Evaluation of Cytokine Levels in Cardiac Transthyretin and Immunoglobulin Light Chain Amyloidosis and Their Correlation with Myocardial Inflammatory Cells and MACE
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Study Participants, Recruitment, and Pre-Analytics
2.2. Endomyocardial Biopsies
2.3. Cytokine Analysis
2.4. Statistical Analysis
2.5. Ethics
3. Results
3.1. Study Population
3.2. Cytokine Levels and Their Association with the Myocardial Presence of Inflammatory Cells in Cardiac Amyloidosis
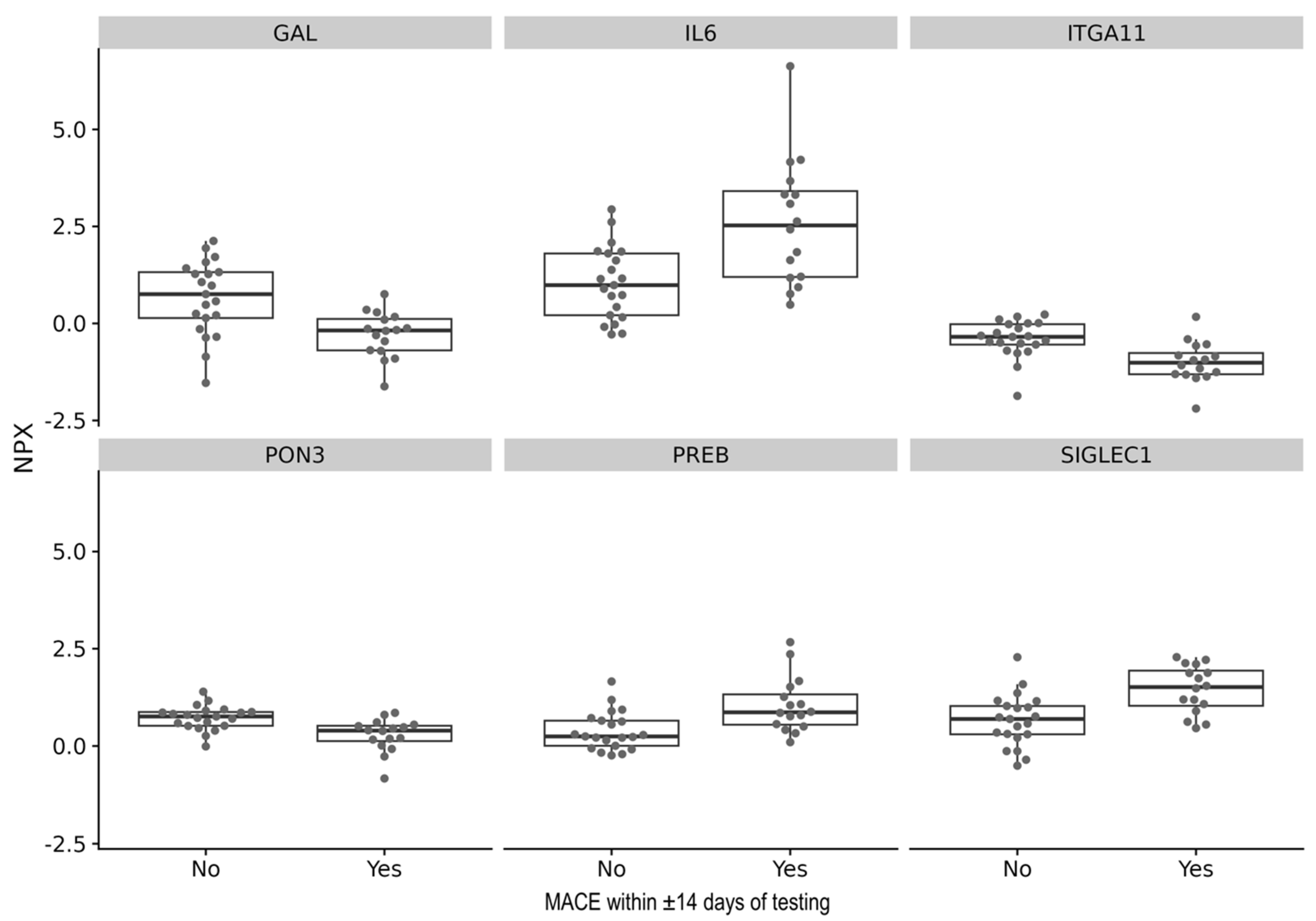
3.3. Cytokine Levels as Predictors of MACE in Cardiac Amyloidosis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia-Pavia, P.; Rapezzi, C.; Adler, Y.; Arad, M.; Basso, C.; Brucato, A.; Burazor, I.; Caforio, A.L.P.; Damy, T.; Eriksson, U.; et al. Diagnosis and treatment of cardiac amyloidosis: A position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2021, 42, 1554–1568. [Google Scholar] [CrossRef]
- Maurer, M.S.; Schwartz, J.H.; Gundapaneni, B.; Elliott, P.M.; Merlini, G.; Waddington-Cruz, M.; Kristen, A.V.; Grogan, M.; Witteles, R.; Damy, T.; et al. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N. Engl. J. Med. 2018, 379, 1007–1016. [Google Scholar] [CrossRef]
- Rapezzi, C.; Elliott, P.; Damy, T.; Nativi-Nicolau, J.; Berk, J.L.; Velazquez, E.J.; Boman, K.; Gundapaneni, B.; Patterson, T.A.; Schwartz, J.H.; et al. Efficacy of Tafamidis in Patients With Hereditary and Wild-Type Transthyretin Amyloid Cardiomyopathy: Further Analyses From ATTR-ACT. JACC Heart Fail. 2021, 9, 115–123. [Google Scholar] [CrossRef]
- Fontana, M.; Berk, J.L.; Gillmore, J.D.; Witteles, R.M.; Grogan, M.; Drachman, B.; Damy, T.; Garcia-Pavia, P.; Taubel, J.; Solomon, S.D.; et al. Vutrisiran in Patients with Transthyretin Amyloidosis with Cardiomyopathy. N. Engl. J. Med. 2025, 392, 33–44. [Google Scholar] [CrossRef]
- Garcia-Pavia, P.; Aus dem Siepen, F.; Donal, E.; Lairez, O.; van der Meer, P.; Kristen, A.V.; Mercuri, M.F.; Michalon, A.; Frost, R.J.A.; Grimm, J.; et al. Phase 1 Trial of Antibody NI006 for Depletion of Cardiac Transthyretin Amyloid. N. Engl. J. Med. 2023, 389, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Musigk, N.; Heidecker, B. Transthyretin amyloidosis: The picture is getting clearer. Eur. J. Heart Fail. 2022, 24, 1697–1699. [Google Scholar] [CrossRef] [PubMed]
- Rubin, J.; Maurer, M.S. Cardiac Amyloidosis: Overlooked, Underappreciated, and Treatable. Annu. Rev. Med. 2020, 71, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.L.; Butler, J.; Heidecker, B. Emerging therapies in transthyretin amyloidosis—A new wave of hope after years of stagnancy? Eur. J. Heart Fail. 2020, 22, 39–53. [Google Scholar] [CrossRef]
- Musigk, N.; Suwalski, P.; Golpour, A.; Fairweather, D.; Klingel, K.; Martin, P.; Frustaci, A.; Cooper, L.T.; Lüscher, T.F.; Landmesser, U.; et al. The inflammatory spectrum of cardiomyopathies. Front. Cardiovasc. Med. 2024, 11, 1251780. [Google Scholar] [CrossRef]
- Siegismund, C.S.; Escher, F.; Lassner, D.; Kühl, U.; Gross, U.; Fruhwald, F.; Wenzel, P.; Münzel, T.; Frey, N.; Linke, R.P.; et al. Intramyocardial inflammation predicts adverse outcome in patients with cardiac AL amyloidosis. Eur. J. Heart Fail. 2018, 20, 751–757. [Google Scholar] [CrossRef]
- Müller, M.L.; Brand, A.; Mattig, I.; Spethmann, S.; Messroghli, D.; Hahn, K.; Violano, M.; Mitchell, J.D.; Hare, J.M.; Frustaci, A.; et al. Myocardial Inflammation in Cardiac Transthyretin Amyloidosis: Prevalence and Potential Prognostic Implications. Circ. Heart Fail. 2025, 18, e012146. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Kalman, J.; Mayer, L.; Fillit, H.M.; Packer, M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N. Engl. J. Med. 1990, 323, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.L. Innate immunity and the failing heart: The cytokine hypothesis revisited. Circ. Res. 2015, 116, 1254–1268. [Google Scholar] [CrossRef] [PubMed]
- Adamo, L.; Rocha-Resende, C.; Prabhu, S.D.; Mann, D.L. Reappraising the role of inflammation in heart failure. Nat. Rev. Cardiol. 2020, 17, 269–285. [Google Scholar] [CrossRef]
- Pieske, B.; Tschöpe, C.; de Boer, R.A.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.P.; et al. How to diagnose heart failure with preserved ejection fraction: The HFA–PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 40, 3297–3317. [Google Scholar] [CrossRef]
- Van Linthout, S.; Tschöpe, C. Inflammation-Cause or Consequence of Heart Failure or Both? Curr. Heart Fail. Rep. 2017, 14, 251–265. [Google Scholar] [CrossRef]
- Klingel, K.; Hehn, A.; Sauter, M. P5403The impact of inflammation on the outcome of cardiac amyloidosis. Eur. Heart J. 2017, 38 (Suppl. S1), ehx493.P5403. [Google Scholar] [CrossRef]
- Lundberg, M.; Eriksson, A.; Tran, B.; Assarsson, E.; Fredriksson, S. Homogeneous antibody-based proximity extension assays provide sensitive and specific detection of low-abundant proteins in human blood. Nucleic Acids Res. 2011, 39, e102. [Google Scholar] [CrossRef]
- Rickham, P.P. HUMAN EXPERIMENTATION. CODE OF ETHICS OF THE WORLD MEDICAL ASSOCIATION. DECLARATION OF HELSINKI. Br. Med. J. 1964, 2, 177. [Google Scholar] [CrossRef]
- Caforio, A.L.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648, 2648a–2648d. [Google Scholar] [CrossRef]
- Halushka, M.K.; d’Amati, G.; Bois, M.C.; De Gaspari, M.; Giordano, C.; Klingel, K.; Leduc, C.; Ohta-Ogo, K.; Ozcan, I.; Rizzo, S.; et al. The frequency of CD3+ lymphocytes in non-myocarditis endomyocardial biopsies. Cardiovasc. Pathol. 2025, 77, 107733. [Google Scholar] [CrossRef]
- Fairweather, D.; Kaya, Z.; Shellam, G.R.; Lawson, C.M.; Rose, N.R. From infection to autoimmunity. J. Autoimmun. 2001, 16, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.; Kottwitz, J.; Baltensperger, N.; Kissel, C.K.; Lovrinovic, M.; Mehra, T.; Scherff, F.; Schmied, C.; Templin, C.; Lüscher, T.F.; et al. Cardiac Magnetic Resonance Imaging in Myocarditis Reveals Persistent Disease Activity Despite Normalization of Cardiac Enzymes and Inflammatory Parameters at 3-Month Follow-Up. Circ. Heart Fail. 2017, 10, e004262. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Menger, J.; Collini, V.; Gröschel, J.; Adler, Y.; Brucato, A.; Christian, V.; Ferreira, V.M.; Gandjbakhch, E.; Heidecker, B.; Kerneis, M.; et al. 2025 ESC Guidelines for the management of myocarditis and pericarditis: Developed by the task force for the management of myocarditis and pericarditis of the European Society of Cardiology (ESC)Endorsed by the Association for European Paediatric and Congenital Cardiology (AEPC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2025, ehaf192. [Google Scholar] [CrossRef]
- Kottam, A.; Hanneman, K.; Schenone, A.; Daubert, M.A.; Sidhu, G.D.; Gropler, R.J.; Garcia, M.J.; on behalf of the American Heart Association Council on Cardiovascular Radiology and Intervention. State-of-the-Art Imaging of Infiltrative Cardiomyopathies: A Scientific Statement From the American Heart Association . Circ. Cardiovasc. Imaging 2023, 16, e000081. [Google Scholar] [CrossRef]
- Lurz, P.; Luecke, C.; Eitel, I.; Föhrenbach, F.; Frank, C.; Grothoff, M.; Waha, S.d.; Rommel, K.-P.; Lurz, J.A.; Klingel, K.; et al. Comprehensive Cardiac Magnetic Resonance Imaging in Patients With Suspected Myocarditis. JACC 2016, 67, 1800–1811. [Google Scholar] [CrossRef]
- Hein, S.J.; Knoll, M.; Aus dem Siepen, F.; Furkel, J.; Schoenland, S.; Hegenbart, U.; Katus, H.A.; Kristen, A.V.; Konstandin, M. Elevated interleukin-6 levels are associated with impaired outcome in cardiac transthyretin amyloidosis. World J. Cardiol. 2021, 13, 55–67. [Google Scholar] [CrossRef]
- Lugitsch, J.; Hoeller, V.; Schwegel, N.; Santner, V.; Gollmer, J.; Kolesnik, E.; Lipp, R.; Niedrist, T.; Rainer, P.; Zirlik, A.; et al. Interleukin-6 plasma levels predict mortality and heart failure events in cardiac transthyretin amyloidosis. Eur. Heart J. 2023, 44 (Suppl. 2), ehad655.1884. [Google Scholar] [CrossRef]
- Ghosh, S.; Villacorta-Martin, C.; Lindstrom-Vautrin, J.; Kenney, D.; Golden, C.S.; Edwards, C.V.; Sanchorawala, V.; Connors, L.H.; Giadone, R.M.; Murphy, G.J. Mapping cellular response to destabilized transthyretin reveals cell- and amyloidogenic protein-specific signatures. Amyloid 2023, 30, 379–393. [Google Scholar] [CrossRef]
- Jordan, T.L.; Maar, K.; Redhage, K.R.; Misra, P.; Blancas-Mejia, L.M.; Dick, C.J.; Wall, J.S.; Williams, A.; Dietz, A.B.; van Wijnen, A.J.; et al. Light chain amyloidosis induced inflammatory changes in cardiomyocytes and adipose-derived mesenchymal stromal cells. Leukemia 2020, 34, 1383–1393. [Google Scholar] [CrossRef]
- Golpour, A.; Suwalski, P.; Landmesser, U.; Heidecker, B. Case report: Magnetocardiography as a potential method of therapy monitoring in amyloidosis. Front. Cardiovasc. Med. 2023, 10, 1224578. [Google Scholar] [CrossRef]
- Brala, D.; Thevathasan, T.; Grahl, S.; Barrow, S.; Violano, M.; Bergs, H.; Golpour, A.; Suwalski, P.; Poller, W.; Skurk, C.; et al. Application of Magnetocardiography to Screen for Inflammatory Cardiomyopathy and Monitor Treatment Response. J. Am. Heart Assoc. 2023, 12, e027619. [Google Scholar] [CrossRef]
- Müller, M.L.; Suwalski, P.; Wilke, F.; Latinova, E.; Satilmis, G.; Musigk, N.; Brand, A.; Mattig, I.; Knebel, F.; Messroghli, D.; et al. Magnetocardiography for the non-invasive diagnosis of myocardial inflammation in cardiac amyloidosis: A proof-of-concept study. ESC Heart Fail. 2025. [Google Scholar] [CrossRef]

| Analysis 1: Correlation of Cytokines with Myocardial Presence of Inflammatory Cells | Analysis 2: Correlation of Cytokines with MACE | |||||
|---|---|---|---|---|---|---|
| EMB-Positive for Myocardial Presence of Inflammatory Cells (n = 17) | EMB-Negative for Myocardial Presence of Inflammatory Cells (n = 10) | p-Value | MACE ± 14 Days of a Study Visit (n = 16) | No MACE ± 14 Days of a Study Visit (n = 21) | p-Value | |
| ATTR (n, %) | 10 (58.8%) | 7 (70.0%) | 0.574 | 7 (43.8%) | 15 (71.4%) | 0.574 |
| AL (n, %) | 7 (41.2%) | 3 (30.0%) | 9 (56.2%) | 6 (28.6%) | ||
| Age in years (median, IQR) | 77.0 (73.0–83.0) | 76.5 (72.8–81.8) | 1 | 78.0 (74.5–83.0) | 78.0 (68.0–82.0) | 0.684 |
| Sex female (n, %) | 5 (29.4%) | 2 (20.0%) | 0.932 | 5 (31.3%) | 4 (19.0%) | 0.933 |
| BMI in kg/m2 (median, IQR) | 23.9 (22.6–25.3) | 25.4 (22.4–26.7) | 0.359 | 24.6 (21.3–27.3) | 23.9 (21.8–25.9) | 0.255 |
| hs-cTn in ng/L (median, IQR) | 69.0 (44.0–159.0) | 55.0 (41.0–78.0) | 0.590 | 81.5 (57.3–129.0) | 57.0 (35.0–79.5) | 0.182 |
| NT-proBNP in ng/L (median, IQR) | 5008.0 (2553.0–9448.0) | 2112.5 (1163.3–5370.0) | 0.204 | 6428.5 (3673.5–16,363.5) | 2114.0 (1010.0–5008.0) | 0.132 |
| Creatinine in mg/dL (median, IQR) | 1.2 (1.1–1.4) | 1.3 (1.1–1.5) | 0.530 | 1.3 (1.0–1.6) | 1.2 (1.1–1.4) | 0.049 |
| Assay | Estimate | R2 | p-Value | FDR |
|---|---|---|---|---|
| CD244 | −0.47 | 0.28 | 0.0045 | 1 |
| ADGRE2 | −0.49 | 0.24 | 0.0101 | 1 |
| AOC1 | −1.13 | 0.22 | 0.0134 | 1 |
| CLEC4D | −1.01 | 0.22 | 0.0142 | 1 |
| IL1RL2 | −0.41 | 0.22 | 0.0143 | 1 |
| CD84 | −0.37 | 0.21 | 0.0166 | 1 |
| PRKAB1 | −0.76 | 0.21 | 0.0167 | 1 |
| OSCAR | −0.57 | 0.20 | 0.0207 | 1 |
| RGS8 | −1.04 | 0.18 | 0.0251 | 1 |
| CD200 | −0.31 | 0.18 | 0.0285 | 1 |
| Assay | Estimate | R2 | p-Value | FDR |
|---|---|---|---|---|
| PON3 | −0.45 | 0.28 | 0.00075 | 0.28 |
| SIGLEC1 | 0.79 | 0.28 | 0.00077 | 0.28 |
| IL6 | 1.55 | 0.28 | 0.00086 | 0.31 |
| ITGA11 | −0.59 | 0.27 | 0.00107 | 0.39 |
| GAL | −0.95 | 0.26 | 0.00136 | 0.49 |
| PREB | 0.66 | 0.24 | 0.00193 | 0.70 |
| PRKCQ | 0.73 | 0.24 | 0.00196 | 0.71 |
| EPCAM | −0.81 | 0.23 | 0.00250 | 0.90 |
| PNPT1 | 1.04 | 0.22 | 0.00320 | 1.00 |
| TNFRSF13C | 0.80 | 0.22 | 0.00330 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musigk, N.; Suwalski, P.; Müller, M.; Violano, M.; Klingel, K.; Weiner, J., 3rd; Beule, D.; Landmesser, U.; Heidecker, B. Evaluation of Cytokine Levels in Cardiac Transthyretin and Immunoglobulin Light Chain Amyloidosis and Their Correlation with Myocardial Inflammatory Cells and MACE. Biomedicines 2025, 13, 2254. https://doi.org/10.3390/biomedicines13092254
Musigk N, Suwalski P, Müller M, Violano M, Klingel K, Weiner J 3rd, Beule D, Landmesser U, Heidecker B. Evaluation of Cytokine Levels in Cardiac Transthyretin and Immunoglobulin Light Chain Amyloidosis and Their Correlation with Myocardial Inflammatory Cells and MACE. Biomedicines. 2025; 13(9):2254. https://doi.org/10.3390/biomedicines13092254
Chicago/Turabian StyleMusigk, Nicolas, Phillip Suwalski, Maximilian Müller, Michele Violano, Karin Klingel, January Weiner, 3rd, Dieter Beule, Ulf Landmesser, and Bettina Heidecker. 2025. "Evaluation of Cytokine Levels in Cardiac Transthyretin and Immunoglobulin Light Chain Amyloidosis and Their Correlation with Myocardial Inflammatory Cells and MACE" Biomedicines 13, no. 9: 2254. https://doi.org/10.3390/biomedicines13092254
APA StyleMusigk, N., Suwalski, P., Müller, M., Violano, M., Klingel, K., Weiner, J., 3rd, Beule, D., Landmesser, U., & Heidecker, B. (2025). Evaluation of Cytokine Levels in Cardiac Transthyretin and Immunoglobulin Light Chain Amyloidosis and Their Correlation with Myocardial Inflammatory Cells and MACE. Biomedicines, 13(9), 2254. https://doi.org/10.3390/biomedicines13092254







