventilator dependence, and length of stay. Eur. J. Med. Res. 2025, 30, 315. [Google Scholar] [CrossRef]
- Atyeo, C.; Alter, G. The multifaceted roles of breast milk antibodies. Cell 2021, 184, 1486–1499. [Google Scholar] [CrossRef] [PubMed]
- Meek, J.Y.; Noble, L.; Section on Breastfeeding. Policy Statement: Breastfeeding and the Use of Human Milk. Pediatrics 2022, 150, e2022057988. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, C.P.; Ahmad, R.; Fulton, W.B.; Lopez, C.M.; Eke, B.O.; Scheese, D.; Duess, J.W.; Steinway, S.N.; Raouf, Z.; Moore, H.; et al. Human milk oligosaccharides reduce necrotizing enterocolitis-induced neuroinflammation and cognitive impairment in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2023, 325, G23–G41. [Google Scholar] [CrossRef] [PubMed]
- Schanler, R.J.; Shulman, R.J.; Lau, C. Feeding strategies for premature infants: Beneficial outcomes of feeding fortified human milk versus preterm formula. Pediatrics 1999, 103 Pt 1, 1150–1157. [Google Scholar] [CrossRef]
- Adamkin, D.H. Use of human milk and fortification in the NICU. J. Perinatol. 2023, 43, 551–559. [Google Scholar] [CrossRef]
- Premkumar, M.H.; Pammi, M.; Suresh, G. Human milk-derived fortifier versus bovine milk-derived fortifier for prevention of mortality and morbidity in preterm neonates. Cochrane Database Syst. Rev. 2019, 11, CD013145. [Google Scholar] [CrossRef]
- Playford, R.J.; Weiser, M.J. Bovine Colostrum: Its Constituents and Uses. Nutrients 2021, 13, 265. [Google Scholar] [CrossRef]
- Yan, X.; Pan, X.; Ding, L.; Dai, Y.; Chen, J.; Yang, Y.; Li, Y.; Hao, H.; Qiu, H.; Ye, Z.; et al. Bovine colostrum to supplement the first feeding of very preterm infants: The PreColos randomized controlled trial. Clin. Nutr. 2023, 42, 1408–1417. [Google Scholar] [CrossRef]
- Bakshi, S.; Paswan, V.K.; Yadav, S.P.; Bhinchhar, B.K.; Kharkwal, S.; Rose, H.; Kanetkar, P.; Kumar, V.; Al-Zamani, Z.A.S.; Bunkar, D.S. A comprehensive review on infant formula: Nutritional and functional constituents, recent trends in processing and its impact on infants’ gut microbiota. Front. Nutr. 2023, 10, 1194679. [Google Scholar] [CrossRef]
- Werts, A.D.; Fulton, W.B.; Ladd, M.R.; Saad-Eldin, A.; Chen, Y.X.; Kovler, M.L.; Jia, H.; Banfield, E.C.; Buck, R.H.; Goehring, K.; et al. A Novel Role for Necroptosis in the Pathogenesis of Necrotizing Enterocolitis. Cell Mol. Gastroenterol. Hepatol. 2020, 9, 403–423. [Google Scholar] [CrossRef] [PubMed]
- Hackam, D.J.; Sodhi, C.P. Bench to bedside-new insights into the pathogenesis of necrotizing enterocolitis. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Frazer, L.C.; Yamaguchi, Y.; Jania, C.M.; Lanik, W.E.; Gong, Q.; Singh, D.K.; Mackay, S.; Akopyants, N.S.; Good, M. Microfluidic Model of Necrotizing Enterocolitis Incorporating Human Neonatal Intestinal Enteroids and a Dysbiotic Microbiome. J. Vis. Exp. 2023, 197, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Bautista, G.M.; Cera, A.J.; Chaaban, H.; McElroy, S.J. State-of-the-art review and update of in vivo models of necrotizing enterocolitis. Front. Pediatr. 2023, 11, 1161342. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Olson, J.; Yang, J.; Besner, G.E. Heparin-binding EGF-like growth factor promotes neuronal nitric oxide synthase expression and protects the enteric nervous system after necrotizing enterocolitis. Pediatr. Res. 2017, 82, 490–500. [Google Scholar] [CrossRef]
- Sangild, P.T.; Thymann, T.; Schmidt, M.; Stoll, B.; Burrin, D.G.; Buddington, R.K. Invited review: The preterm pig as a model in pediatric gastroenterology. J. Anim. Sci. 2013, 91, 4713–4729. [Google Scholar] [CrossRef]
- Sangild, P.T.; Siggers, R.H.; Schmidt, M.; Elnif, J.; Bjornvad, C.R.; Thymann, T.; Grondahl, M.L.; Hansen, A.K.; Jensen, S.K.; Boye, M.; et al. Diet- and colonization-dependent intestinal dysfunction predisposes to necrotizing enterocolitis in preterm pigs. Gastroenterology 2006, 130, 1776–1792. [Google Scholar] [CrossRef]
- Waligora-Dupriet, A.J.; Dugay, A.; Auzeil, N.; Nicolis, I.; Rabot, S.; Huerre, M.R.; Butel, M.J. Short-chain fatty acids and polyamines in the pathogenesis of necrotizing enterocolitis: Kinetics aspects in gnotobiotic quails. Anaerobe 2009, 15, 138–144. [Google Scholar] [CrossRef]
- Bozeman, A.P.; Dassinger, M.S.; Birusingh, R.J.; Burford, J.M.; Smith, S.D. An animal model of necrotizing enterocolitis (NEC) in preterm rabbits. Fetal Pediatr. Pathol. 2013, 32, 113–122. [Google Scholar] [CrossRef]
- Kelleher, M.A.; Liu, Z.; Wang, X.; Kroenke, C.D.; Houser, L.A.; Dozier, B.L.; Martin, L.D.; Waites, K.B.; McEvoy, C.; Schelonka, R.L.; et al. Beyond the uterine environment: A nonhuman primate model to investigate maternal-fetal and neonatal outcomes following chronic intrauterine infection. Pediatr. Res. 2017, 82, 244–252. [Google Scholar] [CrossRef][Green Version]
- Suntsova, M.V.; Buzdin, A.A. Differences between human and chimpanzee genomes and their implications in gene expression, protein functions and biochemical properties of the two species. BMC Genomics 2020, 21 (Suppl. S7), 535. [Google Scholar] [CrossRef]
- Namachivayam, K.; Blanco, C.L.; MohanKumar, K.; Jagadeeswaran, R.; Vasquez, M.; McGill-Vargas, L.; Garzon, S.A.; Jain, S.K.; Gill, R.K.; Freitag, N.E.; et al. Smad7 inhibits autocrine expression of TGF-beta2 in intestinal epithelial cells in baboon necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G167–G180. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, C.P.; Neal, M.D.; Siggers, R.; Sho, S.; Ma, C.; Branca, M.F.; Prindle, T., Jr.; Russo, A.M.; Afrazi, A.; Good, M.; et al. Intestinal epithelial Toll-like receptor 4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology 2012, 143, 708–718.e5. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, C.P.; Wipf, P.; Yamaguchi, Y.; Fulton, W.B.; Kovler, M.; Nino, D.F.; Zhou, Q.; Banfield, E.; Werts, A.D.; Ladd, M.R.; et al. Insights image for “The human milk oligosaccharides 2’-fucosyllactose and 6’-sialyllactose protect against the development of necrotizing enterocolitis by inhibiting toll-like receptor 4 signaling”. Pediatr. Res. 2021, 89, 248. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.S.; Liu, Y.; Wang, X.W.; Xu, B.; Lin, Y.L.; Song, Y.; Dong, Y.; Liu, J.L.; Wang, X.J.; Liu, S.; et al. LPS Enhances the Chemosensitivity of Oxaliplatin in HT29 Cells via GSDMD-Mediated Pyroptosis. Cancer Manag. Res. 2020, 12, 10397–10409. [Google Scholar] [CrossRef]
- Smith, H.S. In vitro properties of epithelial cell lines established from human carcinomas and nonmalignant tissue. J. Natl. Cancer Inst. 1979, 62, 225–230. [Google Scholar]
- Day, R.S., 3rd; Ziolkowski, C.H. Human brain tumour cell strains with deficient host-cell reactivation of N-methyl-N’-nitro-N-nitrosoguanidine-damaged adenovirus 5. Nature 1979, 279, 797–799. [Google Scholar] [CrossRef]
- Scheese, D.; Lu, P.; Moore, H.; Tsuboi, K.; Tragesser, C.; Duess, J.; Raouf, Z.; Sampah, M.F.; Klerk, D.; El Baassiri, M.; et al. Cytomegalovirus Worsens Necrotizing Enterocolitis Severity in Mice via Increased Toll-Like Receptor 4 Signaling. Cell Mol. Gastroenterol. Hepatol. 2025, 19, 101473. [Google Scholar] [CrossRef]
- Valiei, A.; Aminian-Dehkordi, J.; Mofrad, M.R.K. Gut-on-a-chip models for dissecting the gut microbiology and physiology. APL Bioeng. 2023, 7, 011502. [Google Scholar] [CrossRef]
- Lanik, W.E.; Luke, C.J.; Nolan, L.S.; Gong, Q.; Frazer, L.C.; Rimer, J.M.; Gale, S.E.; Luc, R.; Bidani, S.S.; Sibbald, C.A.; et al. Microfluidic device facilitates in vitro modeling of human neonatal necrotizing enterocolitis-on-a-chip. JCI Insight 2023, 8, e146496. [Google Scholar] [CrossRef]
- Vallender, E.J.; Hotchkiss, C.E.; Lewis, A.D.; Rogers, J.; Stern, J.A.; Peterson, S.M.; Ferguson, B.; Sayers, K. Nonhuman primate genetic models for the study of rare diseases. Orphanet J. Rare Dis. 2023, 18, 20. [Google Scholar] [CrossRef]
- Weaver, L.T.; Laker, M.F.; Nelson, R. Intestinal permeability in the newborn. Arch. Dis. Child. 1984, 59, 236–241. [Google Scholar] [CrossRef] [PubMed]
- van Elburg, R.M.; Fetter, W.P.; Bunkers, C.M.; Heymans, H.S. Intestinal permeability in relation to birth weight and gestational and postnatal age. Arch. Dis. Child. Fetal Neonatal Ed. 2003, 88, F52–F55. [Google Scholar] [CrossRef] [PubMed]
- Adegboye, C.; Emeonye, C.; Wu, Y.S.; Kwon, J.; Oliveira, L.F.S.; Raveeniraraj, S.; O’Connell, A.E. Necrotizing enterocolitis causes increased ileal goblet cell loss in Wnt2b KO mice. bioRxiv 2025. [Google Scholar] [CrossRef]
- Ireland, H.; Houghton, C.; Howard, L.; Winton, D.J. Cellular inheritance of a Cre-activated reporter gene to determine Paneth cell longevity in the murine small intestine. Dev. Dyn. 2005, 233, 1332–1336. [Google Scholar] [CrossRef]
- Coutinho, H.B.; da Mota, H.C.; Coutinho, V.B.; Robalinho, T.I.; Furtado, A.F.; Walker, E.; King, G.; Mahida, Y.R.; Sewell, H.F.; Wakelin, D. Absence of lysozyme (muramidase) in the intestinal Paneth cells of newborn infants with necrotising enterocolitis. J. Clin. Pathol. 1998, 51, 512–514. [Google Scholar] [CrossRef]
- Lueschow, S.R.; Stumphy, J.; Gong, H.; Kern, S.L.; Elgin, T.G.; Underwood, M.A.; Kalanetra, K.M.; Mills, D.A.; Wong, M.H.; Meyerholz, D.K.; et al. Loss of murine Paneth cell function alters the immature intestinal microbiome and mimics changes seen in neonatal necrotizing enterocolitis. PLoS ONE 2018, 13, e0204967. [Google Scholar] [CrossRef]
- Meyer, A.R.; Brown, M.E.; McGrath, P.S.; Dempsey, P.J. Injury-Induced Cellular Plasticity Drives Intestinal Regeneration. Cell Mol. Gastroenterol. Hepatol. 2022, 13, 843–856. [Google Scholar] [CrossRef]
- Afrazi, A.; Branca, M.F.; Sodhi, C.P.; Good, M.; Yamaguchi, Y.; Egan, C.E.; Lu, P.; Jia, H.; Shaffiey, S.; Lin, J.; et al. Toll-like receptor 4-mediated endoplasmic reticulum stress in intestinal crypts induces necrotizing enterocolitis. J. Biol. Chem. 2014, 289, 9584–9599. [Google Scholar] [CrossRef]
- Sodhi, C.P.; Shi, X.H.; Richardson, W.M.; Grant, Z.S.; Shapiro, R.A.; Prindle, T., Jr.; Branca, M.; Russo, A.; Gribar, S.C.; Ma, C.; et al. Toll-like receptor-4 inhibits enterocyte proliferation via impaired beta-catenin signaling in necrotizing enterocolitis. Gastroenterology 2010, 138, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Neal, M.D.; Sodhi, C.P.; Jia, H.; Dyer, M.; Egan, C.E.; Yazji, I.; Good, M.; Afrazi, A.; Marino, R.; Slagle, D.; et al. Toll-like receptor 4 is expressed on intestinal stem cells and regulates their proliferation and apoptosis via the p53 up-regulated modulator of apoptosis. J. Biol. Chem. 2012, 287, 37296–37308. [Google Scholar] [CrossRef] [PubMed]
- Neal, M.D.; Sodhi, C.P.; Dyer, M.; Craig, B.T.; Good, M.; Jia, H.; Yazji, I.; Afrazi, A.; Richardson, W.M.; Beer-Stolz, D.; et al. A critical role for TLR4 induction of autophagy in the regulation of enterocyte migration and the pathogenesis of necrotizing enterocolitis. J. Immunol. 2013, 190, 3541–3551. [Google Scholar] [CrossRef]
- Pammi, M.; Cope, J.; Tarr, P.I.; Warner, B.B.; Morrow, A.L.; Mai, V.; Gregory, K.E.; Kroll, J.S.; McMurtry, V.; Ferris, M.J.; et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: A systematic review and meta-analysis. Microbiome 2017, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R.F. Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front. Immunol. 2022, 13, 812774. [Google Scholar] [CrossRef]
- Takeuchi, O.; Hoshino, K.; Kawai, T.; Sanjo, H.; Takada, H.; Ogawa, T.; Takeda, K.; Akira, S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 1999, 11, 443–451. [Google Scholar] [CrossRef]
- Cario, E.; Gerken, G.; Podolsky, D.K. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology 2007, 132, 1359–1374. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, L.; Fatheree, N.Y.; Liu, X.; Pacheco, S.E.; Tatevian, N.; Rhoads, J.M. Changes in intestinal Toll-like receptors and cytokines precede histological injury in a rat model of necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G442–G450. [Google Scholar] [CrossRef][Green Version]
- Chassaing, B.; Ley, R.E.; Gewirtz, A.T. Intestinal epithelial cell toll-like receptor 5 regulates the intestinal microbiota to prevent low-grade inflammation and metabolic syndrome in mice. Gastroenterology 2014, 147, 1363–1377.e17. [Google Scholar] [CrossRef]
- Pedersen, G.; Andresen, L.; Matthiessen, M.W.; Rask-Madsen, J.; Brynskov, J. Expression of Toll-like receptor 9 and response to bacterial CpG oligodeoxynucleotides in human intestinal epithelium. Clin. Exp. Immunol. 2005, 141, 298–306. [Google Scholar] [CrossRef]
- Gribar, S.C.; Sodhi, C.P.; Richardson, W.M.; Anand, R.J.; Gittes, G.K.; Branca, M.F.; Jakub, A.; Shi, X.H.; Shah, S.; Ozolek, J.A.; et al. Reciprocal expression and signaling of TLR4 and TLR9 in the pathogenesis and treatment of necrotizing enterocolitis. J. Immunol. 2009, 182, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Kovler, M.L.; Gonzalez Salazar, A.J.; Fulton, W.B.; Lu, P.; Yamaguchi, Y.; Zhou, Q.; Sampah, M.; Ishiyama, A.; Prindle, T., Jr.; Wang, S.; et al. Toll-like receptor 4-mediated enteric glia loss is critical for the development of necrotizing enterocolitis. Sci. Transl. Med. 2021, 13, eabg3459. [Google Scholar] [CrossRef] [PubMed]
- Yazji, I.; Sodhi, C.P.; Lee, E.K.; Good, M.; Egan, C.E.; Afrazi, A.; Neal, M.D.; Jia, H.; Lin, J.; Ma, C.; et al. Endothelial TLR4 activation impairs intestinal microcirculatory perfusion in necrotizing enterocolitis via eNOS-NO-nitrite signaling. Proc. Natl. Acad. Sci. USA 2013, 110, 9451–9456. [Google Scholar] [CrossRef]
- Bowker, R.M.; Yan, X.; De Plaen, I.G. Intestinal microcirculation and necrotizing enterocolitis: The vascular endothelial growth factor system. Semin. Fetal Neonatal Med. 2018, 23, 411–415. [Google Scholar] [CrossRef]
- Senarathna, J.; Kovler, M.; Prasad, A.; Bhargava, A.; Thakor, N.V.; Sodhi, C.P.; Hackam, D.J.; Pathak, A.P. In vivo phenotyping of the microvasculature in necrotizing enterocolitis with multicontrast optical imaging. Microcirculation 2022, 29, e12768. [Google Scholar] [CrossRef]
- Jones, I.H.; Collins, J.E.; Hall, N.J.; Heinson, A.I. Transcriptomic analysis of the effect of remote ischaemic conditioning in an animal model of necrotising enterocolitis. Sci. Rep. 2024, 14, 10783. [Google Scholar] [CrossRef]
- Boskabadi, H.; Ghayour-Mobarhan, M.; Saeidinia, A. Serum pro-oxidant/antioxidant balance in term versus preterm neonates. Medicine 2022, 101, e31381. [Google Scholar] [CrossRef]
- Lee, J.W.; Davis, J.M. Future applications of antioxidants in premature infants. Curr. Opin. Pediatr. 2011, 23, 161–166. [Google Scholar] [CrossRef]
- Xiong, X.; Wu, L.; Liu, X.; Wang, J.; Xiao, J.; Chen, K.; Zhuansun, D.; Meng, X.; Feng, J.; Chen, X. Comprehensive Characterization of the Oxidative Stress Profiles in Neonatal Necrotizing Enterocolitis. Int. J. Med. Sci. 2025, 22, 2139–2154. [Google Scholar] [CrossRef]
- Sodhi, C.P.; Gonzalez Salazar, A.J.; Kovler, M.L.; Fulton, W.B.; Yamaguchi, Y.; Ishiyama, A.; Wang, S.; Prindle, T., Jr.; Vurma, M.; Das, T.; et al. The administration of a pre-digested fat-enriched formula prevents necrotising enterocolitis-induced lung injury in mice. Br. J. Nutr. 2022, 128, 1050–1063. [Google Scholar] [CrossRef]
- Chokshi, N.K.; Guner, Y.S.; Hunter, C.J.; Upperman, J.S.; Grishin, A.; Ford, H.R. The role of nitric oxide in intestinal epithelial injury and restitution in neonatal necrotizing enterocolitis. Semin. Perinatol. 2008, 32, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Nam, Y.W.; Kim, S.; Oh, D.B.; Song, J. Necroptosis molecular mechanisms: Recent findings regarding novel necroptosis regulators. Exp. Mol. Med. 2021, 53, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.E.; Price, D.R.; Ryter, S.W.; Choi, A.M.K. Necroptosis: A crucial pathogenic mediator of human disease. JCI Insight 2019, 4, e128834. [Google Scholar] [CrossRef]
- Ferrand, A.; Al Nabhani, Z.; Tapias, N.S.; Mas, E.; Hugot, J.P.; Barreau, F. NOD2 Expression in Intestinal Epithelial Cells Protects Toward the Development of Inflammation and Associated Carcinogenesis. Cell Mol. Gastroenterol. Hepatol. 2019, 7, 357–369. [Google Scholar] [CrossRef]
- Hartel, C.; Hartz, A.; Pagel, J.; Rupp, J.; Stein, A.; Kribs, A.; Muller, A.; Haase, R.; Gille, C.; Bottger, R.; et al. NOD2 Loss-of-Function Mutations and Risks of Necrotizing Enterocolitis or Focal Intestinal Perforation in Very Low-birth-weight Infants. Inflamm. Bowel Dis. 2016, 22, 249–256. [Google Scholar] [CrossRef][Green Version]
- Zhu, F.; Wang, L.; Gong, Z.; Wang, Y.; Gao, Y.; Cai, W.; Wu, J. Blockage of NLRP3 inflammasome activation ameliorates acute inflammatory injury and long-term cognitive impairment induced by necrotizing enterocolitis in mice. J. Neuroinflammation 2021, 18, 66. [Google Scholar] [CrossRef]
- Netea, M.G.; Schlitzer, A.; Placek, K.; Joosten, L.A.B.; Schultze, J.L. Innate and Adaptive Immune Memory: An Evolutionary Continuum in the Host’s Response to Pathogens. Cell Host Microbe 2019, 25, 13–26. [Google Scholar] [CrossRef]
- Nino, D.F.; Sodhi, C.P.; Hackam, D.J. Necrotizing enterocolitis: New insights into pathogenesis and mechanisms. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 590–600. [Google Scholar] [CrossRef]
- Sampah, M.E.S.; Hackam, D.J. Dysregulated Mucosal Immunity and Associated Pathogeneses in Preterm Neonates. Front. Immunol. 2020, 11, 899. [Google Scholar] [CrossRef]
- Van Belkum, M.; Mendoza Alvarez, L.; Neu, J. Preterm neonatal immunology at the intestinal interface. Cell Mol. Life Sci. 2020, 77, 1209–1227. [Google Scholar] [CrossRef]
- Klinke, M.; Chaaban, H.; Boettcher, M. The role of neutrophil extracellular traps in necrotizing enterocolitis. Front. Pediatr. 2023, 11, 1121193. [Google Scholar] [CrossRef]
- Zhang, W.; He-Yang, J.; Zhuang, W.; Liu, J.; Zhou, X. Causative role of mast cell and mast cell-regulatory function of disialyllacto-N-tetraose in necrotizing enterocolitis. Int. Immunopharmacol. 2021, 96, 107597. [Google Scholar] [CrossRef] [PubMed]
- Christensen, R.D.; Lambert, D.K.; Gordon, P.V.; Baer, V.L.; Gerday, E.; Henry, E. Neonates presenting with bloody stools and eosinophilia can progress to two different types of necrotizing enterocolitis. J. Perinatol. 2012, 32, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Sampah, M.E.S.; Hackam, D.J. Prenatal Immunity and Influences on Necrotizing Enterocolitis and Associated Neonatal Disorders. Front. Immunol. 2021, 12, 650709. [Google Scholar] [CrossRef] [PubMed]
- Mass, E.; Ballesteros, I.; Farlik, M.; Halbritter, F.; Gunther, P.; Crozet, L.; Jacome-Galarza, C.E.; Handler, K.; Klughammer, J.; Kobayashi, Y.; et al. Specification of tissue-resident macrophages during organogenesis. Science 2016, 353, aaf4238. [Google Scholar] [CrossRef]
- Palis, J.; Yoder, M.C. Yolk-sac hematopoiesis: The first blood cells of mouse and man. Exp. Hematol. 2001, 29, 927–936. [Google Scholar] [CrossRef]
- Viola, M.F.; Boeckxstaens, G. Niche-specific functional heterogeneity of intestinal resident macrophages. Gut 2021, 70, 1383–1395. [Google Scholar] [CrossRef]
- Shaw, T.N.; Houston, S.A.; Wemyss, K.; Bridgeman, H.M.; Barbera, T.A.; Zangerle-Murray, T.; Strangward, P.; Ridley, A.J.L.; Wang, P.; Tamoutounour, S.; et al. Tissue-resident macrophages in the intestine are long lived and defined by Tim-4 and CD4 expression. J. Exp. Med. 2018, 215, 1507–1518. [Google Scholar] [CrossRef]
- De Schepper, S.; Verheijden, S.; Aguilera-Lizarraga, J.; Viola, M.F.; Boesmans, W.; Stakenborg, N.; Voytyuk, I.; Schmidt, I.; Boeckx, B.; Dierckx de Casterle, I.; et al. Self-Maintaining Gut Macrophages Are Essential for Intestinal Homeostasis. Cell 2018, 175, 400–415.e13. [Google Scholar] [CrossRef]
- Bain, C.C.; Bravo-Blas, A.; Scott, C.L.; Perdiguero, E.G.; Geissmann, F.; Henri, S.; Malissen, B.; Osborne, L.C.; Artis, D.; Mowat, A.M. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat. Immunol. 2014, 15, 929–937. [Google Scholar] [CrossRef]
- Tokuyama, H.; Ueha, S.; Kurachi, M.; Matsushima, K.; Moriyasu, F.; Blumberg, R.S.; Kakimi, K. The simultaneous blockade of chemokine receptors CCR2, CCR5 and CXCR3 by a non-peptide chemokine receptor antagonist protects mice from dextran sodium sulfate-mediated colitis. Int. Immunol. 2005, 17, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Qualls, J.E.; Kaplan, A.M.; van Rooijen, N.; Cohen, D.A. Suppression of experimental colitis by intestinal mononuclear phagocytes. J. Leukoc. Biol. 2006, 80, 802–815. [Google Scholar] [CrossRef] [PubMed]
- Platt, A.M.; Bain, C.C.; Bordon, Y.; Sester, D.P.; Mowat, A.M. An independent subset of TLR expressing CCR2-dependent macrophages promotes colonic inflammation. J. Immunol. 2010, 184, 6843–6854. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, E.; Varol, C.; Farache, J.; Elmaliah, E.; Satpathy, A.T.; Friedlander, G.; Mack, M.; Shpigel, N.; Boneca, I.G.; Murphy, K.M.; et al. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity 2012, 37, 1076–1090. [Google Scholar] [CrossRef]
- Neudecker, V.; Haneklaus, M.; Jensen, O.; Khailova, L.; Masterson, J.C.; Tye, H.; Biette, K.; Jedlicka, P.; Brodsky, K.S.; Gerich, M.E.; et al. Myeloid-derived miR-223 regulates intestinal inflammation via repression of the NLRP3 inflammasome. J. Exp. Med. 2017, 214, 1737–1752. [Google Scholar] [CrossRef]
- Becker, F.; Holthoff, C.; Anthoni, C.; Rijcken, E.; Alexander, J.S.; Gavins, F.N.; Spiegel, H.U.; Senninger, N.; Vowinkel, T. Downregulation of CX(3)CR1 ameliorates experimental colitis: Evidence for CX(3)CL1-CX(3)CR1-mediated immune cell recruitment. Int. J. Colorectal Dis. 2017, 32, 315–324. [Google Scholar] [CrossRef]
- Managlia, E.; Liu, S.X.L.; Yan, X.; Tan, X.D.; Chou, P.M.; Barrett, T.A.; De Plaen, I.G. Blocking NF-kappaB Activation in Ly6c(+) Monocytes Attenuates Necrotizing Enterocolitis. Am. J. Pathol. 2019, 189, 604–618. [Google Scholar] [CrossRef]
- Maheshwari, A.; Kelly, D.R.; Nicola, T.; Ambalavanan, N.; Jain, S.K.; Murphy-Ullrich, J.; Athar, M.; Shimamura, M.; Bhandari, V.; Aprahamian, C.; et al. TGF-beta2 suppresses macrophage cytokine production and mucosal inflammatory responses in the developing intestine. Gastroenterology 2011, 140, 242–253. [Google Scholar] [CrossRef]
- MohanKumar, K.; Namachivayam, K.; Chapalamadugu, K.C.; Garzon, S.A.; Premkumar, M.H.; Tipparaju, S.M.; Maheshwari, A. Smad7 interrupts TGF-beta signaling in intestinal macrophages and promotes inflammatory activation of these cells during necrotizing enterocolitis. Pediatr. Res. 2016, 79, 951–961. [Google Scholar] [CrossRef]
- Olaloye, O.O.; Liu, P.; Toothaker, J.M.; McCourt, B.T.; McCourt, C.C.; Xiao, J.; Prochaska, E.; Shaffer, S.; Werner, L.; Faculty, U.N.; et al. CD16+CD163+ monocytes traffic to sites of inflammation during necrotizing enterocolitis in premature infants. J. Exp. Med. 2021, 218, e20200344. [Google Scholar] [CrossRef]
- Hui, L.; Dai, Y.; Guo, Z.; Zhang, J.; Zheng, F.; Bian, X.; Wu, Z.; Jiang, Q.; Guo, M.; Ma, K.; et al. Immunoregulation effects of different gammadeltaT cells and toll-like receptor signaling pathways in neonatal necrotizing enterocolitis. Medicine 2017, 96, e6077. [Google Scholar] [CrossRef]
- Dingle, B.M.; Liu, Y.; Fatheree, N.Y.; Min, J.; Rhoads, J.M.; Tran, D.Q. FoxP3(+) regulatory T cells attenuate experimental necrotizing enterocolitis. PLoS ONE 2013, 8, e82963. [Google Scholar] [CrossRef] [PubMed]
- Weitkamp, J.H.; Rosen, M.J.; Zhao, Z.; Koyama, T.; Geem, D.; Denning, T.L.; Rock, M.T.; Moore, D.J.; Halpern, M.D.; Matta, P.; et al. Small intestinal intraepithelial TCRgammadelta+ T lymphocytes are present in the premature intestine but selectively reduced in surgical necrotizing enterocolitis. PLoS ONE 2014, 9, e99042. [Google Scholar] [CrossRef] [PubMed]
- Emami, C.N.; Mittal, R.; Wang, L.; Ford, H.R.; Prasadarao, N.V. Role of neutrophils and macrophages in the pathogenesis of necrotizing enterocolitis caused by Cronobacter sakazakii. J. Surg. Res. 2012, 172, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Musemeche, C.; Caplan, M.; Hsueh, W.; Sun, X.; Kelly, A. Experimental necrotizing enterocolitis: The role of polymorphonuclear neutrophils. J. Pediatr. Surg. 1991, 26, 1047–1049; discussion 1049–1050. [Google Scholar] [CrossRef]
- Klinke, M.; Vincent, D.; Trochimiuk, M.; Appl, B.; Tiemann, B.; Reinshagen, K.; Pagerols Raluy, L.; Boettcher, M. Development of an improved murine model of necrotizing enterocolitis shows the importance of neutrophils in NEC pathogenesis. Sci. Rep. 2020, 10, 8049. [Google Scholar] [CrossRef]
- Vincent, D.; Klinke, M.; Eschenburg, G.; Trochimiuk, M.; Appl, B.; Tiemann, B.; Bergholz, R.; Reinshagen, K.; Boettcher, M. NEC is likely a NETs dependent process and markers of NETosis are predictive of NEC in mice and humans. Sci. Rep. 2018, 8, 12612. [Google Scholar] [CrossRef]
- Marcos, V.; Nussbaum, C.; Vitkov, L.; Hector, A.; Wiedenbauer, E.M.; Roos, D.; Kuijpers, T.; Krautgartner, W.D.; Genzel-Boroviczeny, O.; Sperandio, M.; et al. Delayed but functional neutrophil extracellular trap formation in neonates. Blood 2009, 114, 4908–4911; author reply 4911–4912. [Google Scholar] [CrossRef]
- Juul, S.E.; Haynes, J.W.; McPherson, R.J. Evaluation of eosinophilia in hospitalized preterm infants. J. Perinatol. 2005, 25, 182–188. [Google Scholar] [CrossRef]
- Travers, J.; Rothenberg, M.E. Eosinophils in mucosal immune responses. Mucosal Immunol. 2015, 8, 464–475. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Alysandratos, K.D.; Angelidou, A.; Delivanis, D.A.; Sismanopoulos, N.; Zhang, B.; Asadi, S.; Vasiadi, M.; Weng, Z.; Miniati, A.; et al. Mast cells and inflammation. Biochim. Biophys. Acta 2012, 1822, 21–33. [Google Scholar] [CrossRef]
- Walsh, K.T.; Zemper, A.E. The Enteric Nervous System for Epithelial Researchers: Basic Anatomy, Techniques, and Interactions With the Epithelium. Cell Mol. Gastroenterol. Hepatol. 2019, 8, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, J.; Watkins, D.J.; Boomer, L.A.; Matthews, M.A.; Su, Y.; Besner, G.E. Enteric nervous system abnormalities are present in human necrotizing enterocolitis: Potential neurotransplantation therapy. Stem Cell Res. Ther. 2013, 4, 157. [Google Scholar] [CrossRef] [PubMed]
- More, K.; Hanumantharaju, A.; Amrit, A.; Nimbalkar, S.M.; Patole, S. Use of Probiotics for Preventing Necrotizing Enterocolitis in Preterm Infants: A Survey of Current Practices Among Indian Neonatologists. Cureus 2024, 16, e73923. [Google Scholar] [CrossRef] [PubMed]
- Morvaridzadeh, M.; Sadeghi, E.; Agah, S.; Nachvak, S.M.; Fazelian, S.; Moradi, F.; Persad, E.; Heshmati, J. Effect of melatonin supplementation on oxidative stress parameters: A systematic review and meta-analysis. Pharmacol. Res. 2020, 161, 105210. [Google Scholar] [CrossRef]
- Provitera, L.; Tomaselli, A.; Raffaeli, G.; Crippa, S.; Arribas, C.; Amodeo, I.; Gulden, S.; Amelio, G.S.; Cortesi, V.; Manzoni, F.; et al. Human Bone Marrow-Derived Mesenchymal Stromal Cells Reduce the Severity of Experimental Necrotizing Enterocolitis in a Concentration-Dependent Manner. Cells 2023, 12, 760. [Google Scholar] [CrossRef]
- Balsamo, F.; Tian, Y.; Pierro, A.; Li, B. Amniotic fluid stem cells: A novel treatment for necrotizing enterocolitis. Front. Pediatr. 2022, 10, 1020986. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Wan, Q.; Bu, C.; Jin, W.; Yuan, F.; Zhou, W. Intestinal stem cell-derived extracellular vesicles ameliorate necrotizing enterocolitis injury. Mol. Cell Probes 2025, 79, 101997. [Google Scholar] [CrossRef]
- Maltais-Bilodeau, C.; Henckel, E.; Deguise, M.O.; Lesage, F.; Cobey, K.D.; Ahmadzai, N.; Skidmore, B.; Ferretti, E.; Thebaud, B. Cell-based therapies in preclinical models of necrotizing enterocolitis: A systematic review and meta-analysis. Stem Cells Transl. Med. 2025, 14, szae102. [Google Scholar] [CrossRef]
- Deianova, N.; El Manouni El Hassani, S.; Struijs, E.A.; Jansen, E.E.W.; Bakkali, A.; van de Wiel, M.A.; de Boode, W.P.; Hulzebos, C.V.; van Kaam, A.H.; Kramer, B.W.; et al. Fecal amine metabolite analysis before onset of severe necrotizing enterocolitis in preterm infants: A prospective case-control study. Sci. Rep. 2022, 12, 12310. [Google Scholar] [CrossRef]
- Oshima, K.; Hinoki, A.; Uchida, H.; Tanaka, Y.; Okuno, Y.; Go, Y.; Shirota, C.; Tainaka, T.; Sumida, W.; Yokota, K.; et al. Single-cell RNA sequencing of intestinal immune cells in neonatal necrotizing enterocolitis. Pediatr. Surg. Int. 2023, 39, 179. [Google Scholar] [CrossRef]
- Calvo, L.N.; Greenberg, R.G.; Gray, K.D. Safety and Effectiveness of Probiotics in Preterm Infants with Necrotizing Enterocolitis. Neoreviews 2024, 25, e193–e206. [Google Scholar] [CrossRef]
- Arnold, M.J. Predicting and Preventing Preterm Birth: Recommendations From ACOG. Am. Fam. Physician 2022, 106, 337–339. [Google Scholar]
- McDougall, A.R.A.; Hastie, R.; Goldstein, M.; Tuttle, A.; Ammerdorffer, A.; Gulmezoglu, A.M.; Vogel, J.P. New medicines for spontaneous preterm birth prevention and preterm labour management: Landscape analysis of the medicine development pipeline. BMC Pregnancy Childbirth 2023, 23, 525. [Google Scholar] [CrossRef]
| Parameter | Summary Data | Key Trends | References |
|---|---|---|---|
| Overall incidence | 2–7% of very low birth weight (VLBW; <1500 g) infants | Higher rates in extremely preterm (<28 weeks) | [1] |
| Gestational age | <28 weeks: 7–10% incidence; 28–32 weeks: 2–5%; >32 weeks: <1% | Strong inverse correlation between gestational age and NEC risk | [1,45,46,47,48] |
| Birth weight | <1000 g: 8–12%; 1000–1500 g: 3–6%; >1500 g: <1% | Lower birth weight associated with greater severity and mortality | [45] |
| Mortality | 15–30% overall; higher in surgical NEC (>50% in ELBW infants) | Mortality has remained high despite advances in NICU care | [1,35,49] |
| Racial/ethnic disparities | Higher incidence in non-Hispanic Black infants vs. White infants; disparities persist after adjusting for GA/BW | Suggests contributions from socioeconomic, healthcare access, and feeding practices | [2,35] |
| Long-term outcomes | 20–50% risk of neurodevelopmental impairment in survivors; increased risk of short bowel syndrome and growth failure | NEC is a leading cause of post-NICU morbidity | [5,6,7,8] |
| Economic burden | Median additional cost per NEC case: $70,000–$180,000 USD; national annual cost >$500 million USD | Surgical NEC associated with 3–4 × higher costs than medical NEC | [1] |
| Model Type | Key Features | Advantages | Limitations | References |
|---|---|---|---|---|
| Small Animal Rodent Models (Mouse, Rat) | Formula feeding combined with hypoxia or cold stress, with or without LPS or NEC-causing bacteria. | Low cost, short gestation period, large litter sizes, and availability of genetic knockouts, especially mouse mutants (KO, transgenic, e.g., TLR4). | Limited resemblance to human pathophysiology, Less tissue availability for analysis | [15,18,22,87,88] |
| Preterm Piglet | Delivered by C-section at 90–95% gestation; fed formula with optional bacterial inoculation. | Closest to human preterm gut anatomy, physiology, and immune development; allows clinically relevant feeding studies. | High costs, specialized facilities, ethical and logistical constraints | [15,77,87,89,90] |
| Quails | Germ-free neonatal quails fed orally with Clostridium butyricum. | Offers a spontaneous way to study NEC, mimics key human like NEC features (cecal wall thickening, pneumatosis, hemorrhage, and mucosal necrosis). | Uncommon, species-specific immune features and anatomical differences | [91] |
| Rabbit | Preterm cesarean, exposure to enteral feeding, Enterobacter cloacae colonization, pharmacological agents (ranitidine, indomethacin) | Histopathological changes mirror human NEC, ranging from villous tip sloughing to transmural necrosis | Moderate survival rates, limited genetic manipulations, and more expansive compared to rodents. | [92] |
| Non-human Primates—rhesus macaques | Chronic intra-amniotic infection with Ureaplasma parvum induces preterm birth, systemic fetal inflammation, and NEC like pathology. | Offer unparalleled physiological relevance for studying human NEC, 95–98.5% genetic similarity to humans | Rare or nearly nonexistent, expensive, and regulatory barriers | [93,94] |
| Non-human Primates Baboon | Premature 125 days of gestation, 67% of the term, equivalent to 26–27 weeks in humans), managed with neonatal intensive care protocols, develop spontaneous NEC. | Offers a highly translational non-human primate system with clinical, radiologic, and histopathologic features that closely resemble those of human disease. | High cost and logistical challenges, expensive, and regulatory barriers | [95] |
| IEC6, Caco-2, HT-29, HTB-38 | Epithelial cell lines, In vitro models treated with stimulants such as LPS, NEC bacteria, H2O2, Pro-inflammatory cytokines | Allows highly controlled treatments to study cell-specific effects. | Treatments do mimic in vivo models, low translational value | [18,96,97,98,99,100,101] |
| Ex vivo intestine | Isolated intestinal tissue exposed to LPS, cytokines, or formula | Allows controlled mechanistic studies; preserves tissue architecture | Short viability; no systemic immune or vascular contribution | [18,97] |
| Intestinal Organoids | 3D cultures derived from human or animal stem cells; exposed to inflammatory stimuli. | Human-derived tissues, Scalable for multiplexing, and suitable for molecular manipulation. | Lack immune, vascular, and nervous components; immature phenotype | [84] |
| NEC-on-a-Chip | Microfluidic devices mimicking gut epithelium, microbiota, immune interactions | High control over microenvironment; real-time imaging; potential for personalized testing | Still experimental; may not fully replicate in vivo complexity | [86,102,103] |
| Intervention/Molecule | Preventative/Therapeutic/Mechanism of Action | Translational Stage | References |
|---|---|---|---|
| Human Breast milk/Donor milk | Mother’s own milk or donor milk provides the highest protection, naturally rich in immunomodulatory and anti-inflammatory agents (e.g., lactoferrin, sIgA, cytokines, EGF, TGF, HMOs). | Fully translational and widely accepted | [2,25,26,27,28,29,76,77] |
| HMOs fortification | Prebiotic effect, block pathogen adhesion, modulate immunity, inhibit TLR4 signaling | Clinical, HMOs fortified infant formulae | [25,26,27,28,29,77,78] |
| Probiotics (Bifidobacterium, Lactobacillus) | Modulate gut microbiota, enhance barrier function, reduce inflammation | Clinical trials (multiple RCTs) | [22,23,177] |
| Bovine Colostrum | Immune-modulatory proteins, growth factors, enhance barrier repair | Early-phase clinical | [79,81] |
| TLR4 Antagonists (e.g., Compound-34) | Block TLR4 signaling to reduce inflammation and apoptosis | Pre-clinical | [19,96,112] |
| Stem Cell Therapy (amniotic fluid–derived MSCs) | Promote mucosal repair, immunomodulation | Pre-clinical | [179,180,181,182] |
| Lactoferrin | Antimicrobial, anti-inflammatory, iron-binding | Clinical trials | [83] |
| Antioxidants (N-acetylcysteine, melatonin) | Reduce oxidative stress, protect mitochondria | Pre-clinical | [7,27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishiyama, A.; Jang, H.-S.; Dintaman, J.M.; Duess, J.W.; Tragesser, C.; Sodhi, C.P. Necrotizing Enterocolitis: A Comprehensive Review on Toll-like Receptor 4-Mediated Pathophysiology, Clinical, and Therapeutic Insights. Biomedicines 2025, 13, 2288. https://doi.org/10.3390/biomedicines13092288
Ishiyama A, Jang H-S, Dintaman JM, Duess JW, Tragesser C, Sodhi CP. Necrotizing Enterocolitis: A Comprehensive Review on Toll-like Receptor 4-Mediated Pathophysiology, Clinical, and Therapeutic Insights. Biomedicines. 2025; 13(9):2288. https://doi.org/10.3390/biomedicines13092288
Chicago/Turabian StyleIshiyama, Asuka, Hee-Seong Jang, Jay M. Dintaman, Johannes W. Duess, Cody Tragesser, and Chhinder P. Sodhi. 2025. "Necrotizing Enterocolitis: A Comprehensive Review on Toll-like Receptor 4-Mediated Pathophysiology, Clinical, and Therapeutic Insights" Biomedicines 13, no. 9: 2288. https://doi.org/10.3390/biomedicines13092288
APA StyleIshiyama, A., Jang, H.-S., Dintaman, J. M., Duess, J. W., Tragesser, C., & Sodhi, C. P. (2025). Necrotizing Enterocolitis: A Comprehensive Review on Toll-like Receptor 4-Mediated Pathophysiology, Clinical, and Therapeutic Insights. Biomedicines, 13(9), 2288. https://doi.org/10.3390/biomedicines13092288







