Association of LPCAT1*rs9728 Variant with Reduced Susceptibility to Neonatal Respiratory Distress Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Considerations
2.2. Study Participants and Eligibility Criteria
2.3. Clinical Signs of NRDS
2.4. Definition of Study Variables
2.4.1. Maternal and Obstetric Characteristics
2.4.2. Neonatal Characteristics of Preterm RDS
2.5. Selection of the LPCAT1 Genetic Variant
2.6. Allelic Discrimination Analysis for LPCAT1*rs9728 Variant
2.7. Statistical Analysis
3. Results
3.1. Maternal and Obstetric Characteristics of the Studied Participants
3.2. Preterm Neonatal Characteristics of the Studied Participants
3.3. Basic Characteristics of NRDS Classes Stratified by Prematurity
3.4. RDS Preterm Neonatal Outcomes Based on Gestational Age Classes
3.5. Analysis of LPCAT1 (rs9728; c.*1668T>C) Variant
3.6. Impact of LPCAT1 (rs9728; c.*1668T>C) Variant with Susceptibility to NRDS
3.7. Implication of the LPCAT1 Variant with Demographic and Clinical Data Among RDS Neonates
3.8. Effect of the LPCAT1 Variant on Neonatal Outcomes Among RDS Neonates
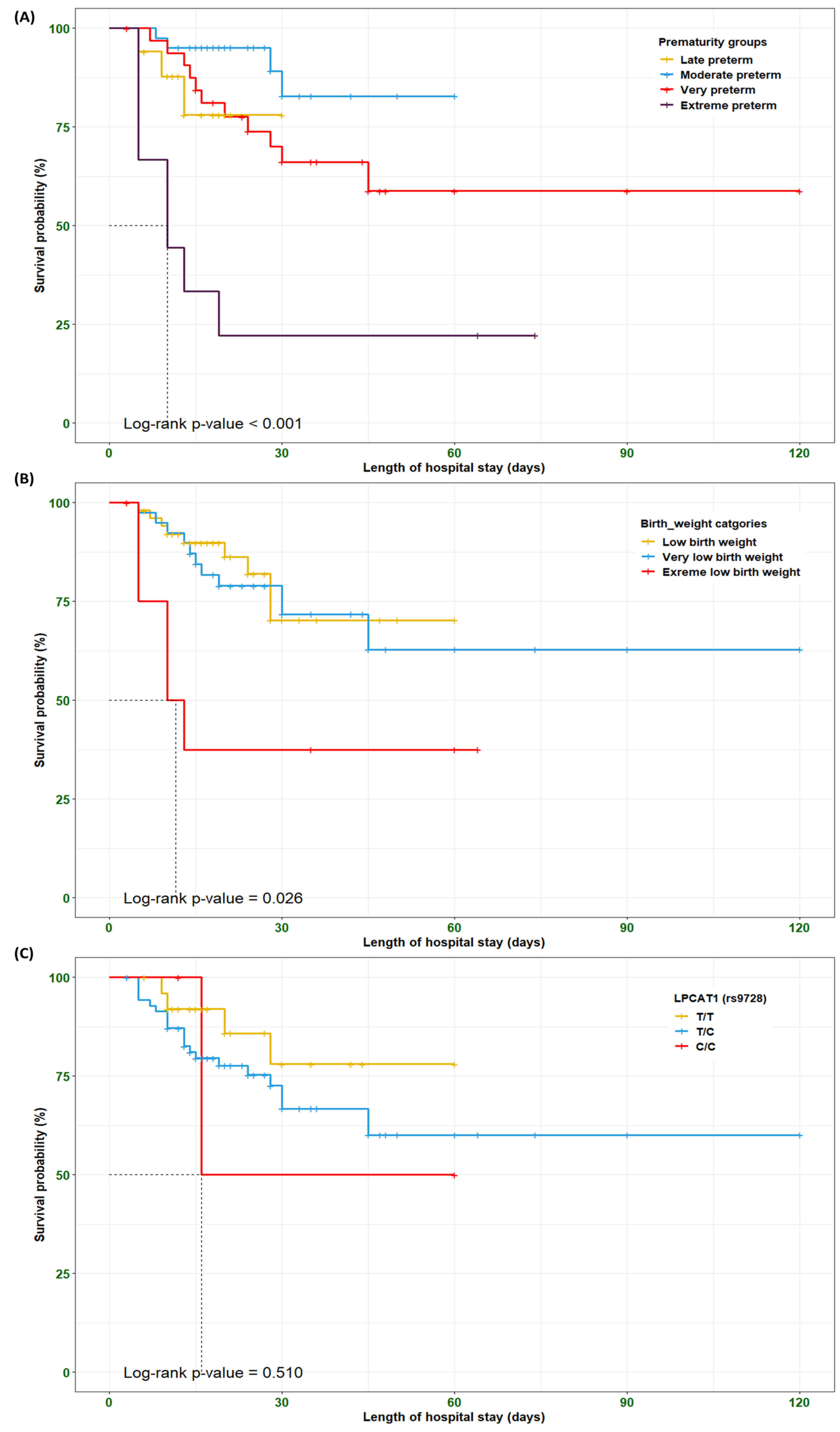
3.9. Survival Analysis Among the RDS Preterm Neonates
3.10. In Silico Data Analysis for the LPCAT1 (rs9728; c.*1668T>C) Variant
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LPCAT1 | Lysophosphatidylcholine acyltransferase 1 |
| NRDS | Neonatal Respiratory Distress Syndrome |
| LCAT | Lecithin–cholesterol acyltransferase |
| DPPC | Dipalmitoyl-phosphatidylcholine |
References
- Fang, K.; Yue, S.; Wang, S.; Wang, M.; Yu, X.; Ding, Y.; Lv, M.; Liu, Y.; Cao, C.; Liao, Z. The association between sex and neonatal respiratory distress syndrome. BMC Pediatr. 2024, 24, 129. [Google Scholar] [CrossRef]
- Sweet, L.R.; Keech, C.; Klein, N.P.; Marshall, H.S.; Tagbo, B.N.; Quine, D.; Kaur, P.; Tikhonov, I.; Nisar, M.I.; Kochhar, S.; et al. Respiratory distress in the neonate: Case definition & guidelines for data collection, analysis, and presentation of maternal immunization safety data. Vaccine 2017, 35, 6506–6517. [Google Scholar] [CrossRef]
- Tochie, J.N.; Sibetcheu, A.T.; Arrey-Ebot, P.E.; Choukem, S.P. Global, Regional and National Trends in the Burden of Neonatal Respiratory Failure and essentials of its diagnosis and management from 1992 to 2022: A scoping review. Eur. J. Pediatr. 2024, 183, 9–50. [Google Scholar] [CrossRef]
- Marinonio, A.S.S.; Costa-Nobre, D.T.; Miyoshi, M.H.; Balda, R.d.C.X.; Areco, K.C.N.; Konstantyner, T.; Kawakami, M.D.; Sanudo, A.; Bandiera-Paiva, P.; de Freitas, R.M.V.; et al. Clusters of preterm live births and respiratory distress syndrome-associated neonatal deaths: Spatial distribution and cooccurrence patterns. BMC Public Health 2022, 22, 1226. [Google Scholar] [CrossRef]
- Bulimba, M.; Cosmas, J.; Abdallah, Y.; Massawe, A.; Manji, K. Early outcomes of preterm neonates with respiratory distress syndrome admitted at Muhimbili National Hospital, a prospective study. BMC Pediatr. 2022, 22, 731. [Google Scholar] [CrossRef] [PubMed]
- De Luca, D. Respiratory distress syndrome in preterm neonates in the era of precision medicine: A modern critical care-based approach. Pediatr. Neonatol. 2021, 62 (Suppl. S1), S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Baseer, K.A.A.; Mohamed, M.; Abd-Elmawgood, E.A. Risk Factors of Respiratory Diseases Among Neonates in Neonatal Intensive Care Unit of Qena University Hospital, Egypt. Ann. Glob. Health 2020, 86, 22. [Google Scholar] [CrossRef] [PubMed]
- Elkabany, Z.A.; El-Farrash, R.A.; Shinkar, D.M.; Ismail, E.A.; Nada, A.S.; Farag, A.S.; Elsayed, M.A.; Salama, D.H.; Macken, E.L.; Gaballah, S.A. Oxidative stress markers in neonatal respiratory distress syndrome: Advanced oxidation protein products and 8-hydroxy-2-deoxyguanosine in relation to disease severity. Pediatr. Res. 2020, 87, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Sweet, D.G.; Carnielli, V.; Greisen, G.; Hallman, M.; Ozek, E.; Te Pas, A.; Plavka, R.; Roehr, C.C.; Saugstad, O.D.; Simeoni, U.; et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome—2019 Update. Neonatology 2019, 115, 432–450. [Google Scholar] [CrossRef]
- Whitsett, J.A.; Alenghat, T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat. Immunol. 2015, 16, 27–35. [Google Scholar] [CrossRef]
- Ariki, S.; Nishitani, C.; Kuroki, Y. Diverse functions of pulmonary collectins in host defense of the lung. J. Biomed. Biotechnol. 2012, 2012, 532071. [Google Scholar] [CrossRef]
- Whitsett, J.A.; Wert, S.E.; Weaver, T.E. Alveolar Surfactant Homeostasis and the Pathogenesis of Pulmonary Disease. Annu. Rev. Med. 2010, 61, 105–119. [Google Scholar] [CrossRef]
- Reuter, S.; Moser, C.; Baack, M. Respiratory distress in the newborn. Pediatr. Rev. 2014, 35, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, W. Lung surfactant: Function and composition in the context of development and respiratory physiology. Ann. Anat.-Anat. Anz. 2016, 208, 146–150. [Google Scholar] [CrossRef]
- Dushianthan, A.; Goss, V.; Cusack, R.; Grocott, M.P.; Postle, A.D. Phospholipid composition and kinetics in different endobronchial fractions from healthy volunteers. BMC Pulm. Med. 2014, 14, 10. [Google Scholar] [CrossRef]
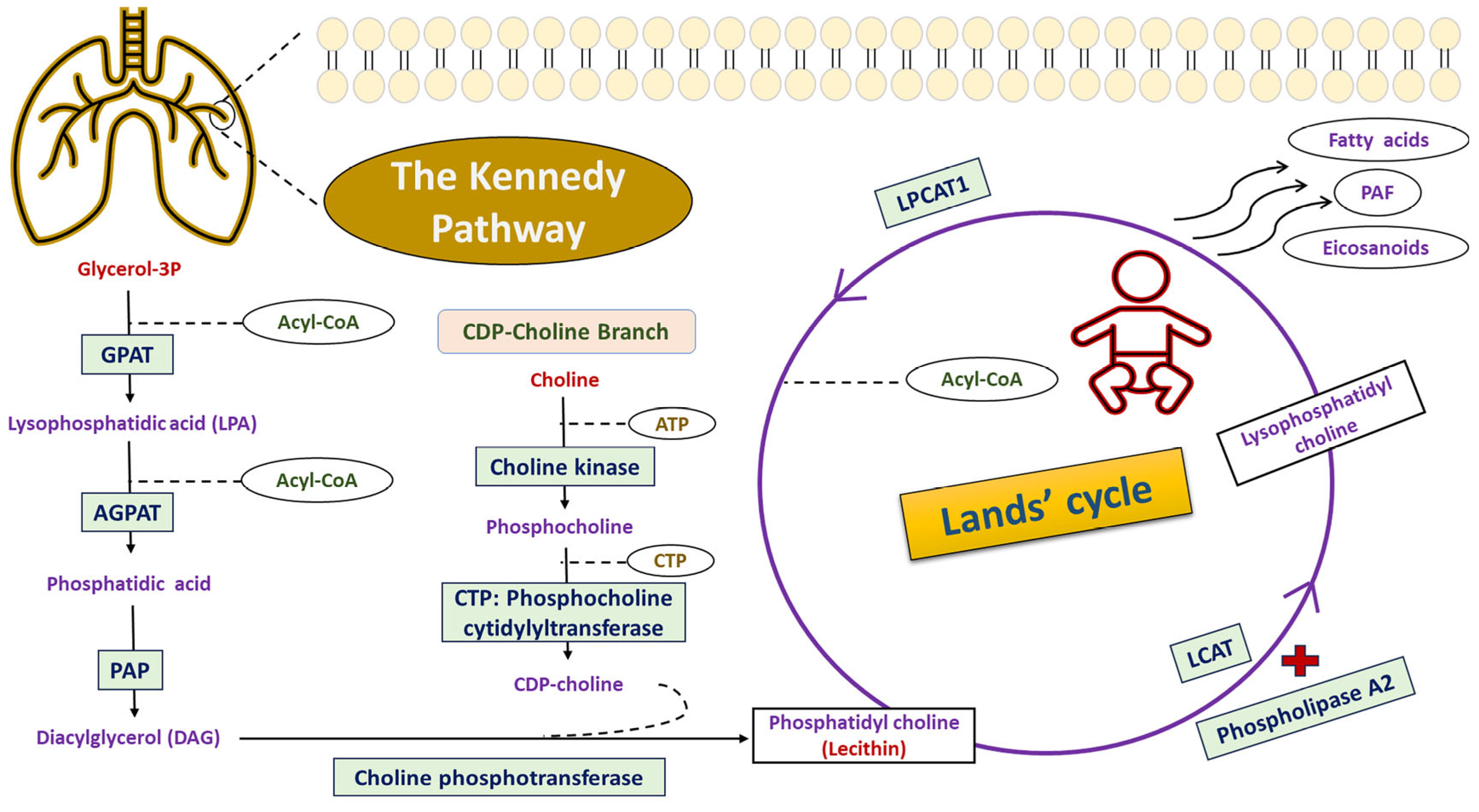
- Moessinger, C.; Klizaite, K.; Steinhagen, A.; Philippou-Massier, J.; Shevchenko, A.; Hoch, M.; Ejsing, C.S.; Thiele, C. Two different pathways of phosphatidylcholine synthesis, the Kennedy Pathway and the Lands Cycle, differentially regulate cellular triacylglycerol storage. BMC Cell Biol. 2014, 15, 43. [Google Scholar] [CrossRef] [PubMed]
- Shindou, H.; Shimizu, T. Acyl-CoA:Lysophospholipid Acyltransferases. J. Biol. Chem. 2009, 284, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Shimizu, T. Platelet-activating factor (PAF) receptor and genetically engineered PAF receptor mutant mice. Prog. Lipid Res. 2000, 39, 41–82. [Google Scholar] [CrossRef]
- Kennedy, E.P.; Weiss, S.B. The function of cytidine coenzymes in the biosynthesis of phospholipides. J. Biol. Chem. 1956, 222, 193–214. [Google Scholar] [CrossRef]
- Yu, J.; Loh, K.; Song, Z.-y.; Yang, H.-q.; Zhang, Y.; Lin, S. Update on glycerol-3-phosphate acyltransferases: The roles in the development of insulin resistance. Nutr. Diabetes 2018, 8, 34. [Google Scholar] [CrossRef]
- Takeuchi, K.; Reue, K. Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1195–E1209. [Google Scholar] [CrossRef] [PubMed]
- Carman, G.M.; Han, G.S. Phosphatidic acid phosphatase, a key enzyme in the regulation of lipid synthesis. J. Biol. Chem. 2009, 284, 2593–2597. [Google Scholar] [CrossRef]
- Fagone, P.; Jackowski, S. Phosphatidylcholine and the CDP-choline cycle. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2013, 1831, 523–532. [Google Scholar] [CrossRef]
- Gibellini, F.; Smith, T.K. The Kennedy pathway—De novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB Life 2010, 62, 414–428. [Google Scholar] [CrossRef]
- Chen, Z.X.; Liang, L.; Huang, H.Q.; Li, J.D.; He, R.Q.; Huang, Z.G.; Song, R.; Chen, G.; Li, J.J.; Cai, Z.W.; et al. LPCAT1 enhances the invasion and migration in gastric cancer: Based on computational biology methods and in vitro experiments. Cancer Med. 2023, 12, 13438–13454. [Google Scholar] [CrossRef] [PubMed]
- Abdelzaher, E.; Mostafa, M.F. Lysophosphatidylcholine acyltransferase 1 (LPCAT1) upregulation in breast carcinoma contributes to tumor progression and predicts early tumor recurrence. Tumour Biol. 2015, 36, 5473–5483. [Google Scholar] [CrossRef] [PubMed]
- Rousset, X.; Shamburek, R.; Vaisman, B.; Amar, M.; Remaley, A.T. Lecithin cholesterol acyltransferase: An anti- or pro-atherogenic factor? Curr. Atheroscler. Rep. 2011, 13, 249–256. [Google Scholar] [CrossRef]
- O’Donnell, V.B. New appreciation for an old pathway: The Lands Cycle moves into new arenas in health and disease. Biochem. Soc. Trans. 2022, 50, 1–11. [Google Scholar] [CrossRef]
- Shida-Sakazume, T.; Endo-Sakamoto, Y.; Unozawa, M.; Fukumoto, C.; Shimada, K.; Kasamatsu, A.; Ogawara, K.; Yokoe, H.; Shiiba, M.; Tanzawa, H.; et al. Lysophosphatidylcholine Acyltransferase1 Overexpression Promotes Oral Squamous Cell Carcinoma Progression via Enhanced Biosynthesis of Platelet-Activating Factor. PLoS ONE 2015, 10, e0120143. [Google Scholar] [CrossRef]
- Liu, R.; Yin, C.; Zhao, P.; Guo, B.; Ke, W.; Zheng, X.; Xie, D.; Wang, Y.; Wang, G.; Jia, Y.; et al. Nuclear respiratory factor 1 drives hepatocellular carcinoma progression by activating LPCAT1-ERK1/2-CREB axis. Biol. Direct 2023, 18, 67. [Google Scholar] [CrossRef]
- Zhao, T.; Sun, R.; Ma, X.; Wei, L.; Hou, Y.; Song, K.; Jiang, J. Overexpression of LPCAT1 enhances endometrial cancer stemness and metastasis by changing lipid components and activating the TGF/β-Smad2/3 signaling pathway. Acta Biochim. Biophys. Sin. 2022, 54, 904–916. [Google Scholar] [CrossRef]
- Wei, C.; Dong, X.; Lu, H.; Tong, F.; Chen, L.; Zhang, R.; Dong, J.; Hu, Y.; Wu, G.; Dong, X. LPCAT1 promotes brain metastasis of lung adenocarcinoma by up-regulating PI3K/AKT/MYC pathway. J. Exp. Clin. Cancer Res. 2019, 38, 95. [Google Scholar] [CrossRef]
- Lebok, P.; von Hassel, A.; Meiners, J.; Hube-Magg, C.; Simon, R.; Höflmayer, D.; Hinsch, A.; Dum, D.; Fraune, C.; Göbel, C.; et al. Up-regulation of lysophosphatidylcholine acyltransferase 1 (LPCAT1) is linked to poor prognosis in breast cancer. Aging 2019, 11, 7796–7804. [Google Scholar] [CrossRef]
- Shen, W.; Kuang, P.; Wang, B.; Zeng, Q.; Chen, C.; Lin, X. Genetic Polymorphisms of LPCAT1, CHPT1 and PCYT1B and Risk of Neonatal Respiratory Distress Syndrome among a Chinese Han Population. Pediatr. Neonatol. 2020, 61, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Amer, K.; Soliman, N.A.; Soror, S.; Gad, Y.Z.; Moustafa, A.; Elmonem, M.A.; Amer, M.; Ragheb, A.; Kotb, A.; Taha, T.; et al. Egypt Genome: Towards an African new genomic era. J. Adv. Res. 2025, 71, 415–427. [Google Scholar] [CrossRef]
- Wohlers, I.; Künstner, A.; Munz, M.; Olbrich, M.; Fähnrich, A.; Calonga-Solís, V.; Ma, C.; Hirose, M.; El-Mosallamy, S.; Salama, M.; et al. An integrated personal and population-based Egyptian genome reference. Nat. Commun. 2020, 11, 4719. [Google Scholar] [CrossRef] [PubMed]
- Ekhaguere, O.A.; Okonkwo, I.R.; Batra, M.; Hedstrom, A.B. Respiratory distress syndrome management in resource limited settings-Current evidence and opportunities in 2022. Front. Pediatr. 2022, 10, 961509. [Google Scholar] [CrossRef]
- Pramanik, A.K.; Rangaswamy, N.; Gates, T. Neonatal Respiratory Distress: A Practical Approach to Its Diagnosis and Management. Pediatr. Clin. N. Am. 2015, 62, 453–469. [Google Scholar] [CrossRef]
- Demircubuk, A.G.; Coskun, M.Y.; Demiryurek, S.; Dokuyucu, R.; Oztuzcu, S.; Taviloglu, Z.S.; Arslan, A.; Sivasli, E. Endothelial NOS gene Glu298Asp polymorphism in preterm neonates with respiratory distress syndrome. Pediatr. Pulmonol. 2013, 48, 976–980. [Google Scholar] [CrossRef]
- Souza, R.T.; Costa, M.L.; Mayrink, J.; Feitosa, F.E.; Rocha Filho, E.A.; Leite, D.F.; Vettorazzi, J.; Calderon, I.M.; Sousa, M.H.; Passini, R., Jr.; et al. Perinatal outcomes from preterm and early term births in a multicenter cohort of low risk nulliparous women. Sci. Rep. 2020, 10, 8508. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.S.; Papageorghiou, A.; Culhane, J.; Bhutta, Z.; Goldenberg, R.L.; Gravett, M.; Iams, J.D.; Conde-Agudelo, A.; Waller, S.; Barros, F.; et al. Challenges in defining and classifying the preterm birth syndrome. Am. J. Obstet. Gynecol. 2012, 206, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Ballard, J.L.; Khoury, J.C.; Wedig, K.; Wang, L.; Eilers-Walsman, B.L.; Lipp, R. New Ballard Score, expanded to include extremely premature infants. J. Pediatr. 1991, 119, 417–423. [Google Scholar] [CrossRef]
- Rozycki, H.J.; Yitayew, M. The Apgar score in clinical research: For what, how and by whom it is used. J. Perinat. Med. 2023, 51, 580–585. [Google Scholar] [CrossRef]
- Apgar, V. A proposal for a new method of evaluation of the newborn infant. Curr. Res. Anesth. Analg. 1953, 32, 260–267. [Google Scholar] [CrossRef]
- Hedstrom, A.B.; Gove, N.E.; Mayock, D.E.; Batra, M. Performance of the Silverman Andersen Respiratory Severity Score in predicting PCO2 and respiratory support in newborns: A prospective cohort study. J. Perinatol. 2018, 38, 505–511. [Google Scholar] [CrossRef]
- Karnati, S.; Kollikonda, S.; Abu-Shaweesh, J. Late preterm infants—Changing trends and continuing challenges. Int. J. Pediatr. Adolesc. Med. 2020, 7, 36–44. [Google Scholar] [CrossRef]
- Mohamed, A.S.; Salama, A.F.; Sabaa, M.A.; Toraih, E.; Elshazli, R.M. GEMIN4 Variants: Risk Profiling, Bioinformatics, and Dynamic Simulations Uncover Susceptibility to Bladder Carcinoma. Arch. Med. Res. 2024, 55, 102970. [Google Scholar] [CrossRef]
- Elshazli, R.M.; Kassab, H.M.; Salama, A.F.; Okasha, K.M. Genetic Variants of AGO1*rs595961 and AGO2*rs4961280 with Susceptibility to Bladder Carcinoma. Indian. J. Clin. Biochem. 2024, 40, 576–587. [Google Scholar] [CrossRef]
- Seif Eldin, W.R.; Saad, E.A.; Monier, A.; Elshazli, R.M. Association of TERT (rs2736098 and rs2736100) genetic variants with elevated risk of hepatocellular carcinoma: A retrospective case-control study. Sci. Rep. 2023, 13, 18382. [Google Scholar] [CrossRef] [PubMed]
- Elsalahaty, M.I.; Salama, A.F.; Diab, T.; Ghazy, M.; Toraih, E.; Elshazli, R.M. Unleash Multifunctional Role of miRNA Biogenesis Gene Variants (XPO5*rs34324334 and RAN*rs14035) with Susceptibility to Hepatocellular Carcinoma. J. Pers. Med. 2023, 13, 959. [Google Scholar] [CrossRef] [PubMed]
- Sakran, M.I.; Alalawy, A.I.; Alharbi, A.A.; El-Hefnawy, M.E.; Alzahrani, S.M.; Alfuraydi, A.; Alzuaibr, F.M.; Zidan, N.S.; Elsaid, A.M.; Toraih, E.A.; et al. The blockage signal for PD-L1/CD274 gene variants and their potential impact on lung carcinoma susceptibility. Int. Immunopharmacol. 2023, 125, 111180. [Google Scholar] [CrossRef]
- Toraih, E.A.; Hussein, M.H.; Al Ageeli, E.; Ellaban, M.; Kattan, S.W.; Moroz, K.; Fawzy, M.S.; Kandil, E. Matrix Metalloproteinase 9/microRNA-145 Ratio: Bridging Genomic and Immunological Variabilities in Thyroid Cancer. Biomedicines 2023, 11, 2953. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Ye, D.; Wang, J. Analysis of diagnosing neonatal respiratory distress syndrome with lung ultrasound score. Pak. J. Med. Sci. 2022, 38, 1101–1106. [Google Scholar] [CrossRef]
- Legesse, B.T.; Abera, N.M.; Alemu, T.G.; Atalell, K.A. Incidence and predictors of mortality among neonates with respiratory distress syndrome admitted at West Oromia Referral Hospitals, Ethiopia, 2022. Multi-centred institution based retrospective follow-up study. PLoS ONE 2023, 18, e0289050. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Tontonoz, P. Phospholipid Remodeling in Physiology and Disease. Annu. Rev. Physiol. 2019, 81, 165–188. [Google Scholar] [CrossRef] [PubMed]
- Bridges, J.P.; Ikegami, M.; Brilli, L.L.; Chen, X.; Mason, R.J.; Shannon, J.M. LPCAT1 regulates surfactant phospholipid synthesis and is required for transitioning to air breathing in mice. J. Clin. Investig. 2010, 120, 1736–1748. [Google Scholar] [CrossRef]
- Lin, S.; Ikegami, M.; Moon, C.; Naren, A.P.; Shannon, J.M. Lysophosphatidylcholine Acyltransferase 1 (LPCAT1) Specifically Interacts with Phospholipid Transfer Protein StarD10 to Facilitate Surfactant Phospholipid Trafficking in Alveolar Type II Cells. J. Biol. Chem. 2015, 290, 18559–18574. [Google Scholar] [CrossRef]
- Wilcox, E.H.; Webb, R.F.; Tshering, K.C.; Hughes, M.Y.; Cavé, H.; DiStefano, M.T.; Dziadzio, H.; Garber, K.; Gelb, B.D.; Gripp, K.W.; et al. Updated ACMG/AMP specifications for variant interpretation and gene curations from the ClinGen RASopathy expert panels. Genet. Med. Open 2025, 3, 103430. [Google Scholar] [CrossRef]
- Chakraborty, M.; Kotecha, S. Pulmonary surfactant in newborn infants and children. Breathe 2013, 9, 476–488. [Google Scholar] [CrossRef]
- Aslamzai, M.; Froogh, B.A.; Mukhlis, A.H.; Faizi, O.A.; Sajid, S.A.; Hakimi, Z. Factors associated with respiratory distress syndrome in preterm neonates admitted to a tertiary hospital in Kabul city: A retrospective cross-sectional study. Glob. Pediatr. 2023, 3, 100035. [Google Scholar] [CrossRef]
- Sun, H.; Xu, F.; Xiong, H.; Kang, W.; Bai, Q.; Zhang, Y.; Zhou, C.; Zhuang, F.; Wang, X.; Zhu, C. Characteristics of respiratory distress syndrome in infants of different gestational ages. Lung 2013, 191, 425–433. [Google Scholar] [CrossRef]
- Shen, W.; Du, J.; Wang, B.; Zeng, Q. Analysis of nitric oxide synthase gene polymorphisms in neonatal respiratory distress syndrome among the Chinese Han population. Ital. J. Pediatr. 2014, 40, 27. [Google Scholar] [CrossRef] [PubMed]
- Welch, R.A.; Shaw, M.K.; Welch, K.C. Amniotic fluid LPCAT1 mRNA correlates with the lamellar body count. J. Perinat. Med. 2016, 44, 531–532. [Google Scholar] [CrossRef]
- Welch, R.A.; Recanati, M.A.; Welch, K.C.; Shaw, M.K. Maternal plasma LPCAT 1 mRNA correlates with lamellar body count. J. Perinat. Med. 2018, 46, 429–431. [Google Scholar] [CrossRef]
- Tanosaki, T.; Mikami, Y.; Shindou, H.; Suzuki, T.; Hashidate-Yoshida, T.; Hosoki, K.; Kagawa, S.; Miyata, J.; Kabata, H.; Masaki, K.; et al. Lysophosphatidylcholine Acyltransferase 1 Deficiency Promotes Pulmonary Emphysema via Apoptosis of Alveolar Epithelial Cells. Inflammation 2022, 45, 1765–1779. [Google Scholar] [CrossRef]
- Guo, Y.; Xue, W.; Sun, S.; Chen, X.; Li, H.; Yan, C. Circular RNA circZCCHC6 contributes to tumorigenesis by regulating LPCAT1 via miR-433-3p in non-small cell lung cancer. Clin. Exp. Med. 2022, 22, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Oliver, G.R.; Marcano-Bonilla, S.; Quist, J.; Tolosa, E.J.; Iguchi, E.; Swanson, A.A.; Hoppman, N.L.; Schwab, T.; Sigafoos, A.; Prodduturi, N.; et al. LPCAT1-TERT fusions are uniquely recurrent in epithelioid trophoblastic tumors and positively regulate cell growth. PLoS ONE 2021, 16, e0250518. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | Levels | Study Participants | p | |
|---|---|---|---|---|
| Non-RDS Neonates | RDS Neonates | |||
| (n = 100) | (n = 100) | |||
| Demographic and clinical characteristics | ||||
| Maternal age (years) | M ± SD | 26.1 ± 5.26 | 27.6 ± 5.85 | 0.058 |
| Maternal age groups | ≤20 years, n (%) | 13 (13.0) | 9 (9.0) | 0.090 |
| 21–34 years, n (%) | 79 (79.0) | 73 (73.0) | ||
| ≥35 years, n (%) | 8 (8.0) | 18 (18.0) | ||
| Consanguinity (yes/no) | n (%)/n (%) | 20 (20.0)/80 (80.0) | 22 (22.0)/78 (78.0) | 0.728 |
| Obstetric factors | ||||
| Previous preterm birth (yes/no) | n (%)/n (%) | 18 (18.0)/82 (82.0) | 14 (14.0)/86 (86.0) | 0.440 |
| Use of antenatal steroids (yes/no) | n (%)/n (%) | 90 (90.0)/10 (10.0) | 75 (75.0)/25 (25.0) | 0.005 |
| PPROM (yes/no) | n (%)/n (%) | 25 (25.0)/75 (75.0) | 36 (36.0)/64 (64.0) | 0.091 |
| Modes of delivery (CS/VD) | n (%)/n (%) | 91 (91.0)/9 (9.0) | 78 (78.0)/22 (22.0) | 0.011 |
| Multiple pregnancy (yes/no) | n (%)/n (%) | 2 (2.0)/98 (98.0) | 44 (44.0)/56 (56.0) | <0.001 |
| Maternal comorbidities | ||||
| GDM (yes/no) | n (%)/n (%) | 8 (8.0)/92 (92.0) | 7 (7.0)/93 (93.0) | 0.788 |
| PIH (yes/no) | n (%)/n (%) | 12 (12.0)/88 (88.0) | 32 (32.0)/68 (68.0) | <0.001 |
| Antepartum hemorrhage (yes/no) | n (%)/n (%) | 3 (3.0)/97 (97.0) | 13 (13.0)/87 (87.0) | 0.009 |
| Infections (yes/no) | n (%)/n (%) | 2 (2.0)/98 (98.0) | 6 (6.0)/94 (94.0) | 0.149 |
| Characteristics | Levels | Study Participants | p | |
|---|---|---|---|---|
| Non-RDS Neonates | RDS Neonates | |||
| (n = 100) | (n = 100) | |||
| Demographic data | ||||
| Gender (males/females) | n (%)/n (%) | 63 (63.0)/37 (37.0) | 53 (53.0)/47 (47.0) | 0.152 |
| Gestational births (Singleton/Multiple) | n (%)/n (%) | 98 (98.0)/2 (2.0) | 56 (56.0)/44 (44.0) | <0.001 |
| Gestational age (weeks) | M ± SD | 32.4 ± 1.62 | 31.2 ± 2.54 | 0.452 |
| Birth weight (grams) | M ± SD | 1564.2 ± 152.3 | 1491.5 ± 419.3 | 0.645 |
| Clinical data | ||||
| Apgar score | Apgar score (1 min), M ± SD | 8.02 ± 0.87 | 6.54 ± 1.07 | <0.001 |
| Apgar score (5 min), M ± SD | 9.99 ± 0.10 | 8.91 ± 1.44 | <0.001 | |
| Silverman–Andersen respiratory severity score (RSS) | ||||
| RSS | M ± SD | -- | 7.74 ± 2.75 | NA |
| RD grades based on RSS | None or mild score (0–3), n (%) | -- | 17 (17.0) | NA |
| Moderate score (4–6), n (%) | -- | 24 (24.0) | ||
| Severe score (7–10), n (%) | -- | 59 (59.0) | ||
| Use of surfactant (yes/no) | n (%)/n (%) | -- | 16 (16.0)/84 (84.0) | NA |
| Characteristics | Levels | Prematurity Based on Gestational Age | p | |||
|---|---|---|---|---|---|---|
| Late Preterm (34–36 Weeks) | Moderate Preterm (32–33 Weeks) | Very Preterm (28–31 Weeks) | Extreme Preterm (<28 Weeks) | |||
| (n = 17) | (n = 41) | (n = 33) | (n = 9) | |||
| Demographic data | ||||||
| Gender (males/females) | n (%)/n (%) | 8 (47.1)/9 (52.9) | 26 (63.4)/15 (36.6) | 17 (51.5)/16 (48.5) | 2 (22.2)/7 (77.8) | 0.140 |
| Gestation births (Singleton/Multiple) | n (%)/n (%) | 7 (41.2)/10 (58.8) | 19 (46.3)/22 (53.7) | 14 (42.4)/19 (57.6) | 4 (44.4)/5 (55.6) | 0.981 |
| Gestational age (weeks) | M ± SD | 34.4 ± 0.70 | 32.5 ± 0.51 | 29.4 ± 0.94 | 26.0 ± 0.87 | <0.001 |
| Birth weight (grams) | M ± SD | 1954.4 ± 400.7 | 1571.5 ± 298.8 | 1300.7 ± 296.6 | 951.7 ± 299.4 | <0.001 |
| Birth weight classes | ||||||
| LBW (≥1500–<2500) | n (%) | 14 (82.4) | 26 (63.4) | 11 (33.3) | 1 (11.1) | <0.001 |
| VLBW (≥1000–<1500) | n (%) | 3 (17.6) | 15 (36.6) | 18 (54.6) | 3 (33.3) | |
| ELBW (<1000) | n (%) | 0 (0.0) | 0 (0.0) | 4 (12.1) | 5 (55.6) | |
| Clinical data | ||||||
| Apgar score | Apgar score (1 min.), M ± SD | 6.76 ± 0.66 | 6.75 ± 0.77 | 6.39 ± 1.32 | 5.67 ± 1.41 | 0.026 |
| Apgar score (5 min.), M ± SD | 9.65 ± 0.99 | 9.29 ± 1.27 | 8.51 ± 1.46 | 7.22 ± 1.20 | <0.001 | |
| Silverman–Andersen respiratory severity score (RSS) | ||||||
| RSS | M ± SD | 5.41 ± 2.72 | 6.97 ± 2.81 | 9.27 ± 1.57 | 10.0 ± 0.01 | <0.001 |
| RD grades based on RSS | None or mild score (0–3), n (%) | 8 (47.1) | 9 (22.0) | 0 (0.0) | 0 (0.0) | <0.001 |
| Moderate score (4–6), n (%) | 5 (29.4) | 13 (31.7) | 6 (18.2) | 0 (0.0) | ||
| Severe score (7–10), n (%) | 4 (23.5) | 19 (46.3) | 27 (81.8) | 9 (100.0) | ||
| Use of surfactant (yes/no) | n (%)/n (%) | 2 (11.8)/15 (88.2) | 6 (14.6)/35 (85.4) | 7 (21.2)/26 (78.8) | 1 (11.1)/8 (88.9) | 0.774 |
| LPCAT1-rs9728 Variant | Non-RDS Neonates | RDS Neonates | OR (95% CI) | p | ||
|---|---|---|---|---|---|---|
| Genotypic Frequencies | n (%) = 100 | n (%) 100 | ||||
| T/T | 29 (29.0) | 26 (26.0) | 1.0 | |||
| T/C | 56 (56.0) | 71 (71.0) | 1.41 (0.75–2.67) | 0.332 | ||
| C/C | 15 (15.0) | 3 (3.0) | 0.22 (0.06–0.86) | 0.027 | ||
| HWE | χ2 = 2.03, p = 0.220 | χ2 = 24.9, p < 0.001 | ||||
| Allelic Frequencies | n (%) 200 | n (%) 200 | ||||
| T allele | 114 (57.0) | 123 (61.5) | 1.0 | |||
| C allele | 86 (43.0) | 77 (38.5) | 0.83 (0.56–1.24) | 0.416 | ||
| Genetic Association Models | Non-RDS Neonates | RDS Neonates | Adjusted OR (95% CI) | p | AIC | |
| Model | Genotypes | n (%) 100 | n (%) 100 | |||
| Codominant | T/T | 29 (29.0) | 26 (26.0) | 1.0 | 0.006 | 273.1 |
| T/C | 56 (56.0) | 71 (71.0) | 1.37 (0.72–2.60) | |||
| C/C | 15 (15.0) | 3 (3.0) | 0.22 (0.06–0.86) | |||
| Dominant | T/T | 29 (29.0) | 26 (26.0) | 1.0 | 0.706 | 281.1 |
| T/C + C/C | 71 (71.0) | 74 (74.0) | 1.13 (0.60–2.11) | |||
| Recessive | T/T + T/C | 85 (85.0) | 97 (97.0) | 1.0 | 0.002 | 272.0 |
| C/C | 15 (15.0) | 3 (3.0) | 0.18 (0.05–0.64) | |||
| Log-additive | -- | -- | -- | 0.75 (0.46–1.22) | 0.249 | 279.9 |
| Parameter | Levels | LPCAT1 (rs9728; c.*1668T>C) | p | ||
|---|---|---|---|---|---|
| T/T (n = 26) | T/C (n = 71) | C/C (n = 3) | |||
| Respiratory outcomes | |||||
| Duration of MV (Days) | M ± SD | 8.06 ± 4.44 | 9.31 ± 4.71 | 7.00 ± 0.01 | 0.568 |
| Duration of oxygen therapy (Days) | M ± SD | 14.1 ± 8.47 | 14.8 ± 8.62 | 11.3 ± 4.62 | 0.762 |
| LOS (Days) | M ± SD | 25.2 ± 15.0 | 26.2 ± 19.6 | 29.3 ± 26.6 | 0.928 |
| Neonatal comorbidities | |||||
| PDA (yes/no) | n (%)/n (%) | 2 (7.7)/24 (92.3) | 4 (5.6)/67 (94.4) | 0 (0.0)/3 (100.0) | 0.843 |
| BPD (yes/no) | n (%)/n (%) | 0 (0.0)/26 (100.0) | 2 (2.8)/69 (97.2) | 0 (0.0)/3 (100.0) | 0.659 |
| ROP (yes/no) | n (%)/n (%) | 7 (26.9)/19 (73.1) | 17 (23.9)/54 (76.1) | 0 (0.0)/3 (100.0) | 0.586 |
| IVH (yes/no) | n (%)/n (%) | 1 (3.9)/25 (96.1) | 6 (8.4)/65 (91.6) | 0 (0.0)/3 (100.0) | 0.653 |
| Pulmonary hemorrhage (yes/no) | n (%)/n (%) | 0 (0.0)/26 (100.0) | 4 (5.6)/67 (94.4) | 0 (0.0)/3 (100.0) | 0.427 |
| Pneumothorax (yes/no) | n (%)/n (%) | 2 (7.7)/24 (92.3) | 7 (9.9)/64 (90.1) | 0 (0.0)/3 (100.0) | 0.813 |
| Late-onset sepsis (yes/no) | n (%)/n (%) | 12 (46.2)/14 (53.8) | 45 (63.4)/26 (36.6) | 2 (66.7)/1 (33.3) | 0.301 |
| Survival status | |||||
| Survived | n (%) | 22 (84.6) | 51 (71.8) | 2 (66.7) | 0.412 |
| Dead | n (%) | 4 (15.4) | 20 (28.2) | 1 (33.3) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorgham, S.; Yahia, S.; Shahin, D.; Eita, A.M.; Toraih, E.A.; Elshazli, R.M. Association of LPCAT1*rs9728 Variant with Reduced Susceptibility to Neonatal Respiratory Distress Syndrome. Biomedicines 2025, 13, 2237. https://doi.org/10.3390/biomedicines13092237
Dorgham S, Yahia S, Shahin D, Eita AM, Toraih EA, Elshazli RM. Association of LPCAT1*rs9728 Variant with Reduced Susceptibility to Neonatal Respiratory Distress Syndrome. Biomedicines. 2025; 13(9):2237. https://doi.org/10.3390/biomedicines13092237
Chicago/Turabian StyleDorgham, Shimaa, Sohier Yahia, Doaa Shahin, Ahmad M. Eita, Eman A. Toraih, and Rami M. Elshazli. 2025. "Association of LPCAT1*rs9728 Variant with Reduced Susceptibility to Neonatal Respiratory Distress Syndrome" Biomedicines 13, no. 9: 2237. https://doi.org/10.3390/biomedicines13092237
APA StyleDorgham, S., Yahia, S., Shahin, D., Eita, A. M., Toraih, E. A., & Elshazli, R. M. (2025). Association of LPCAT1*rs9728 Variant with Reduced Susceptibility to Neonatal Respiratory Distress Syndrome. Biomedicines, 13(9), 2237. https://doi.org/10.3390/biomedicines13092237






