Sex-Stratified Prediction Models for 5-Year Nonalcoholic Fatty Liver Disease Risk in Thyroid Cancer Patients: A Nationwide Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Design & Population
2.3. Model Construction
2.4. Model Evaluation
2.5. Risk Stratification
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
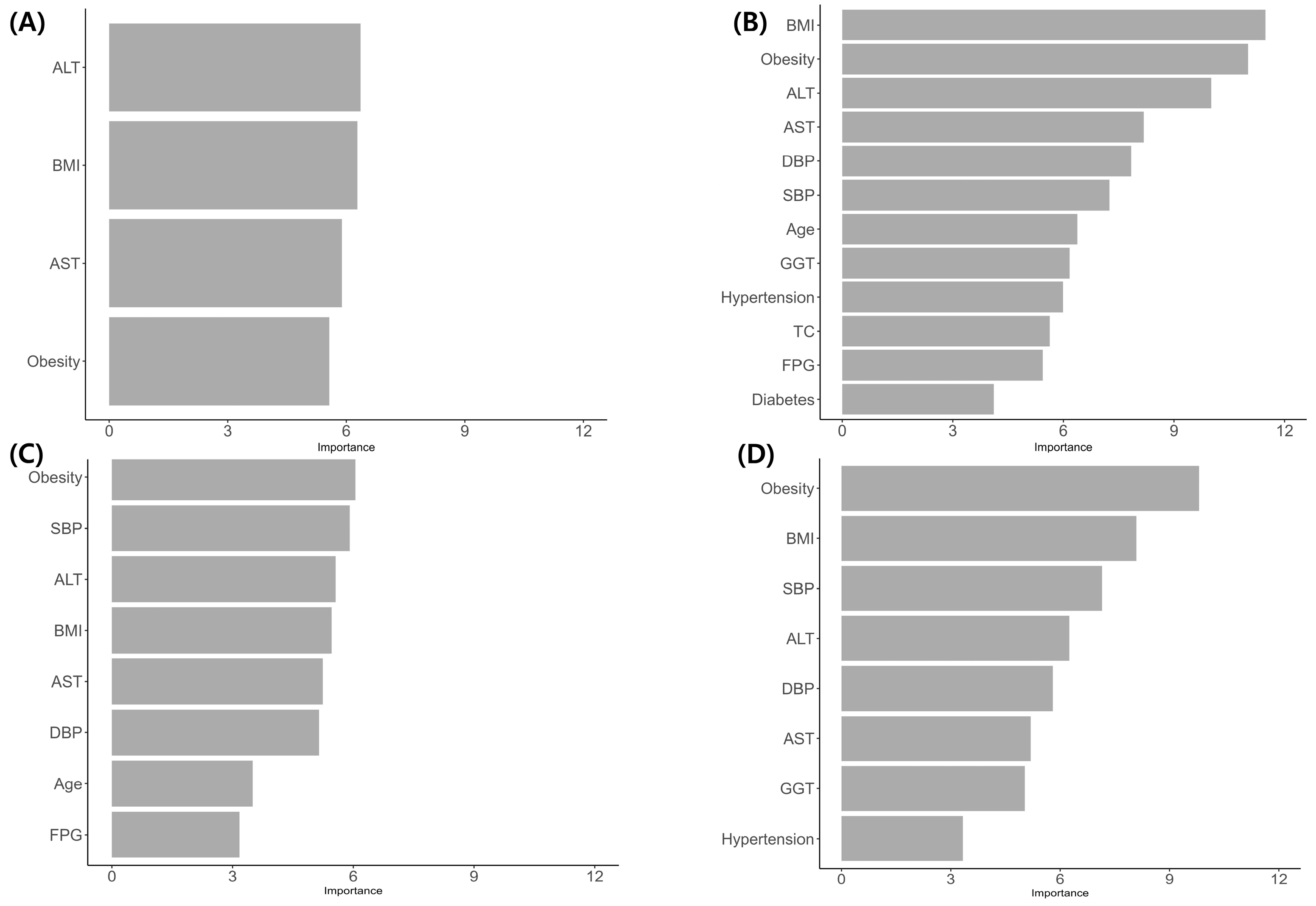
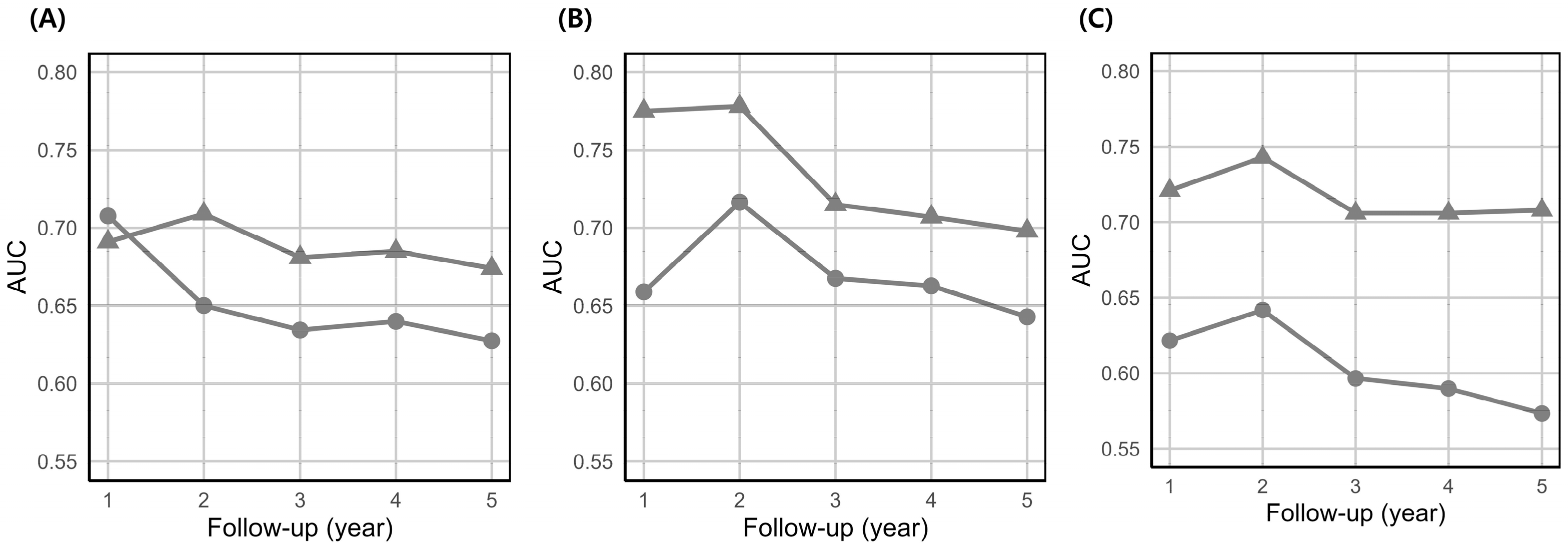
3.2. Model Performance
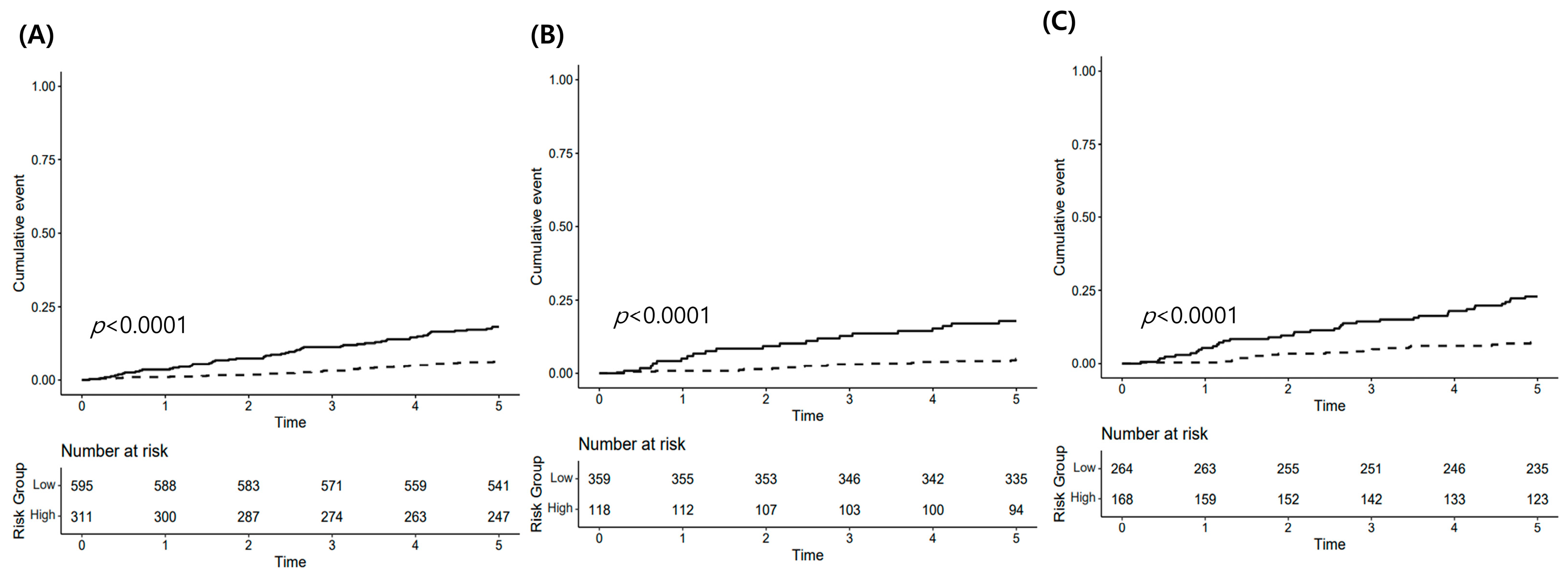
3.3. Risk Stratification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NAFLD | Nonalcoholic fatty liver disease |
| RSF | Random survival forest |
| Cox | Cox proportional hazards regression |
| NHIS | National Health Insurance Service |
| AUC | Area under the ROC curve |
References
- Wiltshire, J.J.; Drake, T.M.; Uttley, L.; Balasubramanian, S.P. Systematic review of trends in the incidence rates of thyroid Cancer. Thyroid 2016, 26, 1541–1552. [Google Scholar] [CrossRef]
- Jung, K.-W.; Kang, M.J.; Park, E.H.; Yun, E.H.; Kim, H.-J.; Kong, H.-J.; Im, J.-S.; Seo, H.G. Prediction of Cancer Incidence and Mortality in Korea, 2023. Cancer Res. Treat. 2023, 55, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Lee, J.-H.; Back, K.; Park, K.S.; Chung, Y.S. Changes in the Trend of Thyroid Cancer Epidemiology According to South Korean Nationwide Database, 1999–2020. J. Endocr. Surg. 2024, 24, 31–38. [Google Scholar] [CrossRef]
- Cho, Y.B.; Park, K.S. Increased risk of nonalcoholic fatty liver disease in patients with thyroid cancer: A nationwide cohort study. BMC Cancer. 2025, 25, 1093. [Google Scholar] [CrossRef]
- Tsai, M.-C.; Hsieh, C.-T.; Hsu, H.-Y.; Yeh, T.-L.; Lee, W.-C.; Chiang, C.-J.; Hsiao, B.-Y.; Jhuang, J.-R.; Tsai, W.-H.; Cheng, S.-P.; et al. Association between thyroid cancer and cardiovascular disease risk: A nationwide observation study. Sci. Rep. 2022, 12, 18438. [Google Scholar] [CrossRef] [PubMed]
- Baliram, R.; Latif, R.; Zaidi, M.; Davies, T.F. Expanding the role of thyroid-stimulating hormone in skeletal physiology. Front. Endocrinol. 2017, 8, 252. [Google Scholar] [CrossRef]
- Jia, Y.; Li, D.; You, Y.; Yu, J.; Jiang, W.; Liu, Y.; Zeng, R.; Wan, Z.; Lei, Y.; Liao, X. Multi-system diseases and death trajectory of metabolic dysfunction-associated fatty liver disease: Findings from the UK Biobank. BMC Med. 2023, 21, 398. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Henry, L. Understanding the Burden of Nonalcoholic Fatty Liver Disease: Time for Action. Diabetes Spectr. 2024, 37, 9–19. [Google Scholar] [CrossRef]
- Battistella, S.; D’Arcangelo, F.; Grasso, M.; Zanetto, A.; Gambato, M.; Germani, G.; Senzolo, M.; Russo, F.P.; Burra, P. Liver transplantation for non-alcoholic fatty liver disease: Indications and post-transplant management. Clin. Mol. Hepatol. 2023, 29, S286–S301. [Google Scholar] [CrossRef]
- Xiang, L.-L.; Cao, Y.-T.; Sun, J.; Li, R.-H.; Qi, F.; Zhang, Y.-J.; Zhang, W.-H.; Yan, L.; Zhou, X.-Q. Association between thyroid function and nonalcoholic fatty liver disease: A dose-response meta-analysis. Front. Endocrinol. 2024, 15, 1399517. [Google Scholar] [CrossRef]
- Hatziagelaki, E.; Paschou, S.A.; Schön, M.; Psaltopoulou, T.; Roden, M. NAFLD and thyroid function: Pathophysiological and therapeutic considerations. Trends Endocrinol. Metab. 2022, 33, 755–768. [Google Scholar] [CrossRef]
- Bano, A.; Chaker, L.; Plompen, E.P.; Hofman, A.; Dehghan, A.; Franco, O.H.; Harry, L.A.J.; Murad, S.D.; Peeters, R.P. Thyroid Function and the Risk of Nonalcoholic Fatty Liver Disease: The Rotterdam Study. J. Clin. Endocrinol. Metab. 2016, 101, 3204–3211. [Google Scholar] [CrossRef] [PubMed]
- Schini, M.; Johansson, H.; Harvey, N.C.; Lorentzon, M.; Kanis, J.A.; McCloskey, E.V. An overview of the use of the fracture risk assessment tool (FRAX) in osteoporosis. J. Endocrinol. Investig. 2024, 47, 501–511. [Google Scholar] [CrossRef]
- Hippisley-Cox, J.; Coupland, C.; Brindle, P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: Prospective cohort study. BMJ 2017, 357, j2099. [Google Scholar] [CrossRef]
- Paulino, Â.D.C.; Guimarães, L.N.F.; Shiguemori, E.H. Hybrid adaptive computational intelligence-based multisensor data fusion applied to realtime UAV autonomous navigation. Intel. Artif. 2019, 22, 162–195. [Google Scholar] [CrossRef]
- Berkowitz, M.; Altman, R.M.; Loughin, T.M. Random forests for survival data: Which methods work best and under what conditions? Int. J. Biostat. 2024, 20, 315–345. [Google Scholar] [CrossRef] [PubMed]
- Deo, S.V.; Deo, V.; Sundaram, V. Survival analysis-part 2: Cox proportional hazards model. Indian J. Thorac. Cardiovasc. Surg. 2021, 37, 229–233. [Google Scholar] [CrossRef]
- Li, L.W.; Liu, X.; Shen, M.L.; Zhao, M.J.; Liu, H. Development and validation of a random survival forest model for predicting long-term survival of early-stage young breast cancer patients based on the SEER database and an external validation cohort. Am. J. Cancer Res. 2024, 14, 1609–1621. [Google Scholar] [CrossRef]
- Touraine, C.; Winter, A.; Castan, F.; Azria, D.; Gourgou, S. Time-Dependent ROC Curve Analysis for Assessing the Capability of Radiation-Induced CD8 T-Lymphocyte Apoptosis to Predict Late Toxicities after Adjuvant Radiotherapy of Breast Cancer Patients. Cancers 2023, 15, 4676. [Google Scholar] [CrossRef] [PubMed]
- Ogłuszka, M.; Orzechowska, M.; Jędroszka, D.; Witas, P.; Bednarek, A.K. Evaluate Cutpoints: Adaptable continuous data distribution system for determining survival in Kaplan-Meier estimator. Comput. Methods Programs Biomed. 2019, 177, 133–139. [Google Scholar] [CrossRef]
- Fresneda, S.; Abbate, M.; Busquets-Cortés, C.; López-González, A.; Fuster-Parra, P.; Bennasar-Veny, M.; Yáñez, A.M. Sex and age differences in the association of fatty liver index-defined non-alcoholic fatty liver disease with cardiometabolic risk factors: A cross-sectional study. Biol. Sex Differ. 2022, 13, 64. [Google Scholar] [CrossRef]
- Jaroenlapnopparat, A.; Charoenngam, N.; Ponvilawan, B.; Mariano, M.; Thongpiya, J.; Yingchoncharoen, P. Menopause is associated with increased prevalence of nonalcoholic fatty liver disease: A systematic review and meta-analysis. Menopause 2023, 30, 348–354. [Google Scholar] [CrossRef]
- Li, L.; Liu, D.W.; Yan, H.Y.; Wang, Z.Y.; Zhao, S.H.; Wang, B. Obesity is an independent risk factor for non-alcoholic fatty liver disease: Evidence from a meta-analysis of 21 cohort studies. Obes. Rev. 2016, 17, 510–519. [Google Scholar] [CrossRef]
- Xuan, Y.; Wu, D.; Zhang, Q.; Yu, Z.; Yu, J.; Zhou, D. Elevated ALT/AST ratio as a marker for NAFLD risk and severity: Insights from a cross-sectional analysis in the United States. Front. Endocrinol. 2024, 15, 1457598. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Ryu, S.; Sung, E.; Jang, Y. Higher concentrations of alanine aminotransferase within the reference interval predict nonalcoholic fatty liver disease. Clin. Chem. 2007, 53, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Lu, H.; Ran, Y. Advancing non-alcoholic fatty liver disease prediction: A comprehensive machine learning approach integrating SHAP interpretability and multi-cohort validation. Front. Endocrinol. 2024, 15, 1450317. [Google Scholar] [CrossRef]
- Yuan, M.; He, J.; Hu, X.; Yao, L.; Chen, P.; Wang, Z.; Pingji, L.; Zhiyu, X.; Yingan, J.; Lanjuan, L. Hypertension and NAFLD risk: Insights from the NHANES 2017–2018 and Mendelian randomization analyses. Chin. Med. J. 2024, 137, 457–464. [Google Scholar] [CrossRef]
- Ciardullo, S.; Monti, T.; Sala, I.; Grassi, G.; Mancia, G.; Perseghin, G. Nonalcoholic Fatty Liver Disease and Advanced Fibrosis in US Adults Across Blood Pressure Categories. Hypertension 2020, 76, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Rogal, S.S.; Yakovchenko, V.; Morgan, T.; Bajaj, J.S.; Gonzalez, R.; Park, A.; Beste, L.; Miech, E.J.; Lamorte, C.; Neely, B.; et al. Getting to implementation: A protocol for a Hybrid III stepped wedge cluster randomized evaluation of using data-driven implementation strategies to improve cirrhosis care for Veterans. Implement. Sci. 2020, 15, 92. [Google Scholar] [CrossRef]
- Eddington, H.S.; Trickey, A.W.; Shah, V.; Harris, A.H.S. Tutorial: Implementing and visualizing machine learning (ML) clinical prediction models into web-accessible calculators using Shiny R. Ann. Transl. Med. 2022, 10, 1414. [Google Scholar] [CrossRef]
- Ayaz, M.; Pasha, M.F.; Alzahrani, M.Y.; Budiarto, R.; Stiawan, D. The Fast Health Interoperability Resources (FHIR) Standard: Systematic Literature Review of Implementations, Applications, Challenges and Opportunities. JMIR Med. Inform. 2021, 9, e21929. [Google Scholar] [CrossRef]
- Fox, R.K.; Chu, J.N.; Goldman, M.L.; Islam, K.B.; Brandman, D. Prospective study of a case-finding algorithm to detect NAFLD with advanced fibrosis in primary care patients. Hepatol. Commun. 2023, 7, e0024. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wong, V.W.-S.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef] [PubMed]

| Variables | All (N = 3644) | Male (N = 635) | Female (N = 3009) | p Value |
|---|---|---|---|---|
| Age | 50.0 (42.0–56.0) | 47.0 (39.0–55.5) | 50.0 (42.0–57.0) | <0.001 |
| Income | <0.001 | |||
| Low | 931 (25.5) | 80 (12.6) | 851 (28.3) | |
| Middle | 972 (26.7) | 146 (23.0) | 826 (27.5) | |
| High | 1741 (47.8) | 409 (64.4) | 1332 (44.3) | |
| Residence | 0.853 | |||
| Urban | 1827 (50.1) | 321 (50.6) | 1506 (50.0) | |
| Rural | 1817 (49.9) | 314 (49.4) | 1503 (50.0) | |
| Disability | 131 (3.6) | 28 (4.4) | 103 (3.4) | 0.273 |
| Insurance type | <0.001 | |||
| Self-employed | 978 (26.8) | 113 (17.8) | 865 (28.7) | |
| Work-employed | 2666 (73.2) | 522 (82.2) | 2144 (71.3) | |
| Smoking status | <0.001 | |||
| Never | 3164 (86.8) | 248 (39.1) | 2916 (96.9) | |
| Ex | 240 (6.6) | 200 (31.5) | 40 (1.3) | |
| Current | 240 (6.6) | 187 (29.4) | 53 (1.8) | |
| Alcohol intake | <0.001 | |||
| 0 | 2755 (75.6) | 268 (42.2) | 2487 (82.7) | |
| 1 | 715 (19.6) | 256 (40.3) | 459 (15.3) | |
| 2 | 139 (3.8) | 92 (14.5) | 47 (1.6) | |
| ≥3 | 35 (1.0) | 19 (3.0) | 16 (0.5) | |
| Alcohol binge | 1342 (36.8) | 353 (55.6) | 989 (32.9) | <0.001 |
| Regular exercise | 490 (13.4) | 83 (13.1) | 407 (13.5) | 0.809 |
| Health examination | ||||
| BMI (kg/m2) | 23.4 (21.5–25.6) | 24.8 (23.1–26.7) | 23.1 (21.2–25.3) | <0.001 |
| BMI | <0.001 | |||
| Underweight | 128 (3.5) | 9 (1.4) | 119 (4.0) | |
| Normal | 1455 (39.9) | 145 (22.8) | 1310 (43.5) | |
| Overweight | 883 (24.2) | 182 (28.7) | 701 (23.3) | |
| Obese | 1178 (32.3) | 299 (47.1) | 879 (29.2) | |
| SBP (mmHg) | 120 (110–130) | 124 (116–131) | 120 (110–130) | <0.001 |
| DBP (mmHg) | 75 (70–80) | 80 (70–85) | 74 (69–80) | <0.001 |
| FPG (mg/dL) | 92 (85–100) | 94 (87–105) | 92 (85–100) | <0.001 |
| TC (mg/dL) | 191 (168–218) | 192 (170–219) | 191(168–218) | 0.661 |
| AST (IU/L) | 21 (18–26) | 24 (20–29) | 21 (17–25) | <0.001 |
| ALT (IU/L) | 18 (14–26) | 25 (19–35) | 17 (13–24) | <0.001 |
| GGT (IU/L) | 18 (13–27) | 32 (22–48) | 17 (12–23) | <0.001 |
| Comorbidities | ||||
| Dyslipidemia | 1784 (49.0) | 325 (51.2) | 1459 (48.5) | 0.234 |
| Diabetes | 949 (26.0) | 174 (27.4) | 775 (25.8) | 0.419 |
| Hypertension | 1515 (41.6) | 352 (55.4) | 1163 (38.7) | <0.001 |
| Obesity | 1802 (49.5) | 409 (64.4) | 1393 (46.3) | <0.001 |
| CCI | 0.440 | |||
| ≤2 | 350 (55.1) | 1605 (53.3) | 1955 (53.6) | |
| >2 | 285 (44.9) | 1404 (46.7) | 1689 (46.4) | |
| Thyroidectomy type | 0.737 | |||
| Lobectomy | 663 (18.2) | 119 (18.7) | 544 (18.1) | |
| Total thyroidectomy | 2981 (81.8) | 516 (81.3) | 2465 (81.9) | |
| Outcome | ||||
| NAFLD | 371 (10.2) | 64 (10.1) | 307 (10.2) | 0.983 |
| RSF | Cox | |||
|---|---|---|---|---|
| C-Index (95% CI) | p Value | C-Index (95% CI) | p Value | |
| Male | 0.59 (0.48–0.71) | 0.046 | 0.64 (0.51–0.76) | 0.541 |
| Female | 0.62 (0.57–0.68) | 0.005 | 0.67 (0.61–0.72) | <0.001 |
| ≤50 years | 0.64 (0.55–0.74) | 0.011 | 0.69 (0.61–0.78) | 0.004 |
| >50 years | 0.57 (0.50–0.64) | 0.110 | 0.70 (0.64–0.76) | 0.003 |
| Total | Event (%) | 1000 PY | HR (95% CI) | p Value | ||
|---|---|---|---|---|---|---|
| All female | Low risk | 595 | 37 (6.22) | 4704.14 | 1.00 | |
| High risk | 311 | 56 (18.01) | 14,588.72 | 3.11 (2.05–4.71) | <0.001 | |
| ≤50 years | Low risk | 359 | 18 (5.01) | 3764.73 | 1.00 | |
| High risk | 118 | 21 (17.80) | 14,496.59 | 3.84 (2.04–7.20) | <0.001 | |
| >50 years | Low risk | 264 | 19 (7.20) | 5477.56 | 1.00 | |
| High risk | 168 | 38 (22.62) | 18,942.98 | 3.47 (2.00–6.02) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, Y.B.; Park, K.S. Sex-Stratified Prediction Models for 5-Year Nonalcoholic Fatty Liver Disease Risk in Thyroid Cancer Patients: A Nationwide Cohort Study. Biomedicines 2025, 13, 2250. https://doi.org/10.3390/biomedicines13092250
Cho YB, Park KS. Sex-Stratified Prediction Models for 5-Year Nonalcoholic Fatty Liver Disease Risk in Thyroid Cancer Patients: A Nationwide Cohort Study. Biomedicines. 2025; 13(9):2250. https://doi.org/10.3390/biomedicines13092250
Chicago/Turabian StyleCho, Young Bin, and Kyoung Sik Park. 2025. "Sex-Stratified Prediction Models for 5-Year Nonalcoholic Fatty Liver Disease Risk in Thyroid Cancer Patients: A Nationwide Cohort Study" Biomedicines 13, no. 9: 2250. https://doi.org/10.3390/biomedicines13092250
APA StyleCho, Y. B., & Park, K. S. (2025). Sex-Stratified Prediction Models for 5-Year Nonalcoholic Fatty Liver Disease Risk in Thyroid Cancer Patients: A Nationwide Cohort Study. Biomedicines, 13(9), 2250. https://doi.org/10.3390/biomedicines13092250







