Valemetostat–SAHA-Driven Acetylation of p53 via SET/TAF-Iβ Displacement and p300 Activation Modulates Cell Cycle Regulators in Pancreatic Cancer Cells
Abstract
1. Introduction
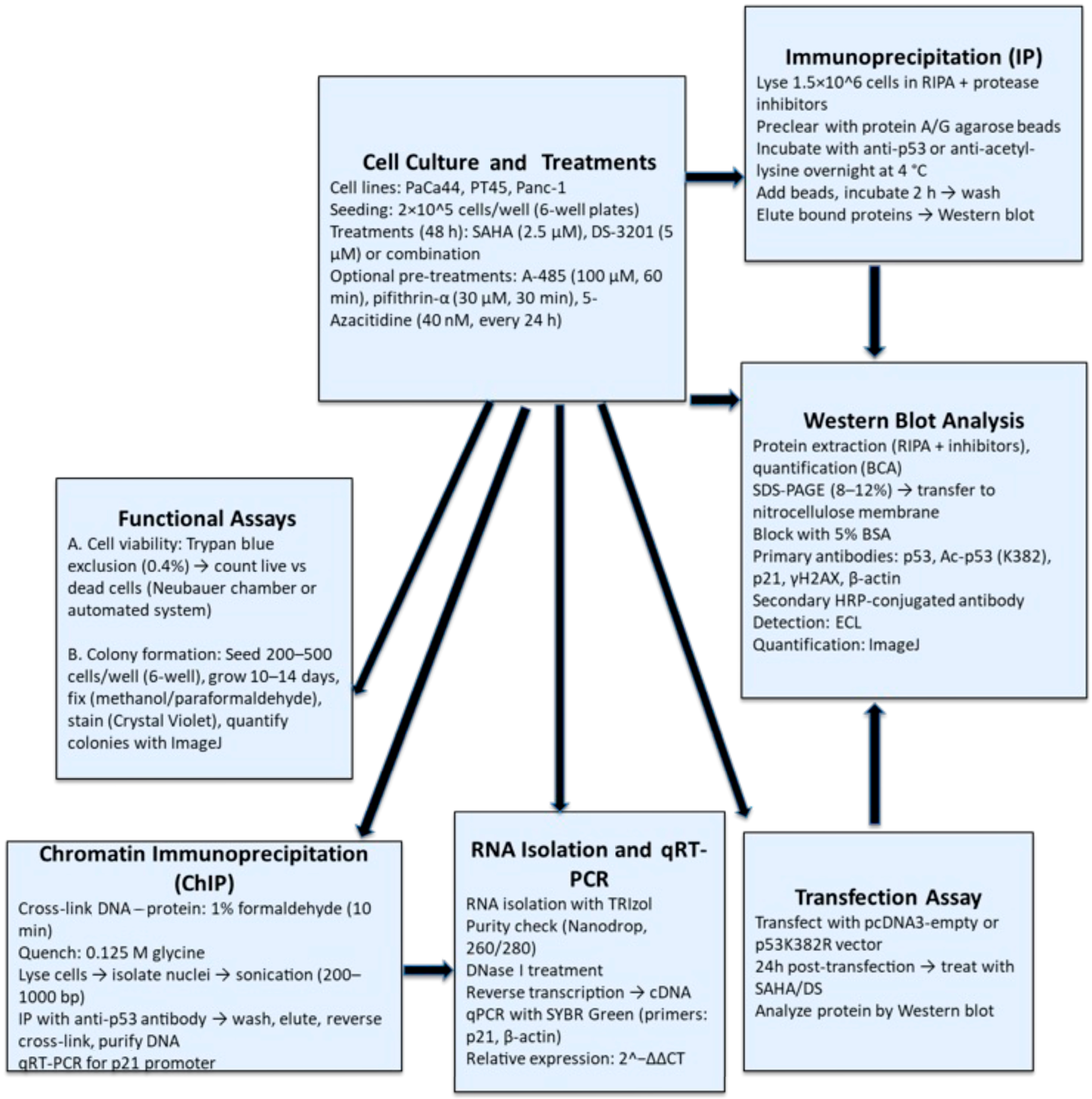
2. Materials and Methods
2.1. Cell Cultures and Treatments
2.2. Chromatin Immunoprecipitation (ChIP) Assay
2.3. Immunoprecipitation Assay
2.4. Western Blotting Analysis
2.5. Antibodies for Western Blotting Analysis
2.6. Densitometric Analysis
2.7. RNA Isolation and Quantitative Real-Time PCR Analysis
- P21 forw:5′-TACATCTCCCATTTCACCTAC-3′;
- P21 rev: 5′CGTAATTTCTCCAAGATCTCC-3′;
- ACT forw: 5′-TCACCCACACTGTGCCATCTACGA-3′;
- ACT rev: 5′-CAGCGGAACCGCTCATTGCCAATGG-3′.
2.8. Transfection with p53 K382R Vector
2.9. Cell Assay Viability
2.10. Colony Forming Assays
2.11. Cell Cycle Analysis
2.12. Statistical Analysis
3. Results
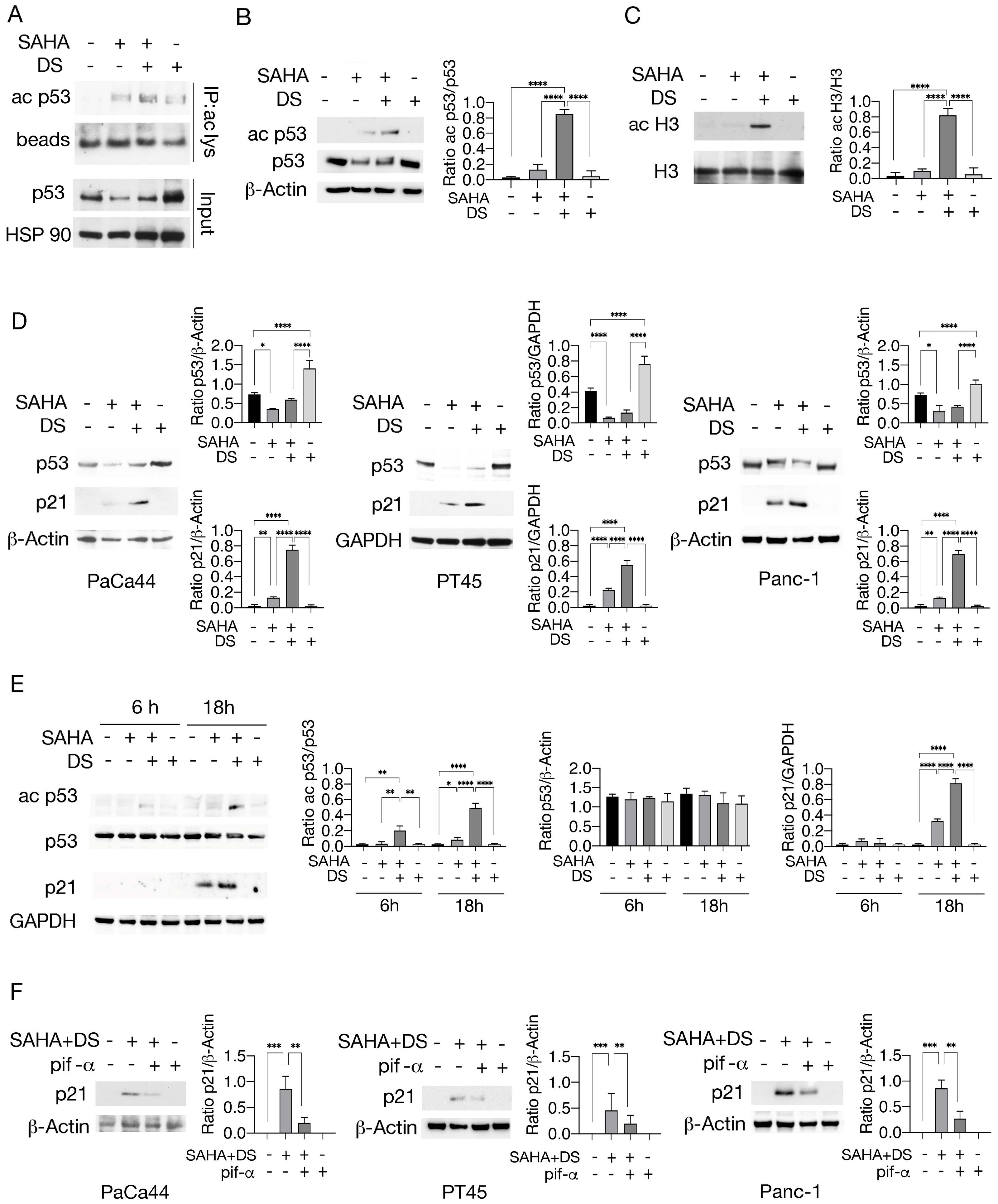
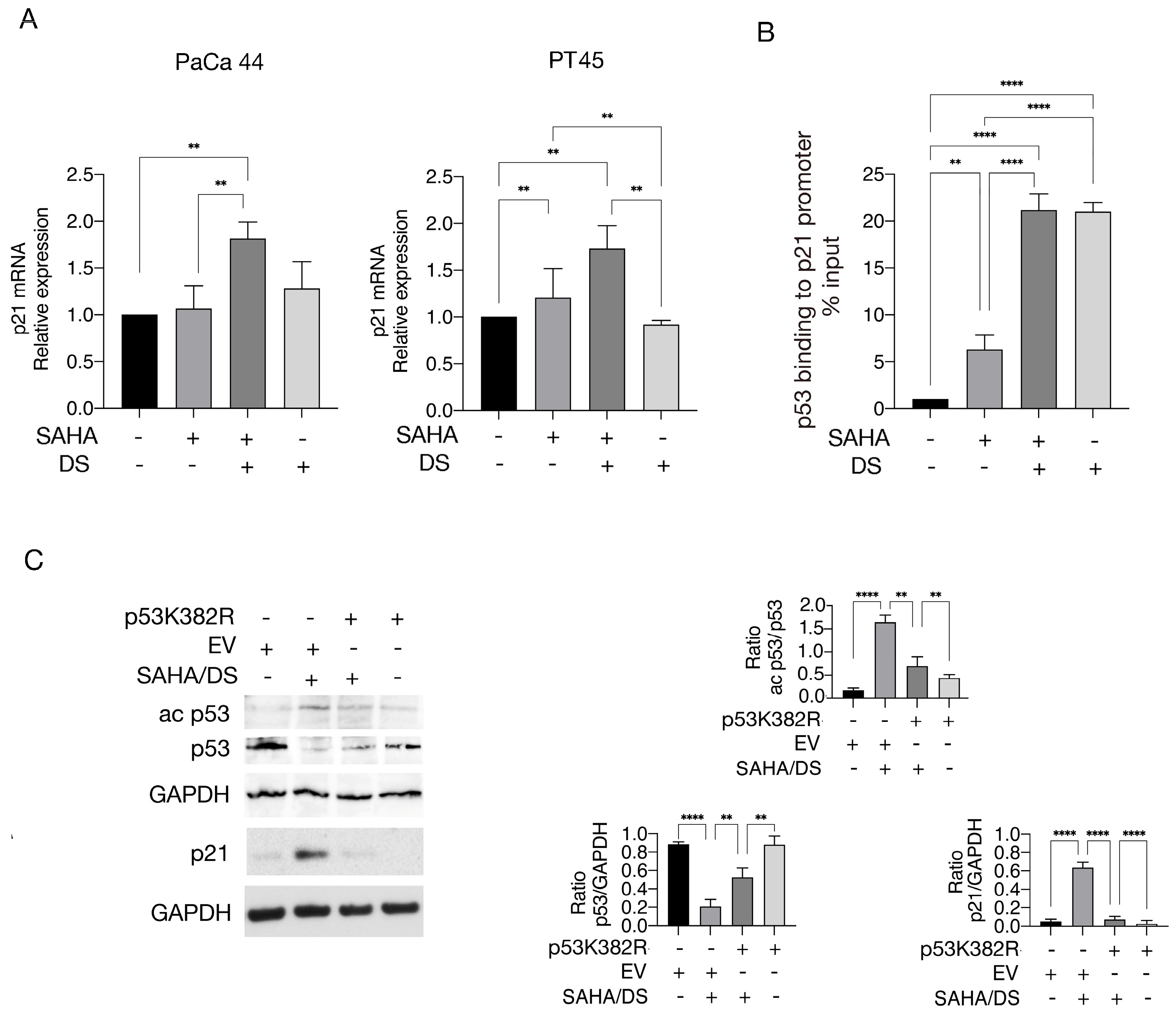
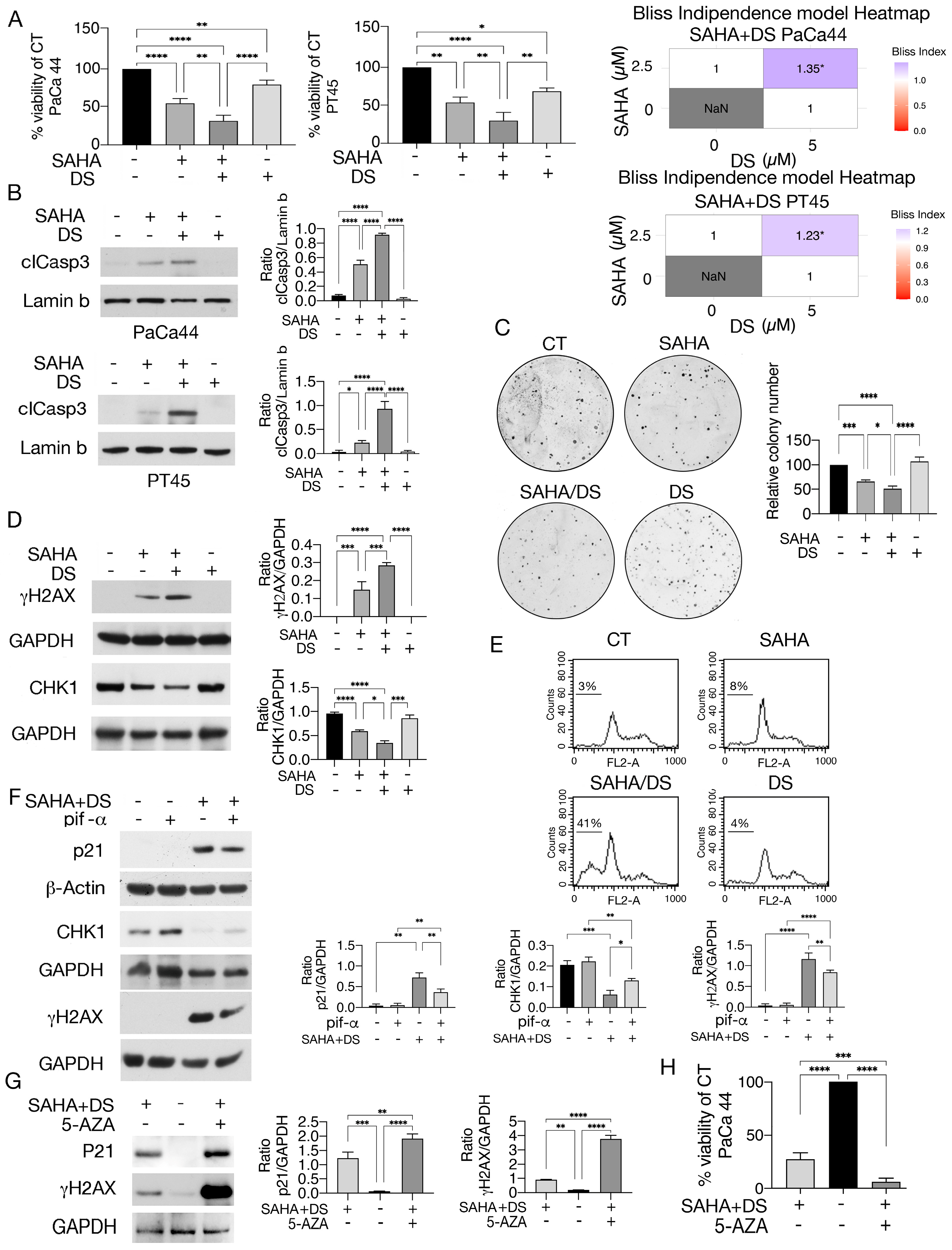
3.1. Valemetostat in Combination with SAHA Increases Lysine 373/382 Acetylation of p53 and Upregulates p21
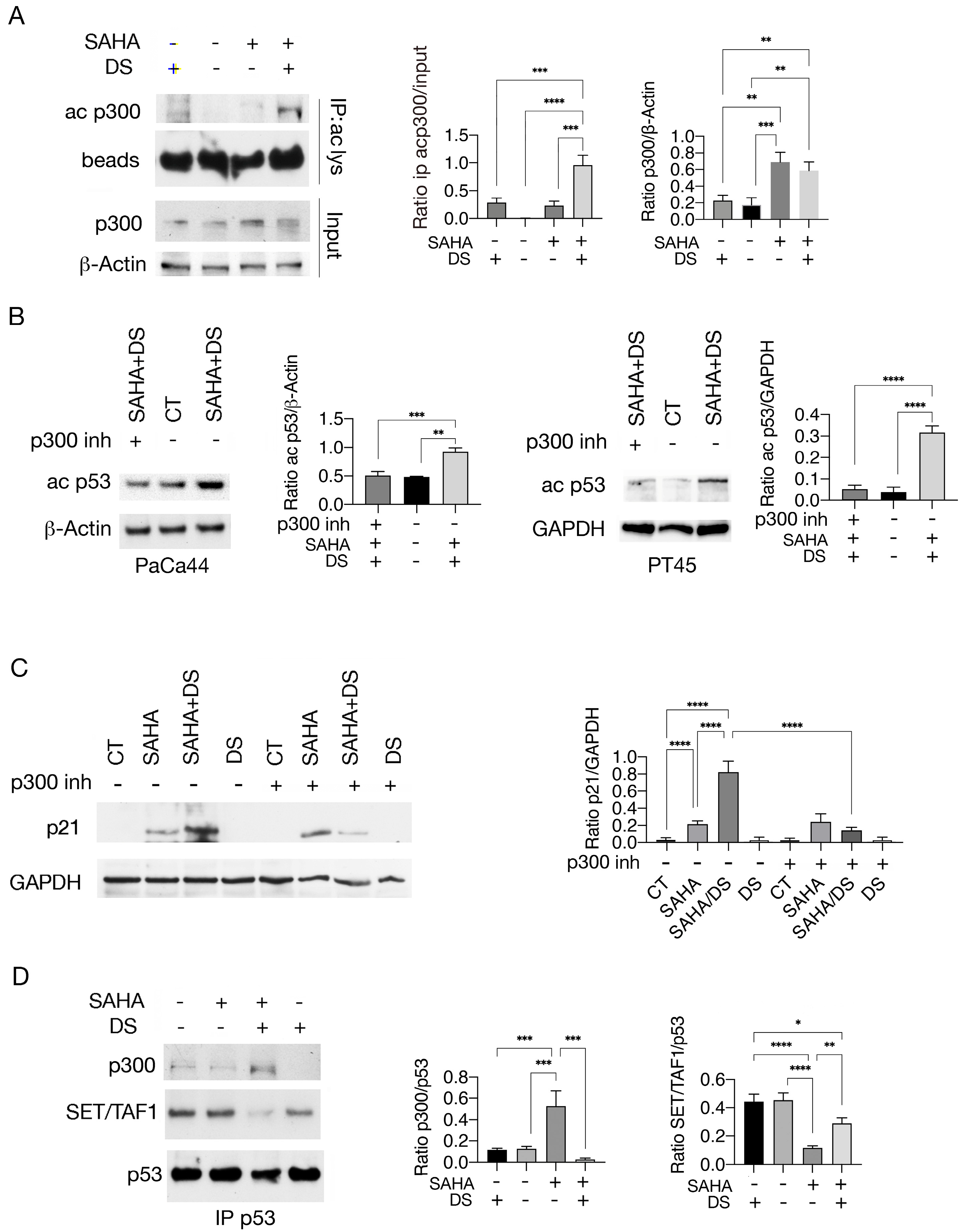
3.2. Valemetostat–SAHA Combination Enhances p53 Acetylation by Increasing Its Interaction with p300 While Reducing That with the Cochaperone SET/TAF-Iβ
3.3. Valemetostat–SAHA, Even More in Combination with 5-AZA, Impairs Pancreatic Cancer Cell Survival, Reducing CHK1 and Increasing DNA Damage
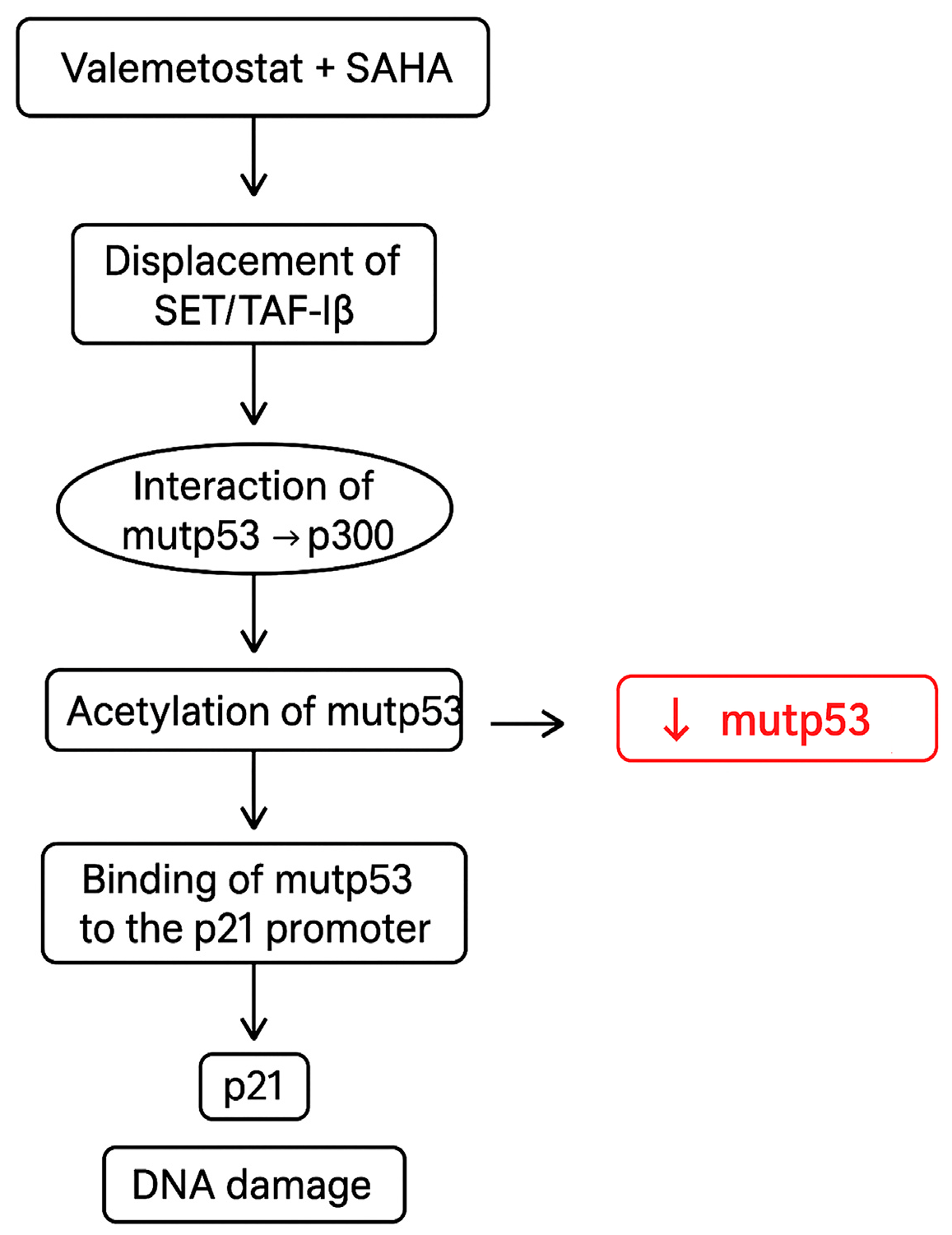
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saiki, Y.; Jiang, C.; Ohmuraya, M.; Furukawa, T. Genetic Mutations of Pancreatic Cancer and Genetically Engineered Mouse Models. Cancers 2021, 14, 71. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, M.; Wang, S.; Abbas, S.J.; Zhang, J.; Li, Y.; Shao, R.; Liu, Y. An Overview of Epigenetic Methylation in Pancreatic Cancer Progression. Front. Oncol. 2022, 12, 854773. [Google Scholar] [CrossRef]
- Zhang, T.; Cooper, S.; Brockdorff, N. The interplay of histone modifications—Writers that read. EMBO Rep. 2015, 16, 1467–1481. [Google Scholar] [CrossRef]
- Audia, J.E.; Campbell, R.M. Histone Modifications and Cancer. Cold Spring Harb. Perspect. Biol. 2016, 8, a019521. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, F.; Collavin, L.; Del Sal, G. Mutant p53 as a guardian of the cancer cell. Cell Death Differ. 2019, 26, 199–212. [Google Scholar] [CrossRef]
- Li, D.; Marchenko, N.D.; Moll, U.M. SAHA shows preferential cytotoxicity in mutant p53 cancer cells by destabilizing mutant p53 through inhibition of the HDAC6-Hsp90 chaperone axis. Cell Death Differ. 2011, 18, 1904–1913. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, R.; Di Crosta, M.; D’Orazi, G.; Cirone, M. Post-Translational Modifications (PTMs) of mutp53 and Epigenetic Changes Induced by mutp53. Biology 2024, 13, 508. [Google Scholar] [CrossRef] [PubMed]
- Di Crosta, M.; Ragone, F.C.; Benedetti, R.; D’Orazi, G.; Gilardini Montani, M.S.; Cirone, M. SAHA/5-AZA Enhances Acetylation and Degradation of mutp53, Upregulates p21 and Downregulates c-Myc and BRCA-1 in Pancreatic Cancer Cells. Int. J. Mol. Sci. 2024, 25, 7020. [Google Scholar] [CrossRef]
- Xu, D.; Qian, W.; Yang, Z.; Zhang, Z.; Sun, P.; Wan, Q.; Yin, Y.; Hu, Y.; Gong, L.; Zhang, B.; et al. Acetylation halts missense mutant p53 aggregation and rescues tumor suppression in non-small cell lung cancers. iScience 2023, 26, 107003. [Google Scholar] [CrossRef]
- Meek, D.W.; Anderson, C.W. Posttranslational modification of p53: Cooperative integrators of function. Cold Spring Harb. Perspect. Biol. 2009, 1, a000950. [Google Scholar] [CrossRef]
- Ivanov, G.S.; Ivanova, T.; Kurash, J.; Ivanov, A.; Chuikov, S.; Gizatullin, F.; Herrera-Medina, E.M.; Rauscher, F., 3rd; Reinberg, D.; Barlev, N.A. Methylation-acetylation interplay activates p53 in response to DNA damage. Mol. Cell. Biol. 2007, 27, 6756–6769. [Google Scholar] [CrossRef]
- Kurash, J.K.; Lei, H.; Shen, Q.; Marston, W.L.; Granda, B.W.; Fan, H.; Wall, D.; Li, E.; Gaudet, F. Methylation of p53 by Set7/9 mediates p53 acetylation and activity in vivo. Mol. Cell 2008, 29, 392–400. [Google Scholar] [CrossRef]
- Ito, A.; Lai, C.H.; Zhao, X.; Saito, S.; Hamilton, M.H.; Appella, E.; Yao, T.P. p300/CBP-mediated p53 acetylation is commonly induced by p53-activating agents and inhibited by MDM2. EMBO J. 2001, 20, 1331–1340. [Google Scholar] [CrossRef]
- Kaypee, S.; Sahadevan, S.A.; Patil, S.; Ghosh, P.; Roy, N.S.; Roy, S.; Kundu, T.K. Mutant and Wild-Type Tumor Suppressor p53 Induces p300 Autoacetylation. iScience 2018, 4, 260–272. [Google Scholar] [CrossRef]
- Kawai, H.; Nie, L.; Wiederschain, D.; Yuan, Z.M. Dual role of p300 in the regulation of p53 stability. J. Biol. Chem. 2001, 276, 45928–45932. [Google Scholar] [CrossRef]
- Liu, Y.; Denlinger, C.E.; Rundall, B.K.; Smith, P.W.; Jones, D.R. Suberoylanilide hydroxamic acid induces Akt-mediated phosphorylation of p300, which promotes acetylation and transcriptional activation of RelA/p65. J. Biol. Chem. 2006, 281, 31359–31368. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, K.S.; Seol, J.E.; Yu, K.; Chakravarti, D.; Seo, S.B. Inhibition of p53 acetylation by INHAT subunit SET/TAF-Ibeta represses p53 activity. Nucleic Acids Res. 2012, 40, 75–87. [Google Scholar] [CrossRef]
- Kim, K.B.; Kim, D.W.; Park, J.W.; Jeon, Y.J.; Kim, D.; Rhee, S.; Chae, J.I.; Seo, S.B. Inhibition of Ku70 acetylation by INHAT subunit SET/TAF-Ibeta regulates Ku70-mediated DNA damage response. Cell. Mol. Life Sci. 2014, 71, 2731–2745. [Google Scholar] [CrossRef]
- Hwang, I.J.; Park, J.; Seo, S.B. Non-canonical transcriptional regulation of INHAT subunit SET/TAF-Ibeta by EZH2. Biochem. Biophys. Res. Commun. 2022, 635, 136–143. [Google Scholar] [CrossRef]
- Romeo, M.A.; Gilardini Montani, M.S.; Benedetti, R.; Arena, A.; Maretto, M.; Bassetti, E.; Caiazzo, R.; D’Orazi, G.; Cirone, M. Anticancer effect of AZD2461 PARP inhibitor against colon cancer cells carrying wt or dysfunctional p53. Exp. Cell Res. 2021, 408, 112879. [Google Scholar] [CrossRef]
- Puca, R.; Nardinocchi, L.; Sacchi, A.; Rechavi, G.; Givol, D.; D’Orazi, G. HIPK2 modulates p53 activity towards pro-apoptotic transcription. Mol. Cancer 2009, 8, 85. [Google Scholar] [CrossRef]
- Ryu, H.W.; Shin, D.H.; Lee, D.H.; Choi, J.; Han, G.; Lee, K.Y.; Kwon, S.H. HDAC6 deacetylates p53 at lysines 381/382 and differentially coordinates p53-induced apoptosis. Cancer Lett. 2017, 391, 162–171. [Google Scholar] [CrossRef]
- Knowell, A.E.; Patel, D.; Morton, D.J.; Sharma, P.; Glymph, S.; Chaudhary, J. Id4 dependent acetylation restores mutant-p53 transcriptional activity. Mol. Cancer 2013, 12, 161. [Google Scholar] [CrossRef]
- Li, Y.; Prives, C. Are interactions with p63 and p73 involved in mutant p53 gain of oncogenic function? Oncogene 2007, 26, 2220–2225. [Google Scholar] [CrossRef]
- Kim, S.H.; Kang, H.J.; Na, H.; Lee, M.O. Trichostatin A enhances acetylation as well as protein stability of ERalpha through induction of p300 protein. Breast Cancer Res. 2010, 12, R22. [Google Scholar] [CrossRef]
- Zamperla, M.G.; Illi, B.; Barbi, V.; Cencioni, C.; Santoni, D.; Gagliardi, S.; Garofalo, M.; Zingale, G.A.; Pandino, I.; Sbardella, D.; et al. HDAC6 inhibition disrupts HDAC6-P300 interaction reshaping the cancer chromatin landscape. Clin. Epigenetics 2024, 16, 109. [Google Scholar] [CrossRef]
- Wang, D.; Kon, N.; Lasso, G.; Jiang, L.; Leng, W.; Zhu, W.G.; Qin, J.; Honig, B.; Gu, W. Acetylation-regulated interaction between p53 and SET reveals a widespread regulatory mode. Nature 2016, 538, 118–122. [Google Scholar] [CrossRef]
- Jiang, K.; Deng, M.; Du, W.; Liu, T.; Zhou, Y. Functions and inhibitors of CHK1 in cancer therapy. Med. Drug Discov. 2024, 22, 100185. [Google Scholar] [CrossRef]
- Gottifredi, V.; Karni-Schmidt, O.; Shieh, S.S.; Prives, C. p53 down-regulates CHK1 through p21 and the retinoblastoma protein. Mol. Cell. Biol. 2001, 21, 1066–1076. [Google Scholar] [CrossRef]
- Pasini, D.; Malatesta, M.; Jung, H.R.; Walfridsson, J.; Willer, A.; Olsson, L.; Skotte, J.; Wutz, A.; Porse, B.; Jensen, O.N.; et al. Characterization of an antagonistic switch between histone H3 lysine 27 methylation and acetylation in the transcriptional regulation of Polycomb group target genes. Nucleic Acids Res. 2010, 38, 4958–4969. [Google Scholar] [CrossRef]
- Hogg, S.J.; Motorna, O.; Cluse, L.A.; Johanson, T.M.; Coughlan, H.D.; Raviram, R.; Myers, R.M.; Costacurta, M.; Todorovski, I.; Pijpers, L.; et al. Targeting histone acetylation dynamics and oncogenic transcription by catalytic P300/CBP inhibition. Mol. Cell 2021, 81, 2183–2200.e13. [Google Scholar] [CrossRef]
- He, A.; Shen, X.; Ma, Q.; Cao, J.; von Gise, A.; Zhou, P.; Wang, G.; Marquez, V.E.; Orkin, S.H.; Pu, W.T. PRC2 directly methylates GATA4 and represses its transcriptional activity. Genes Dev. 2012, 26, 37–42. [Google Scholar] [CrossRef]
- Karimian, A.; Ahmadi, Y.; Yousefi, B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair. 2016, 42, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ding, L.; Wang, D.; Ye, Z.; He, Y.; Ma, L.; Zhu, R.; Pan, Y.; Wu, Q.; Pang, K.; et al. EZH2 cooperates with gain-of-function p53 mutants to promote cancer growth and metastasis. EMBO J. 2019, 38, e99599. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhao, H.; Wang, R.; Chen, Y.; Ouyang, X.; Li, W.; Sun, Y.; Peng, A. Cancer epigenetics: From laboratory studies and clinical trials to precision medicine. Cell Death Discov. 2024, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yan, J.; Zhang, M.; Wang, Y.; Chen, Y.; Fu, X.; Wei, R.; Zheng, X.L.; Liu, Z.; Zhang, X.; et al. Targeting Epigenetic Crosstalk as a Therapeutic Strategy for EZH2-Aberrant Solid Tumors. Cell 2018, 175, 186–199.e19. [Google Scholar] [CrossRef]
- Coulter, J.B.; Easwaran, H. Combining EZH2 and HDAC inhibitors to target castration-resistant prostate cancers. PLoS Biol. 2023, 21, e3002081. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Crosta, M.; Ragone, F.C.; Benedetti, R.; D’Orazi, G.; Santarelli, R.; Gilardini Montani, M.S.; Cirone, M. Valemetostat–SAHA-Driven Acetylation of p53 via SET/TAF-Iβ Displacement and p300 Activation Modulates Cell Cycle Regulators in Pancreatic Cancer Cells. Biomedicines 2025, 13, 2279. https://doi.org/10.3390/biomedicines13092279
Di Crosta M, Ragone FC, Benedetti R, D’Orazi G, Santarelli R, Gilardini Montani MS, Cirone M. Valemetostat–SAHA-Driven Acetylation of p53 via SET/TAF-Iβ Displacement and p300 Activation Modulates Cell Cycle Regulators in Pancreatic Cancer Cells. Biomedicines. 2025; 13(9):2279. https://doi.org/10.3390/biomedicines13092279
Chicago/Turabian StyleDi Crosta, Michele, Francesca Chiara Ragone, Rossella Benedetti, Gabriella D’Orazi, Roberta Santarelli, Maria Saveria Gilardini Montani, and Mara Cirone. 2025. "Valemetostat–SAHA-Driven Acetylation of p53 via SET/TAF-Iβ Displacement and p300 Activation Modulates Cell Cycle Regulators in Pancreatic Cancer Cells" Biomedicines 13, no. 9: 2279. https://doi.org/10.3390/biomedicines13092279
APA StyleDi Crosta, M., Ragone, F. C., Benedetti, R., D’Orazi, G., Santarelli, R., Gilardini Montani, M. S., & Cirone, M. (2025). Valemetostat–SAHA-Driven Acetylation of p53 via SET/TAF-Iβ Displacement and p300 Activation Modulates Cell Cycle Regulators in Pancreatic Cancer Cells. Biomedicines, 13(9), 2279. https://doi.org/10.3390/biomedicines13092279





