Polymorphisms in VEGF Signaling Pathway Genes and Their Potential Impact on Type 2 Diabetes Mellitus and Associated Complications: A Scoping Review
Abstract
1. Introduction
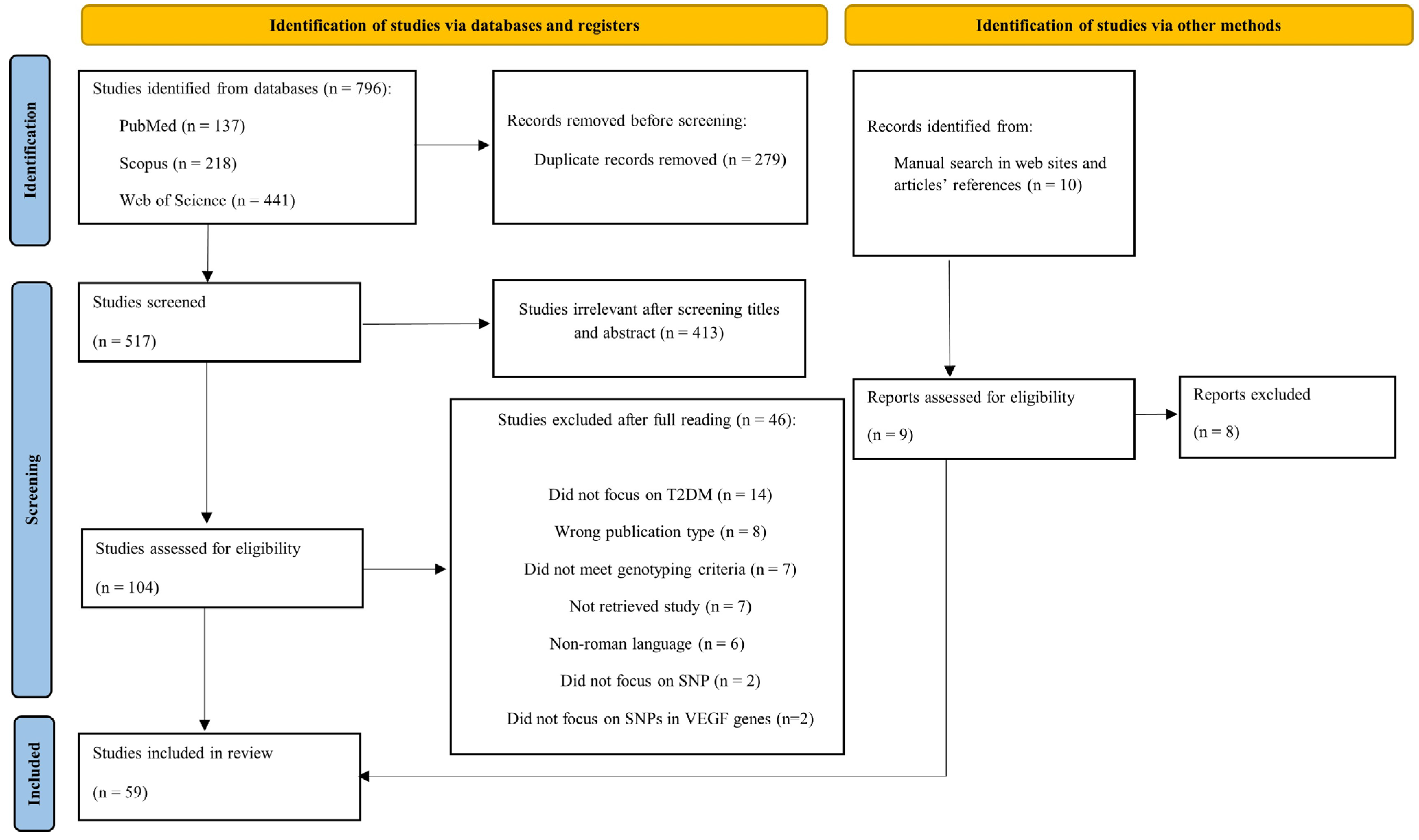
2. Methods
2.1. Research Strategy
2.2. Eligibility Criteria
2.3. Study Selection
2.4. Data Extraction
3. Results and Discussion
3.1. Characteristics of Included Studies
3.2. Association Between Polymorphisms in the VEGF Gene and VEGFR2 and the Occurrence of TDM2 or Related Complications
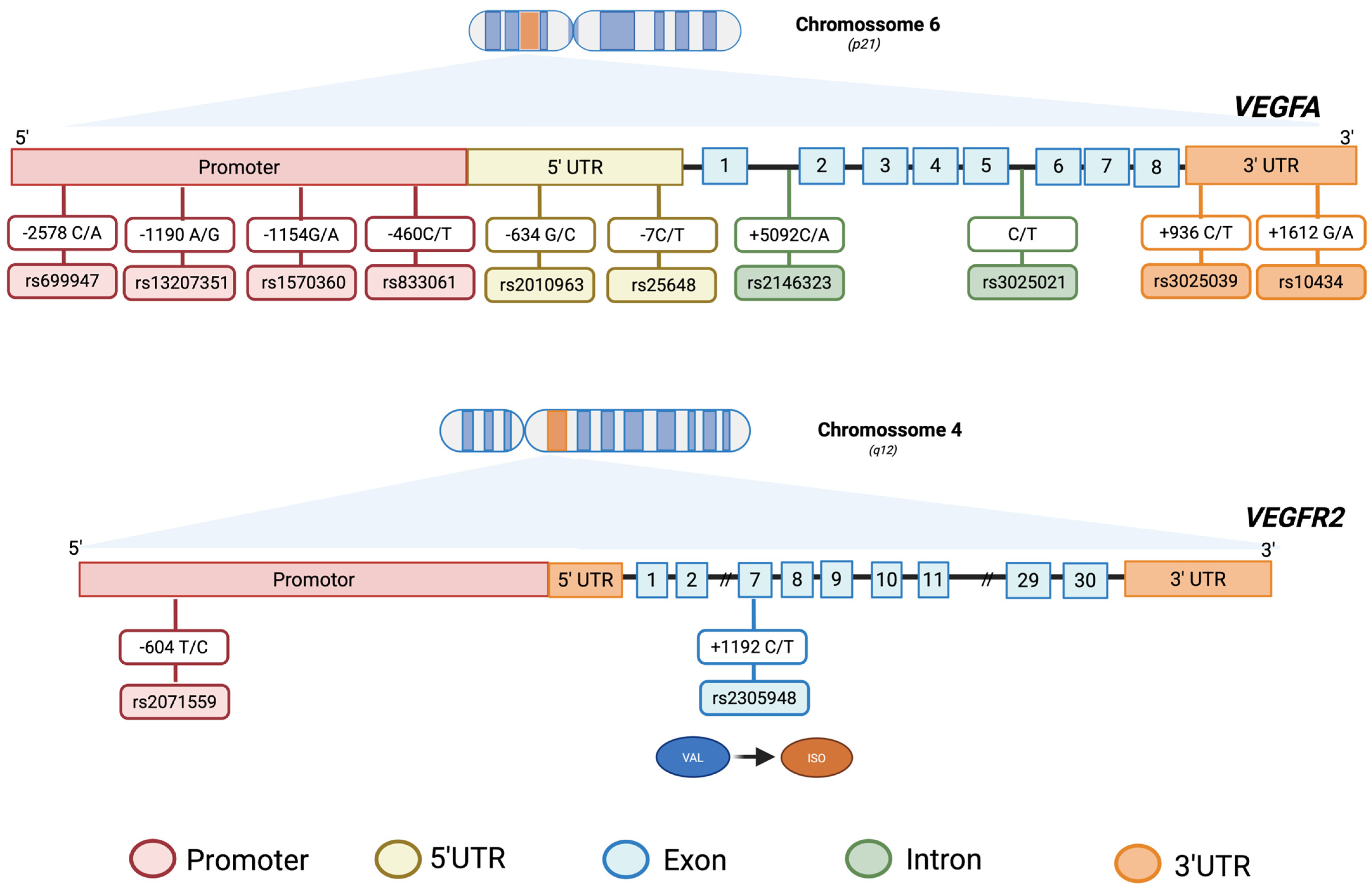
3.2.1. VEGFA Polymorphisms
VEGFA rs2010963
VEGFA rs699947
VEGFA rs3025039
VEGFA rs833061
VEGFA rs1570360
VEGFA rs13207351
VEGFA rs2146323
VEGFA rs25648
VEGFA rs10434
VEGFA rs3025021
VEGFA rs3025020
VEGFA rs3025035
VEGFA rs6921438
VEGFA rs833069
3.2.2. VEGFR2 Polymorphisms
VEGFR2 rs2071559
VEGFR2 rs2305948
3.2.3. VEGFR1 Polymorphisms
VEGFR1 rs7993418
3.2.4. VEGFB Polymorphisms
VEGFB rs12366035
3.2.5. VEGFC Polymorphisms
VEGFC rs7664413
3.2.6. Other Polymorphisms
3.3. Expert Opinion and Future Perspectives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARMS-PCR | Amplification Refractory Mutation System–Polymerase Chain Reaction |
| Ca2+ | Calcium Ion |
| CNPq | Conselho Nacional de Desenvolvimento Científico e Tecnológico |
| DAG | Diacylglycerol |
| DFU | Diabetic Foot Ulcer |
| DM | Diabetes Mellitus |
| DNP | Diabetic Nephropathy |
| DPN | Diabetic Peripheral Neuropathy |
| DR | Diabetic Retinopathy |
| ERK | Extracellular signal-Regulated Kinase |
| FAK | Focal Adhesion Kinase |
| GDM | Gestational Diabetes Mellitus |
| IP3 | Inositol 1,4,5-Trisphosphate |
| KASP | Kompetitive Allele Specific PCR |
| MAPK | Mitogen-Activated Protein Kinase |
| MEK | MAPK/ERK Kinase |
| mTOR | Mammalian Target of Rapamycin |
| NPDR | Non-Proliferative Diabetic Retinopathy |
| OSF | Open Science Framework |
| PDR | Proliferative Diabetic Retinopathy |
| PCR | Polymerase Chain Reaction |
| PCR/LDR | Polymerase Chain Reaction–Ligase Detection Reaction |
| PCR-RFLP | Polymerase Chain Reaction–Restriction Fragment Length Polymorphism |
| PI3K | Phosphoinositide 3-Kinase |
| PKC | Protein Kinase C |
| PLCγ | Phospholipase C gamma |
| PRISMA-ScR | Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews |
| SNP | Single-Nucleotide Polymorphism |
| T1DM | Type 1 Diabetes Mellitus |
| T2DM | Type 2 Diabetes Mellitus |
| TAQMAN | TaqMan Allelic Discrimination Assay |
| UTR | Untranslated Region |
| VEGF | Vascular Endothelial Growth Factor |
| VEGFA | Vascular Endothelial Growth Factor A |
| VEGFB | Vascular Endothelial Growth Factor B |
| VEGFC | Vascular Endothelial Growth Factor C |
| VEGFD | Vascular Endothelial Growth Factor D |
| VEGFR1 | Vascular Endothelial Growth Factor Receptor 1 |
| VEGFR2 | Vascular Endothelial Growth Factor Receptor 2 |
| VEGFR3 | Vascular Endothelial Growth Factor Receptor 3 |
References
- Lohela, M.; Bry, M.; Tammela, T.; Alitalo, K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr. Opin. Cell Biol. 2009, 21, 154–165. [Google Scholar] [CrossRef]
- Hokkanen, K.; Tirronen, A.; Ylä-Herttuala, S. Intestinal lymphatic vessels and their role in chylomicron absorption and lipid homeostasis. Curr. Opin. Lipidol. 2019, 30, 370–376. [Google Scholar] [CrossRef]
- Shibuya, M. VEGF-VEGFR System as a Target for Suppressing Inflammation and other Diseases. Endocr. Metab. Immune Disord. Drug Targets 2015, 15, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Morita, T. Molecular and functional diversity of vascular endothelial growth factors. Mol. Divers. 2006, 10, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, G.; Cohen, T.; Gengrinovitch, S.; Poltorak, Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999, 13, 9–22. [Google Scholar] [CrossRef]
- Shibuya, M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J. Biochem. Mol. Biol. 2006, 39, 469–478. [Google Scholar] [CrossRef]
- Hamrah, P.; Chen, L.; Cursiefen, C.; Zhang, Q.; Joyce, N.C.; Dana, M.R. Expression of vascular endothelial growth factor receptor-3 (VEGFR-3) on monocytic bone marrow-derived cells in the conjunctiva. Exp. Eye Res. 2004, 79, 553–561. [Google Scholar] [CrossRef]
- Olsson, A.-K.; Dimberg, A.; Kreuger, J.; Claesson-Welsh, L. VEGF receptor signalling? In control of vascular function. Nat. Rev. Mol. Cell Biol. 2006, 7, 359–371. [Google Scholar] [CrossRef]
- Simons, M.; Gordon, E.; Claesson-Welsh, L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 2016, 17, 611–625. [Google Scholar] [CrossRef]
- Niu, G.; Chen, X. Vascular endothelial growth factor as an anti-angiogenic target for cancer therapy. Curr. Drug Targets 2010, 11, 1000–1017. [Google Scholar] [CrossRef]
- Hoeben, A.; Landuyt, B.; Highley, M.S.; Wildiers, H.; Van Oosterom, A.T.; De Bruijn, E.A. Vascular endothelial growth factor and angiogenesis. Pharmacol. Rev. 2004, 56, 549–580. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Gerber, H.P.; Vu, T.H.; Ryan, A.M.; Kowalski, J.; Werb, Z.; Ferrara, N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat. Med. 1999, 5, 623–628. [Google Scholar] [CrossRef]
- Ferrara, N.; Carver-Moore, K.; Chen, H.; Dowd, M.; Lu, L.; O’Shea, K.S.; Powell-Braxton, L.; Hillan, K.J.; Moore, M.W. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 1996, 380, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Chintalgattu, V.; Nair, D.M.; Katwa, L.C. Cardiac myofibroblasts: A novel source of vascular endothelial growth factor (VEGF) and its receptors Flt-1 and KDR. J. Mol. Cell Cardiol. 2003, 35, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, L.F.; Tomaselli, K.J. Extracellular matrix molecules and their receptors: Functions in neural development. Annu. Rev. Neurosci. 1991, 14, 531–570. [Google Scholar] [CrossRef]
- Jiang, X.; Nicolls, M.R.; Tian, W.; Rockson, S.G. Lymphatic Dysfunction, Leukotrienes, and Lymphedema. Annu. Rev. Physiol. 2018, 80, 49–70. [Google Scholar] [CrossRef] [PubMed]
- Alkim, C.; Alkim, H.; Koksal, A.R.; Boga, S.; Sen, I. Angiogenesis in Inflammatory Bowel Disease. Int. J. Inflamm. 2015, 2015, 970890. [Google Scholar] [CrossRef]
- Martin, A.; Komada, M.R.; Sane, D.C. Abnormal angiogenesis in diabetes mellitus. Med. Res. Rev. 2003, 23, 117–145. [Google Scholar] [CrossRef]
- Kolluru, G.K.; Bir, S.C.; Kevil, C.G. Endothelial dysfunction and diabetes: Effects on angiogenesis, vascular remodeling, and wound healing. Int. J. Vasc. Med. 2012, 2012, 918267. [Google Scholar] [CrossRef] [PubMed]
- Terzić, R.; Cilenšek, I.; Pleskovič, R.Z.; Mankoč, S.; Milutinović, A. Vascular endothelial growth factor (VEGF)-related single nucleotide polymorphisms rs10738760 and rs6921438 are not associated with diabetic retinopathy (DR) in Slovenian patients with type 2 diabetes mellitus (T2DM). Biomol. Biomed. 2017, 17, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Harvey, N.L.; Srinivasan, R.S.; Dillard, M.E.; Johnson, N.C.; Witte, M.H.; Boyd, K.; Sleeman, M.W.; Oliver, G. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat. Genet. 2005, 37, 1072–1081. [Google Scholar] [CrossRef]
- Aspelund, A.; Robciuc, M.R.; Karaman, S.; Makinen, T.; Alitalo, K. Lymphatic System in Cardiovascular Medicine. Circ. Res. 2016, 118, 515–530. [Google Scholar] [CrossRef]
- Schiekofer, S.; Galasso, G.; Sato, K.; Kraus, B.J.; Walsh, K. Impaired revascularization in a mouse model of type 2 diabetes is associated with dysregulation of a complex angiogenic-regulatory network. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1603–1609. [Google Scholar] [CrossRef]
- Waltenberger, J. VEGF resistance as a molecular basis to explain the angiogenesis paradox in diabetes mellitus. Biochem. Soc. Trans. 2009, 37, 1167–1170. [Google Scholar] [CrossRef]
- Awata, T.; Inoue, K.; Kurihara, S.; Ohkubo, T.; Watanabe, M.; Inukai, K.; Inoue, I.; Katayama, S. A common polymorphism in the 5′-untranslated region of the VEGF gene is associated with diabetic retinopathy in type 2 diabetes. Diabetes 2002, 51, 1635–1639. [Google Scholar] [CrossRef]
- Dong, P.P. Association of vascular endothelial growth factor expression and polymorphisms with the risk of gestational diabetes mellitus. J. Clin. Lab. Anal. 2019, 33, e22686. [Google Scholar] [CrossRef]
- Molina-Ayala, M.A.; Rodríguez-Amador, V.; Suárez-Sánchez, R.; León-Solís, L.; Gómez-Zamudio, J.; Mendoza-Zubieta, V.; Cruz, M.; Suárez-Sánchez, F. Expression of obesity- and type-2 diabetes-associated genes in omental adipose tissue of individuals with obesity. Gene 2022, 815, 146181. [Google Scholar] [CrossRef]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2019, 10, 1607. [Google Scholar] [CrossRef] [PubMed]
- ADA. Standards of Care in Diabetes—2025. Diabetes Care 2025, 48, s1–s352. [Google Scholar] [CrossRef]
- Sociedade Brasileira de Diabetes (SBD). Diretriz Oficial da Sociedade Brasileira de Diabetes; SBD: Sao Paulo, Brazil, 2021. [Google Scholar] [CrossRef]
- Aikaeli, F.; Njim, T.; Gissing, S.; Moyo, F.; Alam, U.; Mfinanga, S.G.; Okebe, J.; Ramaiya, K.; Webb, E.L.; Jaffar, S.; et al. Prevalence of microvascular and macrovascular complications of diabetes in newly diagnosed type 2 diabetes in low-and-middle-income countries: A systematic review and meta-analysis. PLoS Glob. Public Health 2022, 2, e0000599. [Google Scholar] [CrossRef]
- Buraczynska, M.; Ksiazek, P.; Baranowicz-Gaszczyk, I.; Jozwiak, L. Association of the VEGF gene polymorphism with diabetic retinopathy in type 2 diabetes patients. Nephrol. Dial. Transplant. 2007, 22, 827–832. [Google Scholar] [CrossRef]
- Mahdy, R.A.; Nada, W.M.; Hadhoud, K.M.; El-Tarhony, S.A. The role of vascular endothelial growth factor in the progression of diabetic vascular complications. Eye 2010, 24, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Luo, J.; Peng, H. Associations Between Genetic Polymorphisms in the VEGFA, ACE, and SOD2 Genes and Susceptibility to Diabetic Nephropathy in the Han Chinese. Genet. Test. Mol. Biomark. 2019, 23, 644–651. [Google Scholar] [CrossRef]
- Yap, R.W.K.; Lin, M.H.; Shidoji, Y.; Yap, W.S. Association of Stress, Mental Health, and VEGFR-2 Gene Polymorphisms with Cardiometabolic Risk in Chinese Malaysian Adults. Nutrients 2019, 11, 1140. [Google Scholar] [CrossRef]
- Stranger, B.E.; Forrest, M.S.; Dunning, M.; Ingle, C.E.; Beazley, C.; Thorne, N.; Redon, R.; Bird, C.P.; de Grassi, A.; Lee, C.; et al. Relative Impact of Nucleotide and Copy Number Variation on Gene Expression Phenotypes. Science 2007, 315, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhang, L.; Xing, W.; Zhuo, R.; Lin, X.; Hao, Y.; Wu, Q.; Zhao, J. The Associations between VEGF Gene Polymorphisms and Diabetic Retinopathy Susceptibility: A Meta-Analysis of 11 Case-Control Studies. J. Diabetes Res. 2014, 2014, 805801. [Google Scholar] [CrossRef]
- Elfaki, I.; Mir, R.; Duhier, F.M.A.; Alotaibi, M.A.; Alalawy, A.I.; Barnawi, J.; Babakr, A.T.; Mir, M.M.; Altayeb, F.; Mirghani, H.; et al. Clinical Implications of MiR128, Angiotensin I Converting Enzyme and Vascular Endothelial Growth Factor Gene Abnormalities and Their Association with T2D. Curr. Issues Mol. Biol. 2021, 43, 1859–1875. [Google Scholar] [CrossRef] [PubMed]
- Porojan, M.D.; Cătană, A.; Popp, R.A.; Dumitrascu, D.L.; Bala, C. The role of NOS2A −954G/C and vascular endothelial growth factor +936C/T polymorphisms in type 2 diabetes mellitus and diabetic nonproliferative retinopathy risk management. Ther. Clin. Risk Manag. 2015, 11, 1743–1748. [Google Scholar] [CrossRef]
- Liao, P.-Y.; Lee, K.H. From SNPs to functional polymorphism: The insight into biotechnology applications. Biochem. Eng. J. 2010, 49, 149–158. [Google Scholar] [CrossRef]
- Polychronakos, C.; Alriyami, M. Diabetes in the post-GWAS era. Nat. Genet. 2015, 47, 1373–1374. [Google Scholar] [CrossRef]
- Choudhuri, S.; Chowdhury, I.H.; Das, S.; Dutta, D.; Saha, A.; Sarkar, R.; Mandal, L.K.; Mukherjee, S.; Bhattacharya, B. Role of NF-κB activation and VEGF gene polymorphisms in VEGF up regulation in non-proliferative and proliferative diabetic retinopathy. Mol. Cell. Biochem. 2015, 405, 265–279. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, Z.; Jiang, H.; Song, X. Relationship between single nucleotide polymorphisms in the 3’-untranslated region of the vascular endothelial growth factor gene and susceptibility to diabetic peripheral neuropathy in China. Arch. Med. Sci. 2014, 10, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Gazal, S.; Weissbrod, O.; Hormozdiari, F.; Dey, K.K.; Nasser, J.; Jagadeesh, K.A.; Weiner, D.J.; Shi, H.; Fulco, C.P.; O’Connor, L.J.; et al. Combining SNP-to-gene linking strategies to identify disease genes and assess disease omnigenicity. Nat. Genet. 2022, 54, 827–836. [Google Scholar] [CrossRef]
- Sisodiya, S.M. Precision medicine and therapies of the future. Epilepsia 2021, 62, S90–S105. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.D.; Godfrey, C.M.; Khalil, H.; McInerney, P.; Parker, D.; Soares, C.B. Guidance for conducting systematic scoping reviews. Int. J. Evid. Based Healthc. 2015, 13, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Suganthalakshmi, B.; Anand, R.; Kim, R.; Mahalakshmi, R.; Karthikprakash, S.; Namperumalsamy, P.; Sundaresan, P. Association of VEGF and eNOS gene polymorphisms in type 2 diabetic retinopathy. Mol. Vis. 2006, 12, 336–341. [Google Scholar]
- Errera, F.I.V.; Canani, L.H.; Silva, M.E.R.; Yeh, E.; Takahashi, W.; Santos, K.G.; Souto, K.E.P.; Tschiedel, B.; Roisenberg, I.; Gross, J.L.; et al. Functional Vascular Endothelial Growth Factor −634G>C SNP Is Associated with Proliferative Diabetic Retinopathy: A case-control study in a Brazilian population of European ancestry. Diabetes Care 2007, 30, 275–279. [Google Scholar] [CrossRef]
- Petrovic, D.; Verhovec, R.; Globocnik Petrovic, M.; Osredkar, J.; Peterlin, B. Association of vascular endothelial growth factor gene polymorphism with myocardial infarction in patients with type 2 diabetes. Cardiology 2007, 107, 291–295. [Google Scholar] [CrossRef]
- Szaflik, J.P.; Wysocki, T.; Kowalski, M.; Majsterek, I.; Borucka, A.I.; Blasiak, J.; Szaflik, J. An association between vascular endothelial growth factor gene promoter polymorphisms and diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2008, 246, 39–43. [Google Scholar] [CrossRef]
- Petrovic, M.G.; Korosec, P.; Kosnik, M.; Osredkar, J.; Hawlina, M.; Peterlin, B.; Petrovic, D. Local and genetic determinants of vascular endothelial growth factor expression in advanced proliferative diabetic retinopathy. Mol. Vis. 2008, 14, 1382–1387. [Google Scholar]
- Uthra, S.; Raman, R.; Mukesh, B.N.; Rajkumar, S.A.; Padmaja, K.R.; Paul, P.G.; Lakshmipathy, P.; Gnanamoorthy, P.; Sharma, T.; McCarty, C.A.; et al. Association of VEGF gene polymorphisms with diabetic retinopathy in a south Indian cohort. Ophthalmic Genet. 2008, 29, 11–15. [Google Scholar] [CrossRef]
- Abhary, S.; Burdon, K.P.; Gupta, A.; Lake, S.; Selva, D.; Petrovsky, N.; Craig, J.E. Common Sequence Variation in the VEGFA Gene Predicts Risk of Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5552–5558. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Ko, G.J.; Kang, Y.S.; Lee, M.H.; Song, H.K.; Kim, H.K.; Cha, D.R. Role of the VEGF 936 C/T polymorphism in diabetic microvascular complications in type 2 diabetic patients. Nephrology 2009, 14, 681–688. [Google Scholar] [CrossRef]
- Nakamura, S.; Iwasaki, N.; Funatsu, H.; Kitano, S.; Iwamoto, Y. Impact of variants in the VEGF gene on progression of proliferative diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2009, 247, 21–26. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Prasad, P.; B, K.T.; Kumar, K.M.; Ammini, A.C.; Gupta, A.; Gupta, R. Oxidative stress pathway genes and chronic renal insufficiency in Asian Indians with Type 2 diabetes. J. Diabetes Complicat. 2009, 23, 102–111. [Google Scholar] [CrossRef]
- Chun, M.Y.; Hwang, H.S.; Cho, H.Y.; Chun, H.J.; Woo, J.T.; Lee, K.W.; Nam, M.S.; Baik, S.H.; Kim, Y.S.; Park, Y. Association of vascular endothelial growth factor polymorphisms with nonproliferative and proliferative diabetic retinopathy. J. Clin. Endocrinol. Metab. 2010, 95, 3547–3551. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Andresen, B.T.; Yang, K.; Zhang, Y.; Li, X.; Li, X.; Wang, H. Association of vascular endothelial growth factor -634C/G polymorphism and diabetic retinopathy in type 2 diabetic Han Chinese. Exp. Biol. Med. 2010, 235, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Feghhi, M.; Nikzamir, A.; Esteghamati, A.; Mahmoudi, T.; Yekaninejad, M.S. Relationship of vascular endothelial growth factor (VEGF) +405 G/C polymorphism and proliferative retinopathy in patients with type 2 diabetes. Transl. Res. 2011, 158, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Amoli, M.M.; Hasani-Ranjbar, S.; Roohipour, N.; Sayahpour, F.A.; Amiri, P.; Zahedi, P.; Mehrab-Mohseni, M.; Heshmat, R.; Larijani, B.; Tavakkoly-Bazzaz, J. VEGF gene polymorphism association with diabetic foot ulcer. Diabetes Res. Clin. Pract. 2011, 93, 215–219. [Google Scholar] [CrossRef]
- Yang, X.; Deng, Y.; Gu, H.; Lim, A.; Altankhuyag, A.; Jia, W.; Ma, K.; Xu, J.; Zou, Y.; Snellingen, T.; et al. Polymorphisms in the vascular endothelial growth factor gene and the risk of diabetic retinopathy in Chinese patients with type 2 diabetes. Mol. Vis. 2011, 17, 3088–3096. [Google Scholar]
- Bleda, S.; De Haro, J.; Varela, C.; Esparza, L.; Ferruelo, A.; Acin, F. Vascular endothelial growth factor polymorphisms are involved in the late vascular complications in Type II diabetic patients. Diabetes Vasc. Dis. Res. 2012, 9, 68–74. [Google Scholar] [CrossRef]
- Nikzamir, A.; Esteghamati, A.; Hammedian, A.A.; Mahmoudi, T. The role of vascular endothelial growth factor +405 G/C polymorphism and albuminuria in patients with type 2 diabetes mellitus. Mol. Biol. Rep. 2012, 39, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Paine, S.K.; Basu, A.; Mondal, L.K.; Sen, A.; Choudhuri, S.; Chowdhury, I.H.; Saha, A.; Bhadhuri, G.; Mukherjee, A.; Bhattacharya, B. Association of vascular endothelial growth factor, transforming growth factor beta, and interferon gamma gene polymorphisms with proliferative diabetic retinopathy in patients with type 2 diabetes. Mol. Vis. 2012, 18, 2749–2757. [Google Scholar] [PubMed]
- Bonnefond, A.; Saulnier, P.-J.; Stathopoulou, M.G.; Grarup, N.; Ndiaye, N.C.; Roussel, R.; Nezhad, M.A.; Dechaume, A.; Lantieri, O.; Hercberg, S.; et al. What Is the Contribution of Two Genetic Variants Regulating VEGF Levels to Type 2 Diabetes Risk and to Microvascular Complications? PLoS ONE 2013, 8, e55921. [Google Scholar] [CrossRef]
- El-Shazly, S.F.; El-Bradey, M.H.; Tameesh, M.K. Vascular endothelial growth factor gene polymorphism prevalence in patients with diabetic macular oedema and its correlation with anti-vascular endothelial growth factor treatment outcomes. Clin. Exp. Ophthalmol. 2014, 42, 369–378. [Google Scholar] [CrossRef]
- Fan, X.; Wu, Q.; Li, Y.; Hao, Y.; Ning, N.; Kang, Z.; Cui, Y.; Liu, R.; Han, L. Association of polymorphisms in the vascular endothelial growth factor gene and its serum levels with diabetic retinopathy in Chinese patients with type 2 diabetes: A cross-sectional study. Chin. Med. J. 2014, 127, 651–657. [Google Scholar] [CrossRef]
- Kariž, S.; Petrovič, D. Minor association of kinase insert domain-containing receptor gene polymorphism (rs2071559) with myocardial infarction in Caucasians with type 2 diabetes mellitus: Case-control cross-sectional study. Clin. Biochem. 2014, 47, 192–196. [Google Scholar] [CrossRef]
- Pirie, F.J.; Maharaj, S.; Esterhuizen, T.M.; Paruk, I.M.; Motala, A.A. Retinopathy in subjects with type 2 diabetes at a tertiary diabetes clinic in Durban, South Africa: Clinical, biochemical and genetic factors. J. Clin. Transl. Endocrinol. 2014, 1, e9–e12. [Google Scholar] [CrossRef]
- Yang, X.; Deng, Y.; Gu, H.; Ren, X.; Li, N.; Lim, A.; Snellingen, T.; Liu, X.; Wang, N.; Liu, N. Candidate gene association study for diabetic retinopathy in Chinese patients with type 2 diabetes. Mol. Vis. 2014, 20, 200–214. [Google Scholar]
- Yuan, Y.; Wen, Z.; Guan, Y.; Sun, Y.; Yang, J.; Fan, X.; Yang, X.; Liu, R. The relationships between type 2 diabetic retinopathy and VEGF-634G/C and VEGF-460C/T polymorphisms in Han Chinese subjects. J. Diabetes Its Complicat. 2014, 28, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Ghisleni, M.M.; Biolchi, V.; Jordon, B.C.; Rempel, C.; Genro, J.P.; Pozzobon, A. Association study of C936T polymorphism of the VEGF gene and the C242T polymorphism of the p22phox gene with diabetes mellitus type 2 and distal diabetic polyneuropathy. Mol. Med. Rep. 2015, 12, 4626–4633. [Google Scholar] [CrossRef] [PubMed]
- Moradzadegan, A.; Vaisi-Raygani, A.; Nikzamir, A.; Rahimi, Z. Angiotensin converting enzyme insertion/deletion (I/D) (rs4646994) and Vegf polymorphism (+405G/C; rs2010963) in type II diabetic patients: Association with the risk of coronary artery disease. J. Renin-Angiotensin-Aldosterone Syst. 2015, 16, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.F.; Liou, S.W.; Wu, H.H.; Lin, C.H.; Huang, L.S.; Woung, L.C.; Tsai, C.Y. Regulatory SNPs Alter the Gene Expression of Diabetic Retinopathy Associated Secretary Factors. Int. J. Med. Sci. 2016, 13, 717–723. [Google Scholar] [CrossRef]
- Fattah, R.A.A.; Eltanamly, R.M.; Nabih, M.H.; Kamal, M.M. Vascular endothelial growth factor gene polymorphism is not associated with diabetic retinopathy in Egyptian Patients. Middle East. Afr. J. Ophthalmol. 2016, 23, 75–78. [Google Scholar] [CrossRef]
- Kamal, A.; Abu Eleinen, K.; Siam, I. Association of vascular endothelial growth factor -634G/C and receptor for advanced glycation end products G82S gene polymorphisms with diabetic retinopathy. Int. J. Ophthalmol. 2016, 9, 1106–1111. [Google Scholar] [CrossRef]
- Merlo, S.; Starčević, J.N.; Mankoč, S.; Šantl Letonja, M.; Cokan Vujkovac, A.; Zorc, M.; Petrovič, D. Vascular Endothelial Growth Factor Gene Polymorphism (rs2010963) and Its Receptor, Kinase Insert Domain-Containing Receptor Gene Polymorphism (rs2071559), and Markers of Carotid Atherosclerosis in Patients with Type 2 Diabetes Mellitus. J. Diabetes Res. 2016, 2016, 1482194. [Google Scholar] [CrossRef]
- Gonzalez-Salinas, R.; Solis-Sainz, J.C.; Garcia-Gutierrez, M.C.; Garcia-Aguirre, G.; Soberon, V.; Gonzalez, V.; Lechuga, R.; Garcia-Solis, V.; Morales, V. In search of association between the VEGF gene polymorphisms: rs3025035, rs3025021, rs2010963 and proliferative diabetic retinopathy in Mexican population. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1463. [Google Scholar]
- Zhuang, J.; Li, J.; Yao, Y.; Jing, B.; Shan, P.; Han, P.; Wang, H. VEGF gene promoter polymorphisms are associated with diabetic foot ulcer. Biomed. Res. 2017, 28, 1689–1692. [Google Scholar]
- Barus, J.; Setyopranoto, I.; Sadewa, A.H.; Wibowo, S. Vascular Endothelial Growth Factor 936 C/T Gene Polymorphism in Indonesian Subjects with Diabetic Polyneuropathy. Open Access Maced. J. Med. Sci. 2018, 6, 1784–1789. [Google Scholar] [CrossRef]
- Li, X. The association between MCP-1, VEGF polymorphisms and their serum levels in patients with diabetic foot ulcer. Medicine 2018, 97, e10959. [Google Scholar] [CrossRef]
- Li, X.; Lu, Y.; Wei, P. Association between VEGF genetic variants and diabetic foot ulcer in Chinese Han population: A case-control study. Medicine 2018, 97, e10672. [Google Scholar] [CrossRef]
- Arredondo-García, V.K.; Cepeda-Nieto, A.C.; Batallar-Gómez, T.; Salinas-Santander, M.; Zugasti-Cruz, A.; Ramírez-Calvillo, L.; Maldonado-Sánchez, K.; Morlett-Chávez, J.; Barajas-Martínez, H. Association of the Vascular Endothelial Growth Factor Gene Polymorphism +936 C/T with Diabetic Neuropathy in Patients with Type 2 Diabetes Mellitus. Arch. Med. Res. 2019, 50, 181–186. [Google Scholar] [CrossRef]
- Muhammad Dahlan, K.; Pratama, D.; Muradi, A.; Anita Suryandari, D.; Yunaini, L.; Boediningsih, S. Association of vascular endothelial growth factor gene +405 C>G and -460 C>T polymorphism with diabetic foot ulcer in Indonesia. J. Phys. Conf. Ser. 2019, 1246, 012008. [Google Scholar] [CrossRef]
- Yari, H.; Jahangir Sooltani, N.; Saremi, M.A. Investigating the relationship between VEGF gene C936T-rs3025039 polymorphism and type 2 diabetes. Pers. Med. J. 2020, 5, 21–24. [Google Scholar] [CrossRef]
- Khan, N.; Paterson, A.D.; Roshandel, D.; Raza, A.; Ajmal, M.; Waheed, N.K.; Azam, M.; Qamar, R. Association of IGF1 and VEGFA polymorphisms with diabetic retinopathy in Pakistani population. Acta Diabetol. 2020, 57, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Abdelghany, A.A.; Toraih, E.A.; Mohamed, A.A.; Lashine, R.M.; Mohammad, M.H.S.; Nafie, M.S.; Fawzy, M.S. Association of VEGF Gene Family Variants with Central Macular Thickness and Visual Acuity After Aflibercept Short-Term Treatment in Diabetic Patients: A Pilot Study. Ophthalmic Res. 2021, 64, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Jiang, D.; Ding, Z.; Xiong, Y.; Zeng, X.; Liao, M.; Zheng, L.; Yang, B. Association of four gene polymorphisms in Chinese Guangxi population with diabetic retinopathy in type 2 diabetic patients. BMC Ophthalmol. 2021, 21, 383. [Google Scholar] [CrossRef] [PubMed]
- Imbaby, S.A.E.; Badrah, M.; Fattah, Y.H.A. The relation between IL-10 gene (-1082G/A) and VEGF gene 936 C/T polymorphism and diabetic polyneuropathy in a cohort of Egyptian patients with type 2 diabetes. Clin. Diabetol. 2021, 10, 420–427. [Google Scholar]
- Wijaya, A.R.; Surudarma, I.W.; Wihandani, D.M.; Putra, I. Polymorphisms of vascular endothelial growth factor-2578C/A rs699947 are risk factors for diabetic retinopathy in type-2 diabetes mellitus patients in Bali, Indonesia. Biomedicine 2021, 11, 11–17. [Google Scholar] [CrossRef]
- Mohamed, M.K.; Atef, A.A.; Moemen, L.A.; Abdel Azeem, A.A.; Mohalhal, I.A.; Taha, A.M. Association study of HIF-1α rs11549465 and VEGF rs3025039 genetic variants with diabetic retinopathy in Egyptian patients: Crosslinks with angiogenic, inflammatory, and anti-inflammatory markers. J. Genet. Eng. Biotechnol. 2022, 20, 122. [Google Scholar] [CrossRef] [PubMed]
- Omar, T.A.; El-Saeed, G.K.; Khodeer, S.A.; Dawood, A.A.; El-Deeb, S.M.; Elshemy, A.M.; Montaser, B.A. Vascular endothelial growth factor A with two genetic variants for prediction of mixed microvascular diabetic complications. Egypt. J. Med. Hum. Genet. 2022, 23, 93. [Google Scholar] [CrossRef]
- Rabbind Singh, A.; Rahul, G.; Manish, S.; Anupreeti, J.; Shukla, D. Association of VEGFA promoter polymorphisms rs699947 and rs35569394 with diabetic retinopathy among North-Central Indian subjects: A case-control study. Ophthalmic Genet. 2022, 43, 80–87. [Google Scholar] [CrossRef]
- Alnaji, H.A.; Omran, R.; Hasan, A.H.; Al Nuwaini, M.Q. Diabetic retinopathy progression associated with haplotypes of two VEGFA SNPs rs2010963 and rs699947. Egypt. J. Basic. Appl. Sci. 2023, 10, 123–134. [Google Scholar] [CrossRef]
- Del Cuore, A.; Pipitone, R.M.; Casuccio, A.; Mazzola, M.M.; Puleo, M.G.; Pacinella, G.; Riolo, R.; Maida, C.; Di Chiara, T.; Di Raimondo, D.; et al. Metabolic memory in diabetic foot syndrome (DFS): MICRO-RNAS, single nucleotide polymorphisms (SNPs) frequency and their relationship with indices of endothelial function and adipo-inflammatory dysfunction. Cardiovasc. Diabetol. 2023, 22, 148. [Google Scholar] [CrossRef] [PubMed]
- Jehanzeb, M.; Khan, N.U.; Hussain, M.; Subrina, J.; Ayub, S.; Mustafa, A. Association of candidate genes (ALR2, RAGE, and VEGF) polymorphisms with diabetic retinopathy in type 2 diabetic patients of Khyber Pakhtunkhwa, Pakistan. Mol. Biol. Rep. 2023, 50, 227–234. [Google Scholar] [CrossRef]
- Qayyum, S.; Afzal, M.; Naveed, A.K.; Butt, I.A.; Sajjad, M.; Azam, M. Association of vascular endothelial growth factor a gene (VEGFA) polymorphisms, rs699947 and rs1570360, with diabetic retinopathy and altered VEGF secretion in the Pakistani patients with type 2 diabetes mellitus: A casecontrol study. J. Pak. Med. Assoc. 2023, 73, 2348–2356. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Shao, C.; Guan, Y.; Lu, H.; Wang, D.; Zhang, S. Association between the VEGFR-2 -604T/C polymorphism (rs2071559) and type 2 diabetic retinopathy. Open Life Sci. 2023, 18, 20220081. [Google Scholar] [CrossRef]
- Nussdorfer, P.; Petrovič, D.; Alibegović, A.; Cilenšek, I.; Petrovič, D. The KDR Gene rs2071559 and the VEGF Gene rs6921438 May Be Associated with Diabetic Nephropathy in Caucasians with Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2024, 25, 9439. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Liu, S.; Gao, M.; Wang, W.; Chen, K.; Huang, L.; Liu, Y. Diabetic vascular diseases: Molecular mechanisms and therapeutic strategies. Signal Transduct. Target. Ther. 2023, 8, 152. [Google Scholar] [CrossRef] [PubMed]
- Warren, C.M.; Ziyad, S.; Briot, A.; Der, A.; Iruela-Arispe, M.L. A ligand-independent VEGFR2 signaling pathway limits angiogenic responses in diabetes. Sci. Signal. 2014, 7, ra1. [Google Scholar] [CrossRef]
- Saifullah; Tsukahara, T. Genotyping of single nucleotide polymorphisms using the SNP-RFLP method. Biosci. Trends 2018, 12, 240–246. [Google Scholar] [CrossRef]
- Medrano, R.F.V.; de Oliveira, C.A. Guidelines for the Tetra-Primer ARMS–PCR Technique Development. Mol. Biotechnol. 2014, 56, 599–608. [Google Scholar] [CrossRef]
- Millis, M.P. Medium-throughput SNP genotyping using mass spectrometry: Multiplex SNP genotyping using the iPLEX® Gold assay. Methods Mol. Biol. 2011, 700, 61–76. [Google Scholar] [CrossRef]
- Chu, H.; Cheng, C.; Tu, R.; Zhou, J.; Niu, F.; Sun, B.; Li, Y.; Yao, Y.; Huang, Y.; Luo, Z.; et al. A PCR/LDR-based functional molecular marker of rice (Oryza sativa L.) aroma allele BADH2-E2. Rice Genom. Genet. 2020, 11, 1–6. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Nolan, T.; Pfaffl, M.W. Quantitative real-time RT-PCR—A perspective. J. Mol. Endocrinol. 2005, 34, 597–601. [Google Scholar] [CrossRef]
- Hosking, L.; Lumsden, S.; Lewis, K.; Yeo, A.; McCarthy, L.; Bansal, A.; Riley, J.; Purvis, I.; Xu, C.F. Detection of genotyping errors by Hardy-Weinberg equilibrium testing. Eur. J. Hum. Genet. 2004, 12, 395–399. [Google Scholar] [CrossRef]
- Vincenti, V.; Cassano, C.; Rocchi, M.; Persico, G. Assignment of the vascular endothelial growth factor gene to human chromosome 6p21.3. Circulation 1996, 93, 1493–1495. [Google Scholar] [CrossRef]
- Harper, S.J.; Bates, D.O. VEGF-A splicing: The key to anti-angiogenic therapeutics? Nat. Rev. Cancer 2008, 8, 880–887. [Google Scholar] [CrossRef]
- Mamer, S.B.; Wittenkeller, A.; Imoukhuede, P.I. VEGF-A splice variants bind VEGFRs with differential affinities. Sci. Rep. 2020, 10, 14413. [Google Scholar] [CrossRef]
- Onesto, C.; Berra, E.; Grépin, R.; Pagès, G. Poly(A)-binding protein-interacting protein 2, a strong regulator of vascular endothelial growth factor mRNA. J. Biol. Chem. 2004, 279, 34217–34226. [Google Scholar] [CrossRef]
- Levy, N.S.; Chung, S.; Furneaux, H.; Levy, A.P. Hypoxic stabilization of vascular endothelial growth factor mRNA by the RNA-binding protein HuR. J. Biol. Chem. 1998, 273, 6417–6423. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Wei, D.; Shi, Q.; Huang, S. Constitutive and inducible expression and regulation of vascular endothelial growth factor. Cytokine Growth Factor. Rev. 2004, 15, 297–324. [Google Scholar] [CrossRef]
- Shahbazi, M.; Fryer, A.A.; Pravica, V.; Brogan, I.J.; Ramsay, H.M.; Hutchinson, I.V.; Harden, P.N. Vascular endothelial growth factor gene polymorphisms are associated with acute renal allograft rejection. J. Am. Soc. Nephrol. 2002, 13, 260–264. [Google Scholar] [CrossRef]
- Lambrechts, D.; Storkebaum, E.; Morimoto, M.; Del-Favero, J.; Desmet, F.; Marklund, S.L.; Wyns, S.; Thijs, V.; Andersson, J.; Van Marion, I.; et al. VEGF is a modifier of amyotrophic lateral sclerosis in mice and humans and protects motoneurons against ischemic death. Nat. Genet. 2003, 34, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Awata, T.; Kurihara, S.; Takata, N.; Neda, T.; Iizuka, H.; Ohkubo, T.; Osaki, M.; Watanabe, M.; Nakashima, Y.; Inukai, K.; et al. Functional VEGF C-634G polymorphism is associated with development of diabetic macular edema and correlated with macular retinal thickness in type 2 diabetes. Biochem. Biophys. Res. Commun. 2005, 333, 679–685. [Google Scholar] [CrossRef]
- Watson, C.J.; Webb, N.J.; Bottomley, M.J.; Brenchley, P.E. Identification of polymorphisms within the vascular endothelial growth factor (VEGF) gene: Correlation with variation in VEGF protein production. Cytokine 2000, 12, 1232–1235. [Google Scholar] [CrossRef] [PubMed]
- Renner, W.; Kotschan, S.; Hoffmann, C.; Obermayer-Pietsch, B.; Pilger, E. A common 936 C/T mutation in the gene for vascular endothelial growth factor is associated with vascular endothelial growth factor plasma levels. J. Vasc. Res. 2000, 37, 443–448. [Google Scholar] [CrossRef]
- Koukourakis, M.I.; Papazoglou, D.; Giatromanolaki, A.; Bougioukas, G.; Maltezos, E.; Sivridis, E. VEGF gene sequence variation defines VEGF gene expression status and angiogenic activity in non-small cell lung cancer. Lung Cancer 2004, 46, 293–298. [Google Scholar] [CrossRef]
- Stevens, A.; Soden, J.; Brenchley, P.E.; Ralph, S.; Ray, D.W. Haplotype analysis of the polymorphic human vascular endothelial growth factor gene promoter. Cancer Res. 2003, 63, 812–816. [Google Scholar]
- Al-Habboubi, H.H.; Sater, M.S.; Almawi, A.W.; Al-Khateeb, G.M.; Almawi, W.Y. Contribution of VEGF polymorphisms to variation in VEGF serum levels in a healthy population. Eur. Cytokine Netw. 2011, 22, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.P.; Hud, E.; Shea, E.; Shearman, C.W. Vitreous fluid of db/db mice exhibits alterations in angiogenic and metabolic factors consistent with early diabetic retinopathy. Ophthalmic Res. 2008, 40, 5–9. [Google Scholar] [CrossRef]
- Katsura, Y.; Okano, T.; Matsuno, K.; Osako, M.; Kure, M.; Watanabe, T.; Iwaki, Y.; Noritake, M.; Kosano, H.; Nishigori, H.; et al. Erythropoietin is highly elevated in vitreous fluid of patients with proliferative diabetic retinopathy. Diabetes Care 2005, 28, 2252–2254. [Google Scholar] [CrossRef]
- Takagi, H.; Watanabe, D.; Suzuma, K.; Kurimoto, M.; Suzuma, I.; Ohashi, H.; Ojima, T.; Murakami, T. Novel role of erythropoietin in proliferative diabetic retinopathy. Diabetes Res. Clin. Pract. 2007, 77, S62–S64. [Google Scholar] [CrossRef]
- Wasada, T.; Kawahara, R.; Katsumori, K.; Naruse, M.; Omori, Y. Plasma concentration of immunoreactive vascular endothelial growth factor and its relation to smoking. Metabolism 1998, 47, 27–30. [Google Scholar] [CrossRef]
- Debette, S.; Visvikis-Siest, S.; Chen, M.H.; Ndiaye, N.C.; Song, C.; Destefano, A.; Safa, R.; Azimi Nezhad, M.; Sawyer, D.; Marteau, J.B.; et al. Identification of cis- and trans-acting genetic variants explaining up to half the variation in circulating vascular endothelial growth factor levels. Circ. Res. 2011, 109, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, T.; Hashiguchi, T.; Horinouchi, S.; Uto, T.; Oku, H.; Kimura, K.; Makisumi, K.; Arimura, K. Serum VEGF increases in diabetic polyneuropathy, particularly in the neurologically active symptomatic stage. Diabet. Med. 2009, 26, 247–252. [Google Scholar] [CrossRef]
- Feener, E.P.; King, G.L. Vascular dysfunction in diabetes mellitus. Lancet 1997, 350, S9–S13. [Google Scholar] [CrossRef] [PubMed]
- Silha, J.V.; Krsek, M.; Sucharda, P.; Murphy, L.J. Angiogenic factors are elevated in overweight and obese individuals. Int. J. Obes. 2005, 29, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, G.; Lobello, R.; Scopacasa, F.; Contaldo, F.; Pasanisi, F.; Cirillo, M.; De Caterina, M.; Conca, P.; Terracciano, D.; Gennarelli, N. The contribution of omental adipose tissue to adipokine concentrations in patients with the metabolic syndrome. Clin. Investig. Med. 2007, 30, E192–E199. [Google Scholar] [CrossRef]
- Huez, I.; Bornes, S.; Bresson, D.; Creancer, L.; Prats, H. New vascular endothelial growth factor isoform generated by internal ribosome entry site-driven CUG translation initiation. Mol. Endocrinol. 2001, 15, 2197–2210. [Google Scholar] [CrossRef] [PubMed]
- Mateo, I.; Llorca, J.; Infante, J.; Rodríguez-Rodríguez, E.; Sánchez-Quintana, C.; Sánchez-Juan, P.; Berciano, J.; Combarros, O. Case-control study of vascular endothelial growth factor (VEGF) genetic variability in Alzheimer’s disease. Neurosci. Lett. 2006, 401, 171–173. [Google Scholar] [CrossRef]
- Vailati, F.B.; Crispim, D.; Sortica, D.A.; Souza, B.M.; Brondani, L.A.; Canani, L.H. The C Allele of −634G/C Polymorphism in the VEGFA Gene Is Associated with Increased VEGFA Gene Expression in Human Retinal Tissue. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6411–6415. [Google Scholar] [CrossRef] [PubMed]
- Valiatti, F.B.; Crispim, D.; Benfica, C.; Valiatti, B.B.; Kramer, C.K.; Canani, L.H. Papel do fator de crescimento vascular endotelial na angiogênese e na retinopatia diabética. Arq. Bras. Endocrinol. Metabol. 2011, 55, 106–113. [Google Scholar] [CrossRef]
- Antonetti, D.A.; Silva, P.S.; Stitt, A.W. Current understanding of the molecular and cellular pathology of diabetic retinopathy. Nat. Rev. Endocrinol. 2021, 17, 195–206. [Google Scholar] [CrossRef]
- Wong, T.Y.; Cheung, C.M.; Larsen, M.; Sharma, S.; Simó, R. Diabetic retinopathy. Nat. Rev. Dis. Primers 2016, 2, 16012. [Google Scholar] [CrossRef]
- Duh, E.J.; Sun, J.K.; Stitt, A.W. Diabetic retinopathy: Current understanding, mechanisms, and treatment strategies. JCI Insight 2017, 2, e93751. [Google Scholar] [CrossRef]
- Gonzalez-Salinas, R.; Garcia-Gutierrez, M.C.; Garcia-Aguirre, G.; Morales-Canton, V.; Velez-Montoya, R.; Soberon-Ventura, V.R.; Gonzalez, V.; Lechuga, R.; Garcia-Solis, P.; Garcia-Gutierrez, D.G.; et al. Evaluation of VEGF gene polymorphisms and proliferative diabetic retinopathy in Mexican population. Int. J. Ophthalmol. 2017, 10, 135–139. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; The Executive Group on behalf of the Joint European Society of Cardiology/American College of Cardiology/American Heart Association/World Heart Federation Task Force for the Universal Definition of Myocardial. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef]
- Levey, A.S.; Atkins, R.; Coresh, J.; Cohen, E.P.; Collins, A.J.; Eckardt, K.U.; Nahas, M.E.; Jaber, B.L.; Jadoul, M.; Levin, A.; et al. Chronic kidney disease as a global public health problem: Approaches and initiatives—A position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007, 72, 247–259. [Google Scholar] [CrossRef]
- Meza Letelier, C.E.; San Martín Ojeda, C.A.; Ruiz Provoste, J.J.; Frugone Zaror, C.J. Pathophysiology of diabetic nephropathy: A literature review. Medwave 2017, 17, e6839. [Google Scholar] [CrossRef]
- Figueroa-Romero, C.; Sadidi, M.; Feldman, E.L. Mechanisms of disease: The oxidative stress theory of diabetic neuropathy. Rev. Endocr. Metab. Disord. 2008, 9, 301–314. [Google Scholar] [CrossRef]
- Shakher, J.; Stevens, M.J. Update on the management of diabetic polyneuropathies. Diabetes Metab. Syndr. Obes. 2011, 4, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Brogan, I.J.; Khan, N.; Isaac, K.; Hutchinson, J.A.; Pravica, V.; Hutchinson, I.V. Novel polymorphisms in the promoter and 5′ UTR regions of the human vascular endothelial growth factor gene. Hum. Immunol. 1999, 60, 1245–1249. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, X.; Song, C.; Bao, S.; Lai, D.; Mou, J.; Jiang, T.; Wang, N. Expression and characterization of a soluble VEGF receptor 2 protein. Cell Biosci. 2014, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, F.; Allen, J.E.; Allen, J.; Alvarez-Jarreta, J.; Amode, M.R.; Armean, I.M.; Austine-Orimoloye, O.; Azov, A.G.; Barnes, I.; Bennett, R.; et al. Ensembl 2022. Nucleic Acids Res. 2021, 50, D988–D995. [Google Scholar] [CrossRef] [PubMed]
- Yoshiji, H.; Gomez, D.E.; Shibuya, M.; Thorgeirsson, U.P. Expression of vascular endothelial growth factor, its receptor, and other angiogenic factors in human breast cancer. Cancer Res. 1996, 56, 2013–2016. [Google Scholar]
- Hu, Y.F.; Lüscher, B.; Admon, A.; Mermod, N.; Tjian, R. Transcription factor AP-4 contains multiple dimerization domains that regulate dimer specificity. Genes. Dev. 1990, 4, 1741–1752. [Google Scholar] [CrossRef]
- Steffensen, K.D.; Waldstrøm, M.; Brandslund, I.; Jakobsen, A. The relationship of VEGF polymorphisms with serum VEGF levels and progression-free survival in patients with epithelial ovarian cancer. Gynecol. Oncol. 2010, 117, 109–116. [Google Scholar] [CrossRef]
- Chen, M.H.; Tzeng, C.H.; Chen, P.M.; Lin, J.K.; Lin, T.C.; Chen, W.S.; Jiang, J.K.; Wang, H.S.; Wang, W.S. VEGF -460T → C polymorphism and its association with VEGF expression and outcome to FOLFOX-4 treatment in patients with colorectal carcinoma. Pharmacogenomics J. 2011, 11, 227–236. [Google Scholar] [CrossRef]
- Ramírez-Bello, J.; Cadena-Sandoval, D.; Fragoso, J.M.; Barbosa-Cobos, R.E.; Moreno-Eutímio, M.A.; Saavedra-Salinas, M.Á.; Valencia-Pacheco, G.; López-Villanueva, R.F.; Jiménez-Morales, S. The VEGFA -1154G/A polymorphism is associated with reduced risk of rheumatoid arthritis but not with systemic lupus erythematosus in Mexican women. J. Gene Med. 2018, 20, e3024. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Lee, C.; Lin, X.; Zhao, C.; Li, X. Novel function of VEGF-B as an antioxidant and therapeutic implications. Pharmacol. Res. 2019, 143, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.K.; Day, C.P. Candidate gene case-control association studies: Advantages and potential pitfalls. Br. J. Clin. Pharmacol. 2001, 52, 489–499. [Google Scholar] [CrossRef]
- Buroker, N.E.; Ning, X.H.; Zhou, Z.N.; Li, K.; Cen, W.J.; Wu, X.F.; Zhu, W.Z.; Scott, C.R.; Chen, S.H. SNPs, linkage disequilibrium, and chronic mountain sickness in Tibetan Chinese. Hypoxia 2017, 5, 67–74. [Google Scholar] [CrossRef]
- Ne, B.; Buroker, N. Identifying Alterations in Transcriptional Factor Binding Sites. Fetal. Neonatal. Dev. Med. 2017, 1, 1. [Google Scholar] [CrossRef]
- Churchill, A.J.; Carter, J.G.; Ramsden, C.; Turner, S.J.; Yeung, A.; Brenchley, P.E.C.; Ray, D.W. VEGF Polymorphisms Are Associated with Severity of Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3611–3616. [Google Scholar] [CrossRef]
- Dyer, S.C.; Austine-Orimoloye, O.; Azov, A.G.; Barba, M.; Barnes, I.; Barrera-Enriquez, V.P.; Becker, A.; Bennett, R.; Beracochea, M.; Berry, A.; et al. Ensembl 2025. Nucleic Acids Res. 2024, 53, D948–D957. [Google Scholar] [CrossRef]
- Liu, Y.; Cox, S.R.; Morita, T.; Kourembanas, S. Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5’ enhancer. Circ. Res. 1995, 77, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.T.; Li, Y.; Duan, C.H.; Zhang, W.; Guo, X.L. Further evidence for the contribution of the vascular endothelial growth factor gene in coronary artery disease susceptibility. Gene 2013, 521, 217–221. [Google Scholar] [CrossRef]
- Ruggiero, D.; Dalmasso, C.; Nutile, T.; Sorice, R.; Dionisi, L.; Aversano, M.; Bröet, P.; Leutenegger, A.-L.; Bourgain, C.; Ciullo, M. Genetics of VEGF Serum Variation in Human Isolated Populations of Cilento: Importance of VEGF Polymorphisms. PLoS ONE 2011, 6, e16982. [Google Scholar] [CrossRef]
- Sajovic, J.; Cilenšek, I.; Mankoč, S.; Tajnšek, Š.; Kunej, T.; Petrovič, D.; Globočnik Petrovič, M. Vascular endothelial growth factor (VEGF)-related polymorphisms rs10738760 and rs6921438 are not risk factors for proliferative diabetic retinopathy (PDR) in patients with type 2 diabetes mellitus (T2DM). Bosn. J. Basic. Med. Sci. 2019, 19, 94–100. [Google Scholar] [CrossRef]
- Galan, A.; Ferlin, A.; Caretti, L.; Buson, G.; Sato, G.; Frigo, A.C.; Foresta, C. Association of Age-related Macular Degeneration with Polymorphisms in Vascular Endothelial Growth Factor and Its Receptor. Ophthalmology 2010, 117, 1769–1774. [Google Scholar] [CrossRef]
- Rahimi, N. Vascular endothelial growth factor receptors: Molecular mechanisms of activation and therapeutic potentials. Exp. Eye Res. 2006, 83, 1005–1016. [Google Scholar] [CrossRef]
- Tetikoğlu, M.; Yüksel, Z.; Aktas, S.; Sağdik, H.M.; Özcura, F. VEGF-A gene polymorphisms and responses to intravitreal ranibizumab treatment in patients with diabetic macular edema. Int. Ophthalmol. 2018, 38, 2381–2388. [Google Scholar] [CrossRef] [PubMed]
- Al-Kateb, H.; Mirea, L.; Xie, X.; Sun, L.; Liu, M.; Chen, H.; Bull, S.B.; Boright, A.P.; Paterson, A.D. Multiple variants in vascular endothelial growth factor (VEGFA) are risk factors for time to severe retinopathy in type 1 diabetes: The DCCT/EDIC genetics study. Diabetes 2007, 56, 2161–2168. [Google Scholar] [CrossRef] [PubMed]
- Sait, S.N.; Dougher-Vermazen, M.; Shows, T.B.; Terman, B.I. The kinase insert domain receptor gene (KDR) has been relocated to chromosome 4q11-->q12. Cytogenet. Cell Genet. 1995, 70, 145–146. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information (NCBI). KDR Kinase Insert Domain Receptor [Homo sapiens (Human)]—Gene ID: 3791. Available online: https://www.ncbi.nlm.nih.gov/gene/3791 (accessed on 18 June 2025).
- Gilbert, R.E.; Vranes, D.; Berka, J.L.; Kelly, D.J.; Cox, A.; Wu, L.L.; Stacker, S.A.; Cooper, M.E. Vascular endothelial growth factor and its receptors in control and diabetic rat eyes. Lab. Investig. 1998, 78, 1017–1027. [Google Scholar] [PubMed]
- Chou, E.; Suzuma, I.; Way, K.J.; Opland, D.; Clermont, A.C.; Naruse, K.; Suzuma, K.; Bowling, N.L.; Vlahos, C.J.; Aiello, L.P.; et al. Decreased cardiac expression of vascular endothelial growth factor and its receptors in insulin-resistant and diabetic States: A possible explanation for impaired collateral formation in cardiac tissue. Circulation 2002, 105, 373–379. [Google Scholar] [CrossRef]
- Ishida, S.; Shinoda, K.; Kawashima, S.; Oguchi, Y.; Okada, Y.; Ikeda, E. Coexpression of VEGF receptors VEGF-R2 and neuropilin-1 in proliferative diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1649–1656. [Google Scholar]
- Witmer, A.N.; Blaauwgeers, H.G.; Weich, H.A.; Alitalo, K.; Vrensen, G.F.; Schlingemann, R.O. Altered expression patterns of VEGF receptors in human diabetic retina and in experimental VEGF-induced retinopathy in monkey. Investig. Ophthalmol. Vis. Sci. 2002, 43, 849–857. [Google Scholar]
- Frazer, K.A.; Ballinger, D.G.; Cox, D.R.; Hinds, D.A.; Stuve, L.L.; Gibbs, R.A.; Belmont, J.W.; Boudreau, A.; Hardenbol, P.; Leal, S.M.; et al. A second generation human haplotype map of over 3.1 million SNPs. Nature 2007, 449, 851–861. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Zhang, W.; Yu, H.; Lou, K.; Zhang, Y.; Qin, Q.; Zhao, B.; Yang, Y.; Hui, R. Polymorphisms of KDR Gene Are Associated with Coronary Heart Disease. J. Am. Coll. Cardiol. 2007, 50, 760–767. [Google Scholar] [CrossRef]
- Wirostko, B.; Wong, T.Y.; Simó, R. Vascular endothelial growth factor and diabetic complications. Prog. Retin. Eye Res. 2008, 27, 608–621. [Google Scholar] [CrossRef] [PubMed]
- Satoh, H.; Yoshida, M.C.; Matsushime, H.; Shibuya, M.; Sasaki, M. Regional localization of the human c-ros-1 on 6q22 and flt on 13q12. Jpn. J. Cancer Res. 1987, 78, 772–775. [Google Scholar] [PubMed]
- Sela, S.; Itin, A.; Natanson-Yaron, S.; Greenfield, C.; Goldman-Wohl, D.; Yagel, S.; Keshet, E. A novel human-specific soluble vascular endothelial growth factor receptor 1: Cell-type-specific splicing and implications to vascular endothelial growth factor homeostasis and preeclampsia. Circ. Res. 2008, 102, 1566–1574. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Shibuya, M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin. Sci. 2005, 109, 227–241. [Google Scholar] [CrossRef]
- Lambrechts, D.; Claes, B.; Delmar, P.; Reumers, J.; Mazzone, M.; Yesilyurt, B.T.; Devlieger, R.; Verslype, C.; Tejpar, S.; Wildiers, H.; et al. VEGF pathway genetic variants as biomarkers of treatment outcome with bevacizumab: An analysis of data from the AViTA and AVOREN randomised trials. Lancet Oncol. 2012, 13, 724–733. [Google Scholar] [CrossRef]
- Ito, N.; Wernstedt, C.; Engström, U.; Claesson-Welsh, L. Identification of vascular endothelial growth factor receptor-1 tyrosine phosphorylation sites and binding of SH2 domain-containing molecules. J. Biol. Chem. 1998, 273, 23410–23418. [Google Scholar] [CrossRef]
- Moeinfar, S.; Mashayekhi, F.; Bahadori, M.H.; Faraji, R.; Salehi, Z. The Association of Soluble VEGFR-1 Serum Level and Genetic (rs7993418) Polymorphism with In Vitro Fertilization and Embryo Transfer Outcome in the Population of Northern Iran. J. Reprod. Infertil. 2023, 24, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Paavonen, K.; Horelli-Kuitunen, N.; Chilov, D.; Kukk, E.; Pennanen, S.; Kallioniemi, O.P.; Pajusola, K.; Olofsson, B.; Eriksson, U.; Joukov, V.; et al. Novel human vascular endothelial growth factor genes VEGF-B and VEGF-C localize to chromosomes 11q13 and 4q34, respectively. Circulation 1996, 93, 1079–1082. [Google Scholar] [CrossRef]
- Hagberg, C.E.; Mehlem, A.; Falkevall, A.; Muhl, L.; Fam, B.C.; Ortsäter, H.; Scotney, P.; Nyqvist, D.; Samén, E.; Lu, L.; et al. Targeting VEGF-B as a novel treatment for insulin resistance and type 2 diabetes. Nature 2012, 490, 426–430. [Google Scholar] [CrossRef]
- Quintanilha, J.C.F.; Geyer, S.; Etheridge, A.S.; Racioppi, A.; Hammond, K.; Crona, D.J.; Peña, C.E.; Jacobson, S.B.; Marmorino, F.; Rossini, D.; et al. KDR genetic predictor of toxicities induced by sorafenib and regorafenib. Pharmacogenomics J. 2022, 22, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Mukai, E.; Ohta, T.; Kawamura, H.; Lee, E.Y.; Morita, A.; Sasase, T.; Miyajima, K.; Inagaki, N.; Iwanaga, T.; Miki, T. Enhanced vascular endothelial growth factor signaling in islets contributes to β cell injury and consequential diabetes in spontaneously diabetic Torii rats. Diabetes Res. Clin. Pract. 2014, 106, 303–311. [Google Scholar] [CrossRef]
- Wu, J.; Wei, H.; Qu, H.; Feng, Z.; Long, J.; Ge, Q.; Deng, H. Plasma vascular endothelial growth factor B levels are increased in patients with newly diagnosed type 2 diabetes mellitus and associated with the first phase of glucose-stimulated insulin secretion function of β-cell. J. Endocrinol. Investig. 2017, 40, 1219–1226. [Google Scholar] [CrossRef]
- Joukov, V.; Pajusola, K.; Kaipainen, A.; Chilov, D.; Lahtinen, I.; Kukk, E.; Saksela, O.; Kalkkinen, N.; Alitalo, K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996, 15, 290–298. [Google Scholar] [CrossRef]
- Rauniyar, K.; Jha, S.K.; Jeltsch, M. Biology of Vascular Endothelial Growth Factor C in the Morphogenesis of Lymphatic Vessels. Front. Bioeng. Biotechnol. 2018, 6, 7. [Google Scholar] [CrossRef]
- Chien, M.H.; Liu, Y.F.; Hsin, C.H.; Lin, C.H.; Shih, C.H.; Yang, S.F.; Cheng, C.W.; Lin, C.W. Impact of VEGF-C gene polymorphisms and environmental factors on oral cancer susceptibility in Taiwan. PLoS ONE 2013, 8, e60283. [Google Scholar] [CrossRef]
- Almawi, W.Y.; Saldanha, F.L.; Mahmood, N.A.; Al-Zaman, I.; Sater, M.S.; Mustafa, F.E. Relationship between VEGFA polymorphisms and serum VEGF protein levels and recurrent spontaneous miscarriage. Hum. Reprod. 2013, 28, 2628–2635. [Google Scholar] [CrossRef] [PubMed]
- Remuzgo-Martínez, S.; Genre, F.; Pulito-Cueto, V.; Atienza-Mateo, B.; Mora Cuesta, V.M.; Iturbe Fernández, D.; Fernández Rozas, S.M.; Lera-Gómez, L.; Alonso Lecue, P.; Ussetti, M.P.; et al. Role of VEGF Polymorphisms in the Susceptibility and Severity of Interstitial Lung Disease. Biomedicines 2021, 9, 458. [Google Scholar] [CrossRef] [PubMed]



| Database | Queries |
|---|---|
| PubMed | ((((((Polymorphism, Single Nucleotide[MeSH Terms]) OR (Polymorphism*[Title/Abstract])) OR (“SINGLE NUCLEOTIDE POLYMORPHISM”[Title/Abstract])) OR (SNP[Title/Abstract])) AND (((((((“Vascular Endothelial Growth Factor A”[MeSH Terms]) OR (“Vascular Endothelial Growth Factor A”[Title/Abstract])) OR (“Vascular Endothelial Growth Factor”[Title/Abstract])) OR (“Vascular Endothelial Growth Factor RECEPTOR”[Title/Abstract])) OR (VEGF[Title/Abstract])) OR (VEGF-A[Title/Abstract])) OR (“Permeability Factor, Vascular”[Title/Abstract]))) AND ((((((“Diabetes Mellitus”[MeSH Terms]) OR (“Diabetes complications”[MeSH Terms])) OR (“DIABETES MELLITUS”[Title/Abstract])) OR (DM[Title/Abstract])) OR (Diabetic[Title/Abstract])) OR (“Diabetic complications”[Title/Abstract]))) AND ((((((((((((neuropathy[Title/Abstract]) OR (Painful[Title/Abstract])) OR (“peripheral neuropathy”[Title/Abstract])) OR (polyneuropathy[Title/Abstract])) OR (“neuropathic pain”[Title/Abstract])) OR (retinopathy[Title/Abstract])) OR (nephropathy[Title/Abstract])) OR (“Microvascular complications”[Title/Abstract])) OR (“Macrovascular complications”[Title/Abstract])) OR (“Cerebrovascular Accident”[Title/Abstract])) OR (Stroke[Title/Abstract])) OR (“Vascular periphery”[Title/Abstract])) |
| Scopus | ((TITLE-ABS-KEY (“polymorphism, single nucleotide”) OR TITLE-ABS-KEY (polymorphism*) OR TITLE-ABS-KEY (“single nucleotide polymorphism”) OR TITLE-ABS-KEY (SNP))) AND ((TITLE-ABS-KEY (“vascular endothelial growth factor a”) OR TITLE-ABS-KEY (“vascular endothelial growth factor”) OR TITLE-ABS-KEY (“vascular endothelial growth factor receptor”) OR TITLE-ABS-KEY (VEGF) OR TITLE-ABS-KEY (VEGF-a) OR TITLE-ABS-KEY (“permeability factor, vascular”))) AND ((TITLE-ABS-KEY (“diabetes mellitus”) OR TITLE-ABS-KEY (“diabetes complications”) OR TITLE-ABS-KEY (dm) OR TITLE-ABS-KEY (diabetic))) AND ((TITLE-ABS-KEY (neuropathy) OR TITLE-ABS-KEY (painful) OR TITLE-ABS-KEY (“peripheral neuropathy”) OR TITLE-ABS-KEY (polyneuropathy) OR TITLE-ABS-KEY (“neuropathic pain”) OR TITLE-ABS-KEY (retinopathy) OR TITLE-ABS-KEY (nephropathy) OR TITLE-ABS-KEY (“microvascular complications”) OR TITLE-ABS-KEY (“macrovascular complications”) OR TITLE-ABS-KEY (“cerebrovascular accident”) OR TITLE-ABS-KEY (stroke) OR TITLE-ABS-KEY (“vascular periphery”))) |
| Web of Science | “Polymorphism, Single Nucleotide” (Topic) or Polymorphism* (Topic) or “SINGLE NUCLEOTIDE POLYMORPHISM” (Topic) or SNP (Topic) AND TS = (“Vascular Endothelial Growth Factor A”) OR TS = (“Vascular Endothelial Growth Factor”) OR TS = (“Vascular Endothelial Growth Factor RECEPTOR”) OR TS = (VEGF) OR ALL = (VEGF-A) OR TS = (“Permeability Factor, Vascular”) AND TS = (“Diabetes Mellitus”) OR TS = (“Diabetes complications”) OR TS = (DM) OR TS = (Diabetic) AND TS = (neuropathy) OR TS = (Painful) OR TS = (“peripheral neuropathy”) OR TS = (polyneuropathy) OR ALL = (“neuropathic pain”) OR TS = (retinopathy) OR TS = (nephropathy) OR TS = (“Microvascular complications”) OR TS = (“Macrovascular complications”) OR TS = (“Cerebrovascular Accident”) OR TS = (Stroke) OR TS = (“Vascular periphery”) |
| Reference | Ethnicity | Gene | SNP ID | Methods | Ctrl (n) | Cases (n) | Result |
|---|---|---|---|---|---|---|---|
| Suganthalakshmi et al., 2006 [50] | Indian | VEGFA | rs2010963 (−634G/C) rs25648 (−7C/T) | PCR-RFLP | 90 | 120 | Associated with DR. The heterozygous genotypes of rs25648 and rs201093 were 4.17 (95% CI: 1.90–9.18, p = 0.0001 and 2.33 (95% CI: 1.24–4.36, p = 0.008), respectively. Significantly higher in the DR group when compared with control. |
| Buraczynska et al., 2007 [34] | Poland | VEGFA | rs2010963 (−634G/C) | PCR-RFLP | 493 | 426 | No association was found between genotype and DNP or DR in the patient population. |
| Errera et al., 2007 [51] | Brazilian | VEGFA | rs2010963 (−634G/C) | PCR-RFLP | 334 | 167 | The CC genotype is an independent risk factor for PDR in T2DM of European ancestry OR = 1.9; IC 95%: 1.01–3.79; p = 0.04 |
| Petrovic et al., 2007 [52] | Slovenian | VEGFA | rs2010963 (−634G/C) | PCR-RFLP | 228 | 143 | The CC genotype may be a risk factor for MI in long-term T2DM patients (OR = 2.1; 95% CI = 1.1–3.9; p = 0.019) |
| Szaflik et al., 2007 [53] | Poland | VEGFA | rs2010963 (−634G/C) rs833061 (−460T/C) | PCR-RFLP | 61 | 72 | The Allele C is associated with increased VEGF gene promoter activity, and the GC genotype predictive factor for the development of DR in rs2010963. No association with rs833061. |
| Petrovic et al., 2008 [54] | Slovenian | VEGFA | rs2010963 (−634G/C) | PCR-RFLP | 143 | 206 | the VEGF –634 C/G polymorphism failed to contribute to the genetic susceptibility to PDR. |
| Uthra et al., 2008 [55] | Indian | VEGFA |
rs2010963 (−634G/C)
rs3025039 (+936C/T) | PCR/Sequenced | 82 | 131 | No association for DR in rs3025039. The GC genotype in rs2010963 increased risk for DR in patients with microalbuminuria (OR = 8.9; 95% CI: 1.4, 58.3). |
| Abhary et al., 2009 [56] | Australian | VEGFA | rs3025021 (C/T) rs10434 (+1612G/A) | iPLEX | 187 | 139 | The C allele of rs3025021 (p = 0.002; OR, 3.8; 95% CI, 1.5–10.0) and the G allele of rs10434 (p = 0.002; OR, 2.6; 95% CI, 1.3–5.3) significantly associated with blinding DR. |
| Kim et al., 2009 [57] | Korean | VEGFA | rs3025039 (+936 C/T) | PCR-RFLP | 526 | 398 | Diabetics with DR showed a higher frequency of TT genotype and T Allele, it is associated with higher levels of VEGF and DR |
| Nakamura et al., 2009 [58] | Japanese | VEGFA | rs2010963 (−634G/C) rs699947 (−2578C/A) | PCR-RFLP | 292 | 177 | The AA genotype of rs699947 is associated with PDR, the risk is 7.7 (95%, CI: 1.8–30.9). No association in rs2010963. |
| Tiwari et al., 2009 [59] | Indian | VEGFA | rs833061 (−460T/C) rs2010963 (−634G/C) | PCR-RFLP | 224 | 194 | Significant association of rs833061 with CRI (p < 0.05) The CT genotype, OR = 2.23 (1.87–4.18). No association in rs2010963 |
| Chun et al., 2010 [60] | Korean | VEGFA | rs699947 (−2578C/A) rs1570360 (−1154G/A) rs2010963 (−634G/C) | TAQMAN | 260 | 387 | The A allele at rs699947 is significant association with DR. |
| Yang et al., 2010 [61] | Chinese | VEGFA | rs2010963 (−634G/C) | TAQMAN | 96 | 285 | The rs2010963 SNP is not associated with T2DM but increases the risk of DR. |
| Feghhi et al., 2011 [62] | Iranian | VEGFA | rs2010963 (−634G/C) | PCR-RFLP | 279 | 119 | The GG genotype associated with the risk of PDR (OR:1.87, 95%, CI (1.034–3.383). |
| Amoli et al., 2011 [63] | Iranian | VEGFA | rs25648 (−7C/T) rs699947 (−2578C/A) | ARMS-PCR | 98 | 488 | The genotype AA of rs699947 was significantly reduced in DFU cases, conferring a protective effect. |
| Yang et al., 2011 [64] | Chinese | VEGFA | rs699947 (−2578C/A) rs833061 (−460T/C) rs13207351 (−1190A/G) rs2010963 (−634G/C) rs2146323 (+5092C/A) rs3025039 (+936C/T) | iPLEX | 139 | 129 | A significant association of DR was observed with the AA genotype of rs699947 (odds ratio (OR) = 3.54, 95% confidence interval (CI): 1.12–11.19), the CC genotype of rs833061 (OR = 3.72, 95% CI: 1.17–11.85) and the AA genotype of rs13207351 (OR = 3.76, 95% CI: 1.21–11.71. |
| Bleda et al., 2012 [65] | Spanish | VEGFA | rs2010963 (−634G/C) rs699947 (−2578C/A) | TAQMAN | 14 | 26 | The CC genotype of rs2010963 was increased in PAD, while the CA genotype of rs699947 was more prevalent in DR (p = 0.016 and p = 0.002, respectively). |
| Nikzamir et al., 2012 [66] | Iranian | VEGFA | rs2010963 (+405G/C) | PCR-RFLP | 235 | 255 | The GG genotype is independently associated with development of DNP [p = 0.014, OR = 1.771, 95% confidence interval (CI) = 1.124–2.790]. The G allele was not associated with albuminuria. |
| Paine et al., 2012 [67] | Indian | VEGFA | rs833061 (−460T/C) | PCR-RFLP | 240 | 253 | The CC genotype significantly associated with PDR (OR [95% CI]3.66(1.35–11.4)) |
| Bonnefond et al., 2013 [68] | French Danish | VEGFA | rs6921438 (A/G) rs10738760 (A/G) | TAQMAN | 3875 3561 | 6920 2623 | Association with the G allele of rs692143 and T2D in the French population |
| El-Shazly et al., 2014 [69] | Egyptians | VEGFA | rs2010963 (−634G/C) | PCR-RFLP | 180 | 212 | The CC genotype is a risk factor for DR. (p < 0.001) |
| Fan et al., 2014 [70] | Chinese | VEGFA | rs699947 (−2578C/A) rs1570360 (−1154G/A) rs833061 (−460T/C) rs2010963 (−634G/C) rs3025039 (+936C/T) | PCR-RFLP | 668 | 372 | No association between the SNP’s and DR. Patients with DR have higher VEGF level and rs699947 and rs2010963 may be important factor for serum VEGF levels. |
| Kariz et al., 2014 [71] | Slovenian | VEGFR2 | rs2071559 (−604 T/C) rs2305948 (+1192C/T) | PCR-RFLP | 850 | 171 | The CC genotype of the rs2071559 is a risk factor for MI (OR = 1.6; 95% CI = 1.1–2.1; p = 0.022). |
| Pirie et al., 2014 [72] | South African | VEGFA | rs2010963 (−634G/C) | PCR-RFLP | 171 | 117 | No significant association found between SNPs and DR in South African population |
| Yang et al., 2014 [73] | Chinese |
VEGFA VEGFR2 | rs699947 (−2578C/A) rs833061 (−460T/C) rs13207351 (−1190A/G) rs2146323 (+5092C/A) rs2071559 (−604T/C) | iPLEX | 284 | 216 | DR showed significant associations with VEGF SNPs—rs699947 (p < 0.001), rs833061 (p = 0.001), rs13207351 (p < 0.001), and rs2146323 (p = 0.006)—as well as with one variant in the VEGFR2 gene, rs2071559 (p = 0.034) |
| Yuan et al., 2014 [74] | Chinese | VEGFA | rs2010963 (−634G/C) rs833061 (−460T/C) | PCR/LDR | 134 | 144 | The rs833061 was correlated with NPDR, and C allele was associated with lower NPDR risk than T allele. The rs2010963 not correlated with NPDR or PDR. |
| Zhang et al., 2014 [45] | Chinese | VEGFA | rs3025039 (+936 C/T) | PCR-RFLP | 240 | 184 | Allele C of rs3025039 may be a genetic marker susceptible to DPN, while allele T may be a protective marker of DPN. |
| Choudhury et al., 2015 [44] | Indian | VEGFA | rs2010963 (−634G/C) rs3025039 (+936C/T) rs1570360 (−1154G/A) rs2071559 (−604T/C) | TAQMAN | 95 | 102 | The rs2010963 C allele and rs3025039 T allele might be associated with PDR occurrence and, in turn, regulate VEGF expression among PDR subjects. |
| Ghisleni et al., 2015 [75] | Brazilians | VEGFA | rs3025039 (+936 C/T) | PCR-RFLP | 104 | 98 | The rs3025039 are not correlated with the risk of developing T2DM or neuropathic signs and symptoms. |
| Moradzadegan et al., 2015 [76] | Iranian | VEGFA | rs2010963 (−634G/C) | PCR-RFLP | 369 | 141 | The G allele of rs2010963 can be an important independent risk factor for susceptibility of CAD in T2DM patients OR (1.75) (p = 0.024). |
| Porojan et al., 2015 [41] | Caucasians | VEGFA | rs3025039 (+936 C/T) | PCR-RFLP | 208 | 200 | The rs3025039 revealed an increased risk of T2DM, is highly associated with DR; therefore, this genetic variant is confirmed to be an independent genetic risk factor for NPDR. |
| Chen et al., 2016 [77] | Taiwanese | VEGFA | rs1570360 (−1154G/A) rs2010963 (−634G/C) | TAQMAN | 31 | 53 | The rs2010963 is an important genetic marker for DR. The experiments provide a direct association between allele C of rs2010963 and serum levels of VEGFA. |
| Fattah et al., 2016 [78] | Egyptians | VEGFA | rs699947 (−2578C/A) rs10434 (+1612G/A) | RT-PCR | 41 | 41 | The rs699947 or rs10434 polymorphism was not associated with DR in Egyptian patients. |
| Kamal et al., 2016 [79] | Egyptians | VEGFA | rs2010963 (−634G/C) | PCR-RFLP | 61 | 61 | Patients carrying allele C have a higher risk of PDR development, so rs2010963 could be used as a predictive marker for PDR in diabetic patients. |
| Merlo et al., 2016 [80] | Slovenian |
VEGFA
VEGFR2 | rs2010963 (−634G/C) rs2071559 (−604T/C) | KASP | 200 | 595 | There were no statistically significant differences in the VEGF rs2010963 and KDR rs2071559 genotype distribution frequencies between T2DM patients and controls. |
| Gonzales-Salinas et al., 2017 [81] | Mexican | VEGFA | rs2010963 (−634G/C) rs3025021 (C/T) rs3025035 (C/T) | TAQMAN | 71 | 71 | None of the polymorphisms studied were significantly associated with PDR. |
| Zhuang et al., 2017 [82] | Chinese | VEGFA | rs833061 (−460T/C) rs1570360 (−1154G/A) | PCR-RFLP | 188 | 209 | The rs833061 is associated with DFU. |
| Barus et al., 2018 [83] | Indonesian | VEGFA | rs3025039 (+936C/T) | PCR-RFLP | 83 | 69 | The CT + TT genotype is associated with a protective effect against DPN (OR = 0.35; p = 0.01). There was also a significant association with VEGFA plasma level and duration of diabetes diagnosis. |
| Xiaolei Li, 2018 [84] | Chinese | VEGFA | rs2010963 (−634G/C) | PCR-RFLP | 108 | 121 | The CC genotype and C allele of rs2010963 were less common in DFU than in T2DM (OR = 0.36 and 0.63, respectively), but CC carriers showed higher VEGF levels (p = 0.007) |
| Li et al., 2018 [85] | Chinese | VEGFA | rs699947 (−2578C/A) rs13207351 (−1190A/G) | PCR-RFLP | 103 | 185 | Association of rs699947 with the occurrence of DFU. The minor A allele might reduce the susceptibility to DFU, while no significant association was detected for rs13207351. |
| Arredondo-García et al., 2019 [86] | Mexican | VEGFA | rs3025039 (+936C/T) | PCR-RFLP | 128 | 90 | The rs3025039 tended to be a risk factor for the development of DPN, and CT genotype showed a protective effect (OR = 0.52; 95% CI = 0.300–0.90; p = 0.019). |
| Dahlan et al., 2019 [87] | Indonesia | VEGFA | rs833061 (−460T/C) rs2010963 (−634G/C) | PCR-RFLP | 101 | 96 | There was no significant relationship between SNPs with DFU. G and T alleles have a potential protective factor against the occurrence of DFU. (OR 0.90, 95% CI; 0.59 to 1.37 and p = 0.641). |
| Luo et al., 2019 [36] | Chinese | VEGFA | rs2010963 (−634G/C) rs699947 (−2578C/A) | PCR-RFLP | 650 | 580 | The rs2010963 and rs699947 may increase the risk of developing DPN. ([OR] = 1.15, confidence interval [95% CI]: 1.03–1.30) |
| Yari et al., 2020 [88] | Iranian | VEGFA | rs3025039 (+936C/T) | PCR-RFLP | 80 | 80 | No association between rs3025039 and T2DM. |
| Khan et al., 2020 [89] | Pakistanis | VEGFA | rs833061 (−460T/C) rs13207351 (−1190A/G) rs1570360 (−1154G/A) rs2010963 (−634G/C) | PCR-RFLP | 348 | 1126 | There was no association between SNPs and T2DM; The rs13207351 rs13207351was associated with NPDR [OR = 1.97 (95% CI 1.28–3.03, p = 9.0 × 10−3)]. |
| Abdelghany et al., 2021 [90] | Egyptians | VEGFA VEGFR2 VEGFB VEGFC VEGFR1 | rs833069 (+450T/C) rs2305948 (+1192C/T) rs12366035 (C/T) rs7664413 (C/T) rs7993418 (A/G) | TAQMAN | 110 | 125 | This study revealed a significant association between the T allele of rs12366035 and rs7664413, and the AG genotype of rs7993418 and T2DM/DR susceptibility. |
| Elfaki et al., 2021 [40] | Arab | VEGFA | rs699947 (−2578C/A) | ARMS-PCR | 126 | 122 | The results showed that the CA genotype of the VEGF rs699947 was associated with T2DM with OR =2.01, p-value = 0.011. |
| Jin et al., 2021 [91] | Chinese | VEGFA | rs2010963 (−634G/C) | KASP | 386 | 316 | Ther s2010963 in the VEGFA gene are related to the risk of PDR. The CG genotypes of rs2010963 were associated with a decreased risk of PDR (the OR was 0.588, with a 95% CI ranging from 0.366 to 0.946). |
| Imbaby et al., 2021 [92] | Egyptians | VEGFA | rs3025039 (+936 C/T) | PCR-RFLP | 40 | 50 | The rs3025039 may be associated with T2DM. However, there is no association with DPN. |
| Wijaya et al., 2021 [93] | Balinese | VEGFA | rs699947 (−2578C/A) | PCR-RFLP | 35 | 33 | The rs699947 as a risk factor of DR in patients with T2DM (OR = 13.05; 95% CI = 2.69–63.18; p = 0.001). |
| Mohamed et al., 2022 [94] | Egyptian | VEGFA | rs3025039 (+936C/T) | PCR-RFLP | 72 | 72 | The rs3025039 genetic variants were not associated with the PDR progression. |
| Omar et al., 2022 [95] | Egyptian | VEGFA | rs3025020 (−583C/T) rs3025039 (+936C/T) | TAQMAN | 26 | 26 | The T allele of rs3025020 (OR = 2.67; p = 0.04) and both the CT genotype and T allele of rs3025039 (OR = 4.08 and 4.02; p = 0.01 and 0.004) were more common in T2DM patients with mixed complications than in controls. |
| Singh et al., 2022 [96] | Indian | VEGFA | rs699947 (−2578C/A) | PCR-RFLP | 51 | 55 | No association with DR. |
| Alnaji et al., 2023 [97] | Iraqis | VEGFA | rs2010963 (−634G/C) rs699947 (−2578C/A) | ARMS-PCR | 36 | 134 | The rs2010963 GG genotype showed a significant link with DR (OR = 10.29; p = 0.004) |
| Del Cuore et al., 2023 [98] | Italians | VEGFA | rs699947 (−2578C/A) rs3025039 (+936C/T) | TAQMAN | 20 | 90 | The CC genotype of rs699947 is associated with DFU. |
| Jehanzeb et al., 2023 [99] | Pakistanis | VEGFA | rs833061 (−460T/C) | ARMS-PCR | 184 | 180 | Significant association of rs833061 SNP with DR on T2DM |
| Quayyum et al., 2023 [100] | Pakistanis | VEGFA | rs699947 (−2578C/A) rs1570360 (−1154G/A) | ARMS-PCR | 150 | 300 | There was a strong association of rs699947 SNP with PDR in T2DM. |
| Yuan et al., 2023 [101] | Chinese | VEGFR2 | rs2071559 (−604T/C) | PCR/LDR | 114 | 123 | No associations between the rs2071559 SNP and DR or PDR. |
| Nussdorfer et al., 2024 [102] | Chinese | VEGFA VEGFR2 VEGFR2 | rs6921438 (G/A) rs2071559 (−604T/C) rs2305948 (+1192C/T) | TAQMAN | 553 | 344 | In Slovenians with T2DM, rs2071559 C (VEGFR2) increased DN risk, whereas rs6921438 G (VEGFA) was protective |
| SNP ID | Gene | Region | Number of Studies | Associated Complications | Association Pattern |
|---|---|---|---|---|---|
| rs2010963 | VEGFA | 5′ UTR | 31 | DR (PDR, NPDR), MI, DFU, DNP, DPN | C allele = risk for PDR, DR, MI; protective for DFU; mixed for DNP/DPN |
| rs699947 | VEGFA | Promoter | 16 | DR, DFU, T2DM/dyslipidemia | The A allele = protective for DFU; risk or no association for DR; CA genotype = altered lipids and ↑ CV risk-C allele lead to ↑ VEGF |
| rs3025039 | VEGFA | 3′ UTR | 15 | DR, DPN, DNP, T2DM, DFU | T allele = protective for DR, DPN, DFU; C allele = risk for DPN and T2DM/DR; T allele and CT genotype = risk for PDR |
| rs833061 | VEGFA | Promoter | 11 | DR (PDR, NPDR), DFU, CRI | C allele lead to ↑ VEGF C allele = increased DR, protective for NPDR, mixed for DFU; CT genotype = risk for CRI |
| rs1570360 | VEGFA | Promoter | 7 | DR(PDR), DFU | AA genotype = risk for PDR. DFU no association |
| rs2071559 | VEGFR2 | Promoter | 6 | DR, DN, MI | CC genotype = risk for DR, MI, and DN; |
| rs13207351 | VEGFA | Promoter | 4 | DR, DFU | AA genotype = risk for DR. No association with T2DM or DFU |
| rs2305948 | VEGFR2 | Exon 7 | 3 | DN | C allele = risk for DN |
| rs2146323 | VEGFA | Intron 2 | 2 | DR | AA genotype = risk for DR |
| rs25648 | VEGFA | 5′ UTR | 2 | DR, DFU | Heterozygous = risk for DR; no association with DFU |
| rs10434 | VEGFA | 3′ UTR | 2 | DR | G allele = risk for DR |
| rs3025021 | VEGFA | Intron 6 | 2 | DR(PDR) | C allele = risk for DR/PDR |
| rs3025020 | VEGFA | Intron 6 | 1 | T2DM | T allele = risk for T2DM |
| rs3025035 | VEGFA | Intron 7 | 1 | DR | No association |
| rs6921438 | VEGFA | Downstream | 1 | T2DM/DN | G allele = protective for T2DM/DN |
| rs833069 | VEGFA | Intron 2 | 1 | DR | No association |
| rs7993418 | VEGFR1 | Exon 28 | 1 | T2DM, DR | AG genotype = risk for T2DM and DR |
| rs12366035 | VEGFB | Exon 5 | 1 | T2DM, DR | T allele = Risk for T2DM and DR |
| rs7664413 | VEGFC | Intron 5 | 1 | T2DM, DR | T allele = Risk for T2D and DR |
| rs10738760 | VEGF-related | Intergenic (VEGF) | 1 | DR | G allele = Risk for DR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, C.M.G.d.; de Melo, R.C.; Souza, N.M.P.; Silva, P.R.; Aguiar, D.F.; Ferreira, L.M.; Volanski, W.; Picheth, G.; Rego, F.G.d.M.; Sari, M.H.M. Polymorphisms in VEGF Signaling Pathway Genes and Their Potential Impact on Type 2 Diabetes Mellitus and Associated Complications: A Scoping Review. Biomedicines 2025, 13, 2242. https://doi.org/10.3390/biomedicines13092242
Lima CMGd, de Melo RC, Souza NMP, Silva PR, Aguiar DF, Ferreira LM, Volanski W, Picheth G, Rego FGdM, Sari MHM. Polymorphisms in VEGF Signaling Pathway Genes and Their Potential Impact on Type 2 Diabetes Mellitus and Associated Complications: A Scoping Review. Biomedicines. 2025; 13(9):2242. https://doi.org/10.3390/biomedicines13092242
Chicago/Turabian StyleLima, Christiane Mayrhofer Grocoske de, Rafaela Cirillo de Melo, Nathalia Marçallo Peixoto Souza, Paula Rothbarth Silva, Dayane Ferreira Aguiar, Luana Mota Ferreira, Waldemar Volanski, Geraldo Picheth, Fabiane Gomes de Moraes Rego, and Marcel Henrique Marcondes Sari. 2025. "Polymorphisms in VEGF Signaling Pathway Genes and Their Potential Impact on Type 2 Diabetes Mellitus and Associated Complications: A Scoping Review" Biomedicines 13, no. 9: 2242. https://doi.org/10.3390/biomedicines13092242
APA StyleLima, C. M. G. d., de Melo, R. C., Souza, N. M. P., Silva, P. R., Aguiar, D. F., Ferreira, L. M., Volanski, W., Picheth, G., Rego, F. G. d. M., & Sari, M. H. M. (2025). Polymorphisms in VEGF Signaling Pathway Genes and Their Potential Impact on Type 2 Diabetes Mellitus and Associated Complications: A Scoping Review. Biomedicines, 13(9), 2242. https://doi.org/10.3390/biomedicines13092242








