Chitosan–Hydrazone-Modified Calcium Phosphate Scaffolds: Fabrication, Characterization, and Drug Delivery Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Mg-, Sr-, and F-Doped Hydroxyapatite Powder Synthesis
2.2. Processing of the Scaffolds
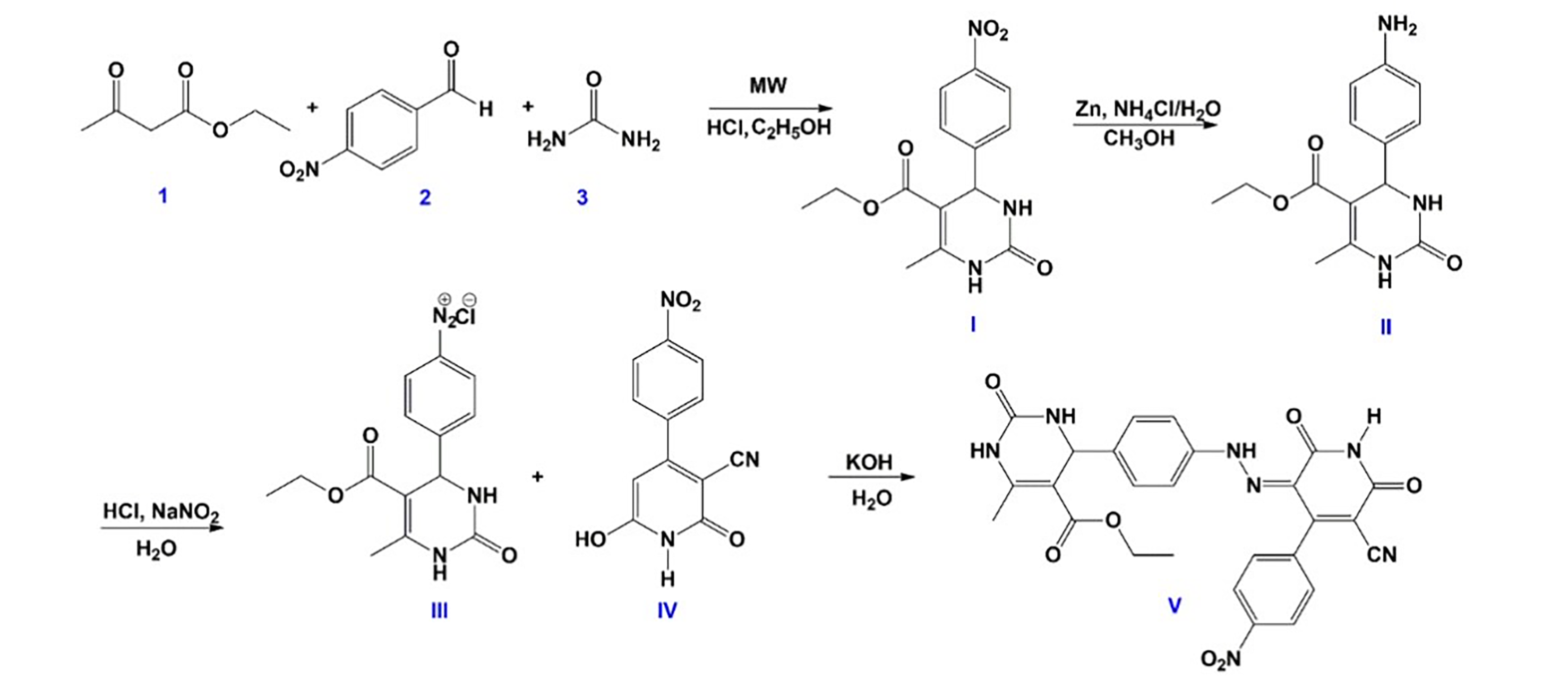
2.3. Synthesis of Hydrazone Drug Candidate
2.4. Antitumor Analysis of Hydrazone Compound
2.4.1. Cell Culture
2.4.2. Preparation of Compound for Antitumor Test
2.4.3. Antitumor Test
2.5. Incorporation of Hydrazone into Chitosan Coating and Scaffold Treatment
2.6. Characterization of Sample Powders
2.6.1. Energy-Dispersive X-Ray (EDX) Analysis
2.6.2. X-Ray Diffraction (XRD) Analysis
2.6.3. Scanning Electron Microscopy (SEM)
2.7. Characterization of Scaffold Samples
2.7.1. Structural Analysis of Mg, Sr, 1F-HAp Scaffolds
2.7.2. Microstructures of Uncoated and Coated Mg, Sr, 1F-HAp Scaffolds
2.7.3. Mechanical Properties of Mg, Sr, 1F-HAp Scaffolds (Uncoated and Coated with Chitosan)
2.7.4. Fourier-Transform Infrared Spectroscopy (FTIR) of Uncoated Mg, Sr, 1F-HAp Scaffolds, Chitosan, and Chitosan-Coated Mg, Sr, 1F-HAp Scaffolds
2.7.5. In Vitro Bioactivity of Scaffolds
2.7.6. In Vitro Cytotoxicity of the Coated Mg, Sr, 1F-HAp Scaffolds
2.7.7. Hydrazone Compound
3. Results and Discussion
3.1. EDX Analysis of Mg, Sr, F-HAp Powders
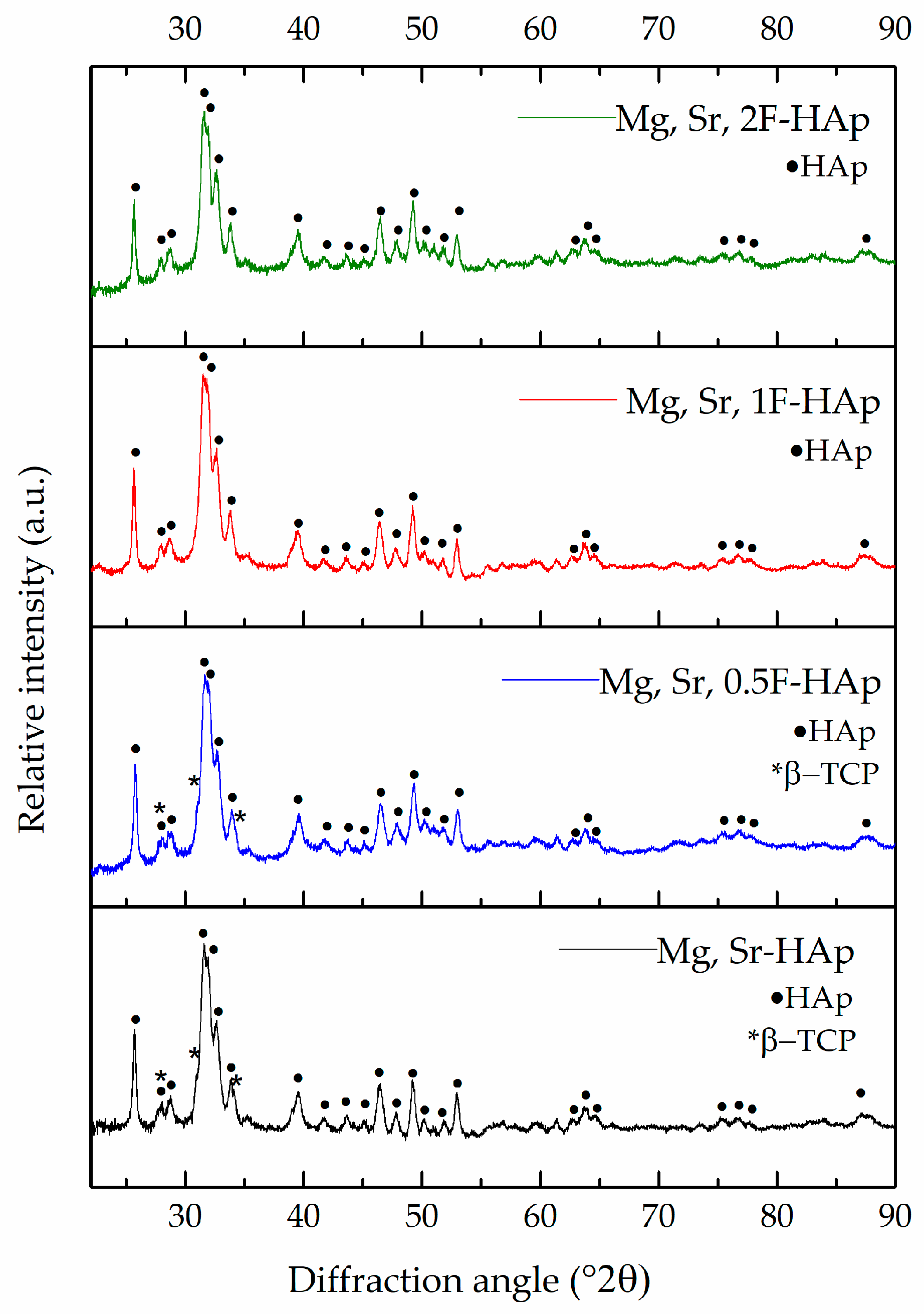
3.2. XRD Analysis of Mg, Sr, F-HAp Powders
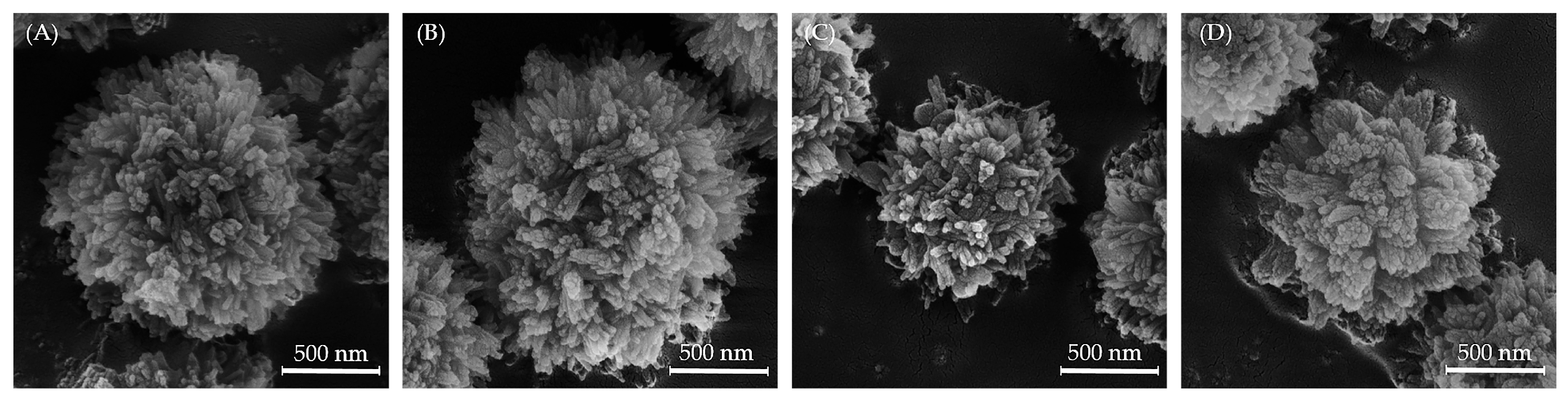
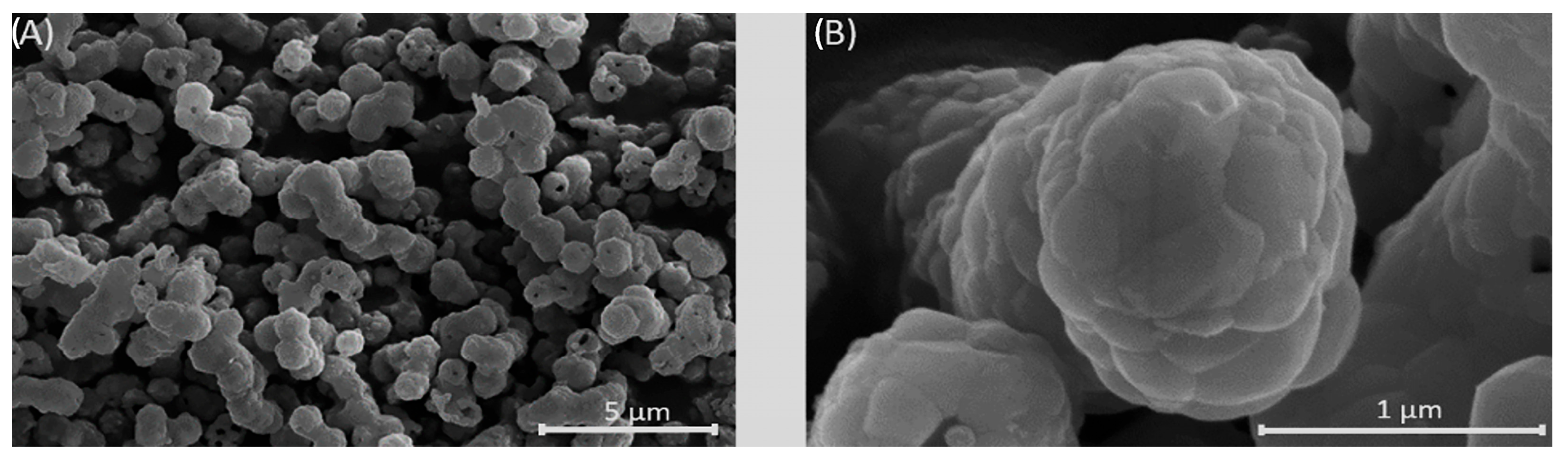
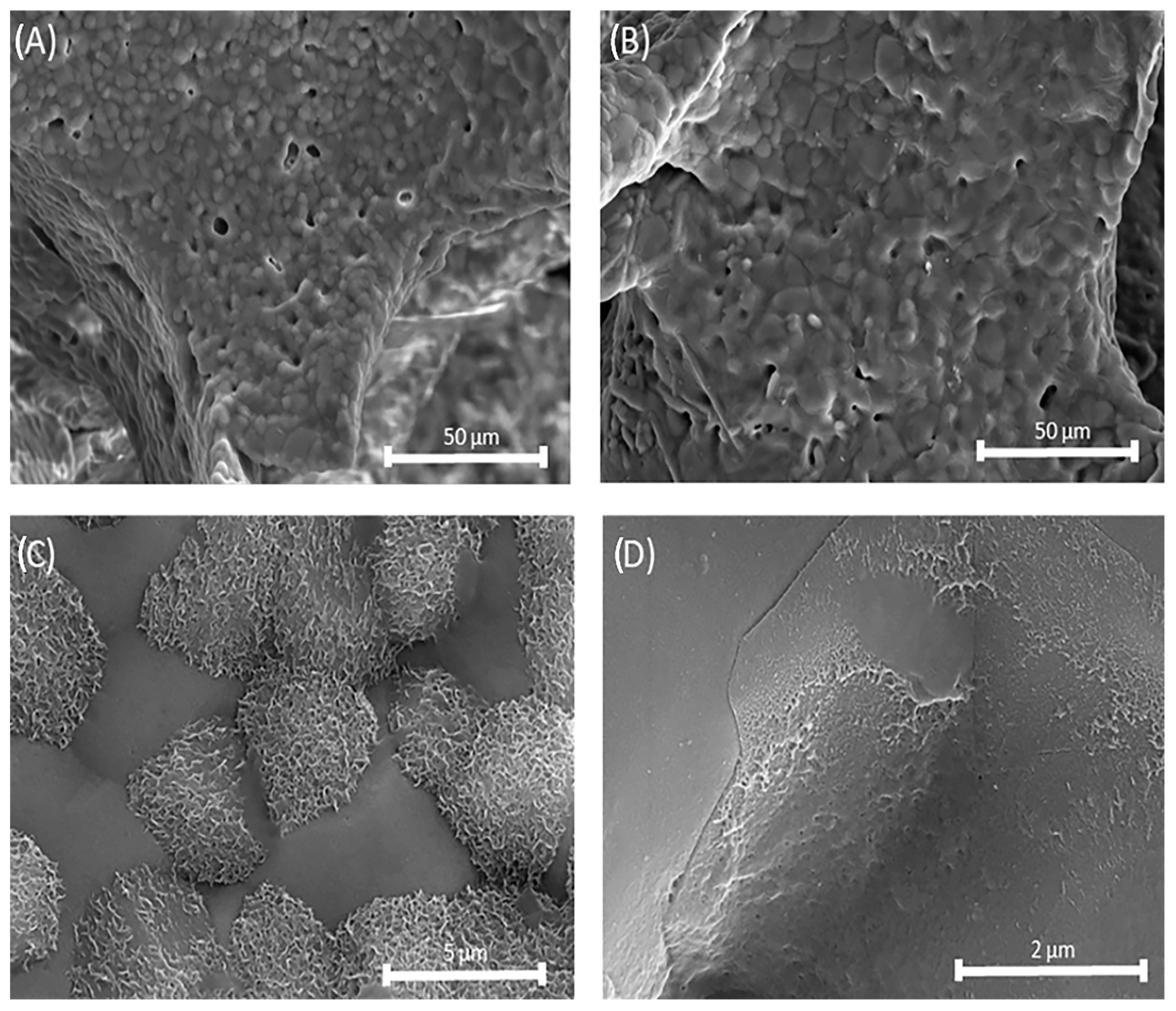
3.3. SEM Analysis of Mg, Sr, F-HAp Powders and Calcined Powder
3.4. XRD Analysis of the Mg, Sr, 1F-HAp Scaffolds
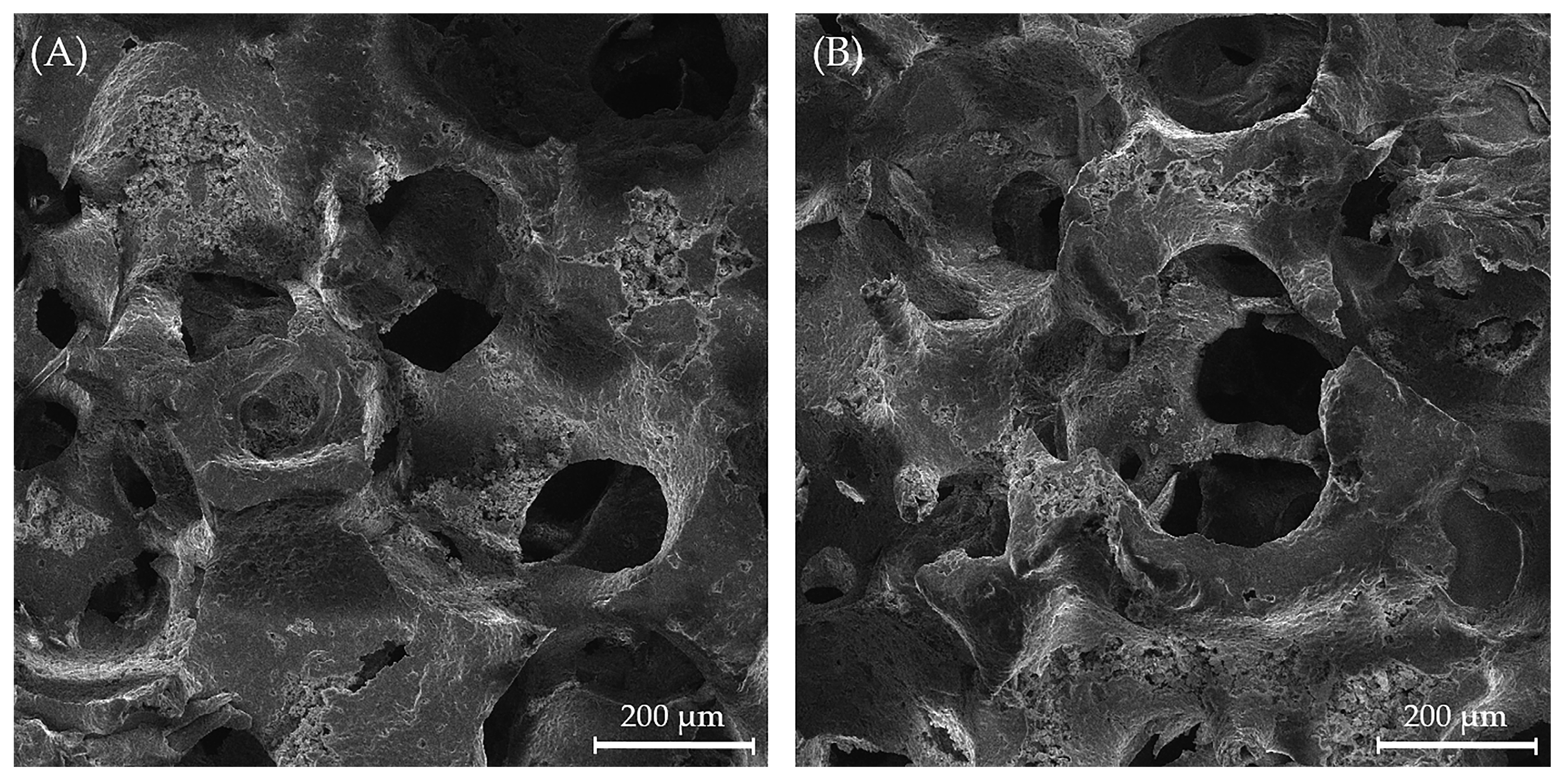
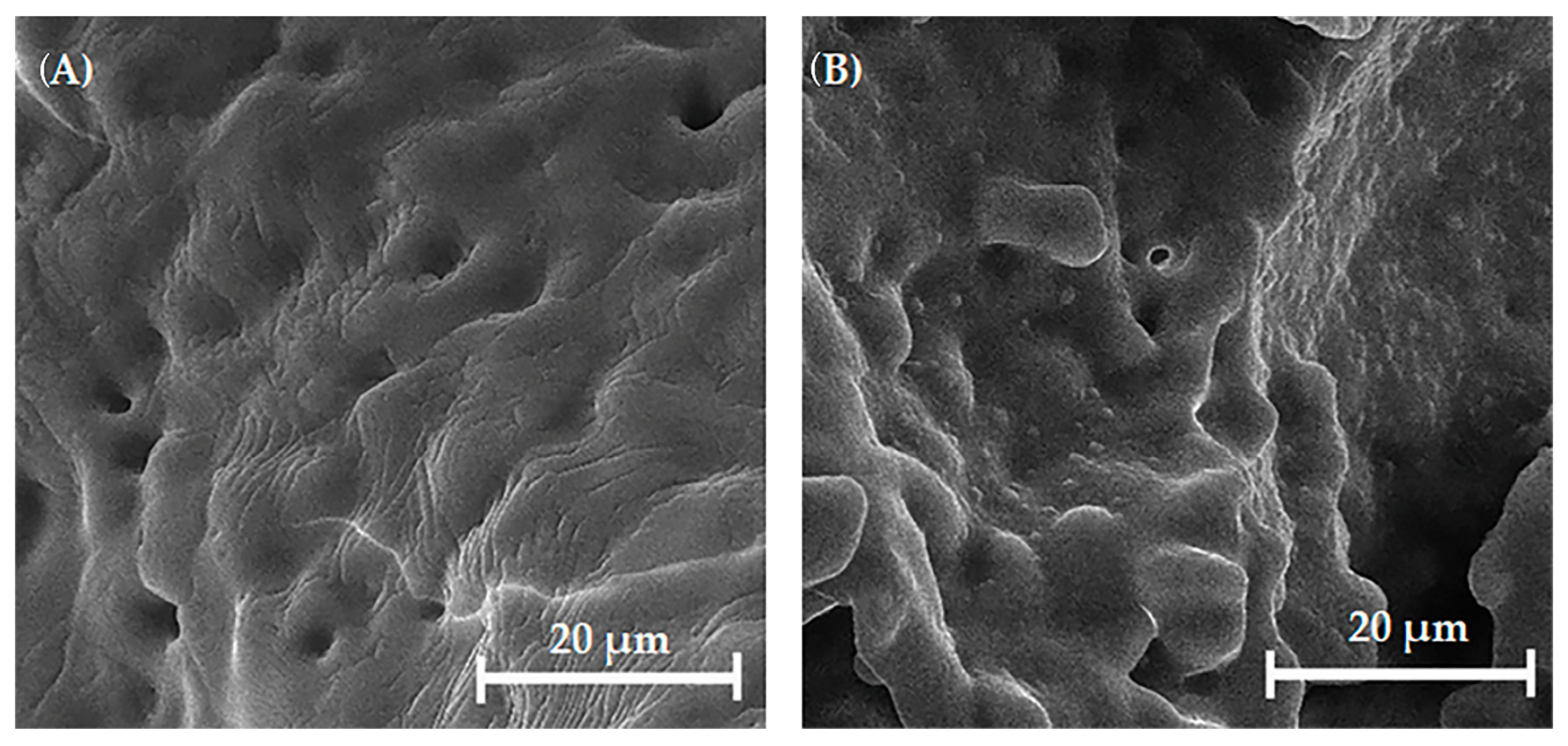
3.5. Structure of the Uncoated and Coated Mg, Sr, 1F-HAp Scaffolds
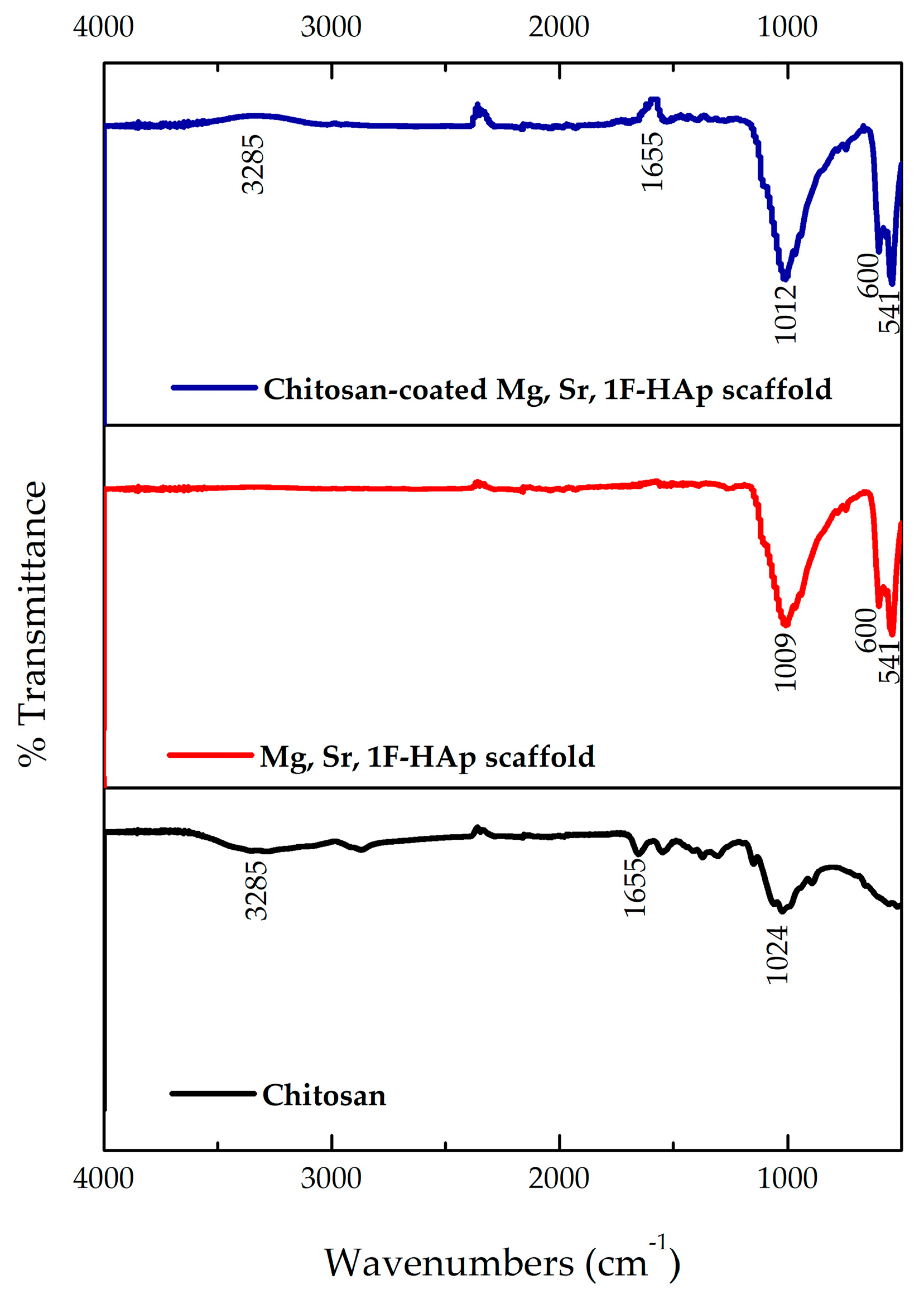
3.6. FTIR Analysis of Uncoated Mg, Sr, 1F-HAp Scaffolds, Chitosan, and Chitosan-Coated Mg, Sr, 1F-HAp Scaffolds
3.7. Mechanical Properties of Uncoated and Coated Mg, Sr, 1F-HAp Scaffolds
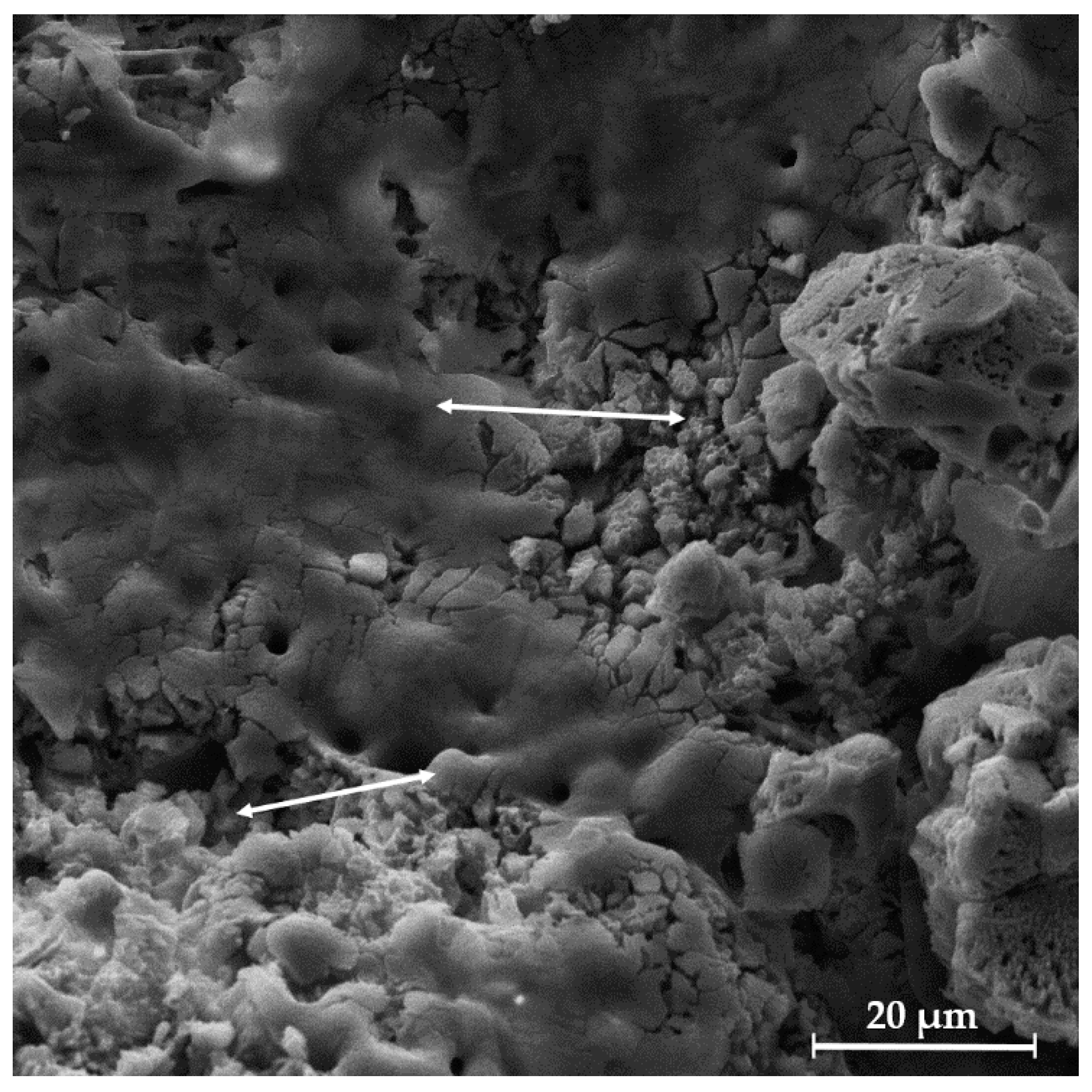
3.8. In Vitro Bioactivity of Uncoated and Coated Scaffolds
3.9. In Vitro Cytotoxicity of Coated Mg, Sr, 1F-HAp Scaffolds
3.10. Structural Characterization of Hydrazone Compound
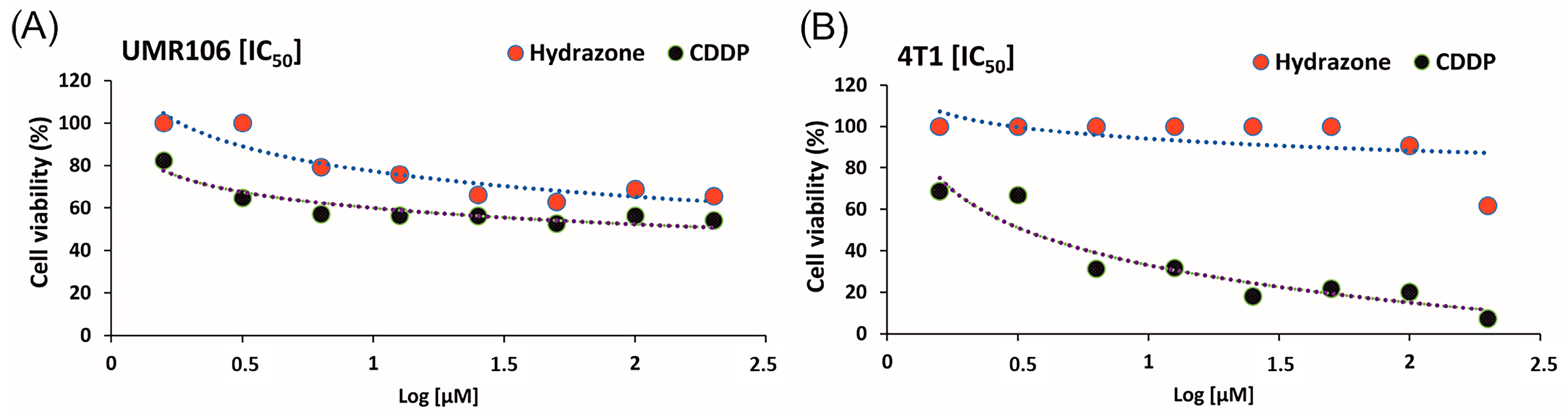
3.11. Antitumor Assessment of Hydrazone Compound
3.12. Hydrazone Compound Release
- Zero-order model:
(k0 = 7.82 × 10−4 mg·day−1; R2 = 0.874)
- 2.
- First-order model:
(k1 = 1.557 × 10−4 day−1; R2 = 0.874)
- 3.
- Higuchi model:
(kH = 2.737 × 10−3 mg·day−1/2; R2 = 0.797)
- 4.
- Korsmeyer–Peppas model:
(kp = 4.1184 × 10−4, n = 0.54; R2 = 0.744),
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahmati, M.; Pennisi, C.P.; Budd, E.; Mobasheri, A.; Mozafari, M. Biomaterials for Regenerative Medicine: Historical Perspectives and Current Trends. In Cell Biology and Translational Medicine, Volume 4: Stem Cells and Cell Based Strategies in Regeneration; Springer: Berlin/Heidelberg, Germany, 2018; Volume 4, pp. 1–19. [Google Scholar]
- Liu, S.; Yu, J.-M.; Gan, Y.-C.; Qiu, X.-Z.; Gao, Z.-C.; Wang, H.; Chen, S.-X.; Xiong, Y.; Liu, G.-H.; Lin, S.-E.; et al. Biomimetic natural biomaterials for tissue engineering and regenerative medicine: New biosynthesis methods, recent advances, and emerging applications. Mil. Med. Res. 2023, 10, 16. [Google Scholar] [CrossRef]
- Dang, M.; Saunders, L.; Niu, X.; Fan, Y.; Ma, P.X. Biomimetic delivery of signals for bone tissue engineering. Bone Res. 2018, 6, 25. [Google Scholar] [CrossRef]
- Bal, Z.; Kaito, T.; Korkusuz, F.; Yoshikawa, H. Bone regeneration with hydroxyapatite-based biomaterials. Emergent Mater. 2020, 3, 521–544. [Google Scholar] [CrossRef]
- Zhu, X.D.; Zhang, H.J.; Fan, H.S.; Li, W.; Zhang, X.D. Effect of phase composition and microstructure of calcium phosphate ceramic particles on protein adsorption. Acta Biomater. 2010, 6, 1536–1541. [Google Scholar] [CrossRef]
- Frank-Kamenetskaya, O.V. Structure, chemistry and synthesis of carbonate apatites—The main components of dental and bone tissues. In Minerals as Advanced Materials I; Springer: Berlin/Heidelberg, Germany, 2008; pp. 241–252. [Google Scholar]
- Noori, A.; Hoseinpour, M.; Kolivand, S.; Lotfibakhshaiesh, N.; Ebrahimi-Barough, S.; Ai, J.; Azami, M. Exploring the various effects of Cu doping in hydroxyapatite nanoparticle. Sci. Rep. 2024, 14, 3421. [Google Scholar] [CrossRef]
- Cuypers, L.A.B.; Bertsch, P.; Wang, R.; Harhangi, H.R.; Joziasse, L.S.; Walboomers, X.F.; van Niftrik, L.; Yang, F.; Leeuwenburgh, S.C.G. The effect of zinc doping on the cytocompatibility and antibacterial efficacy of hydroxyapatite nanoparticles for treatment of bone infection. Open Ceram. 2023, 16, 100488. [Google Scholar] [CrossRef]
- Ivankovic, T.; Turk, H.; Hrenovic, J.; Schauperl, Z.; Ivankovic, M.; Ressler, A. Antibacterial activity of silver doped hydroxyapatite toward multidrug-resistant clinical isolates of Acinetobacter baumannii. J. Hazard. Mater. 2023, 458, 131867. [Google Scholar] [CrossRef] [PubMed]
- Frasnelli, M.; Cristofaro, F.; Sglavo, V.M.; Dirè, S.; Callone, E.; Ceccato, R.; Bruni, G.; Cornaglia, A.I.; Visai, L. Synthesis and characterization of strontium-substituted hydroxyapatite nanoparticles for bone regeneration. Mater. Sci. Eng. C 2017, 71, 653–662. [Google Scholar] [CrossRef]
- Bose, S.; Fielding, G.; Tarafder, S.; Bandyopadhyay, A. Understanding of dopant-induced osteogenesis and angiogenesis in calcium phosphate ceramics. Trends Biotechnol. 2013, 31, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Šupová, M. Substituted hydroxyapatites for biomedical applications: A review. Ceram. Int. 2015, 41, 9203–9231. [Google Scholar] [CrossRef]
- Walker, J.; Shadanbaz, S.; Woodfield, T.B.; Staiger, M.P.; Dias, G.J. Magnesium biomaterials for orthopedic application: A review from a biological perspective. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1316–1331. [Google Scholar] [CrossRef]
- Janackovic, D.; Kostic-Gvozdenovic, L.; Petrovic, R.; Petrovic-Prelevic, I.; Jokanovic, V.; Uskokovic, D. Influence of synthesis parameters on the particle sizes of nanostructured calcium-hydroxyapatite. Key Eng. Mater. 2001, 192, 203–206. [Google Scholar]
- Veljovic, D.; Matic, T.; Stamenic, T.; Kojic, V.; Dimitrijevic-Brankovic, S.; Lukic, M.J.; Jevtic, S.; Radovanovic, Z.; Petrovic, R.; Janackovic, D. Mg/Cu co-substituted hydroxyapatite—Biocompatibility, mechanical properties and antimicrobial activity. Ceram. Int. 2019, 45, 22029–22039. [Google Scholar] [CrossRef]
- Ratnayake, J.T.B.; Mucalo, M.; Dias, G.J. Substituted hydroxyapatites for bone regeneration: A review of current trends. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1285–1299. [Google Scholar] [CrossRef]
- Syazwan, M.N.M.; Marliana, B.I.Y. Physico-chemical properties of Co-Sr doped carbonated hydroxyapatite powders. Mater. Today Proc. 2019, 17, 959–965. [Google Scholar] [CrossRef]
- Marie, P.J. Effects of strontium on bone tissue and bone cells. In Therapeutic Uses of Trace Elements; Springer: Berlin/Heidelberg, Germany, 1996; pp. 277–282. [Google Scholar]
- Matić, T.; Zebić, M.L.; Miletić, V.; Cvijović-Alagić, I.; Petrović, R.; Janaćković, D.J.; Veljović, D.J. Sr,Mg co-doping of calcium hydroxyapatite: Hydrothermal synthesis, processing, characterization and possible application as dentin substitutes. Ceram. Int. 2022, 48, 11155–11165. [Google Scholar] [CrossRef]
- Eriksen, E.F.; Kassem, M. Fluoride stimulates proliferation and differentiation of human marow stromal cells. Bone Miner. 1992, 17, 196. [Google Scholar] [CrossRef]
- Uysal, I.; Severcan, F.; Tezcaner, A.; Evis, Z. Co-doping of hydroxyapatite with zinc and fluoride improves mechanical and biological properties of hydroxyapatite. Prog. Nat. Sci. Mater. Int. 2014, 24, 340–349. [Google Scholar] [CrossRef]
- Bertoni, E.; Bigi, A.; Cojazzi, G.; Gandolfi, M.; Panzavolta, S.; Roveri, N. Nanocrystals of magnesium and fluoride substituted hydroxyapatite. J. Inorg. Biochem. 1998, 72, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Ghaemi, M.H.; Sayenko, S.Y.; Shkuropatenko, V.; Zykova, A.; Ulybkina, K.; Bereznyak, O.; Krupa, A.; Sawczak, M. The effect of Sr and Mg substitutions on structure, mechanical properties and solubility of fluorapatite ceramics for biomedical applications. Process Appl. Ceram. 2022, 16, 218–229. [Google Scholar] [CrossRef]
- Jokić, B.; Mitrić, M.; Radmilović, V.; Drmanić, S.; Petrović, R.; Janaćković, D. Synthesis and characterization of monetite and hydroxyapatite whiskers obtained by a hydrothermal method. Ceram. Int. 2011, 37, 167–173. [Google Scholar] [CrossRef]
- Ma, X.; He, Z.; Han, F.; Zhong, Z.; Chen, L.; Li, B. Preparation of collagen/hydroxyapatite/alendronate hybrid hydrogels as potential scaffolds for bone regeneration. Colloids Surf. B Biointerfaces 2016, 143, 81–87. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, X.; Du, Z.; Jiang, W.; Han, X.; Zhao, D.; Han, D.; Li, Q. Three dimensional printed macroporous polylactic acid/hydroxyapatite composite scaffolds for promoting bone formation in a critical-size rat calvarial defect model. Sci. Technol. Adv. Mater. 2016, 17, 136–148. [Google Scholar] [CrossRef]
- Tripathi, G.; Basu, B. A porous hydroxyapatite scaffold for bone tissue engineering: Physico-mechanical and biological evaluations. Ceram. Int. 2012, 38, 341–349. [Google Scholar] [CrossRef]
- Poinern, G.E.J.; Brundavanam, R.K.; Le, X.; Fawcett, D. The mechanical properties of a porous ceramic derived from a 30 nm sized particle based powder of hydroxyapatite for potential hard tissue engineering. Am. J. Biomed. Eng. 2012, 2, 278–286. [Google Scholar] [CrossRef][Green Version]
- Miri, Z.; Haugen, H.J.; Loca, D.; Rossi, F.; Perale, G.; Moghanian, A.; Ma, Q. Review on the strategies to improve the mechanical strength of highly porous bone bioceramic scaffolds. J. Eur. Ceram. Soc. 2024, 44, 23–42. [Google Scholar] [CrossRef]
- Ignjatović, N.; Wu, V.; Ajduković, Z.; Mihajilov-Krstev, T.; Uskoković, V.; Uskoković, D. Chitosan-PLGA polymer blends as coatings for hydroxyapatite nanoparticles and their effect on antimicrobial properties, osteoconductivity and regeneration of osseous tissues. Mater. Sci. Eng. C 2016, 60, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Kou, S.; Peters, L.M.; Mucalo, M.R. Chitosan: A review of sources and preparation methods. Int. J. Biol. Macromol. 2021, 169, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Harugade, A.; Sherje, A.P.; Pethe, A. Chitosan: A review on properties, biological activities and recent progress in biomedical applications. React. Funct. Polym. 2023, 191, 105634. [Google Scholar] [CrossRef]
- Tadić, J.D.; Lađarević, J.M.; Vitnik, Ž.J.; Vitnik, V.D.; Stanojković, T.P.; Matić, I.Z.; Mijin, D.Ž. Novel azo pyridone dyes based on dihydropyrimidinone skeleton: Synthesis, DFT study and anticancer activity. Dye. Pigment. 2021, 187, 109123. [Google Scholar] [CrossRef]
- Saberian, E.; Jenča, A.; Petrášová, A.; Zare-Zardini, H.; Ebrahimifar, M. Application of Scaffold-Based Drug Delivery in Oral Cancer Treatment: A Novel Approach. Pharmaceutics 2024, 16, 802. [Google Scholar] [CrossRef]
- Marković, M.D.; Tadić, J.D.; Savić, S.I.; Matić, I.Z.; Stanojković, T.P.; Mijin, D.Ž.; Panić, V.V. Soft 3D hybrid network for delivery and controlled release of poorly soluble dihydropyrimidinone compound: An insight into the novel system for potential application in leukemia treatment. J. Biomed. Mater. Res. Part A 2022, 110, 1564–1578. [Google Scholar] [CrossRef]
- Dowaraha, J.; Patelb, D.; Maraka, B.N.; Yadav, U.C.S.; Shahd, P.K.; Shukla, P.K.; Singh, V.P. Green synthesis, structural analysis and anticancer activity of dihydropyrimidinone derivatives. RSC Adv. 2021, 11, 35737–35753. [Google Scholar] [CrossRef]
- Mijin, D.; Bozic Nedeljkovic, B.; Bozic, B.; Kovrlja, I.; Ladarevic, J.; Uscumlic, G. Synthesis, solv Synthesis, solvatochromism, and biological activity of no omism, and biological activity of novel azo. Turk. J. Chem. 2018, 42, 896–907. [Google Scholar]
- Zhang, K.; Liu, Y.; Zhao, Y.; Shi, X.; Zhang, R.; He, Y.; Zhang, H.; Wang, W. Magnesium-Doped Nano-Hydroxyapatite/Polyvinyl Alcohol/Chitosan Composite Hydrogel: Preparation and Characterization. Int. J. Nanomed. 2024, 19, 651–671. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Chen, S.; Zhao, W.; Yang, L.; Yuan, B.; Ioan, V.S.; Iulian, A.V.; Yang, X.; Zhu, X.; Zhang, X. A bioceramic scaffold composed of strontium-doped three-dimensional hydroxyapatite whiskers for enhanced bone regeneration in osteoporotic defects. Theranostics 2020, 10, 1572–1589. [Google Scholar] [CrossRef]
- Herman, K.; Wujczyk, M.; Dobrzynski, M.; Diakowska, D.; Wiglusz, K.; Wiglusz, R.J. In vitro assessment of long-term fluoride ion release from nanofluorapatite. Materials 2021, 14, 3747. [Google Scholar] [CrossRef] [PubMed]
- Kansız, S.; Vurat, M.T.; Parmaksiz, M.; Elçin, A.E.; Elçin, Y.M. Chitosan/PVA reinforced boron/strontium multi-substituted hydroxyapatite-based biocomposites: Effects of synthesis pH and coating on the physicochemical, mechanical, and in vitro biological properties of scaffolds. Mater. Today Chem. 2024, 35, 101865. [Google Scholar] [CrossRef]
- Wu, M.-Y.; Kuo, Y.-T.; Kao, I.-F.; Yen, S.-K. Porous Chitosan/Hydroxyapatite Composite Microspheres for Vancomycin Loading and Releasing. Pharmaceutics 2024, 16, 730. [Google Scholar] [CrossRef] [PubMed]
- Malekmohammadi, S.; Jamshidi, R.; Sadowska, J.M.; Meng, C.; Abeykoon, C.; Akbari, M.; Gong, R.H. Stimuli-Responsive Codelivery System-Embedded Polymeric Nanofibers with Synergistic Effects of Growth Factors and Low-Intensity Pulsed Ultrasound to Enhance Osteogenesis Properties. ACS Appl. Bio Mater. 2024, 7, 4293–4306. [Google Scholar] [CrossRef]
- Veljović, D.; Jančić-Hajneman, R.; Balać, I.; Jokić, B.; Putić, S.; Petrović, R.; Janaćković, D. The effect of the shape and size of the pores on the mechanical properties of porous HAP-based bioceramics. Ceram. Int. 2011, 37, 471–479. [Google Scholar] [CrossRef]
- Veljović, D.; Palcevskis, E.; Dindune, A.; Putić, S.; Balać, I.; Petrović, R.; Janaćković, D. Microwave sintering improves the mechanical properties of biphasic calcium phosphates from hydroxyapatite microspheres produced from hydrothermal processing. J. Mater. Sci 2010, 45, 3175–3183. [Google Scholar] [CrossRef]
- Tadić, J.; Mihajlović, M.; Jovanović, M.; Mijin, D. Continuous flow synthesis of some 6- and 1,6-substituted 3-cyano-4-methyl-2-pyridones. J. Serbian Chem. Soc. 2019, 84, 531–538. [Google Scholar] [CrossRef]
- Rajabi, F.; Hajipour-Verdom, B.; Abdolmaleki, P. Static magnetic field promotes the doxorubicin toxicity effects on osteosarcoma cells. Sci. Rep. 2025, 15, 11902. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ohno, M.; Abe, T. Rapid colorimetric assay for the quantification of leukemia inhibitory factor (LIF) and interleukin-6 (IL-6). J. Immunol. Methods 1991, 145, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Asadian-Ardakani, V.; Saber-Samandari, S.; Saber-Samandari, S. The effect of hydroxyapatite in biopolymer-based scaffolds on release of naproxen sodium. J. Biomed. Mater. Res. Part A 2016, 104, 2992–3003. [Google Scholar] [CrossRef]
- Higuchi, T. Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J. Pharm. Sci. 1963, 52, 1145–1149. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Radulescu, D.E.; Vasile, O.R.; Andronescu, E.; Ficai, A. Latest Research of Doped Hydroxyapatite for Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 13157. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Cacciotti, I.; Lombardi, M.; Montanaro, L.; Bemporad, E.; Sebastiani, M. F-substituted hydroxyapatite nanopowders: Thermal stability, sintering behaviour and mechanical properties. Ceram. Int. 2010, 36, 313–322. [Google Scholar] [CrossRef]
- Nathanael, A.J.; Mangalaraj, D.; Hong, S.I.; Masuda, Y.; Rhee, Y.H.; Kim, H.W. Influence of fluorine substitution on the morphology and structure of hydroxyapatite nanocrystals prepared by hydrothermal method. Mater. Chem. Phys. 2013, 137, 967–976. [Google Scholar] [CrossRef]
- Guo, X.; Yan, H.; Zhao, S.; Li, Z.; Li, Y.; Liang, X. Effect of calcining temperature on particle size of hydroxyapatite synthesized by solid-state reaction at room temperature. Adv. Powder Technol. 2013, 24, 1034–1038. [Google Scholar] [CrossRef]
- Chung, R.J.; Hsieh, M.F.; Huang, K.C.; Chou, F.I.; Perng, L.H. Preparation of porous HA/Beta-TCP biphasic Bioceramic using a molten salt process. Bioceramics 18 2006, 309, 1075–1078. [Google Scholar]
- Matic, T.; Zebić, M.L.; Cvijović-Alagić, I.; Miletić, V.; Petrović, R.; Janaćković, D.; Veljović, D. The effect of calcinated hydroxyapatite and magnesium doped hydroxyapatite as fillers on the mechanical properties of a model BisGMA/TEGDMA dental composite initially and after aging. Metall. Mater. Eng. 2018, 24. [Google Scholar] [CrossRef]
- Pelss, J.; Loca, D.; Berzina-Cimdina, L.; Locs, J.; Lakevics, V. Release of Anticancer Drug Doxorubicin from Biodegradable Polymer Coated Porous Hydroxyapatite Scaffolds. Adv. Mater. Res. 2011, 284, 1770–1773. [Google Scholar] [CrossRef]
- Saeedi, F.; Montazeri, A. Nanoscale hydroxyapatite/chitosan polymer coatings: Synthesis and characterization. Polym. Compos. 2024, 45, 122–136. [Google Scholar] [CrossRef]
- Azeez, S.; Anusha, N.; Sathiyaseelan, A.; Nagarajan, S. Chitosan: A multifaceted biomaterial—Exploring physicochemical insights and diverse drug delivery applications. J. Drug Deliv. Sci. Technol. 2025, 111, 107140. [Google Scholar] [CrossRef]
- Uswatta, S.P.; Okeke, I.U.; Jayasuriya, A.C. Injectable porous nano-hydroxyapatite/chitosan/tripolyphosphate scaffolds with improved compressive strength for bone regeneration. Mater. Sci. Eng. C 2016, 69, 505–512. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Rodrigues, M.T.; Silva, S.S.; Malafaya, P.B.; Gomes, M.E.; Viegas, C.A.; Dias, I.R.; Azevedo, J.T.; Mano, J.F.; Reis, R.L. Novel hydroxyapatite/chitosan bilayered scaffold for osteochondral tissue-engineering applications: Scaffold design and its performance when seeded with goat bone marrow stromal cells. Biomaterials 2006, 27, 6123–6137. [Google Scholar] [CrossRef]
- Pighinelli, L.; Kucharska, M. Chitosan–hydroxyapatite composites. Carbohydr. Polym. 2013, 93, 256–262. [Google Scholar] [CrossRef]
- Galić, A.; Matić, T.; Obradović, N.; Baščarević, Z.; Veljović, Đ. Processing of gelatine coated composite scaffolds based on magnesium and strontium doped hydroxyapatite and yttria-stabilized zirconium oxide. Sci. Sinter. 2023, 55, 469–479. [Google Scholar] [CrossRef]
- Tsiourvas, D.; Sapalidis, A.; Papadopoulos, T. Hydroxyapatite/chitosan-based porous three-dimensional scaffolds with complex geometries. Mater. Today Commun. 2016, 7, 59–66. [Google Scholar] [CrossRef]
- Abert, J.; Bergmann, C.; Fischer, H. Wet chemical synthesis of strontium-substituted hydroxyapatite and its influence on the mechanical and biological properties. Ceram. Int. 2014, 40, 9195–9203. [Google Scholar] [CrossRef]
- Mehrjoo, M.; Javadpour, J.; Shokrgozar, M.A.; Farokhi, M.; Javadian, S.; Bonakdar, S. Effect of magnesium substitution on structural and biological properties of synthetic hydroxyapatite powder. Mater. Express 2015, 5, 41–48. [Google Scholar] [CrossRef]
- Kheradmandfard, M.; Fathi, M.H.; Ansari, F.; Ahmadi, T. Effect of Mg content on the bioactivity and biocompatibility of Mg-substituted fluorapatite nanopowders fabricated via mechanical activation. Mater. Sci. Eng. C 2016, 68, 136–142. [Google Scholar] [CrossRef]
- Wei, M.; Evans, J.H.; Bostrom, T.; Grøndahl, L. Synthesis and characterization of hydroxyapatite, fluoride-substituted hydroxyapatite and fluorapatite. J. Mater. Sci. Mater. Med. 2003, 14, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Jennings, J.A. Controlling chitosan degradation properties in vitro and in vivo. In Chitosan Based Biomaterials Volume 1; Woodhead Publishing: Cambridge, UK, 2017; pp. 159–182. [Google Scholar]
- Zhou, K.; Azaman, F.A.; Cao, Z.; Brennan Fournet, M.; Devine, D.M. Bone tissue engineering scaffold optimisation through modification of chitosan/ceramic composition. Macromol 2023, 3, 326–342. [Google Scholar] [CrossRef]
- Lađarević, J.; Božić, B.; Mtović, L.; Božić-Nedeljković, B.; Mijin, D. Role of the bifurcated intramolecular hydrogen bond on the physico-chemical profile of the novel azo pyridone dyes. Dye. Pigment. 2019, 162, 562–572. [Google Scholar] [CrossRef]
- Porobić, S.; Božić, B.; Dramićanin, M.; Vitnik, V.; Vitnik, Ž.; Marinović-Cincović, M.; Mijin, D. Absorption and fluorescence spectral properties of azo dyes based on 3-amido-6-hydroxy-4-methyl-2-pyridone: Solvent and substituent effects. Dye. Pigment. 2020, 175, 108139. [Google Scholar] [CrossRef]
- Fu, D.; Zhong, L.L.; Xu, J.; Mo, A.; Yang, M. Hydrazone-functionalized nanoscale covalent organic frameworks as a nanocarrier for pH-responsive drug delivery enhanced anticancer activity. RSC Adv. 2024, 14, 20799–20808. [Google Scholar] [CrossRef] [PubMed]
- She, Z.; Zhang, B.; Jin, C.; Feng, Q.; Xu, Y. Preparation and in vitro degradation of porous three-dimensional silk fibroin/chitosan scaffold. Polym. Degrad. Stab. 2008, 93, 1316–1322. [Google Scholar] [CrossRef]




| Sample (Composition) | Ca(NO3)2 × 4H2O (g) | EDTA (g) | Urea (g) | NaH2PO4 × 2H2O (g) | Mg(NO3)2 × 6H2O (g) | Sr(NO3)2 (g) | NaF (g) |
|---|---|---|---|---|---|---|---|
| Mg, Sr-HAp (3.0 Mg2+, 3.0 Sr2+ [mol.%]) | 11.10 | 11.80 | 12.00 | 4.680 | 0.385 | 0.317 | - |
| Mg, Sr, 0.5F-HAp (3.0 Mg2+, 3.0 Sr2+, 0.5 F− [mol.%])) | 11.10 | 11.80 | 12.00 | 4.657 | 0.385 | 0.317 | 0.0063 |
| Mg, Sr, 1F-HAp (3.0 Mg2+, 3.0 Sr2+, 1.0 F− [mol.%])) | 11.10 | 11.80 | 12.00 | 4.633 | 0.385 | 0.317 | 0.0126 |
| Mg, Sr, 2F-HAp (3.0 Mg2+, 3.0 Sr2+, 2 F− [mol.%]) | 11.10 | 11.80 | 12.00 | 4.587 | 0.385 | 0.317 | 0.0252 |
| Samples | Ca [at.%] | P [at.%] | Mg [at.%] | Sr [at.%] | F [at.%] | Ca/P ratio |
|---|---|---|---|---|---|---|
| Mg, Sr-HAp | 13.90 | 11.74 | 0.33 | 1.12 | 0.00 | 1.18 |
| Mg, Sr, 0.5F-HAp | 12.50 | 10.80 | 0.30 | 1.09 | 0.00 | 1.16 |
| Mg, Sr, 1F-HAp | 13.04 | 11.14 | 0.27 | 1.08 | 0.24 | 1.17 |
| Mg, Sr, 2F-HAp | 12.97 | 11.02 | 0.25 | 1.06 | 1.34 | 1.18 |
| Sample | Phases Proportions, wt.% |
|---|---|
| Mg, Sr-HAp | HAp: 92. 50 ± 0.40, β-TCp: 7. 41 ± 0.54 |
| Mg, Sr, 0.5F-HAp | HAp: 93.38 ± 0.01, β-TCp: 6.62 ± 0.01 |
| Mg, Sr. 1F-HAp | HAp: 100 |
| Mg, Sr, 2F-HAp | HAp: 100 |
| Phases | Phases Proportions, wt.% |
|---|---|
| HAp | 33.14 ± 0.03 |
| α-TCP | 4.13 ± 0.01 |
| β-TCP | 1.0 ± 0.01 |
| Sample | Cell Survival of MRC-5 Cells [%], Mean ± SD |
|---|---|
| Control | 100 |
| Mg, Sr, 1F-HAp scaffold coated with chitosan | 106.33 ± 6.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakovljević, T.; Stanisavljević, J.; Stevanović, J.; Petković, M.; Matić, I.Z.; Papić, M.; Živanović, S.; Matić, T.; Ugrinović, V.; Janaćković, D.; et al. Chitosan–Hydrazone-Modified Calcium Phosphate Scaffolds: Fabrication, Characterization, and Drug Delivery Potential. Biomedicines 2025, 13, 2270. https://doi.org/10.3390/biomedicines13092270
Jakovljević T, Stanisavljević J, Stevanović J, Petković M, Matić IZ, Papić M, Živanović S, Matić T, Ugrinović V, Janaćković D, et al. Chitosan–Hydrazone-Modified Calcium Phosphate Scaffolds: Fabrication, Characterization, and Drug Delivery Potential. Biomedicines. 2025; 13(9):2270. https://doi.org/10.3390/biomedicines13092270
Chicago/Turabian StyleJakovljević, Teodora, Jelena Stanisavljević, Julijana Stevanović, Miloš Petković, Ivana Z. Matić, Miloš Papić, Suzana Živanović, Tamara Matić, Vukašin Ugrinović, Djordje Janaćković, and et al. 2025. "Chitosan–Hydrazone-Modified Calcium Phosphate Scaffolds: Fabrication, Characterization, and Drug Delivery Potential" Biomedicines 13, no. 9: 2270. https://doi.org/10.3390/biomedicines13092270
APA StyleJakovljević, T., Stanisavljević, J., Stevanović, J., Petković, M., Matić, I. Z., Papić, M., Živanović, S., Matić, T., Ugrinović, V., Janaćković, D., Ljujić, B., & Veljović, D. (2025). Chitosan–Hydrazone-Modified Calcium Phosphate Scaffolds: Fabrication, Characterization, and Drug Delivery Potential. Biomedicines, 13(9), 2270. https://doi.org/10.3390/biomedicines13092270







