SIK2 Drives Pulmonary Fibrosis by Enhancing Fibroblast Glycolysis and Activation
Abstract
1. Introduction
2. Material and Methods
2.1. Reagents and Antibodies
2.2. Experimental Animals and the Pulmonary Fibrosis Mouse Model
2.3. Human Samples
2.4. Human Precision-Cut Lung Slices
2.5. Primary Lung Fibroblast Culture and Treatment
2.6. Histological and Immunofluorescence Analysis
2.7. Western Blot Analysis
2.8. Quantitative Real-Time Polymerase Chain Reaction
2.9. 2-NBDG Glucose Uptake Detection
2.10. Statistical Analysis
3. Results
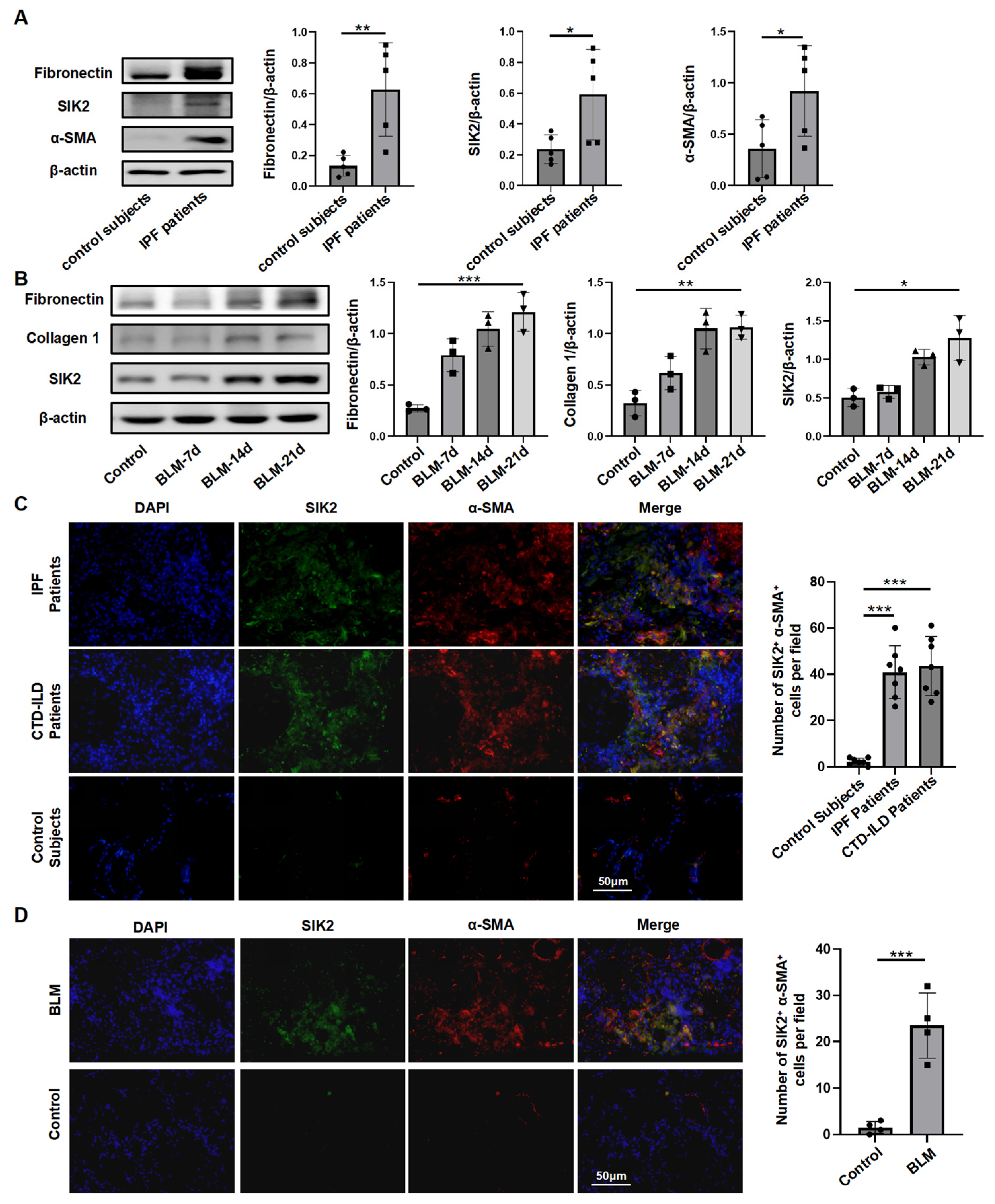
3.1. SIK2 Protein Level Was Elevated in the Pulmonary Fibrosis Lungs
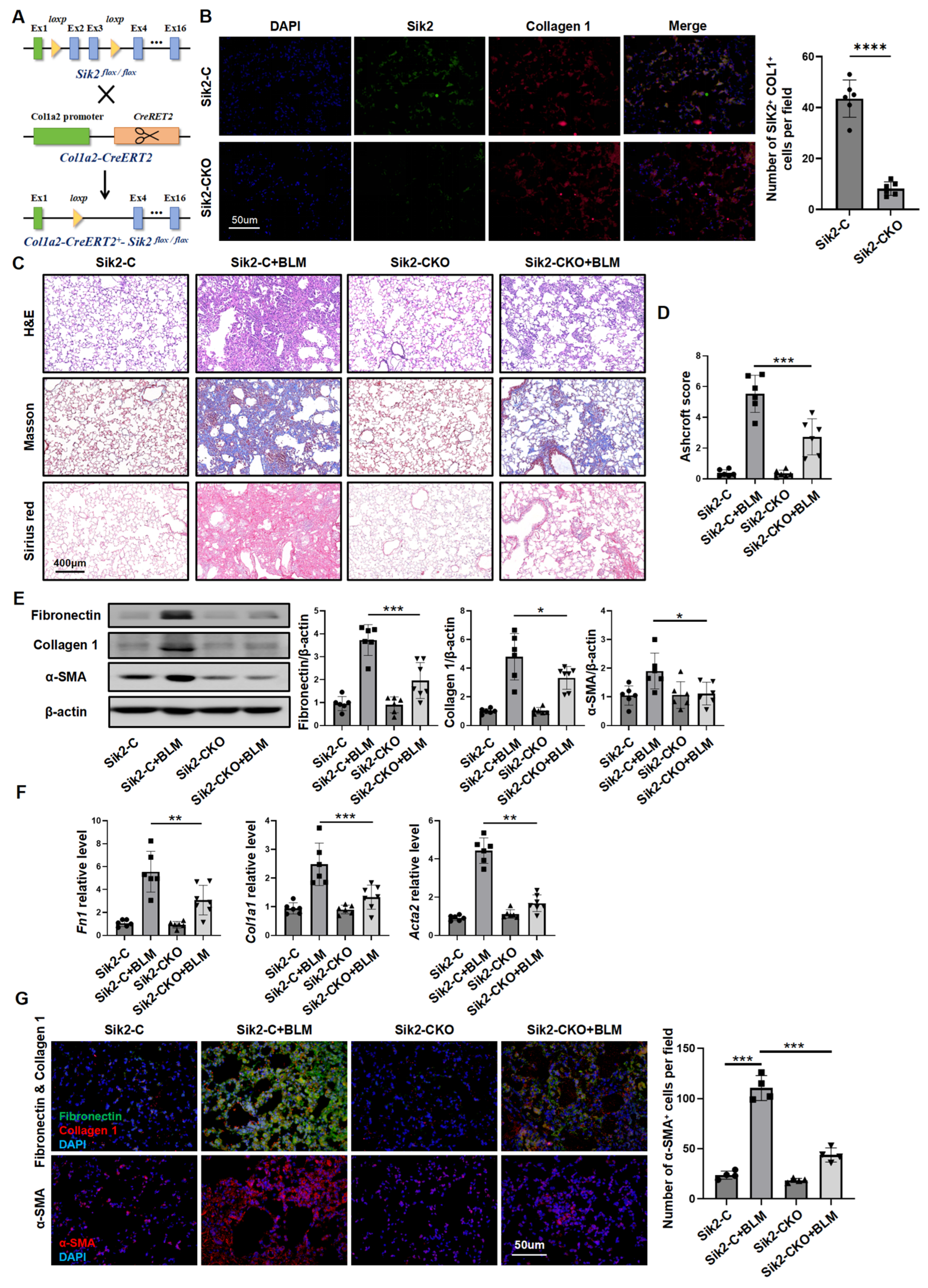
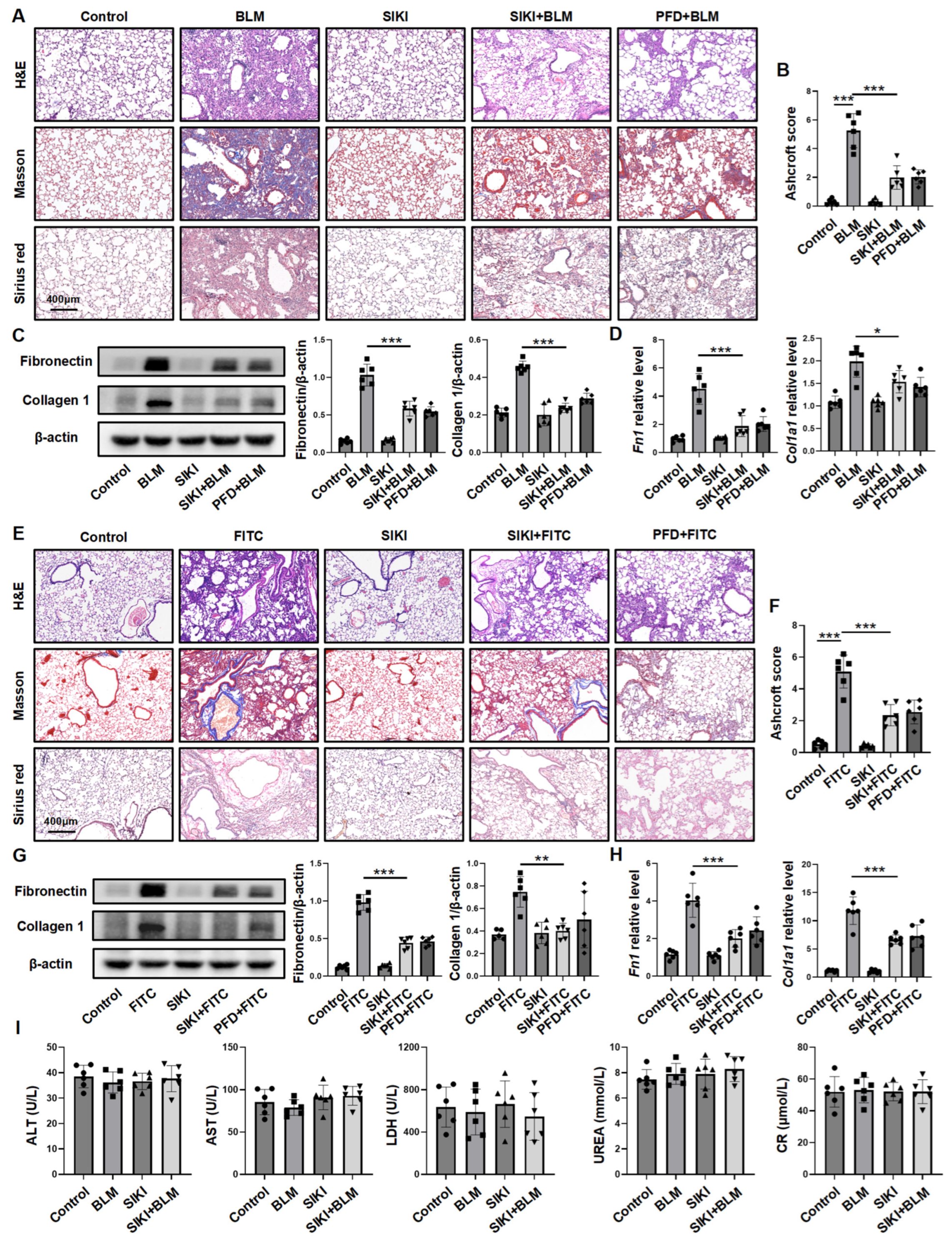
3.2. Fibroblast-Specific Sik2 Knockout Alleviates BLM-Induced Pulmonary Fibrosis in Mice
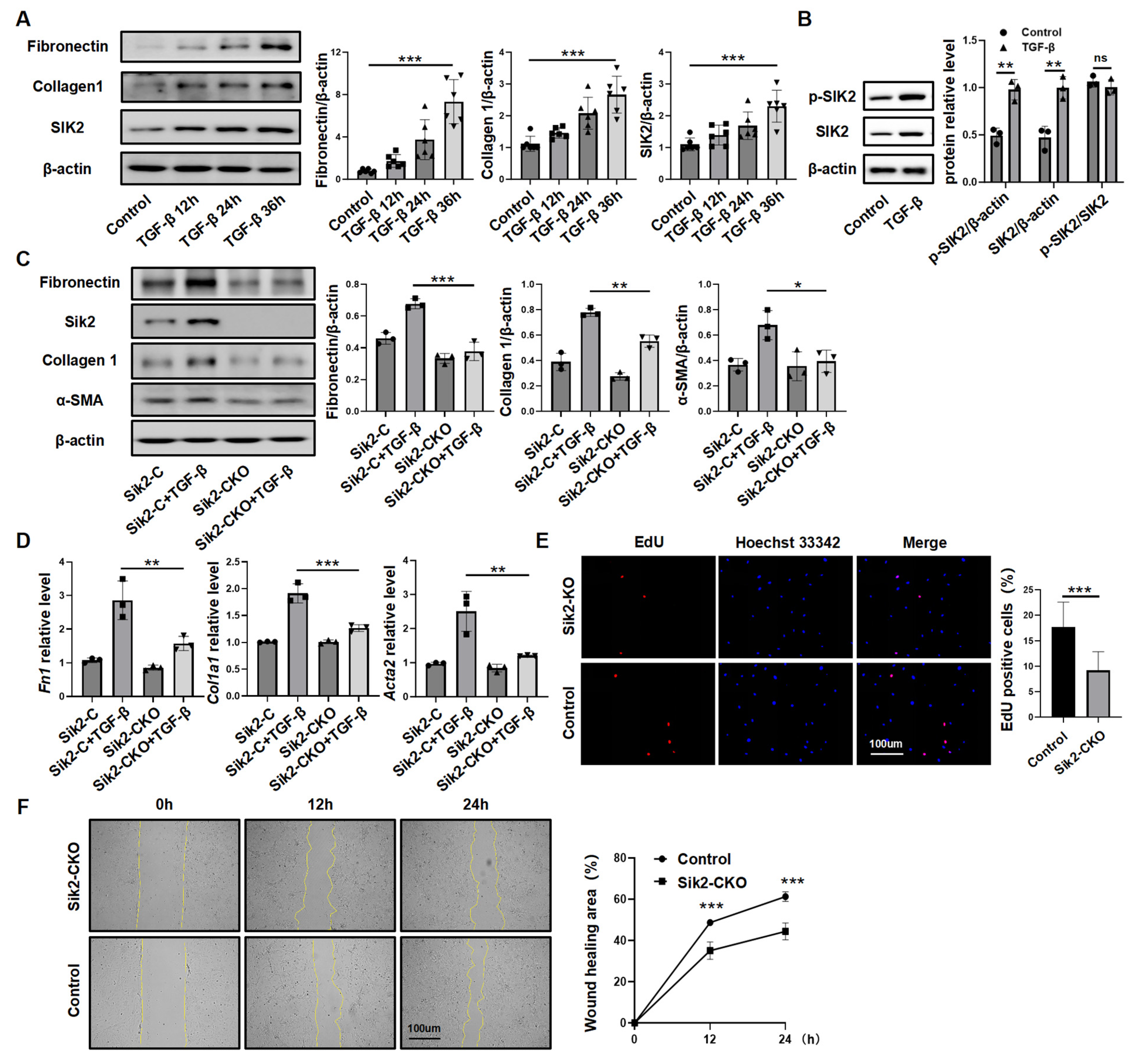
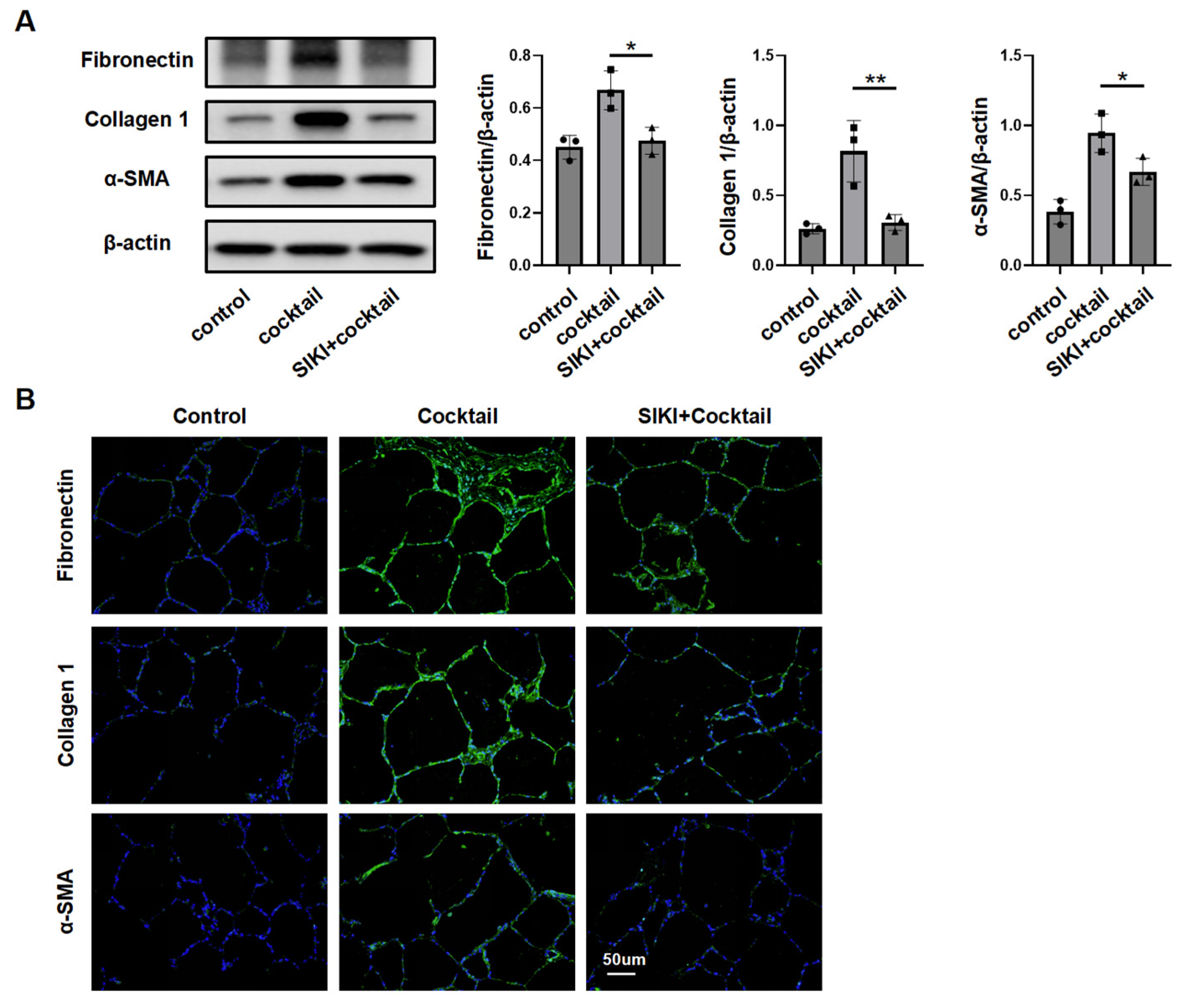
3.3. SIK2 Plays a Crucial Role in Fibroblasts Activation Process
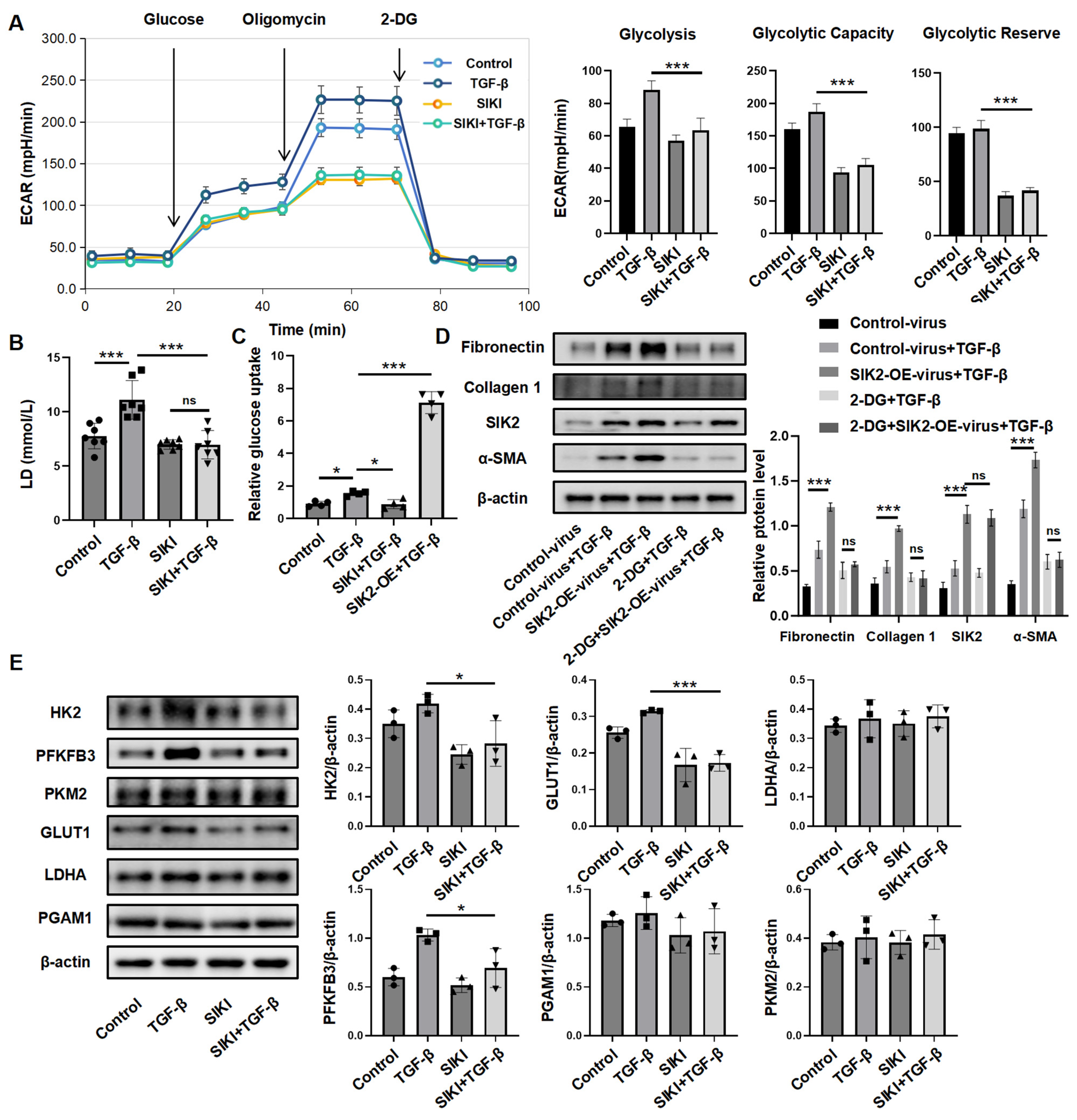
3.4. SIK2 Plays a Significant Role in the Regulation of Fibroblast Glucose Metabolism
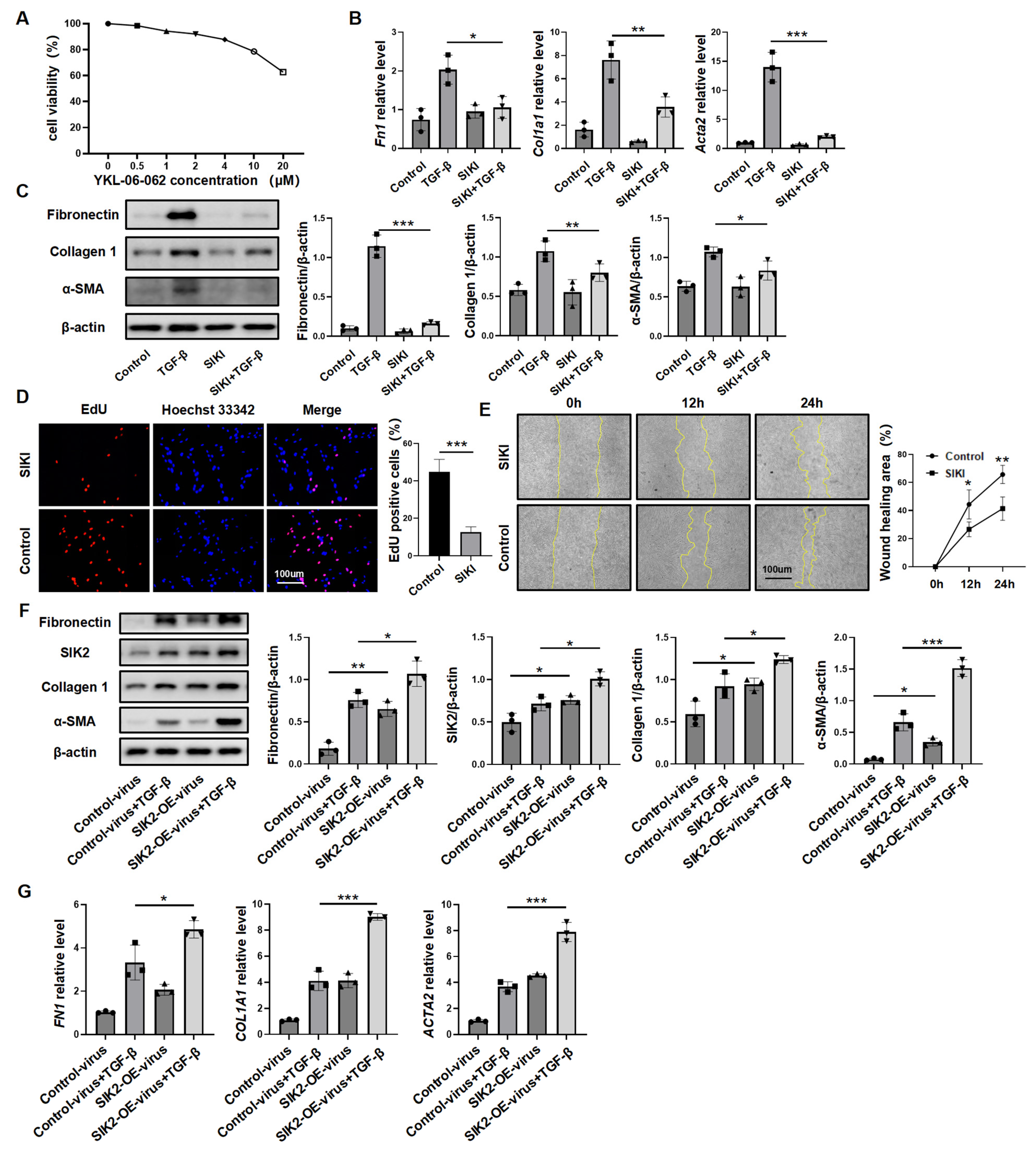
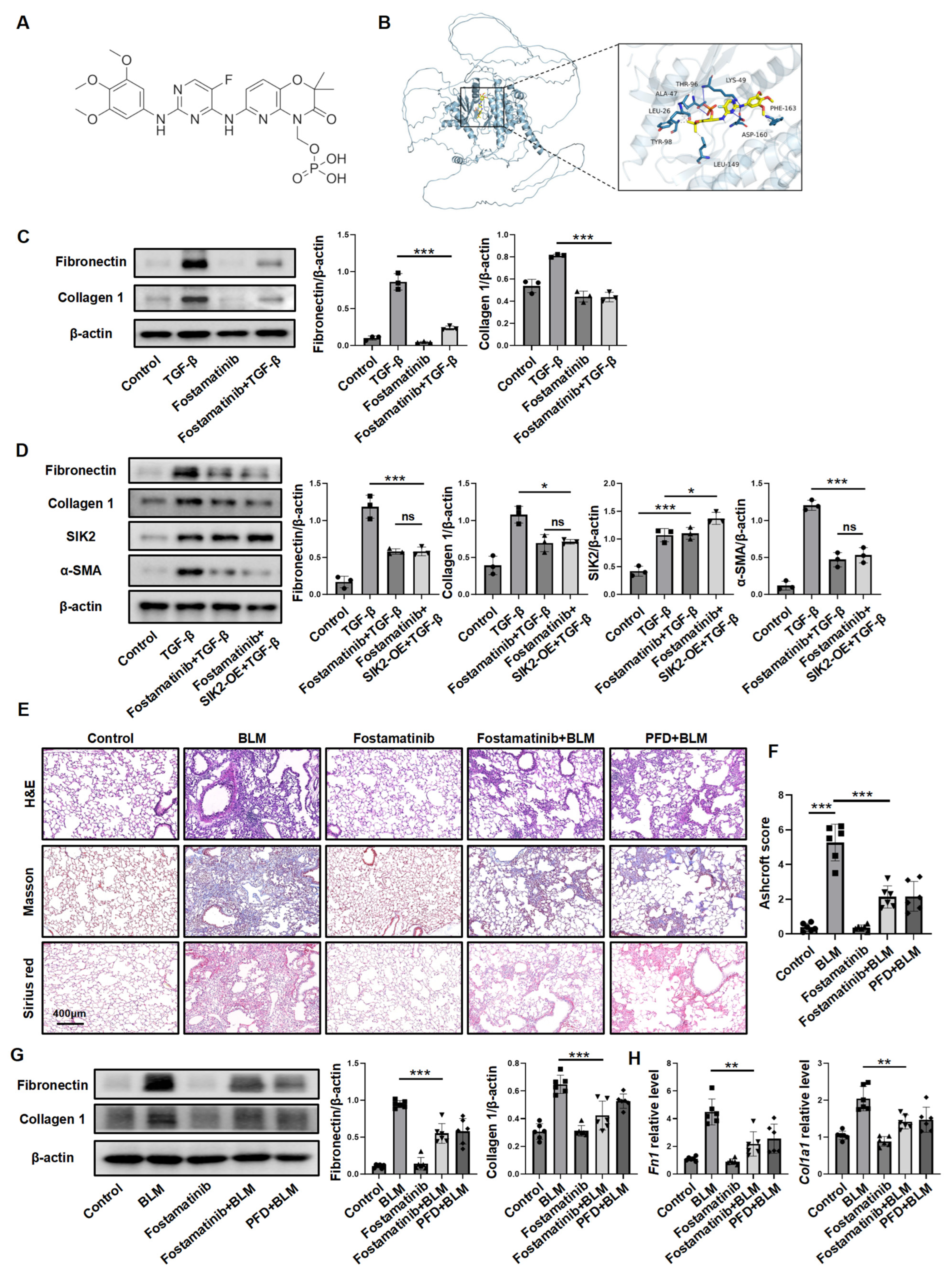
3.5. Inhibiting SIK2 Is a Promising Approach for Anti-Fibrosis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BLM | bleomycin |
| DMEM | Dulbecco’s modified eagle medium |
| DMSO | dimethyl sulfoxide |
| FITC | fluorescein Isothiocyanate |
| HPCLS | human precision-cut lung slices |
| HPFs | human primary pulmonary fibroblasts |
| PDGF | platelet-derived growth factor |
| PF | pulmonary fibrosis |
| PFD | pirfenidone |
| TGF-β | transforming growth factor-beta |
| TNF-α | tumor necrosis factor-alpha |
| 2-DG | 2-deoxy-D-glucose |
References
- Shah Gupta, R.; Koteci, A.; Morgan, A.; George, P.M.; Quint, J.K. Incidence and prevalence of interstitial lung diseases worldwide: A systematic literature review. BMJ Open Respir. Res. 2023, 10, e001291. [Google Scholar] [CrossRef] [PubMed]
- Maher, T.M. Interstitial Lung Disease: A Review. JAMA 2024, 331, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Koudstaal, T.; Funke-Chambour, M.; Kreuter, M.; Molyneaux, P.L.; Wijsenbeek, M.S. Pulmonary fibrosis: From pathogenesis to clinical decision-making. Trends Mol. Med. 2023, 29, 1076–1087. [Google Scholar] [CrossRef] [PubMed]
- Savin, I.A.; Zenkova, M.A.; Sen’kova, A.V. Pulmonary Fibrosis as a Result of Acute Lung Inflammation: Molecular Mechanisms, Relevant In Vivo Models, Prognostic and Therapeutic Approaches. Int. J. Mol. Sci. 2022, 23, 14959. [Google Scholar] [CrossRef]
- Naqvi, M.; Hannah, J.; Lawrence, A.; Myall, K.; West, A.; Chaudhuri, N. Antifibrotic therapy in progressive pulmonary fibrosis: A review of recent advances. Expert Rev. Respir. Med. 2024, 18, 397–407. [Google Scholar] [CrossRef]
- Cruwys, S.; Hein, P.; Humphries, B.; Black, D. Drug discovery and development in idiopathic pulmonary fibrosis: The changing landscape. Drug Discov. Today 2024, 29, 104207. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, L.; Wang, M.; Zhou, S.; Lu, Y.; Cui, H.; Racanelli, A.C.; Zhang, L.; Ye, T.; Ding, B.; et al. Targeting fibrosis, mechanisms and cilinical trials. Signal Transduct. Target. Ther. 2022, 7, 206. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, G.-R.; Yue, H.; Zhou, Q.; Zhang, L.; He, L.; Gu, W.; Gao, R.; Dong, L.; Zhang, H.; et al. Kynurenine acts as a signaling molecule to attenuate pulmonary fibrosis by enhancing the AHR-PTEN axis. J. Adv. Res. 2025, 71, 521–532. [Google Scholar] [CrossRef]
- Younesi, F.S.; Miller, A.E.; Barker, T.H.; Rossi, F.M.V.; Hinz, B. Fibroblast and myofibroblast activation in normal tissue repair and fibrosis. Nat. Rev. Mol. Cell Biol. 2024, 25, 617–638. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Du, C.; Wang, Z.; Wang, J.; Zhou, N.; Wang, B.; Tan, K.; Fan, Y.; Cao, P. Glycolysis and beyond in glucose metabolism: Exploring pulmonary fibrosis at the metabolic crossroads. Front. Endocrinol. 2024, 15, 1379521. [Google Scholar] [CrossRef] [PubMed]
- Roque, W.; Romero, F. Cellular metabolomics of pulmonary fibrosis, from amino acids to lipids. Am. J. Physiol. Cell Physiol. 2021, 320, C689–C695. [Google Scholar] [CrossRef]
- Win, T.; Thomas, B.A.; Lambrou, T.; Hutton, B.F.; Screaton, N.J.; Porter, J.C.; Maher, T.M.; Endozo, R.; Shortman, R.I.; Afaq, A.; et al. Areas of normal pulmonary parenchyma on HRCT exhibit increased FDG PET signal in IPF patients. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 337–342. [Google Scholar] [CrossRef]
- Umeda, Y.; Demura, Y.; Morikawa, M.; Anzai, M.; Kadowaki, M.; Ameshima, S.; Tsuchida, T.; Tsujikawa, T.; Kiyono, Y.; Okazawa, H.; et al. Prognostic Value of Dual-Time-Point 18F-FDG PET for Idiopathic Pulmonary Fibrosis. J. Nucl. Med. 2015, 56, 1869–1875. [Google Scholar] [CrossRef]
- Song, S.E.; Kim, Y.-W.; Kim, J.-Y.; Lee, D.H.; Kim, J.-R.; Park, S.-Y. IGFBP5 mediates high glucose-induced cardiac fibroblast activation. J. Mol. Endocrinol. 2013, 50, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Tan, Z.; Banerjee, S.; Cui, H.; Ge, J.; Liu, R.-M.; Bernard, K.; Thannickal, V.J.; Liu, G. Glycolytic Reprogramming in Myofibroblast Differentiation and Lung Fibrosis. Am. J. Respir. Crit. Care Med. 2015, 192, 1462–1474. [Google Scholar] [CrossRef] [PubMed]
- Townsend, L.K.; Steinberg, G.R. AMPK and the Endocrine Control of Metabolism. Endocr. Rev. 2023, 44, 910–933. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-C.; Hardie, D.G. AMPK: Sensing Glucose as well as Cellular Energy Status. Cell Metab. 2018, 27, 299–313. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Hu, D.; Du, J.; Xing, Y.; Cheng, Y.; He, R.; Liang, X.; Li, H.; Yang, Y. SIK2: A critical glucolipid metabolic reprogramming regulator and potential target in ovarian cancer. J. Obstet. Gynaecol. Res. 2023, 49, 2000–2009. [Google Scholar] [CrossRef]
- Henriksson, E.; Säll, J.; Gormand, A.; Wasserstrom, S.; Morrice, N.A.; Fritzen, A.M.; Foretz, M.; Campbell, D.G.; Sakamoto, K.; Ekelund, M.; et al. SIK2 regulates CRTCs, HDAC4 and glucose uptake in adipocytes. J. Cell Sci. 2015, 128, 472–486. [Google Scholar]
- Ni, X.; Feng, Y.; Fu, X. Role of salt-inducible kinase 2 in the malignant behavior and glycolysis of colorectal cancer cells. Mol. Med. Rep. 2021, 24, 822. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Zhang, X.; Zhao, J.; Zhou, F.; Wang, Y.; Zhao, Z.; Xing, J.; Chen, B.; Li, J.; Liu, S. SIK2 promotes reprogramming of glucose metabolism through PI3K/AKT/HIF-1α pathway and Drp1-mediated mitochondrial fission in ovarian cancer. Cancer Lett. 2020, 469, 89–101. [Google Scholar] [CrossRef]
- Zou, L.; Hong, D.; Li, K.; Jiang, B. Salt-inducible kinase 2 (SIK2) inhibitor ARN-3236 attenuates bleomycin-induced pulmonary fibrosis in mice. BMC Pulm. Med. 2022, 22, 140. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yue, H.; Zhang, S.; Dong, R.; Zhang, F.; Wang, X.; Wang, K.; Zhang, H.; Yang, D.; Dong, Z.; et al. Dehydrocorydaline attenuates bleomycin-induced pulmonary fibrosis by inhibiting fibroblast activation. Respir. Res. 2025, 26, 136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yue, H.; Dong, R.; He, J.; Zhou, L.; Dou, X.; Wang, L.; Zheng, P.; Mao, Z.; Zhu, X.; et al. Trigonelline hydrochloride attenuates silica-induced pulmonary fibrosis by orchestrating fibroblast to myofibroblast differentiation. Respir. Res. 2024, 25, 242. [Google Scholar] [CrossRef]
- Shinde, A.V.; Humeres, C.; Frangogiannis, N.G. The role of α-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef]
- Peixoto, C.; Joncour, A.; Temal-Laib, T.; Tirera, A.; Dos Santos, A.; Jary, H.; Bucher, D.; Laenen, W.; Pereira Fernandes, A.; Lavazais, S.; et al. Discovery of Clinical Candidate GLPG3970: A Potent and Selective Dual SIK2/SIK3 Inhibitor for the Treatment of Autoimmune and Inflammatory Diseases. J. Med. Chem. 2024, 67, 5233–5258. [Google Scholar] [CrossRef]
- van Gijsel-Bonnello, M.; Darling, N.J.; Tanaka, T.; Di Carmine, S.; Marchesi, F.; Thomson, S.; Clark, K.; Kurowska-Stolarska, M.; McSorley, H.J.; Cohen, P.; et al. Salt-inducible kinase 2 regulates fibrosis during bleomycin-induced lung injury. J. Biol. Chem. 2022, 298, 102644. [Google Scholar] [CrossRef]
- Viana, F.; O’Kane, C.M.; Schroeder, G.N. Precision-cut lung slices: A powerful ex vivo model to investigate respiratory infectious diseases. Mol. Microbiol. 2022, 117, 578–588. [Google Scholar] [CrossRef]
- Alsafadi, H.N.; Staab-Weijnitz, C.A.; Lehmann, M.; Lindner, M.; Peschel, B.; Königshoff, M.; Wagner, D.E. An ex vivo model to induce early fibrosis-like changes in human precision-cut lung slices. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 312, L896–L902. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Cornwell, A.; Ziółkowski, H.; Badiei, A. Glucose Transporter Glut1-Dependent Metabolic Reprogramming Regulates Lipopolysaccharide-Induced Inflammation in RAW264.7 Macrophages. Biomolecules 2023, 13, 770. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Choudhury, M.; Kang, J.-H.; Schaefbauer, K.J.; Jung, M.-Y.; Andrianifahanana, M.; Hernandez, D.M.; Leof, E.B. Hexokinase 2 couples glycolysis with the profibrotic actions of TGF-β. Sci. Signal. 2019, 12, eaax4067. [Google Scholar] [CrossRef]
- Traber, G.M.; Yu, A.-M. RNAi-Based Therapeutics and Novel RNA Bioengineering Technologies. J. Pharmacol. Exp. Ther. 2023, 384, 133–154. [Google Scholar] [CrossRef]
- Sarker, A.; Dhama, N.; Gupta, R.D. Dengue virus neutralizing antibody: A review of targets, cross-reactivity, and antibody-dependent enhancement. Front. Immunol. 2023, 14, 1200195. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Rocha, E.; Rusiñol, L.; Puig, L. New and Emerging Oral/Topical Small-Molecule Treatments for Psoriasis. Pharmaceutics 2024, 16, 239. [Google Scholar] [CrossRef]
- Finnerty, J.P.; Ponnuswamy, A.; Dutta, P.; Abdelaziz, A.; Kamil, H. Efficacy of antifibrotic drugs, nintedanib and pirfenidone, in treatment of progressive pulmonary fibrosis in both idiopathic pulmonary fibrosis (IPF) and non-IPF: A systematic review and meta-analysis. BMC Pulm. Med. 2021, 21, 411. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Nielsen, T.E.; Clausen, M.H. FDA-approved small-molecule kinase inhibitors. Trends Pharmacol. Sci. 2015, 36, 422–439. [Google Scholar] [CrossRef]
- Crampon, K.; Giorkallos, A.; Deldossi, M.; Baud, S.; Steffenel, L.A. Machine-learning methods for ligand-protein molecular docking. Drug Discov. Today 2022, 27, 151–164. [Google Scholar] [CrossRef]
- Bussel, J.; Arnold, D.M.; Grossbard, E.; Mayer, J.; Treliński, J.; Homenda, W.; Hellmann, A.; Windyga, J.; Sivcheva, L.; Khalafallah, A.A.; et al. Fostamatinib for the treatment of adult persistent and chronic immune thrombocytopenia: Results of two phase 3, randomized, placebo-controlled trials. Am. J. Hematol. 2018, 93, 921–930. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Dong, R.; Yue, H.; Zhang, F.; Dou, X.; Li, X.; Li, H.; Zhang, H. SIK2 Drives Pulmonary Fibrosis by Enhancing Fibroblast Glycolysis and Activation. Biomedicines 2025, 13, 1919. https://doi.org/10.3390/biomedicines13081919
He J, Dong R, Yue H, Zhang F, Dou X, Li X, Li H, Zhang H. SIK2 Drives Pulmonary Fibrosis by Enhancing Fibroblast Glycolysis and Activation. Biomedicines. 2025; 13(8):1919. https://doi.org/10.3390/biomedicines13081919
Chicago/Turabian StyleHe, Jianhan, Ruihan Dong, Huihui Yue, Fengqin Zhang, Xinran Dou, Xuan Li, Hui Li, and Huilan Zhang. 2025. "SIK2 Drives Pulmonary Fibrosis by Enhancing Fibroblast Glycolysis and Activation" Biomedicines 13, no. 8: 1919. https://doi.org/10.3390/biomedicines13081919
APA StyleHe, J., Dong, R., Yue, H., Zhang, F., Dou, X., Li, X., Li, H., & Zhang, H. (2025). SIK2 Drives Pulmonary Fibrosis by Enhancing Fibroblast Glycolysis and Activation. Biomedicines, 13(8), 1919. https://doi.org/10.3390/biomedicines13081919



