Risk for COVID-19 Vulnerability in Patients with Inflammatory Bowel Disease: Assessing Alterations in ACE2 and TMPRSS2
Abstract
1. Introduction
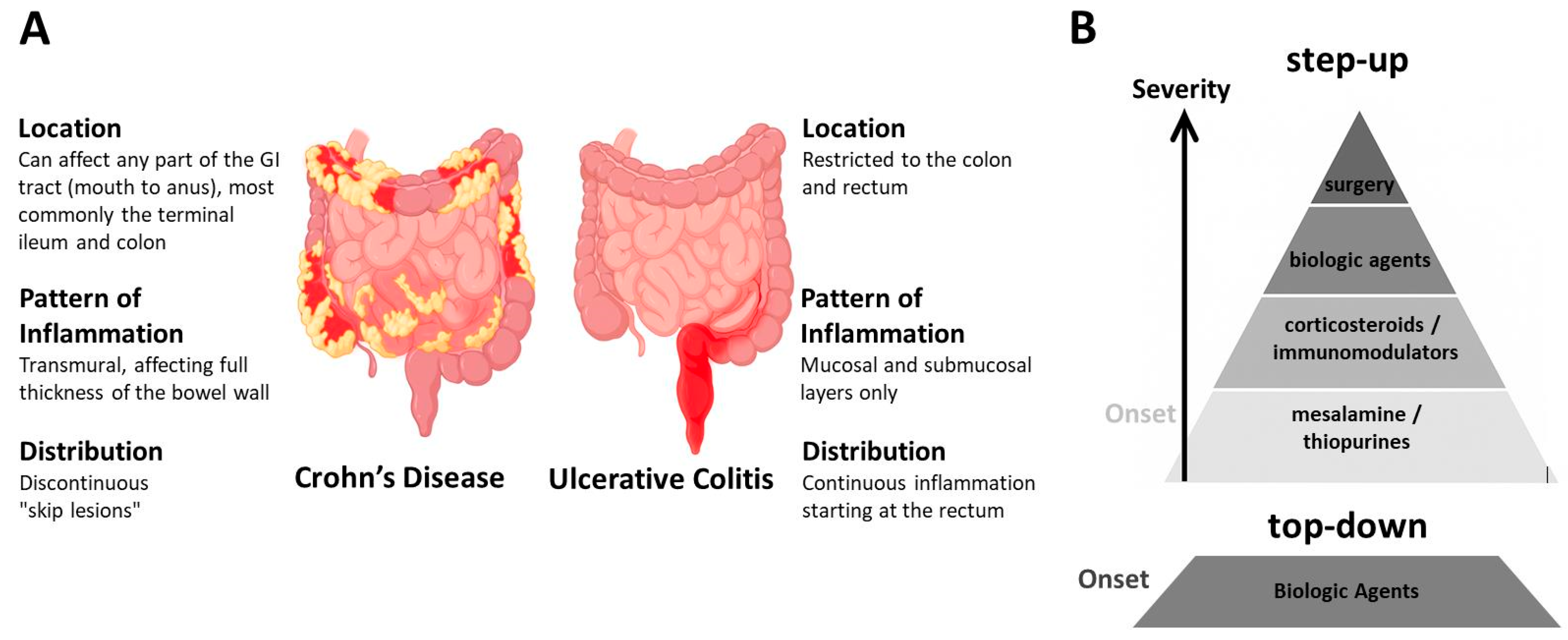
1.1. Inflammatory Bowel Disease (IBD)
1.2. IBD and COVID-19
2. Characterization of ACE2 and TMPRSS2 Molecular Forms and Significance for COVID-19 Risk
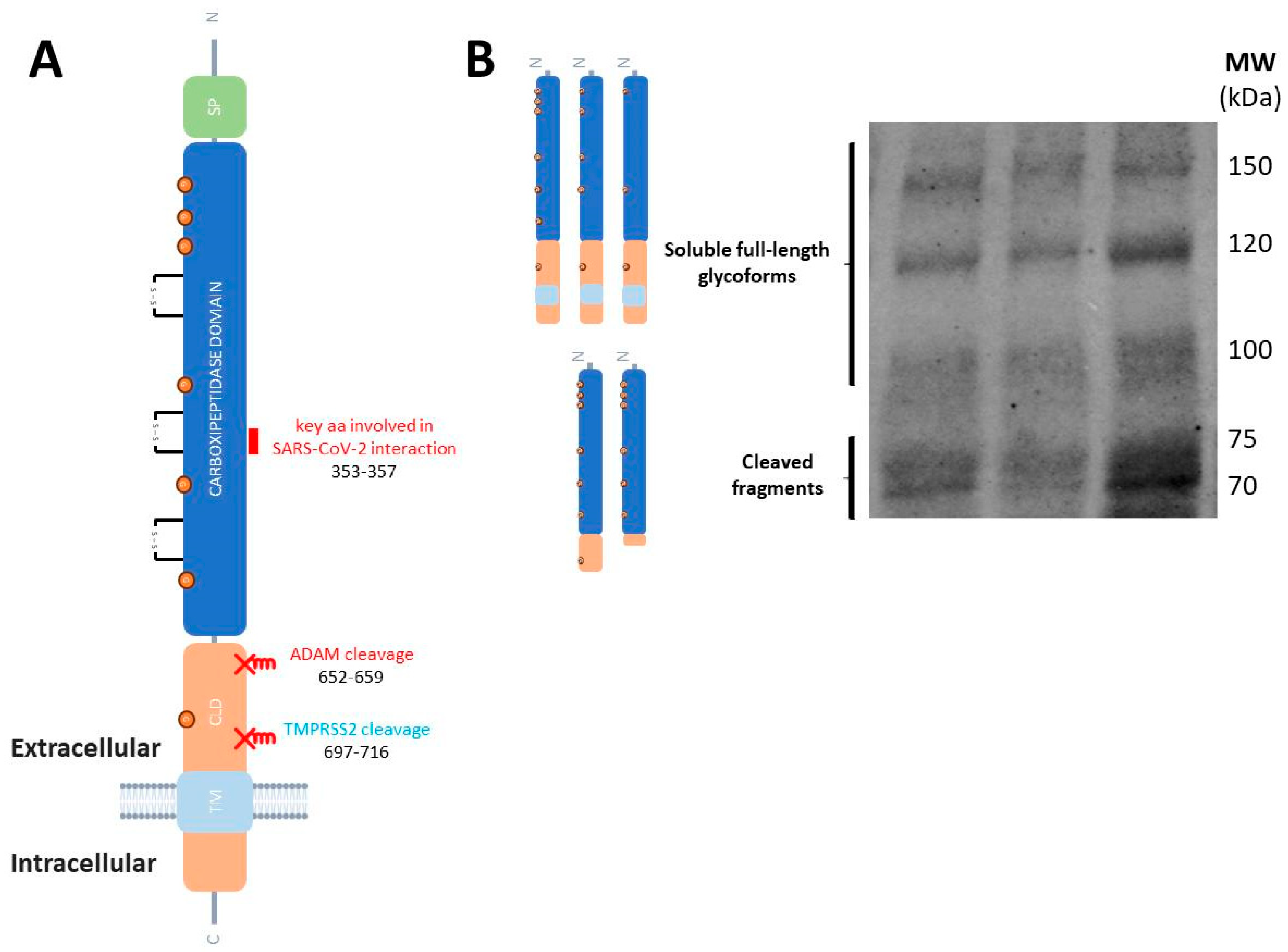
2.1. ACE2 Protein Function and Proteolytic Processing
2.2. TMPRSS2 Protein Function and Proteolytic Processing
2.3. Significance of Particular ACE2 and TMPRSS2 Molecular Species Regarding COVID-19 Vulnerability
3. Levels of ACE2 and TMPRSS2 in IBD Biopsy Tissue
4. Levels of ACE2 and TMPRSS2 in IBD Plasma
5. Effects of IBD Therapies on ACE2 Levels
6. Conclusions and Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE2 | angiotensin-converting enzyme 2 |
| ADAM | a disintegrin and metalloprotease |
| Ang | angiotensin |
| CD | Crohn’s disease |
| CSF | cerebrospinal fluid |
| ELISA | enzyme-linked immunosorbent assays |
| IBD | Inflammatory Bowel Disease |
| JAK | Janus kinase |
| TNFα | tumor necrosis factor-α |
| UC | ulcerative colitis |
References
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Kaplan, G.G. The global burden of inflammatory bowel disease: From 2025 to 2045. Nat. Rev. Gastroenterol. Hepatol. 2025, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fakhoury, M.; Negrulj, R.; Mooranian, A.; Al-Salami, H. Inflammatory bowel disease: Clinical aspects and treatments. J. Inflamm. Res. 2014, 7, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Cosín-Roger, J. Inflammatory Bowel Disease: Immune Function, Tissue Fibrosis and Current Therapies. Int. J. Mol. Sci. 2024, 25, 6416. [Google Scholar] [CrossRef]
- da Silva Júnior, R.T.; Apolonio, J.S.; de Souza Nascimento, J.O.; da Costa, B.T.; Malheiro, L.H.; Luz, M.S.; de Carvalho, L.S.; da Silva Santos, C.; de Melo, F.F. Crohn’s disease and clinical management today: How it does? World J. Methodol. 2023, 13, 399–413. [Google Scholar] [CrossRef]
- Hirschmann, S.; Neurath, M.F. Top-down approach to biological therapy of Crohn’s disease. Expert Opin. Biol. Ther. 2017, 17, 285–293. [Google Scholar] [CrossRef]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68, s1–s106. [Google Scholar] [CrossRef]
- Herrlinger, K.R.; Stange, E.F. Prioritization in inflammatory bowel disease therapy. Expert Rev. Gastroenterol. Hepatol. 2023, 17, 753–767. [Google Scholar] [CrossRef]
- D’Haens, G.; van Bodegraven, A.A. Mesalazine is safe for the treatment of IBD. Gut 2004, 53, 155. [Google Scholar] [CrossRef]
- Axelrad, J.E.; Roy, A.; Lawlor, G.; Korelitz, B.; Lichtiger, S. Thiopurines and inflammatory bowel disease: Current evidence and a historical perspective. World J. Gastroenterol. 2016, 22, 10103–10117. [Google Scholar] [CrossRef]
- Chande, N.; Patton, P.H.; Tsoulis, D.J.; Thomas, B.S.; MacDonald, J.K. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2015, 10, CD000067. [Google Scholar] [CrossRef]
- Rogler, G.; Sandborn, W.J. Is there still a role for thiopurines in Crohn’s disease? Gastroenterology 2013, 145, 714–716. [Google Scholar] [CrossRef] [PubMed]
- Bruscoli, S.; Febo, M.; Riccardi, C.; Migliorati, G. Glucocorticoid Therapy in Inflammatory Bowel Disease: Mechanisms and Clinical Practice. Front. Immunol. 2021, 12, 691480. [Google Scholar] [CrossRef] [PubMed]
- Kapizioni, C.; Desoki, R.; Lam, D.; Balendran, K.; Al-Sulais, E.; Subramanian, S.; Rimmer, J.E.; Negro, J.D.L.R.; Pavey, H.; Pele, L.; et al. Biologic Therapy for Inflammatory Bowel Disease: Real-World Comparative Effectiveness and Impact of Drug Sequencing in 13 222 Patients within the UK IBD BioResource. J. Crohn’s Colitis 2024, 18, 790–800. [Google Scholar] [CrossRef]
- Honap, S.; Agorogianni, A.; Colwill, M.J.; Mehta, S.K.; Donovan, F.; Pollok, R.; Poullis, A.; Patel, K. JAK inhibitors for inflammatory bowel disease: Recent advances. Frontline Gastroenterol. 2023, 15, 59–69. [Google Scholar] [CrossRef]
- Long, D. Crohn’s Disease and Ulcerative Colitis: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines 2024, 12, 689. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, L.; Zheng, Y.; An, J.; Wen, G.; Jin, H.; Tuo, B. Digestive system infection by SARS-CoV-2: Entry mechanism, clinical symptoms and expression of major receptors (Review). Int. J. Mol. Med. 2023, 51, 19. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Matias, J.N.; Flato, U.A.P.; Pilon, J.P.G.; Bitelli, P.; Junior, M.A.P.; de Carvalho, A.C.A.; Haber, J.F.D.S.; Reis, C.H.B.; Goulart, R.A. What Do Influenza and COVID-19 Represent for Patients With Inflammatory Bowel Disease? Gastroenterol. Res. 2021, 14, 1–12. [Google Scholar] [CrossRef]
- Pan, L.; Mu, M.; Yang, P.; Sun, Y.; Wang, R.; Yan, J.; Li, P.; Hu, B.; Wang, J.; Hu, C.; et al. Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am. J. Gastroenterol. 2020, 115, 766–773. [Google Scholar] [CrossRef]
- Lin, L.; Jiang, X.; Zhang, Z.; Huang, S.; Zhang, Z.; Fang, Z.; Gu, Z.; Gao, L.; Shi, H.; Mai, L.; et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut 2020, 69, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Ashktorab, H.; Challa, S.R.; Singh, G.; Nanduri, S.; Ibrahim, M.; Martirosyan, Z.; Whitsell, P.; Chirumamilla, L.G.; Shayegh, N.; Watson, K.; et al. Gastrointestinal Manifestations and Their Association with Neurologic and Sleep Problems in Long COVID-19 Minority Patients: A Prospective Follow-Up Study. Dig. Dis. Sci. 2024, 69, 562–569. [Google Scholar] [CrossRef]
- Aydın, M.F.; Taşdemir, H. Ulcerative Colitis in a COVID-19 Patient: A Case Report. Turk. J. Gastroenterol. 2021, 32, 543–547. [Google Scholar] [CrossRef]
- Gubatan, J.; Levitte, S.; Balabanis, T.; Patel, A.; Sharma, A.; Habtezion, A. SARS-CoV-2 Testing, Prevalence, and Predictors of COVID-19 in Patients with Inflammatory Bowel Disease in Northern California. Gastroenterology 2020, 159, 1141–1144.e2. [Google Scholar] [CrossRef]
- Anushiravani, A.; Saberzadeh-Ardestani, B.; Vahedi, H.; Fakheri, H.; Mansour-Ghanaei, F.; Maleki, I.; Nasseri-Moghaddam, S.; Vosoghinia, H.; Ghadir, M.R.; Hormati, A.; et al. Susceptibility of Patients with Inflammatory Bowel Disease to COVID-19 Compared with Their Households. Middle East J. Dig. Dis. 2022, 14, 182–191. [Google Scholar] [CrossRef]
- Attauabi, M.; Poulsen, A.; Theede, K.; Pedersen, N.; Larsen, L.; Jess, T.; Hansen, M.R.; Verner-Andersen, M.K.; Haderslev, K.V.; Lødrup, A.B.; et al. Prevalence and Outcomes of COVID-19 Among Patients with Inflammatory Bowel Disease-A Danish Prospective Population-based Cohort Study. J. Crohn’s Colitis 2021, 15, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Taxonera, C.; Sagastagoitia, I.; Alba, C.; Mañas, N.; Olivares, D.; Rey, E. 2019 novel coronavirus disease (COVID-19) in patients with inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2020, 52, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Li, H.J.; Wasuwanich, P.; Kim, S.E.; Kim, J.Y.; Jeong, G.H.; Park, S.; Yang, J.W.; Kim, M.S.; Yon, D.K.; et al. COVID-19 susceptibility and clinical outcomes in inflammatory bowel disease: An updated systematic review and meta-analysis. Rev. Med. Virol. 2023, 33, e2414. [Google Scholar] [CrossRef] [PubMed]
- Kogan, L.; Ungaro, R.C.; Caldera, F.; Shah, S.A. Effects of COVID-19 on Patients with Inflammatory Bowel Disease. Rhode Isl. Med. J. 2022, 105, 42–47. [Google Scholar]
- Merad, M.; Blish, C.A.; Sallusto, F.; Iwasaki, A. The immunology and immunopathology of COVID-19. Science 2022, 375, 1122–1127. [Google Scholar] [CrossRef]
- Mangoni, A.A.; Zinellu, A. Systemic inflammation index, disease severity, and mortality in patients with COVID-19: A systematic review and meta-analysis. Front. Immunol. 2023, 14, 1212998. [Google Scholar] [CrossRef]
- Saviano, A.; Brigida, M.; Petruzziello, C.; Zanza, C.; Candelli, M.; Loprete, M.R.M.; Saleem, F.; Ojetti, V. Intestinal Damage, Inflammation and Microbiota Alteration during COVID-19 Infection. Biomedicines 2023, 11, 1014. [Google Scholar] [CrossRef]
- Bourgonje, A.R. Dualistic Implications of Angiotensin-Converting Enzyme 2 (ACE2) Expression in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2025, 16, izaf131. [Google Scholar] [CrossRef]
- Shulla, A.; Heald-Sargent, T.; Subramanya, G.; Zhao, J.; Perlman, S.; Gallagher, T. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J. Virol. 2011, 85, 873–882. [Google Scholar] [CrossRef]
- Bach, M.L.; Jensen, B.L. Effects and regulation of ACE2 and TMPRSS2 abundance in healthy humans and in patients with SARS-CoV-2. Biochem. Soc. Trans. 2025, 53, 775–786. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Lin, W.; Liu, H.; Zhong, W.; Zhang, X.; Zhu, Y.; Wang, X.; Li, J.; Sheng, Q. SARS-CoV-2 Host Receptor ACE2 Protein Expression Atlas in Human Gastrointestinal Tract. Front. Cell Dev. Biol. 2021, 9, 659809. [Google Scholar] [CrossRef] [PubMed]
- Vargas, R.A.V.; Millán, J.M.V.; Bonilla, E.F. Renin-angiotensin system: Basic and clinical aspects-A general perspective. Endocrinol Diabetes Nutr. 2022, 69, 52–62. [Google Scholar] [CrossRef]
- Amraei, R.; Rahimi, N. COVID-19, Renin-Angiotensin System and Endothelial Dysfunction. Cells 2020, 9, 1652. [Google Scholar] [CrossRef] [PubMed]
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B.; Huan, Y.; Yang, P.; Zhang, Y.; Deng, W.; et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005, 11, 875–879. [Google Scholar] [CrossRef]
- Patel, V.B.; Zhong, J.C.; Grant, M.B.; Oudit, G.Y. Role of the ACE2/Angiotensin 1–7 Axis of the Renin-Angiotensin System in Heart Failure. Circ. Res. 2016, 118, 1313–1326. [Google Scholar] [CrossRef]
- Sehn, F.; Büttner, H.; Godau, B.; Müller, M.; Sarcan, S.; Offermann, A.; Perner, S.; Kramer, M.W.; Merseburger, A.S.; Roesch, M.C. The alternative renin-angiotensin-system (RAS) signalling pathway in prostate cancer and its link to the current COVID-19 pandemic. Mol. Biol. Rep. 2023, 50, 1809–1816. [Google Scholar] [CrossRef]
- Verano-Braga, T.; Martins, A.L.V.; Motta-Santos, D.; Campagnole-Santos, M.J.; Santos, R.A.S. ACE2 in the renin-angiotensin system. Clin. Sci. (Lond.) 2020, 134, 3063–3078. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Torres, E.; Oyarzún, A.; Mondaca-Ruff, D.; Azocar, A.; Castro, P.F.; Jalil, J.E.; Chiong, M.; Lavandero, S.; Ocaranza, M.P. ACE2 and vasoactive peptides: Novel players in cardiovascular/renal remodeling and hypertension. Ther. Adv. Cardiovasc. Dis. 2015, 9, 217–237. [Google Scholar] [CrossRef]
- Kuba, K.; Imai, Y.; Ohto-Nakanishi, T.; Penninger, J.M. Trilogy of ACE2: A peptidase in the renin-angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol. Ther. 2010, 128, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.J.; Nalivaeva, N.N. Angiotensin-converting enzyme 2 (ACE2): Two decades of revelations and re-evaluation. Peptides 2022, 151, 170766. [Google Scholar] [CrossRef] [PubMed]
- Khajah, M.A.; Fateel, M.M.; Ananthalakshmi, K.V.; Luqmani, Y.A. Anti-Inflammatory Action of Angiotensin 1–7 in Experimental Colitis. PLoS ONE 2016, 11, e0150861. [Google Scholar] [CrossRef]
- Garg, M.; Royce, S.G.; Tikellis, C.; Shallue, C.; Batu, D.; Velkoska, E.; Burrell, L.M.; Patel, S.K.; Beswick, L.; Jackson, A.; et al. Imbalance of the renin-angiotensin system may contribute to inflammation and fibrosis in IBD: A novel therapeutic target? Gut 2020, 69, 841–851. [Google Scholar] [CrossRef]
- Salmenkari, H.; Korpela, R.; Vapaatalo, H. Renin-angiotensin system in intestinal inflammation-Angiotensin inhibitors to treat inflammatory bowel diseases? Basic Clin. Pharmacol. Toxicol. 2021, 129, 161–172. [Google Scholar] [CrossRef]
- Niehues, R.V.; Wozniak, J.; Wiersch, F.; Lilienthal, E.; Tacken, N.; Schumertl, T.; Garbers, C.; Ludwig, A.; Düsterhöft, S. The collectrin-like part of the SARS-CoV-1 and -2 receptor ACE2 is shed by the metalloproteinases ADAM10 and ADAM17. FASEB J. 2022, 36, e22234. [Google Scholar] [CrossRef]
- Lambert, D.W.; Yarski, M.; Warner, F.J.; Thornhill, P.; Parkin, E.T.; Smith, A.I.; Hooper, N.M.; Turner, A.J. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2). J. Biol. Chem. 2005, 280, 30113–33019. [Google Scholar] [CrossRef]
- Hayashi, T.; Abiko, K.; Mandai, M.; Yaegashi, N.; Konishi, I. Highly conserved binding region of ACE2 as a receptor for SARS-CoV-2 between humans and mammals. Vet. Q. 2020, 40, 243–249. [Google Scholar] [CrossRef]
- Heurich, A.; Hofmann-Winkler, H.; Gierer, S.; Liepold, T.; Jahn, O.; Pöhlmann, S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J. Virol. 2014, 88, 1293–1307. [Google Scholar] [CrossRef]
- García-Ayllón, M.S.; Moreno-Pérez, O.; García-Arriaza, J.; Ramos-Rincón, J.M.; Cortés-Gómez, M.Á.; Brinkmalm, G.; Andrés, M.; León-Ramírez, J.M.; Boix, V.; Gil, J.; et al. Plasma ACE2 species are differentially altered in COVID-19 patients. FASEB J. 2021, 35, e21745. [Google Scholar] [CrossRef]
- Lennol, M.P.; García-Ayllón, M.S.; Avilés-Granados, C.; Trasciatti, C.; Tolassi, C.; Quaresima, V.; Arici, D.; Cristillo, V.; Volonghi, I.; Caprioli, F.; et al. Increased cerebrospinal fluid ACE2 fragments as a read-out of brain infection in COVID-19 encephalopathy patients. J. Infect. Dis. 2025, 26, jiaf093. [Google Scholar]
- Lennol, M.P.; García-Ayllón, M.S.; Esteban, M.; García-Arriaza, J.; Sáez-Valero, J. Serum angiotensin-converting enzyme 2 as a potential biomarker for SARS-CoV-2 infection and vaccine efficacy. Front. Immunol. 2022, 13, 1001951. [Google Scholar] [CrossRef]
- Zang, R.; Castro, M.F.G.; McCune, B.T.; Zeng, Q.; Rothlauf, P.W.; Sonnek, N.M.; Liu, Z.; Brulois, K.F.; Wang, X.; Greenberg, H.B.; et al. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci. Immunol. 2020, 5, eabc3582. [Google Scholar] [CrossRef]
- Afar, D.E.; Vivanco, I.; Hubert, R.S.; Kuo, J.; Chen, E.; Saffran, D.C.; Raitano, A.B.; Jakobovits, A. Catalytic cleavage of the androgen-regulated TMPRSS2 protease results in its secretion by prostate and prostate cancer epithelia. Cancer Res. 2001, 61, 1686–1692. [Google Scholar]
- Chen, Y.W.; Lee, M.S.; Lucht, A.; Chou, F.P.; Huang, W.; Havighurst, T.C.; Kim, K.; Wang, J.K.; Antalis, T.M.; Johnson, M.D.; et al. TMPRSS2, a serine protease expressed in the prostate on the apical surface of luminal epithelial cells and released into semen in prostasomes, is misregulated in prostate cancer cells. Am. J. Pathol. 2010, 176, 2986–2996. [Google Scholar] [CrossRef]
- Wettstein, L.; Kirchhoff, F.; Münch, J. The Transmembrane Protease TMPRSS2 as a Therapeutic Target for COVID-19 Treatment. Int. J. Mol. Sci. 2022, 23, 1351. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Leyva, J.; Lennol, M.P.; Avilés-Granados, C.; García-Ayllón, M.S.; Gutiérrez, A.; Francés, R.; Sáez-Valero, J. Altered plasma levels of the SARS-CoV-2-related proteins ACE2 and TMPRSS2 in patients with Crohn′s disease. Sci. Rep. 2024, 14, 30346. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, U.O.; Kintscher, U. ACE2 and SARS-CoV-2: Tissue or Plasma, Good or Bad? Am. J. Hypertens. 2021, 34, 274–277. [Google Scholar] [CrossRef]
- Beyerstedt, S.; Casaro, E.B.; Rangel, É.B. COVID-19: Angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Fariñas, M.; Tokuyama, M.; Wei, G.; Huang, R.; Livanos, A.; Jha, D.; Levescot, A.; Irizar, H.; Kosoy, R.; Cording, S.; et al. Intestinal Inflammation Modulates the Expression of ACE2 and TMPRSS2 and Potentially Overlaps With the Pathogenesis of SARS-CoV-2-related Disease. Gastroenterology 2021, 160, 287–301.e20. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.K.; Lindstrøm, J.C.; Kalla, R.; Ricanek, P.; Halfvarson, J.; Satsangi, J. Age, Inflammation, and Disease Location Are Critical Determinants of Intestinal Expression of SARS-CoV-2 Receptor ACE2 and TMPRSS2 in Inflammatory Bowel Disease. Gastroenterology 2020, 159, 1151–1154.e2. [Google Scholar] [CrossRef]
- Vickers, C.; Hales, P.; Kaushik, V.; Dick, L.; Gavin, J.; Tang, J.; Godbout, K.; Parsons, T.; Baronas, E.; Hsieh, F.; et al. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J. Biol. Chem. 2002, 277, 14838–14843. [Google Scholar] [CrossRef]
- Ferreira-Duarte, M.; Estevinho, M.M.; Duarte-Araújo, M.; Magro, F.; Morato, M. Unraveling the Role of ACE2, the Binding Receptor for SARS-CoV-2, in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2020, 26, 1787–1795. [Google Scholar] [CrossRef]
- La Rosa, P.; Tiberi, J.; Palermo, E.; Stefanelli, R.; Tiano, S.M.L.; Canterini, S.; Cortese, M.; Hiscott, J.; Fiorenza, M.T. The inactivation of the Niemann Pick C1 cholesterol transporter restricts SARS-CoV-2 entry into host cells by decreasing ACE2 abundance at the plasma membrane. Cell Biosci. 2024, 14, 148. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, D.X.; Tam, J.P. Lipid rafts are involved in SARS-CoV entry into Vero E6 cells. Biochem. Biophys. Res. Commun. 2008, 369, 344–349. [Google Scholar] [CrossRef]
- Lalioti, V.; González-Sanz, S.; Lois-Bermejo, I.; González-Jiménez, P.; Viedma-Poyatos, Á.; Merino, A.; Pajares, M.A.; Pérez-Sala, D. Cell surface detection of vimentin, ACE2 and SARS-CoV-2 Spike proteins reveals selective colocalization at primary cilia. Sci. Rep. 2022, 12, 7063. [Google Scholar] [CrossRef]
- Bolland, W.; Marechal, I.; Petiot, C.; Porrot, F.; Guivel-Benhassine, F.; Brelot, A.; Casartelli, N.; Schwartz, O.; Buchrieser, J. SARS-CoV-2 entry and fusion are independent of ACE2 localization to lipid rafts. J. Virol. 2025, 99, e0182324. [Google Scholar] [CrossRef]
- Hamamoto, Y.; Kawamura, M.; Uchida, H.; Hiramatsu, K.; Katori, C.; Asai, H.; Egawa, S.; Yoshida, K. Increased ACE2 and TMPRSS2 expression in ulcerative colitis. Pathol. Res. Pract. 2024, 254, 155108. [Google Scholar] [CrossRef]
- McAllister, M.J.; Kirkwood, K.; Chuah, S.C.; Thompson, E.J.; Cartwright, J.A.; Russell, C.D.; Dorward, D.A.; Lucas, C.D.; Ho, G.T. Intestinal Protein Characterisation of SARS-CoV-2 Entry Molecules ACE2 and TMPRSS2 in Inflammatory Bowel Disease (IBD) and Fatal COVID-19 Infection. Inflammation 2022, 45, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Patankar, J.V.; Chiriac, M.T.; Lehmann, M.; Kühl, A.A.; Atreya, R.; Becker, C.; Gonzalez-Acera, M.; Schmitt, H.; Gamez-Belmonte, R.; Mahapatro, M.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 Attachment Receptor Angiotensin-Converting Enzyme 2 Is Decreased in Crohn’s Disease and Regulated By Microbial and Inflammatory Signaling. Gastroenterology 2021, 160, 925–928.e4. [Google Scholar] [CrossRef]
- Stocker, N.; Radzikowska, U.; Wawrzyniak, P.; Tan, G.; Huang, M.; Ding, M.; Akdis, C.A.; Sokolowska, M. Regulation of angiotensin-converting enzyme 2 isoforms by type 2 inflammation and viral infection in human airway epithelium. Mucosal Immunol. 2023, 16, 5–16. [Google Scholar] [CrossRef]
- Potdar, A.A.; Dube, S.; Naito, T.; Li, K.; Botwin, G.; Haritunians, T.; Li, D.; Casero, D.; Yang, S.; Bilsborough, J.; et al. Altered Intestinal ACE2 Levels Are Associated With Inflammation, Severe Disease, and Response to Anti-Cytokine Therapy in Inflammatory Bowel Disease. Gastroenterology 2021, 160, 809–822.e7. [Google Scholar] [CrossRef] [PubMed]
- Verstockt, B.; Verstockt, S.; Rahiman, S.A.; Ke, B.J.; Arnauts, K.; Cleynen, I.; Sabino, J.; Ferrante, M.; Matteoli, G.; Vermeire, S. Intestinal Receptor of SARS-CoV-2 in Inflamed IBD Tissue Seems Downregulated by HNF4A in Ileum and Upregulated by Interferon Regulating Factors in Colon. J. Crohn′s Colitis 2021, 15, 485–498. [Google Scholar] [CrossRef]
- Li, X.Z.; Qiu, Y.; Jeffery, L.; Liu, F.; Feng, R.; He, J.S.; Tan, J.Y.; Ye, Z.Y.; Lin, S.N.; Ghosh, S.; et al. Down-Regulation of Colonic ACE2 Expression in Patients With Inflammatory Bowel Disease Responding to Anti-TNF Therapy: Implications for COVID-19. Front. Med. 2021, 7, 613475. [Google Scholar] [CrossRef]
- Toyonaga, T.; Araba, K.C.; Kennedy, M.M.; Keith, B.P.; Wolber, E.A.; Beasley, C.; Steinbach, E.C.; Schaner, M.R.; Jain, A.; Long, M.D.; et al. Increased colonic expression of ACE2 associates with poor prognosis in Crohn’s disease. Sci. Rep. 2021, 11, 13533. [Google Scholar] [CrossRef]
- Pisani, L.F.; Petroni, G.A.; Crespi, G.; Mola, S.; Annunziata, M.L.; Caprioli, F.A.; Porta, C.; Pastorelli, L. Lower Expression of SARS-CoV-2 Host Cell Entry Genes in the Intestinal Mucosa of IBD Patients with Quiescent or Mildly Active Disease. Inflamm. Bowel Dis. 2025, 31, 1690–1701. [Google Scholar] [CrossRef]
- Burgueño, J.F.; Reich, A.; Hazime, H.; Quintero, M.A.; Fernandez, I.; Fritsch, J.; Santander, A.M.; Brito, N.; Damas, O.M.; Deshpande, A.; et al. Expression of SARS-CoV-2 Entry Molecules ACE2 and TMPRSS2 in the Gut of Patients with IBD. Inflamm. Bowel Dis. 2020, 26, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.; Shan, G.; Sun, Z.; Zhang, F.; Xu, C.; Lou, X.; Li, S.; Du, H.; Chen, H.; Xu, G. Quantitative Proteomic Analysis Reveals the Deregulation of Nicotinamide Adenine Dinucleotide Metabolism and CD38 in Inflammatory Bowel Disease. Biomed Res. Int. 2019, 2019, 3950628. [Google Scholar] [CrossRef]
- Park, J.; Jeong, D.; Chung, Y.W.; Kim, D.H.; Cheon, J.H.; Ryu, J.H. Quantitative Proteomic Analysis of the Expression of SARS-CoV-2 Receptors in the Gut of Patients with Chronic Enterocolitis. Yonsei Med. J. 2020, 61, 891–894. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Burrell, L.M.; Velkoska, E.; Griggs, K.; Angus, P.W.; Gibson, P.R.; Lubel, J.S. Upregulation of circulating components of the alternative renin-angiotensin system in inflammatory bowel disease: A pilot study. J. Renin Angiotensin Aldosterone Syst. 2015, 16, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Adamopoulos, P.G.; Bartzoka, N.; Tsiakanikas, P.; Scorilas, A. Characterization of novel ACE2 mRNA transcripts: The potential role of alternative splicing in SARS-CoV-2 infection. Gene 2025, 936, 149092. [Google Scholar] [CrossRef]
- Blaydon, D.C.; Biancheri, P.; Di, W.L.; Plagnol, V.; Cabral, R.M.; Brooke, M.A.; van Heel, D.A.; Ruschendorf, F.; Toynbee, M.; Walne, A.; et al. Inflammatory skin and bowel disease linked to ADAM17 deletion. N. Engl. J. Med. 2011, 365, 1502–1508. [Google Scholar] [CrossRef]
- Imoto, I.; Saito, M.; Suga, K.; Kohmoto, T.; Otsu, M.; Horiuchi, K.; Nakayama, H.; Higashiyama, S.; Sugimoto, M.; Sasaki, A.; et al. Functionally confirmed compound heterozygous ADAM17 missense loss-of-function variants cause neonatal inflammatory skin and bowel disease 1. Sci. Rep. 2021, 11, 9552. [Google Scholar] [CrossRef]
- Kirkegaard, T.; Pedersen, G.; Saermark, T.; Brynskov, J. Tumour necrosis factor-alpha converting enzyme (TACE) activity in human colonic epithelial cells. Clin. Exp. Immunol. 2004, 135, 146–153. [Google Scholar] [CrossRef]
- Brynskov, J.; Foegh, P.; Pedersen, G.; Ellervik, C.; Kirkegaard, T.; Bingham, A.; Saermark, T. Tumour necrosis factor alpha converting enzyme (TACE) activity in the colonic mucosa of patients with inflammatory bowel disease. Gut 2002, 51, 37–43. [Google Scholar] [CrossRef]
- Lykowska-Szuber, L.; Walczak, M.; Skrzypczak-Zielinska, M.; Suszynska-Zajczyk, J.; Stawczyk-Eder, K.; Waszak, K.; Eder, P.; Wozniak, A.; Krela-Kazmierczak, I.; Slomski, R.; et al. Effect of Anti-TNF Therapy on Mucosal Apoptosis Genes Expression in Crohn’s Disease. Front. Immunol. 2021, 12, 615539. [Google Scholar] [CrossRef]
- Franzè, E.; Caruso, R.; Stolfi, C.; Sarra, M.; Cupi, M.L.; Ascolani, M.; Sedda, S.; Antenucci, C.; Ruffa, A.; Caprioli, F.; et al. High expression of the “A Disintegrin And Metalloprotease” 19 (ADAM19), a sheddase for TNF-α in the mucosa of patients with inflammatory bowel diseases. Inflamm. Bowel Dis. 2013, 19, 501–511. [Google Scholar] [CrossRef]
- Robilotti, E.V.; Babady, N.E.; Mead, P.A.; Rolling, T.; Perez-Johnston, R.; Bernardes, M.; Bogler, Y.; Caldararo, M.; Figueroa, C.J.; Glickman, M.S.; et al. Determinants of COVID-19 disease severity in patients with cancer. Nat. Med. 2020, 26, 1218–1223. [Google Scholar] [CrossRef] [PubMed]
- Conley, T.E.; Probert, C.; Subramanian, S. Prevalence of COVID-19 Symptoms Among Inflammatory Bowel Disease Patients Treated with Biological Agents. J. Crohn’s Colitis 2020, 14, 1794–1795. [Google Scholar] [CrossRef]
- Valenzuela, R.; Pedrosa, M.A.; Garrido-Gil, P.; Labandeira, C.M.; Navarro, G.; Franco, R.; Rodriguez-Perez, A.I.; Labandeira-Garcia, J.L. Interactions between ibuprofen, ACE2, renin-angiotensin system, and spike protein in the lung. Implications for COVID-19. Clin. Transl. Med. 2021, 11, e371. [Google Scholar] [CrossRef]
- Bossa, F.; Carparelli, S.; Latiano, A.; Palmieri, O.; Tavano, F.; Panza, A.; Pastore, M.; Marseglia, A.; D’Altilia, M.; Latiano, T.; et al. Impact of the COVID-19 outbreak and the serum prevalence of SARS-CoV-2 antibodies in patients with inflammatory bowel disease treated with biologic drugs. Dig. Liver Dis. 2021, 53, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. COVID-19 and immunomodulation in IBD. Gut 2020, 69, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Fanizzi, F.; D’Amico, F.; Peyrin-Biroulet, L.; Danese, S.; Dignass, A. Treatment targets in IBD: Is it time for new strategies? Best Pract. Res. Clin. Gastroenterol. 2025, 77, 101990. [Google Scholar] [CrossRef]
- De Toma, I.; Dierssen, M. Network analysis of Down syndrome and SARS-CoV-2 identifies risk and protective factors for COVID-19. Sci. Rep. 2021, 11, 1930. [Google Scholar] [CrossRef] [PubMed]
- Souto-Rodríguez, R.; Barreiro-de-Acosta, M.; Domínguez-Muñoz, J.E. Down’s syndrome and inflammatory bowel disease: Is there a real link? Rev. Esp. Enferm. Dig. 2014, 106, 220–222. [Google Scholar]
- Ravel, A.; Mircher, C.; Rebillat, A.S.; Cieuta-Walti, C.; Megarbane, A. Feeding problems and gastrointestinal diseases in Down syndrome. Arch. Pediatr. 2020, 27, 53–60. [Google Scholar] [CrossRef]
- Cianfarini, C.; Hassler, L.; Wysocki, J.; Hassan, A.; Nicolaescu, V.; Elli, D.; Gula, H.; Ibrahim, A.M.; Randall, G.; Henkin, J.; et al. Soluble Angiotensin-Converting Enzyme 2 Protein Improves Survival and Lowers Viral Titers in Lethal Mouse Model of Severe Acute Respiratory Syndrome Coronavirus Type 2 Infection with the Delta Variant. Cells 2024, 13, 203. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, S.; Popowski, K.D.; Liu, S.; Zhu, D.; Mei, X.; Li, J.; Hu, Y.; Dinh, P.C.; Wang, X.; et al. Inhalation of ACE2-expressing lung exosomes provides prophylactic protection against SARS-CoV-2. Nat. Commun. 2024, 15, 2236. [Google Scholar] [CrossRef]
- Seccia, T.M.; Shagjaa, T.; Morpurgo, M.; Caroccia, B.; Sanga, V.; Faoro, S.; Venturini, F.; Iadicicco, G.; Lococo, S.; Mazzitelli, M.; et al. Randomized Clinical Trial Of Nafamostat Mesylate; A Potent Transmembrane Protease Serine 2 (TMPRSS2) Inhibitor, in Patients with COVID-19 Pneumonia. J. Clin. Med. 2023, 12, 6618. [Google Scholar] [CrossRef] [PubMed]
- Boon, A.C.M.; Bricker, T.L.; Fritch, E.J.; Leist, S.R.; Gully, K.; Baric, R.S.; Graham, R.L.; Troan, B.V.; Mahoney, M.; Janetka, J.W. Efficacy of host cell serine protease inhibitor MM3122 against SARS-CoV-2 for treatment and prevention of COVID-19. J. Virol. 2024, 98, e0190323. [Google Scholar] [CrossRef] [PubMed]
- Kintscher, U.; Slagman, A.; Domenig, O.; Röhle, R.; Konietschke, F.; Poglitsch, M.; Möckel, M. Plasma Angiotensin Peptide Profiling and ACE (Angiotensin-Converting Enzyme)-2 Activity in COVID-19 Patients Treated with Pharmacological Blockers of the Renin-Angiotensin System. Hypertension 2020, 76, e34–e36. [Google Scholar] [CrossRef] [PubMed]
- Shahrokh, S.; Ghavami, S.B.; Aghdaei, H.A.; Parigi, T.L.; Farmani, M.; Danese, S.; Daryani, N.E.; Vossoughinia, H.; Balaii, H.; Alborzi, F.; et al. High prevalence of SARS-Coronavirus-2 in patients with inflammatory bowel disease and the role of soluble angiotensin converting Enzyme2. Arch. Physiol. Biochem. 2024, 130, 325–332. [Google Scholar] [CrossRef]
 , residues 255–256) to acquire proteolytic activity. The cleaved protease domain either remains linked to the prodomain via an interdomain disulfide bond or is shed, resulting in a membrane-bound fragment or a cell-free fragment. (B) Representative blot of human plasma samples incubated with an anti-TMPRSS2 ectodomain 14437-1-AP antibody (Proteintech) showing the TMPRSS2 soluble full-length form and both fragments, containing the peptidase domain and the N-terminal prodomain (unpublished blot).
, residues 255–256) to acquire proteolytic activity. The cleaved protease domain either remains linked to the prodomain via an interdomain disulfide bond or is shed, resulting in a membrane-bound fragment or a cell-free fragment. (B) Representative blot of human plasma samples incubated with an anti-TMPRSS2 ectodomain 14437-1-AP antibody (Proteintech) showing the TMPRSS2 soluble full-length form and both fragments, containing the peptidase domain and the N-terminal prodomain (unpublished blot).
 , residues 255–256) to acquire proteolytic activity. The cleaved protease domain either remains linked to the prodomain via an interdomain disulfide bond or is shed, resulting in a membrane-bound fragment or a cell-free fragment. (B) Representative blot of human plasma samples incubated with an anti-TMPRSS2 ectodomain 14437-1-AP antibody (Proteintech) showing the TMPRSS2 soluble full-length form and both fragments, containing the peptidase domain and the N-terminal prodomain (unpublished blot).
, residues 255–256) to acquire proteolytic activity. The cleaved protease domain either remains linked to the prodomain via an interdomain disulfide bond or is shed, resulting in a membrane-bound fragment or a cell-free fragment. (B) Representative blot of human plasma samples incubated with an anti-TMPRSS2 ectodomain 14437-1-AP antibody (Proteintech) showing the TMPRSS2 soluble full-length form and both fragments, containing the peptidase domain and the N-terminal prodomain (unpublished blot).
 ) in the plasma of CD patients, as did the TMPRSS2 prodomain fragment, although both changes were unrelated with vulnerability to SARS-CoV2. For details see [59].
) in the plasma of CD patients, as did the TMPRSS2 prodomain fragment, although both changes were unrelated with vulnerability to SARS-CoV2. For details see [59].
 ) in the plasma of CD patients, as did the TMPRSS2 prodomain fragment, although both changes were unrelated with vulnerability to SARS-CoV2. For details see [59].
) in the plasma of CD patients, as did the TMPRSS2 prodomain fragment, although both changes were unrelated with vulnerability to SARS-CoV2. For details see [59].
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sáez-Leyva, J.; Lennol, M.P.; Avilés-Granados, C.; García-Ayllón, M.-S.; Sáez-Valero, J. Risk for COVID-19 Vulnerability in Patients with Inflammatory Bowel Disease: Assessing Alterations in ACE2 and TMPRSS2. Biomedicines 2025, 13, 2240. https://doi.org/10.3390/biomedicines13092240
Sáez-Leyva J, Lennol MP, Avilés-Granados C, García-Ayllón M-S, Sáez-Valero J. Risk for COVID-19 Vulnerability in Patients with Inflammatory Bowel Disease: Assessing Alterations in ACE2 and TMPRSS2. Biomedicines. 2025; 13(9):2240. https://doi.org/10.3390/biomedicines13092240
Chicago/Turabian StyleSáez-Leyva, Jorge, Matthew P. Lennol, Carlos Avilés-Granados, María-Salud García-Ayllón, and Javier Sáez-Valero. 2025. "Risk for COVID-19 Vulnerability in Patients with Inflammatory Bowel Disease: Assessing Alterations in ACE2 and TMPRSS2" Biomedicines 13, no. 9: 2240. https://doi.org/10.3390/biomedicines13092240
APA StyleSáez-Leyva, J., Lennol, M. P., Avilés-Granados, C., García-Ayllón, M.-S., & Sáez-Valero, J. (2025). Risk for COVID-19 Vulnerability in Patients with Inflammatory Bowel Disease: Assessing Alterations in ACE2 and TMPRSS2. Biomedicines, 13(9), 2240. https://doi.org/10.3390/biomedicines13092240





