Antibody–Drug Conjugates in Breast Cancer: Navigating Innovations, Overcoming Resistance, and Shaping Future Therapies
Abstract
1. Introduction
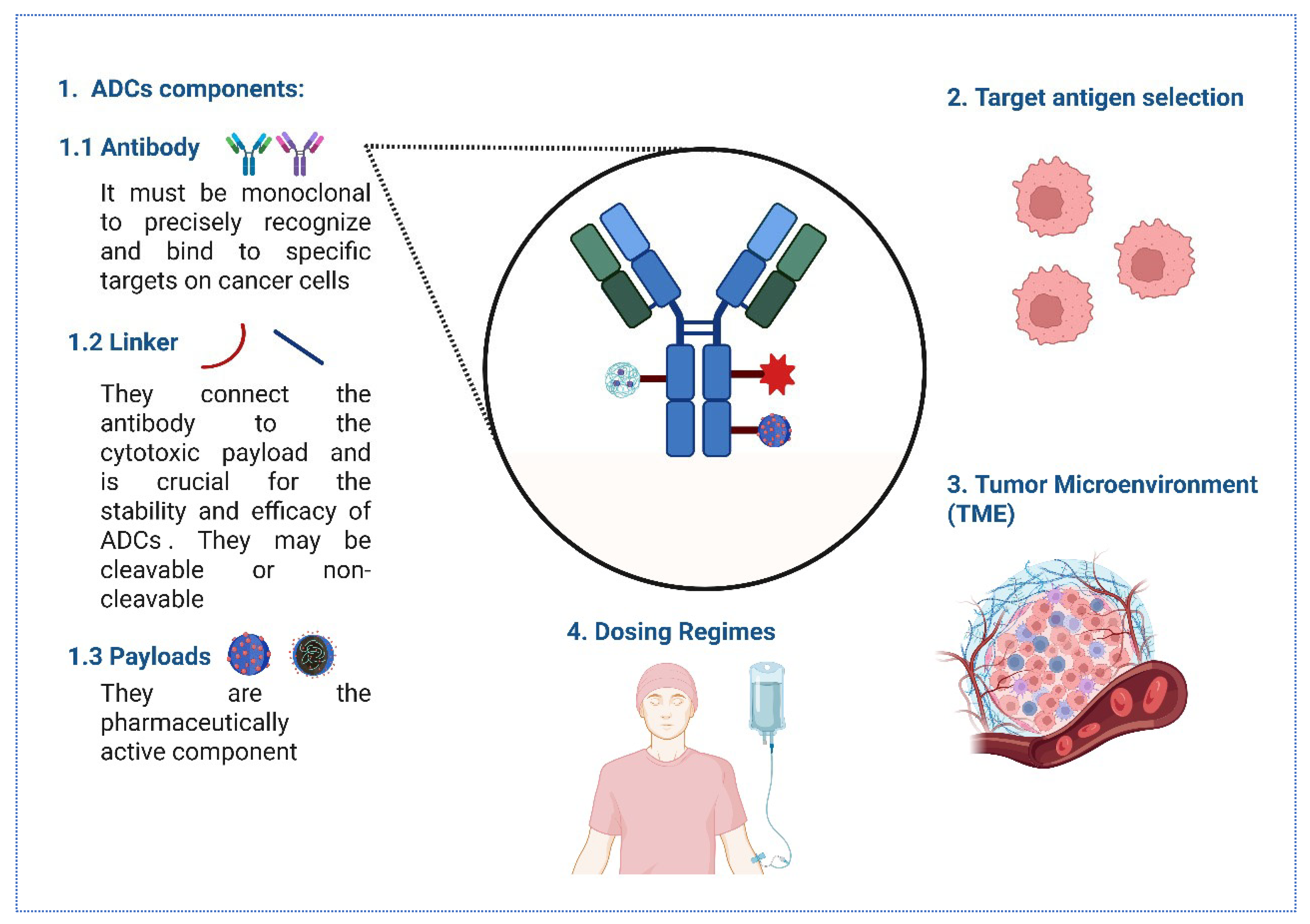
2. Innovations in ADC Development for BC
2.1. Evolving Antibody Targets in BC ADCs
2.2. Advancements in Linker Technology
2.3. Innovative ADC Formats and Conjugation Strategies
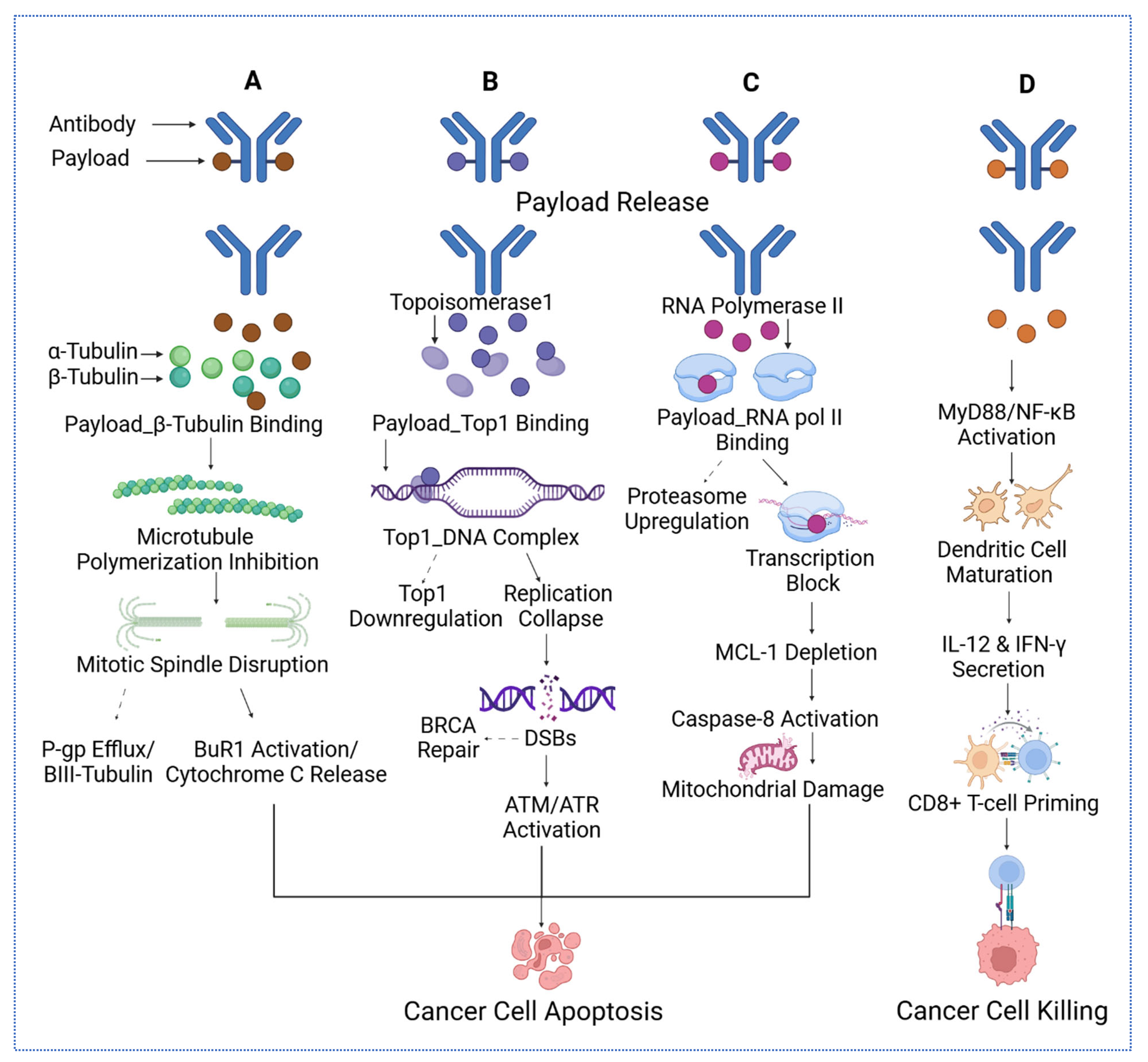
2.4. Next-Generation Cytotoxic Payloads
2.5. Multi-Modal Approaches and Combination Therapies
3. Challenges in ADC Development and Application for BC
3.1. Tumor Heterogeneity and Drug Resistance
3.2. ADC Toxicity and Component Impact
3.3. Patient Selection and Biomarker Development
4. Antibody–Receptor Binding Kinetics and Internalization Pathways
4.1. Intracellular Trafficking and Lysosomal/Endosomal Processing
4.2. The Bystander Effect
| Payload Class | Representative Payload | Mechanism of Cytotoxicity | References |
|---|---|---|---|
| Microtubule Inhibitors | DM1 (in T-DM1), MMAE | Bind to tubulin, inhibit polymerization, arrest mitosis at G2/M phase, trigger apoptosis | [80] |
| DNA Crosslinkers/Alkylators | PBD dimer | Form DNA interstrand crosslinks, block replication/transcription, activate DNA damage response and apoptosis | [81] |
| DNA Cleaving Agents | Calicheamicin | Induce site-specific double-strand DNA breaks via minor groove binding and radical generation | [82] |
| Topoisomerase I Inhibitors | DXd (deruxtecan), SN-38 | Inhibit topoisomerase I, stabilize DNA cleavable complexes, result in replication stress and double-strand breaks | [41] |
| RNA Polymerase II Inhibitors | α-Amanitin | Binds the bridge helix of RNA polymerase II that inhibits transcription, leading to apoptosis in highly active cells | [83] |
| BCL-XL Inhibitor Conjugates | BCL-XL toxin derivatives (experimental) | Promote apoptosis by inhibiting BCL-XL, disrupting mitochondrial membrane potential, enhancing chemosensitivity | [84] |
5. Pharmacokinetics and Pharmacodynamics of ADCs
5.1. Route of Administration and Impact on PK/PD
5.2. Tumor Distribution and Antibody Format
5.3. Metabolism, Catabolism, and Biotransformation
5.4. Linker Chemistry and Payload Release
5.5. PK/PD Variability and Biomarker Considerations
5.6. Strategies for Dose Optimization and PK/PD Modeling
6. Preclinical Evaluation and Translational Challenges
6.1. Preclinical Models for ADC Evaluation
6.2. Biomarker Identification and Validation
6.3. Translational Challenges and Bridging the Gap
| Biomarker | Relevance | Current Application | Drug Targeted | References |
|---|---|---|---|---|
| TROP2 | Trophoblast cell surface antigen 2, overexpressed in various solid tumors, including BC, is effective in triple-negative and hormone receptor-positive, HER2-negative BC | Targeted by ADCs like Sacituzumab Govitecan, approved for metastatic BC | Sacituzumab Govitecan, Datopotamab Deruxtecan | [112,118,119,120] |
| HER3 | Human epidermal growth factor receptor 3, which is overexpressed in various cancers, including BC, and associated with resistance to HER2-targeted therapies | Under investigation in clinical trials for ADCs targeting HER3 | Patritumab deruxtecan (U3-1402) | [121,122] |
| LIV-1 | Zinc transporter LIV-1, overexpressed in BC, particularly in triple-negative BC (TNBC) | Targeted by ADCs in clinical trials, showing promise in TNBC | Ladiratuzumab vedotin | [122,123,124] |
| Claudin-18.2 | A tight junction protein, highly selective expression in tumors, limited in normal tissues | Targeted in gastric and BCs; potential for ADC development | Zolbetuximab (IMAB362) | [125] |
| B7-H3 | An immune checkpoint molecule overexpressed in various cancers, including BC | Targeted by ADCs in clinical trials, with potential for combination with immunotherapy | Enoblituzumab | [117] |
| ROR1 | Receptor tyrosine kinase-like orphan receptor 1 is expressed in various cancers, including BC | Under investigation for ADC targeting; potential in solid tumors | VLS-101 (in development) | [27,112] |
| MUC1 | Mucin protein overexpressed in BC associated with poor prognosis | Targeted by ADCs in clinical trials, showing potential in various subtypes of BC | oportuzumab monatox | [127] |
| CEACAM5 | Overexpressed in several epithelial tumors, including BC, with limited expression in normal tissues | Targeted by ADCs in preclinical and clinical studies | SAR408701 | [126] |
| SPARC | Secreted protein acidic and rich in cysteine, involved in tumor progression and metastasis | Potential targets for ADCs, though specific drugs are still in development | Not specified | [112,127] |
7. Immunogenicity of ADCs in BC
7.1. Factors Influencing ADC Immunogenicity
7.2. Methods for Assessing ADAs
7.3. Impact of ADAs on ADC Pharmacokinetics, Efficacy, and Safety
7.4. Strategies to Mitigate Immunogenicity
8. Future Directions and Perspectives in ADC Development
8.1. Artificial Intelligence and Machine Learning in ADC Design
8.2. Personalized ADC Therapy
8.3. Advancements in Imaging and Diagnostics
8.4. Overcoming Resistance Through Novel Strategies
8.5. Expanding the Therapeutic Window
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADC | Antibody–Drug Conjugate |
| ADCC | Antibody-Dependent Cellular Cytotoxicity |
| ADCP | Antibody-Dependent Cellular Phagocytosis1 |
| ADAs | Anti-Drug Antibodies |
| AI | Artificial Intelligence |
| CDX | Cell Line-Derived Xenografts |
| CDx | Companion Diagnostics |
| CDC | Complement-Dependent Cytotoxicity |
| CDK | Cyclin-Dependent Kinase |
| CNNs | Convolutional Neural Networks |
| ctDNA | Circulating Tumor DNA |
| DAR | Drug-to-Antibody Ratio |
| DDR1 | Discoidin Domain Receptor 1 |
| DL | Deep Learning |
| ECL | Electrochemiluminescence |
| EGFR | Epidermal Growth Factor Receptor |
| ELISAs | Enzyme-Linked Immunosorbent Assays |
| EpCAM | Epithelial Cell Adhesion Molecule |
| ER | Estrogen Receptor |
| FcRn | Neonatal Fc Receptor |
| FISH | Fluorescence In Situ Hybridization |
| GANs | Generative Adversarial Networks |
| GEMMs | Genetically Engineered Mouse Models |
| GNNs | Graph Neural Networks |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| HER3 | Human Epidermal Growth Factor Receptor 3 |
| IHC | Immunohistochemistry |
| iADCs | Immunostimulatory Antibody–Drug Conjugates |
| Igs | Immunoglobulins |
| LC-MS | Liquid Chromatography/Mass Spectrometry |
| LGR5 | Leucine-Rich Repeat-Containing G-Protein Coupled Receptor 5 |
| mAb | Monoclonal Antibody |
| mCTCs | Circulating Tumor Cell-Derived Xenografts |
| mFISHseq | Multiplexed Fluorescence In Situ Hybridization Sequencing |
| ML | Machine Learning |
| MMAE | Monomethyl Auristatin E |
| MOMLIN | Multi-Omics ML Integration |
| PARP | Poly ADP-Ribose Polymerase |
| pCR | Pathological Complete Response |
| PDX | Patient-Derived Xenografts |
| PK | Pharmacokinetics |
| PROTAC | Proteolysis Targeting Chimeras |
| QSP | Quantitative Systems Pharmacology |
| RMSD | Root Mean Square Deviation |
| scFVs | Single-Chain Variable Fragments |
| SG | Sacituzumab Govitecan |
| SNP | Single Nucleotide Polymorphism |
| SPARC | Secreted Protein Acidic and Rich in Cysteine |
| SPR | Surface Plasmon Resonance |
| T-DM1 | Trastuzumab Emtansine |
| T-DXd | Trastuzumab Deruxtecan |
| TILs | Tumor-Infiltrating Lymphocytes |
| TLR | Toll-Like Receptor |
| TME | Tumor Microenvironment |
| TNBC | Triple-Negative Breast Cancer |
| TOP1 | Topoisomerase I |
| TROP2 | Trophoblast Cell Surface Antigen 2 |
| WSIs | Whole-Slide Images |
References
- Wang, J.; Liu, Y.; Zhang, Q.; Li, W.; Feng, J.; Wang, X.; Fang, J.; Han, Y.; Xu, B. Disitamab vedotin, a HER2-directed antibody-drug conjugate, in patients with HER2-overexpression and HER2-low advanced breast cancer: A phase I/Ib study. Cancer Commun. 2024, 44, 833–851. [Google Scholar] [CrossRef]
- Xing, M.; Li, Z.; Cui, Y.; He, M.; Xing, Y.; Yang, L.; Liu, Z.; Luo, L.; Wang, H.; Guo, R. Antibody-drug conjugates for breast cancer: A bibliometric study and clinical trial analysis. Discov. Oncol. 2024, 15, 329. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Huang, R.; Chen, R.; Pan, F.; Shen, X.; Li, H.; Rong, Q.; An, X.; Xue, C.; Shi, Y. Optimal Sequential Strategies for Antibody-Drug Conjugate in Metastatic Breast Cancer: Evaluating Efficacy and Cross-Resistance. Oncologist 2024, 29, e957–e966. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Dai, L.J.; Wu, X.R.; Liu, C.L.; Zhao, S.; Zhang, H.; Chen, L.; Xiao, Y.; Li, M.; Zhao, Y.Z.; et al. Spatial determinants of antibody-drug conjugate SHR-A1811 efficacy in neoadjuvant treatment for HER2-positive breast cancer. Cancer Cell 2025, 43, 1061–1075.e1067. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Sun, S.; Thimmiah, N.; Coates, J.T.; Wu, B.; Abelman, R.O.; Spring, L.; Moy, B.; Ryan, P.; Melkonyan, M.N.; et al. Antibody-Drug Conjugate Sacituzumab Govitecan Enables a Sequential TOP1/PARP Inhibitor Therapy Strategy in Patients with Breast Cancer. Clin. Cancer Res. 2024, 30, 2917–2924. [Google Scholar] [CrossRef]
- Zhou, Z.Z.; Si, Y.; Zhang, J.; Chen, K.; George, A.; Kim, S.; Zhou, L.; Liu, X.M. A Dual-Payload Antibody–Drug Conjugate Targeting CD276/B7-H3 Elicits Cytotoxicity and Immune Activation in Triple-Negative Breast Cancer. Cancer Res. 2024, 84, 3848–3863. [Google Scholar] [CrossRef]
- Zong, H.F.; Li, X.; Han, L.; Wang, L.; Liu, J.J.; Yue, Y.L.; Chen, J.; Ke, Y.; Jiang, H.; Xie, Y.Q.; et al. A novel bispecific antibody drug conjugate targeting HER2 and HER3 with potent therapeutic efficacy against breast cancer. Acta Pharmacol. Sin. 2024, 45, 1727–1739. [Google Scholar] [CrossRef]
- Wiklander, O.P.B.; Mamand, D.R.; Mohammad, D.K.; Zheng, W.; Jawad Wiklander, R.; Sych, T.; Zickler, A.M.; Liang, X.; Sharma, H.; Lavado, A.; et al. Antibody-displaying extracellular vesicles for targeted cancer therapy. Nat. Biomed. Eng. 2024, 8, 1453–1468. [Google Scholar] [CrossRef]
- Li, Q.; Kong, Y.; Zhong, Y.; Huang, A.; Ying, T.; Wu, Y. Half-life extension of single-domain antibody-drug conjugates by albumin binding moiety enhances antitumor efficacy. MedComm 2024, 5, e557. [Google Scholar] [CrossRef]
- Wilding, B.; Woelflingseder, L.; Baum, A.; Chylinski, K.; Vainorius, G.; Gibson, N.; Waizenegger, I.C.; Gerlach, D.; Augsten, M.; Spreitzer, F.; et al. Zongertinib (BI 1810631), an Irreversible HER2 TKI, Spares EGFR Signaling and Improves Therapeutic Response in Preclinical Models and Patients with HER2-Driven Cancers. Cancer Discov. 2025, 15, 119–138. [Google Scholar] [CrossRef]
- Lee, J.; Kida, K.; Koh, J.; Liu, H.; Manyam, G.C.; Gi, Y.J.; Rampa, D.R.; Multani, A.S.; Wang, J.; Jayachandran, G.; et al. The DNA repair pathway as a therapeutic target to synergize with trastuzumab deruxtecan in HER2-targeted antibody-drug conjugate-resistant HER2-overexpressing breast cancer. J. Exp. Clin. Cancer Res. 2024, 43, 236. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.Y.; Lin, C.J.; Wen, S.N.; Wu, Y.C.; Wei, C.Y.; Huang, J.Y.; Tsao, Y.H.; Chen, Y.J.; Tang, W.C.; Wu, Y.C.; et al. Preclinical evaluation of a novel antibody-drug conjugate OBI-992 for Cancer therapy. Sci. Rep. 2025, 15, 8735. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Du, X.; Yuan, J.; Gong, X.; Zhu, Y.; Li, H.; Lin, X.; Zheng, F.; Ran, Y.; Na, Z.; et al. A high-affinity antibody-drug conjugates Actuximab-MMAE for potent and selective targeting of CEACAM5-Positive tumors. Cancer Lett. 2025, 620, 217685. [Google Scholar] [CrossRef]
- Tabariès, S.; Robert, A.; Marcil, A.; Ling, B.; Acchione, M.; Lippens, J.; Pagé, M.; Fortin, A.; Meury, L.; Coutu, M.; et al. Anti-Claudin-2 Antibody-Drug Conjugates for the Treatment of Colorectal Cancer Liver Metastasis. Mol. Cancer Ther. 2024, 23, 1459–1470. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Lu, Y.; Xue, T.; Lai, Q.; Song, H.; Chen, X.; Guo, C.; Yang, J.; Wang, Y. Antibody-drug conjugates targeting DDR1 as a novel strategy for treatment of breast cancer. J. Drug Target. 2024, 32, 1295–1304. [Google Scholar] [CrossRef]
- Loi, S.; Salgado, R. AI-driven biomarkers for antibody-drug conjugates. Cancer Cell 2025, 43, 1000–1002. [Google Scholar] [CrossRef]
- Scheuher, B.; Ghusinga, K.R.; McGirr, K.; Nowak, M.; Panday, S.; Apgar, J.; Subramanian, K.; Betts, A. Towards a platform quantitative systems pharmacology (QSP) model for preclinical to clinical translation of antibody drug conjugates (ADCs). J. Pharmacokinet. Pharmacodyn. 2024, 51, 429–447. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Huang, C.S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef]
- Sakai, H.; Tsurutani, J.; Iwasa, T.; Komoike, Y.; Sakai, K.; Nishio, K.; Nakagawa, K. HER2 genomic amplification in circulating tumor DNA and estrogen receptor positivity predict primary resistance to trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic breast cancer. Breast Cancer 2018, 25, 605–613. [Google Scholar] [CrossRef]
- Filho, O.M.; Viale, G.; Stein, S.; Trippa, L.; Yardley, D.A.; Mayer, I.A.; Abramson, V.G.; Arteaga, C.L.; Spring, L.M.; Waks, A.G. Impact of HER2 heterogeneity on treatment response of early-stage HER2-positive breast cancer: Phase II neoadjuvant clinical trial of T-DM1 combined with pertuzumab. Cancer Discov. 2021, 11, 2474–2487. [Google Scholar] [CrossRef]
- Emens, L.A.; Esteva, F.J.; Beresford, M.; Saura, C.; De Laurentiis, M.; Kim, S.-B.; Im, S.-A.; Wang, Y.; Salgado, R.; Mani, A. Trastuzumab emtansine plus atezolizumab versus trastuzumab emtansine plus placebo in previously treated, HER2-positive advanced breast cancer (KATE2): A phase 2, multicentre, randomised, double-blind trial. Lancet Oncol. 2020, 21, 1283–1295. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Bachelot, T.; Bianchini, G.; Harbeck, N.; Loi, S.; Park, Y.H.; Prat, A.; Gilham, L.; Boulet, T.; Gochitashvili, N.; et al. ASTEFANIA: Adjuvant ado-trastuzumab emtansine and atezolizumab for high-risk, HER2-positive breast cancer. Future Oncol. 2022, 18, 3563–3572. [Google Scholar] [CrossRef] [PubMed]
- Laco, G.S.; Collins, J.R.; Luke, B.T.; Kroth, H.; Sayer, J.M.; Jerina, D.M.; Pommier, Y. Human Topoisomerase I Inhibition: Docking Camptothecin and Derivatives into a Structure-Based Active Site Model. Biochemistry 2002, 41, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Diéras, V.; Deluche, E.; Lusque, A.; Pistilli, B.; Bachelot, T.; Pierga, J.; Viret, F.; Levy, C.; Salabert, L.; Du, F.; et al. Abstract PD8-02: Trastuzumab deruxtecan (T-DXd) for advanced breast cancer patients (ABC), regardless HER2 status: A phase II study with biomarkers analysis (DAISY). Cancer Res. 2022, 82 (Suppl. S4), PD8-02. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef]
- Modi, S.; Park, H.; Murthy, R.K.; Iwata, H.; Tamura, K.; Tsurutani, J.; Moreno-Aspitia, A.; Doi, T.; Sagara, Y.; Redfern, C. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low–expressing advanced breast cancer: Results from a phase Ib study. J. Clin. Oncol. 2020, 38, 1887–1896. [Google Scholar] [CrossRef]
- Guo, Y.; Shen, Z.; Zhao, W.; Lu, J.; Song, Y.; Shen, L.; Lu, Y.; Wu, M.; Shi, Q.; Zhuang, W.; et al. Rational Identification of Novel Antibody-Drug Conjugate with High Bystander Killing Effect against Heterogeneous Tumors. Adv. Sci. 2024, 11, e2306309. [Google Scholar] [CrossRef]
- Skidmore, L.; Sakamuri, S.; Knudsen, N.A.; Hewet, A.G.; Milutinovic, S.; Barkho, W.; Biroc, S.L.; Kirtley, J.; Marsden, R.; Storey, K. ARX788, a site-specific anti-HER2 antibody–drug conjugate, demonstrates potent and selective activity in HER2-low and T-DM1–resistant breast and gastric cancers. Mol. Cancer Ther. 2020, 19, 1833–1843. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Zhang, Q.; Feng, J.; Fang, J.; Chen, X.; Han, Y.; Li, Q.; Zhang, P.; Yuan, P. RC48-ADC, a HER2-targeting antibody-drug conjugate, in patients with HER2-positive and HER2-low expressing advanced or metastatic breast cancer: A pooled analysis of two studies. J. Clin. Oncol. 2021, 39, 1022. [Google Scholar] [CrossRef]
- Zhou, D.-D.; Bai, W.-Q.; Zhai, X.-T.; Sun, L.-P.; Zhen, Y.-S.; Li, Z.-R.; Miao, Q.-F. Excellent effects and possible mechanisms of action of a new antibody–drug conjugate against EGFR-positive triple-negative breast cancer. Mil. Med. Res. 2021, 8, 63. [Google Scholar] [CrossRef]
- Cheung, A.; Chenoweth, A.M.; Johansson, A.; Laddach, R.; Guppy, N.; Trendell, J.; Esapa, B.; Mavousian, A.; Navarro-Llinas, B.; Haider, S.; et al. Anti-EGFR Antibody-Drug Conjugate Carrying an Inhibitor Targeting CDK Restricts Triple-Negative Breast Cancer Growth. Clin. Cancer Res. 2024, 30, 3298–3315. [Google Scholar] [CrossRef] [PubMed]
- Krop, I.E.; Masuda, N.; Mukohara, T.; Takahashi, S.; Nakayama, T.; Inoue, K.; Iwata, H.; Yamamoto, Y.; Alvarez, R.H.; Toyama, T.; et al. Patritumab Deruxtecan (HER3-DXd), a Human Epidermal Growth Factor Receptor 3-Directed Antibody-Drug Conjugate, in Patients With Previously Treated Human Epidermal Growth Factor Receptor 3-Expressing Metastatic Breast Cancer: A Multicenter, Phase I/II Trial. J. Clin. Oncol. 2023, 41, 5550–5560. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, E.P.; Dosunmu, O.; Shastry, M.; Finney, L.; Sellami, D.B.; Sternberg, D.W.; Wright-Browne, V.; Toppmeyer, D.; Gwin, W.R.; Thaddeus, J.T.; et al. A phase 2 study of HER3-DXd in patients (pts) with metastatic breast cancer (MBC). J. Clin. Oncol. 2023, 41 (Suppl. S16), 1004. [Google Scholar] [CrossRef]
- Wahby, S.; Fashoyin-Aje, L.; Osgood, C.L.; Cheng, J.; Fiero, M.H.; Zhang, L.; Tang, S.; Hamed, S.S.; Song, P.; Charlab, R.; et al. FDA Approval Summary: Accelerated Approval of Sacituzumab Govitecan-hziy for Third-line Treatment of Metastatic Triple-negative Breast Cancer. Clin. Cancer Res. 2021, 27, 1850–1854. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef]
- Spring, L.; Tolaney, S.M.; Desai, N.V.; Fell, G.; Trippa, L.; Comander, A.H.; Mulvey, T.M.; McLaughlin, S.; Ryan, P.; Rosenstock, A.S. Phase 2 study of response-guided neoadjuvant sacituzumab govitecan (IMMU-132) in patients with localized triple-negative breast cancer: Results from the NeoSTAR trial. J. Clin. Oncol. 2022, 40, 512. [Google Scholar] [CrossRef]
- Rugo, H.; Bardia, A.; Marmé, F.; Cortés, J.; Schmid, P.; Loirat, D.; Tredan, O.; Ciruelos, E.; Dalenc, F.; Pardo, P.G. LBA76 Overall survival (OS) results from the phase III TROPiCS-02 study of sacituzumab govitecan (SG) vs treatment of physician’s choice (TPC) in patients (pts) with HR+/HER2-metastatic breast cancer (mBC). Ann. Oncol. 2022, 33, S1386. [Google Scholar] [CrossRef]
- Marmé, F.; Stickeler, E.; Furlanetto, J.; Denkert, C.; Schmidt, M.; Reinisch, M.; Reimer, T.; Janni, W.; Untch, M.; Sinn, B.V.; et al. Phase III postneoadjuvant study evaluating sacituzumab govitecan, an antibody drug conjugate in primary HER2-negative breast cancer patients with high relapse risk after standard neoadjuvant treatment: SASCIA. J. Clin. Oncol. 2021, 39 (Suppl. S15), TPS602. [Google Scholar] [CrossRef]
- A Study of Dato-DXd Versus Investigator's Choice Chemotherapy in Patients with Locally Recurrent Inoperable or Metastatic Triple-Negative Breast Cancer, Who Are Not Candidates for PD-1/PD-L1 Inhibitor Therapy (TROPION-Breast02). 2025. Available online: https://clinicaltrials.gov/study/NCT05374512. (accessed on 21 August 2025).
- Xu, B.; Yin, Y.; Fan, Y.; Ouyang, Q.; Song, L.; Wang, X.; Li, W.; Li, M.; Yan, X.; Wang, S.; et al. Sacituzumab tirumotecan (SKB264/MK-2870) in patients (pts) with previously treated locally recurrent or metastatic triple-negative breast cancer (TNBC): Results from the phase III OptiTROP-Breast01 study. J. Clin. Oncol. 2024, 42 (Suppl. S16), 104. [Google Scholar] [CrossRef]
- Okajima, D.; Yasuda, S.; Maejima, T.; Karibe, T.; Sakurai, K.; Aida, T.; Toki, T.; Yamaguchi, J.; Kitamura, M.; Kamei, R. Datopotamab deruxtecan, a novel TROP2-directed antibody–drug conjugate, demonstrates potent antitumor activity by efficient drug delivery to tumor cells. Mol. Cancer Ther. 2021, 20, 2329–2340. [Google Scholar] [CrossRef]
- Sheyi, R.; de la Torre, B.G.; Albericio, F. Linkers: An Assurance for Controlled Delivery of Antibody-Drug Conjugate. Pharmaceutics 2022, 14, 396. [Google Scholar] [CrossRef]
- Shin, S.H.; Park, Y.H.; Park, S.S.; Ju, E.J.; Park, J.; Ko, E.J.; Bae, D.J.; Kim, S.Y.; Chung, C.W.; Song, H.Y.; et al. An Elaborate New Linker System Significantly Enhances the Efficacy of an HER2-Antibody-Drug Conjugate against Refractory HER2-Positive Cancers. Adv. Sci. 2021, 8, e2102414. [Google Scholar] [CrossRef]
- Watanabe, T.; Arashida, N.; Fujii, T.; Shikida, N.; Ito, K.; Shimbo, K.; Seki, T.; Iwai, Y.; Hirama, R.; Hatada, N.; et al. Exo-Cleavable Linkers: Enhanced Stability and Therapeutic Efficacy in Antibody-Drug Conjugates. J. Med. Chem. 2024, 67, 18124–18138. [Google Scholar] [CrossRef]
- Zhou, Q.; Kyazike, J.; Boudanova, E.; Drzyzga, M.; Honey, D.; Cost, R.; Hou, L.; Duffieux, F.; Brun, M.P.; Park, A.; et al. Site-Specific Antibody Conjugation to Engineered Double Cysteine Residues. Pharmaceuticals 2021, 14, 672. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Shi, W.; Dong, Q.; Li, W.; Zhang, J.; Ren, X.; Tang, C.; Liu, B.; Song, Y.; Wu, Y.; et al. A Traceless Site-Specific Conjugation on Native Antibodies Enables Efficient One-Step Payload Assembly. Angew. Chem. Int. Ed. Engl. 2022, 61, e202204132. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Matsuda, Y.; Seki, T.; Shikida, N.; Iwai, Y.; Ooba, Y.; Takahashi, K.; Isokawa, M.; Kawaguchi, S.; Hatada, N.; et al. AJICAP Second Generation: Improved Chemical Site-Specific Conjugation Technology for Antibody-Drug Conjugate Production. Bioconjugate Chem. 2023, 34, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Sheng, W.; Al-Rawe, M.; Mohiuddin, T.M.; Niebert, M.; Zeppernick, F.; Meihold-Heerlein, I.; Hussain, A.F. EpCAM- and EGFR-Specific Antibody Drug Conjugates for Triple-Negative Breast Cancer Treatment. Int. J. Mol. Sci. 2022, 23, 6122. [Google Scholar] [CrossRef]
- Tang, C.; Zeng, Y.; Zhang, J.; Zheng, X.; Tang, F.; Yao, X.; Jiang, Z.X.; Shi, W.; Huang, W. One-Pot Assembly of Dual-Site-Specific Antibody-Drug Conjugates via Glycan Remodeling and Affinity-Directed Traceless Conjugation. Bioconjugate Chem. 2023, 34, 748–755. [Google Scholar] [CrossRef]
- Tan, X.; Fang, P.; Li, K.; You, M.; Cao, Y.; Xu, H.; Zhu, X.; Wang, L.; Wei, X.; Wen, H.; et al. A HER2-targeted antibody-novel DNA topoisomerase I inhibitor conjugate induces durable adaptive antitumor immunity by activating dendritic cells. MAbs 2023, 15, 2220466. [Google Scholar] [CrossRef]
- Fang, S.; Brems, B.M.; Olawode, E.O.; Miller, J.T.; Brooks, T.A.; Tumey, L.N. Design and Characterization of Immune-Stimulating Imidazo [4,5-c]quinoline Antibody-Drug Conjugates. Mol. Pharm. 2022, 19, 3228–3241. [Google Scholar] [CrossRef]
- Huang, L.; Wang, R.; Xie, K.; Zhang, J.; Tao, F.; Pi, C.; Feng, Y.; Gu, H.; Fang, J. A HER2 target antibody drug conjugate combined with anti-PD-(L)1 treatment eliminates hHER2+ tumors in hPD-1 transgenic mouse model and contributes immune memory formation. Breast Cancer Res. Treat. 2022, 191, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Jung, K.H.; Wysocki, P.J.; Jassem, J.; Ma, C.X.; Fernandes, R.; Huisden, R.; Stewart, R.; Vukovic, P.; Tablante Nunes, A.; et al. 166MO Datopotamab deruxtecan (Dato-DXd) + durvalumab (D) as first-line (1L) treatment for unresectable locally advanced/metastatic triple-negative breast cancer (a/mTNBC): Initial results from BEGONIA, a phase Ib/II study. Ann. Oncol. 2022, 33, S199. [Google Scholar] [CrossRef]
- Huppert, L. Avelumab with Binimetinib, Sacituzumab Govitecan, or Liposomal Doxorubicin in Treating Stage IV or Unresectable, Recurrent Triple Negative Breast Cancer (InCITe). Available online: https://clinicaltrials.gov/study/NCT03971409. (accessed on 21 August 2025).
- Laura, M. Study to Evaluate Sacituzumab Govitecan in Combination with Talazoparib in Patients with Metastatic Breast Cancer. 2024. Available online: https://clinicaltrials.gov/study/NCT04039230 (accessed on 21 August 2025).
- Cardillo, T.M.; Zalath, M.B.; Arrojo, R.; Sharkey, R.M.; Govindan, S.V.; Chang, C.H.; Goldenberg, D.M. Sacituzumab govitecan plus platinum-based chemotherapy mediates significant antitumor effects in triple-negative breast, urinary bladder, and small-cell lung carcinomas. Oncotarget 2024, 15, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Tolaney, S.M.; DeMichele, A.; Takano, T.; Rugo, H.S.; Perou, C.; Lynce, F.; Parsons, H.A.; Santa-Maria, C.A.; Rocque, G.B.; Yao, W.; et al. OptimICE-RD: Sacituzumab govitecan + pembrolizumab vs pembrolizumab (±capecitabine) for residual triple-negative breast cancer. Future Oncol. 2024, 20, 2343–2355. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, C.M.; Yamaguchi, A.; Anami, Y.; Xiong, W.; Otani, Y.; Lee, J.; Ueno, N.T.; Zhang, N.; An, Z.; Tsuchikama, K. Antibody-drug conjugates with dual payloads for combating breast tumor heterogeneity and drug resistance. Nat. Commun. 2021, 12, 3528. [Google Scholar] [CrossRef]
- Geukens, T.; De Schepper, M.; Richard, F.; Maetens, M.; Van Baelen, K.; Mahdami, A.; Nguyen, H.L.; Isnaldi, E.; Leduc, S.; Pabba, A.; et al. Intra-patient and inter-metastasis heterogeneity of HER2-low status in metastatic breast cancer. Eur. J. Cancer 2023, 188, 152–160. [Google Scholar] [CrossRef]
- Chen, R.; Herrera, A.F.; Hou, J.; Chen, L.; Wu, J.; Guo, Y.; Synold, T.W.; Ngo, V.N.; Puverel, S.; Mei, M.; et al. Inhibition of MDR1 Overcomes Resistance to Brentuximab Vedotin in Hodgkin Lymphoma. Clin. Cancer Res. 2020, 26, 1034–1044. [Google Scholar] [CrossRef]
- Cabaud, O.; Berger, L.; Crompot, E.; Adélaide, J.; Finetti, P.; Garnier, S.; Guille, A.; Carbuccia, N.; Farina, A.; Agavnian, E.; et al. Overcoming Resistance to Anti-Nectin-4 Antibody-Drug Conjugate. Mol. Cancer Ther. 2022, 21, 1227–1235. [Google Scholar] [CrossRef]
- Coates, J.T.; Sun, S.; Leshchiner, I.; Thimmiah, N.; Martin, E.E.; McLoughlin, D.; Danysh, B.P.; Slowik, K.; Jacobs, R.A.; Rhrissorrakrai, K.; et al. Parallel Genomic Alterations of Antigen and Payload Targets Mediate Polyclonal Acquired Clinical Resistance to Sacituzumab Govitecan in Triple-Negative Breast Cancer. Cancer Discov. 2021, 11, 2436–2445. [Google Scholar] [CrossRef]
- Wang, M.; Ma, Q.; Suthe, S.R.; Hudson, R.E.; Pan, J.Y.; Mikelis, C.; Zhu, M.J.; Wu, Z.G.; Shi, D.R.; Yao, H.P. Humanized dual-targeting antibody-drug conjugates specific to MET and RON receptors as a pharmaceutical strategy for the treatment of cancers exhibiting phenotypic heterogeneity. Acta Pharmacol. Sin. 2025, 46, 1375–1389. [Google Scholar] [CrossRef]
- Ballestín, P.; López de Sá, A.; Díaz-Tejeiro, C.; Paniagua-Herranz, L.; Sanvicente, A.; López-Cade, I.; Pérez-Segura, P.; Alonso-Moreno, C.; Nieto-Jiménez, C.; Ocaña, A. Understanding the Toxicity Profile of Approved ADCs. Pharmaceutics 2025, 17, 258. [Google Scholar] [CrossRef]
- Masters, J.C.; Nickens, D.J.; Xuan, D.; Shazer, R.L.; Amantea, M. Clinical toxicity of antibody drug conjugates: A meta-analysis of payloads. Investig. New Drugs 2018, 36, 121–135. [Google Scholar] [CrossRef]
- Hagerling, C.; Owyong, M.; Sitarama, V.; Wang, C.Y.; Lin, C.; van den Bijgaart, R.J.E.; Koopman, C.D.; Brenot, A.; Nanjaraj, A.; Wärnberg, F.; et al. LGR5 in breast cancer and ductal carcinoma in situ: A diagnostic and prognostic biomarker and a therapeutic target. BMC Cancer 2020, 20, 542. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Tolaney, S.M.; Punie, K.; Loirat, D.; Oliveira, M.; Kalinsky, K.; Zelnak, A.; Aftimos, P.; Dalenc, F.; Sardesai, S.; et al. Biomarker analyses in the phase III ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer. Ann. Oncol. 2021, 32, 1148–1156. [Google Scholar] [CrossRef]
- Paul, E.D.; Huraiová, B.; Valková, N.; Matyasovska, N.; Gábrišová, D.; Gubová, S.; Ignačáková, H.; Ondris, T.; Gala, M.; Barroso, L.; et al. The spatially informed mFISHseq assay resolves biomarker discordance and predicts treatment response in breast cancer. Nat. Commun. 2025, 16, 226. [Google Scholar] [CrossRef]
- Lewis, G.D.; Li, G.; Guo, J.; Yu, S.-F.; Fields, C.T.; Lee, G.; Zhang, D.; Dragovich, P.S.; Pillow, T.; Wei, B.; et al. The HER2-directed antibody-drug conjugate DHES0815A in advanced and/or metastatic breast cancer: Preclinical characterization and phase 1 trial results. Nat. Commun. 2024, 15, 466. [Google Scholar] [CrossRef]
- Cao, X.; Chen, J.; Li, B.; Dang, J.; Zhang, W.; Zhong, X.; Wang, C.; Raoof, M.; Sun, Z.; Yu, J.; et al. Promoting antibody-dependent cellular phagocytosis for effective macrophage-based cancer immunotherapy. Sci. Adv. 2022, 8, eabl9171. [Google Scholar] [CrossRef] [PubMed]
- Pereira, N.A.; Chan, K.F.; Lin, P.C.; Song, Z. The “less-is-more” in therapeutic antibodies: Afucosylated anti-cancer antibodies with enhanced antibody-dependent cellular cytotoxicity. MAbs 2018, 10, 693–711. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Joubert, M.K.; Polozova, A.; De Guzman, R.; Lakamsani, K.; Kinderman, F.; Xiang, D.; Shami, A.; Miscalichi, N.; Flynn, G.C.; et al. Glycan engineering reveals interrelated effects of terminal galactose and core fucose on antibody-dependent cell-mediated cytotoxicity. Biotechnol. Prog. 2020, 36, e3045. [Google Scholar] [CrossRef]
- Joubert, N.; Beck, A.; Dumontet, C.; Denevault-Sabourin, C. Antibody-Drug Conjugates: The Last Decade. Pharmaceuticals 2020, 13, 245. [Google Scholar] [CrossRef]
- Tay, M.Z.; Wiehe, K.; Pollara, J. Antibody-Dependent Cellular Phagocytosis in Antiviral Immune Responses. Front. Immunol. 2019, 10, 332. [Google Scholar] [CrossRef]
- Maass, K.F.; Kulkarni, C.; Betts, A.M.; Wittrup, K.D. Determination of Cellular Processing Rates for a Trastuzumab-Maytansinoid Antibody-Drug Conjugate (ADC) Highlights Key Parameters for ADC Design. Aaps J. 2016, 18, 635–646. [Google Scholar] [CrossRef]
- Ritchie, M.; Tchistiakova, L.; Scott, N. Implications of receptor-mediated endocytosis and intracellular trafficking dynamics in the development of antibody drug conjugates. MAbs 2013, 5, 13–21. [Google Scholar] [CrossRef]
- Durbin, K.R.; Phipps, C.; Liao, X. Mechanistic Modeling of Antibody-Drug Conjugate Internalization at the Cellular Level Reveals Inefficient Processing Steps. Mol. Cancer Ther. 2018, 17, 1341–1351. [Google Scholar] [CrossRef]
- Hammood, M.; Craig, A.W.; Leyton, J.V. Impact of Endocytosis Mechanisms for the Receptors Targeted by the Currently Approved Antibody-Drug Conjugates (ADCs)-A Necessity for Future ADC Research and Development. Pharmaceuticals 2021, 14, 694. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Yang, T.; Zhu, J.; Zhang, Z.; Yang, L.; Zhang, Y.; Hu, C.; Chen, J.; Wang, J.; Tian, X.; et al. Spatiotemporal Quantification of HER2-targeting Antibody-Drug Conjugate Bystander Activity and Enhancement of Solid Tumor Penetration. Clin. Cancer Res. 2024, 30, 984–997. [Google Scholar] [CrossRef] [PubMed]
- Barok, M.; Tanner, M.; Köninki, K.; Isola, J. Trastuzumab-DM1 causes tumour growth inhibition by mitotic catastrophe in trastuzumab-resistant breast cancer cells in vivo. Breast Cancer Res. 2011, 13, R46. [Google Scholar] [CrossRef] [PubMed]
- Hartley, J.A.; Flynn, M.J.; Bingham, J.P.; Corbett, S.; Reinert, H.; Tiberghien, A.; Masterson, L.A.; Antonow, D.; Adams, L.; Chowdhury, S.; et al. Pre-clinical pharmacology and mechanism of action of SG3199, the pyrrolobenzodiazepine (PBD) dimer warhead component of antibody-drug conjugate (ADC) payload tesirine. Sci. Rep. 2018, 8, 10479. [Google Scholar] [CrossRef]
- Ghelli Luserna di Rorà, A.; Jandoubi, M.; Padella, A.; Ferrari, A.; Marranci, A.; Mazzotti, C.; Olimpico, F.; Ghetti, M.; Ledda, L.; Bochicchio, M.T.; et al. Exploring the role of PARP1 inhibition in enhancing antibody-drug conjugate therapy for acute leukemias: Insights from DNA damage response pathway interactions. J. Transl. Med. 2024, 22, 1062. [Google Scholar] [CrossRef]
- Pahl, A.; Lutz, C.; Hechler, T. Amanitins and their development as a payload for antibody-drug conjugates. Drug Discov. Today Technol. 2018, 30, 85–89. [Google Scholar] [CrossRef]
- Judd, A.S.; Bawa, B.; Buck, W.R.; Tao, Z.F.; Li, Y.; Mitten, M.J.; Bruncko, M.; Catron, N.; Doherty, G.; Durbin, K.R.; et al. BCL-X(L)-targeting antibody-drug conjugates are active in preclinical models and mitigate on-mechanism toxicity of small-molecule inhibitors. Sci. Adv. 2024, 10, eado7120. [Google Scholar] [CrossRef]
- Chang, H.P.; Le, H.K.; Shah, D.K. Pharmacokinetics and Pharmacodynamics of Antibody-Drug Conjugates Administered via Subcutaneous and Intratumoral Routes. Pharmaceutics 2023, 15, 1132. [Google Scholar] [CrossRef] [PubMed]
- Pitiot, A.; Heuzé-Vourc’h, N.; Sécher, T. Alternative Routes of Administration for Therapeutic Antibodies-State of the Art. Antibodies 2022, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Wynne, C.; Harvey, V.; Schwabe, C.; Waaka, D.; McIntyre, C.; Bittner, B. Comparison of Subcutaneous and Intravenous Administration of Trastuzumab: A Phase I/Ib Trial in Healthy Male Volunteers and Patients With HER2-Positive Breast Cancer. J. Clin. Pharmacol. 2013, 53, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Aznar, M.A.; Tinari, N.; Rullán, A.J.; Sánchez-Paulete, A.R.; Rodriguez-Ruiz, M.E.; Melero, I. Intratumoral Delivery of Immunotherapy-Act Locally, Think Globally. J. Immunol. 2017, 198, 31–39. [Google Scholar] [CrossRef]
- Zhang, R.; Hao, L.; Chen, P.; Zhang, G.; Liu, N. Multifunctional small-molecule theranostic agents for tumor-specific imaging and targeted chemotherapy. Bioorg. Chem. 2023, 137, 106576. [Google Scholar] [CrossRef]
- Smith, R.A.; Zammit, D.J.; Damle, N.K.; Usansky, H.; Reddy, S.P.; Lin, J.H.; Mistry, M.; Rao, N.S.; Denis, L.J.; Gupta, S. ASN004, A 5T4-targeting scFv-Fc Antibody-Drug Conjugate with High Drug-to-Antibody Ratio, Induces Complete and Durable Tumor Regressions in Preclinical Models. Mol. Cancer Ther. 2021, 20, 1327–1337. [Google Scholar] [CrossRef]
- Nessler, I.; Khera, E.; Vance, S.; Kopp, A.; Qiu, Q.; Keating, T.A.; Abu-Yousif, A.O.; Sandal, T.; Legg, J.; Thompson, L.; et al. Increased Tumor Penetration of Single-Domain Antibody-Drug Conjugates Improves In Vivo Efficacy in Prostate Cancer Models. Cancer Res. 2020, 80, 1268–1278. [Google Scholar] [CrossRef]
- Bordeau, B.M.; Nguyen, T.D.; Polli, J.R.; Chen, P.; Balthasar, J.P. Payload-Binding Fab Fragments Increase the Therapeutic Index of MMAE Antibody-Drug Conjugates. Mol. Cancer Ther. 2023, 22, 459–470. [Google Scholar] [CrossRef]
- Ober, R.J.; Martinez, C.; Vaccaro, C.; Zhou, J.; Ward, E.S. Visualizing the site and dynamics of IgG salvage by the MHC class I-related receptor, FcRn. J. Immunol. 2004, 172, 2021–2029. [Google Scholar] [CrossRef]
- Martin, W.L.; West, A.P., Jr.; Gan, L.; Bjorkman, P.J. Crystal structure at 2.8 A of an FcRn/heterodimeric Fc complex: Mechanism of pH-dependent binding. Mol. Cell 2001, 7, 867–877. [Google Scholar] [CrossRef]
- Wei, B.; Gunzner-Toste, J.; Yao, H.; Wang, T.; Wang, J.; Xu, Z.; Chen, J.; Wai, J.; Nonomiya, J.; Tsai, S.P.; et al. Discovery of Peptidomimetic Antibody–Drug Conjugate Linkers with Enhanced Protease Specificity. J. Med. Chem. 2018, 61, 989–1000. [Google Scholar] [CrossRef]
- Caculitan, N.G.; Dela Cruz Chuh, J.; Ma, Y.; Zhang, D.; Kozak, K.R.; Liu, Y.; Pillow, T.H.; Sadowsky, J.; Cheung, T.K.; Phung, Q.; et al. Cathepsin B Is Dispensable for Cellular Processing of Cathepsin B-Cleavable Antibody-Drug Conjugates. Cancer Res. 2017, 77, 7027–7037. [Google Scholar] [CrossRef]
- Su, D.; Kozak, K.R.; Sadowsky, J.; Yu, S.F.; Fourie-O’Donohue, A.; Nelson, C.; Vandlen, R.; Ohri, R.; Liu, L.; Ng, C.; et al. Modulating Antibody-Drug Conjugate Payload Metabolism by Conjugation Site and Linker Modification. Bioconjugate Chem. 2018, 29, 1155–1167. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Zhang, D. Linker Design Impacts Antibody-Drug Conjugate Pharmacokinetics and Efficacy via Modulating the Stability and Payload Release Efficiency. Front. Pharmacol. 2021, 12, 687926. [Google Scholar] [CrossRef] [PubMed]
- Polson, A.G.; Calemine-Fenaux, J.; Chan, P.; Chang, W.; Christensen, E.; Clark, S.; de Sauvage, F.J.; Eaton, D.; Elkins, K.; Elliott, J.M.; et al. Antibody-Drug Conjugates for the Treatment of Non–Hodgkin’s Lymphoma: Target and Linker-Drug Selection. Cancer Res. 2009, 69, 2358–2364. [Google Scholar] [CrossRef] [PubMed]
- Hamann, P.R.; Hinman, L.M.; Hollander, I.; Beyer, C.F.; Lindh, D.; Holcomb, R.; Hallett, W.; Tsou, H.R.; Upeslacis, J.; Shochat, D.; et al. Gemtuzumab ozogamicin, a potent and selective anti-CD33 antibody-calicheamicin conjugate for treatment of acute myeloid leukemia. Bioconjugate Chem. 2002, 13, 47–58. [Google Scholar] [CrossRef]
- Younes, A.; Bartlett, N.L.; Leonard, J.P.; Kennedy, D.A.; Lynch, C.M.; Sievers, E.L.; Forero-Torres, A. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N. Engl. J. Med. 2010, 363, 1812–1821. [Google Scholar] [CrossRef]
- Kinneer, K.; Meekin, J.; Tiberghien, A.C.; Tai, Y.T.; Phipps, S.; Kiefer, C.M.; Rebelatto, M.C.; Dimasi, N.; Moriarty, A.; Papadopoulos, K.P.; et al. SLC46A3 as a Potential Predictive Biomarker for Antibody-Drug Conjugates Bearing Noncleavable Linked Maytansinoid and Pyrrolobenzodiazepine Warheads. Clin. Cancer Res. 2018, 24, 6570–6582. [Google Scholar] [CrossRef]
- Bosi, C.; Bartha, Á.; Galbardi, B.; Notini, G.; Naldini, M.M.; Licata, L.; Viale, G.; Mariani, M.; Pistilli, B.; Ali, H.R.; et al. Pan-cancer analysis of antibody-drug conjugate targets and putative predictors of treatment response. Eur. J. Cancer 2023, 195, 113379. [Google Scholar] [CrossRef]
- Weddell, J.; Chiney, M.S.; Bhatnagar, S.; Gibbs, J.P.; Shebley, M. Mechanistic Modeling of Intra-Tumor Spatial Distribution of Antibody-Drug Conjugates: Insights into Dosing Strategies in Oncology. Clin. Transl. Sci. 2021, 14, 395–404. [Google Scholar] [CrossRef]
- Liao, M.Z.; Lu, D.; Kågedal, M.; Miles, D.; Samineni, D.; Liu, S.N.; Li, C. Model-Informed Therapeutic Dose Optimization Strategies for Antibody-Drug Conjugates in Oncology: What Can We Learn From US Food and Drug Administration-Approved Antibody-Drug Conjugates? Clin. Pharmacol. Ther. 2021, 110, 1216–1230. [Google Scholar] [CrossRef] [PubMed]
- Palma Chaundler, C.S.; Lu, H.; Fu, R.; Wang, N.; Lou, H.; de Almeida, G.S.; Hadi, L.M.; Aboagye, E.O.; Ghaem-Maghami, S. Kinetics and efficacy of antibody drug conjugates in 3D tumour models. bioRxiv 2023. [Google Scholar] [CrossRef]
- Petersen, M.E.; Brant, M.G.; Lasalle, M.; Das, S.; Duan, R.; Wong, J.; Ding, T.; Wu, K.J.; Siddappa, D.; Fang, C.; et al. Design and Evaluation of ZD06519, a Novel Camptothecin Payload for Antibody Drug Conjugates. Mol. Cancer Ther. 2024, 23, 606–618. [Google Scholar] [CrossRef] [PubMed]
- Parakh, S.; Huynh, N.; Cao, D.D.; Rigopoulos, A.; Gloria, B.; Burvenich, I.J.; Murone, C.; Wichmann, C.W.; Guo, N.Y.; Senko, C.; et al. Characterization of mAb104, a Monoclonal Antibody Targeting a Conformationally Exposed, Tumor-specific epitope of HER2. Mol. Cancer Ther. 2025; ahead of print. [Google Scholar] [CrossRef]
- Aguiar, S.; Dias, J.; Manuel, A.M.; Russo, R.; Gois, P.M.P.; da Silva, F.A.; Goncalves, J. Chimeric Small Antibody Fragments as Strategy to Deliver Therapeutic Payloads. Adv. Protein Chem. Struct. Biol. 2018, 112, 143–182. [Google Scholar] [CrossRef] [PubMed]
- Corti, C.; Boscolo Bielo, L.; Schianca, A.C.; Salimbeni, B.T.; Criscitiello, C.; Curigliano, G. Future potential targets of antibody-drug conjugates in breast cancer. Breast 2023, 69, 312–322. [Google Scholar] [CrossRef]
- Khairani, A.F.; Harmonia, S.; Chou, Y.; Alfarafisa, N.M.; Ramadhanti, J. Optimizing Xenograft Models for Breast Cancer: A Comparative Analysis of Cell-Derived and Patient-Derived Implantation Techniques in Pre-Clinical Research. Breast Cancer 2025, 17, 1–10. [Google Scholar] [CrossRef]
- Schlam, I.; Moges, R.; Morganti, S.; Tolaney, S.M.; Tarantino, P. Next-generation antibody-drug conjugates for breast cancer: Moving beyond HER2 and TROP2. Crit. Rev. Oncol. Hematol. 2023, 190, 104090. [Google Scholar] [CrossRef]
- Nader-Marta, G.; Molinelli, C.; Debien, V.; Martins-Branco, D.; Aftimos, P.; de Azambuja, E.; Awada, A. Antibody-drug conjugates: The evolving field of targeted chemotherapy for breast cancer treatment. Ther. Adv. Med. Oncol. 2023, 15, 17588359231183679. [Google Scholar] [CrossRef]
- Kogai, H.; Tsukamoto, S.; Koga, M.; Miyano, M.; Akagi, T.; Yamaguchi, A.; Mori, K.; Gotoh, K.; Nakazawa, Y. Broad-Spectrum Efficacy of CEACAM6-Targeted Antibody-Drug Conjugate with BET Protein Degrader in Colorectal, Lung, and Breast Cancer Mouse Models. Mol. Cancer Ther. 2025, 24, 392–405. [Google Scholar] [CrossRef]
- Feng, Y.; Lee, J.; Yang, L.; Hilton, M.B.; Morris, K.; Seaman, S.; Edupuganti, V.; Hsu, K.S.; Dower, C.; Yu, G.; et al. Engineering CD276/B7-H3-targeted antibody-drug conjugates with enhanced cancer-eradicating capability. Cell Rep. 2023, 42, 113503. [Google Scholar] [CrossRef]
- Fernández-Santiago, C.; Martínez-Pena, I.; Paramés, M.; Rodríguez-Pérez, M.; Pablo, H.; Abuín, C.; Costa, C.; Dávila-Ibáñez, A.B.; López, R.L.-; Piñeiro, R. “A CTC Model Uncovers Metastatic Drivers and Prognostic Markers in Breast Cancer”. medRxiv 2025. medRxiv:25320597. [Google Scholar] [CrossRef]
- Nucera, S.; Conti, C.; Martorana, F.; Wilson, B.; Genta, S. Antibody-Drug Conjugates to Promote Immune Surveillance: Lessons Learned from Breast Cancer. Biomedicines 2024, 12, 1491. [Google Scholar] [CrossRef]
- Tagawa, S.T.; Balar, A.V.; Petrylak, D.P.; Kalebasty, A.R.; Loriot, Y.; Fléchon, A.; Jain, R.K.; Agarwal, N.; Bupathi, M.; Barthelemy, P.; et al. TROPHY-U-01: A Phase II Open-Label Study of Sacituzumab Govitecan in Patients With Metastatic Urothelial Carcinoma Progressing After Platinum-Based Chemotherapy and Checkpoint Inhibitors. J. Clin. Oncol. 2021, 39, 2474–2485. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Fan, X.; Liu, H.; Liang, T. Advances in Trop-2 targeted antibody-drug conjugates for breast cancer: Mechanisms, clinical applications, and future directions. Front. Immunol. 2024, 15, 1495675. [Google Scholar] [CrossRef]
- Bardia, A.; Rugo, H.S.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Kalinsky, K.; Cortés, J.; Shaughnessy, J.O.; et al. Final Results From the Randomized Phase III ASCENT Clinical Trial in Metastatic Triple-Negative Breast Cancer and Association of Outcomes by Human Epidermal Growth Factor Receptor 2 and Trophoblast Cell Surface Antigen 2 Expression. J. Clin. Oncol. 2024, 42, 1738–1744. [Google Scholar] [CrossRef] [PubMed]
- Naidu, R.; Yadav, M.; Nair, S.; Kutty, M.K. Expression of c-erbB3 protein in primary breast carcinomas. Br. J. Cancer 1998, 78, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Ali, S.; Mata, D.; Lohmann, A.E.; Blanchette, P.S. Antibody-Drug Conjugates in Breast Cancer: Ascent to Destiny and Beyond-A 2023 Review. Curr. Oncol. 2023, 30, 6447–6461. [Google Scholar] [CrossRef]
- Modi, S.; Pusztai, L.; Forero, A.; Mita, M.; Miller, K.; Weise, A.; Krop, I.; Burris, H., III; Kalinsky, K.; Tsai, M.; et al. Abstract PD3-14: Phase 1 study of the antibody-drug conjugate SGN-LIV1A in patients with heavily pretreated triple-negative metastatic breast cancer. Cancer Res. 2018, 78 (Suppl. S4), PD3-14. [Google Scholar] [CrossRef]
- Qu, F.; Lu, R.; Liu, Q.; Wu, X.; Huang, X.; Yin, Y.; Li, W. Antibody-drug conjugates transform the outcome of individuals with low-HER2-expression advanced breast cancer. Cancer 2024, 130, 1392–1402. [Google Scholar] [CrossRef]
- Cao, W.; Xing, H.; Li, Y.; Tian, W.; Song, Y.; Jiang, Z.; Yu, J. Claudin18.2 is a novel molecular biomarker for tumor-targeted immunotherapy. Biomark. Res. 2022, 10, 38. [Google Scholar] [CrossRef]
- Decary, S.; Berne, P.F.; Nicolazzi, C.; Lefebvre, A.M.; Dabdoubi, T.; Cameron, B.; Rival, P.; Devaud, C.; Prades, C.; Bouchard, H.; et al. Preclinical Activity of SAR408701: A Novel Anti-CEACAM5-maytansinoid Antibody-drug Conjugate for the Treatment of CEACAM5-positive Epithelial Tumors. Clin. Cancer Res. 2020, 26, 6589–6599. [Google Scholar] [CrossRef]
- Yang, T.; Li, W.; Huang, T.; Zhou, J. Antibody-Drug Conjugates for Breast Cancer Treatment: Emerging Agents, Targets and Future Directions. Int. J. Mol. Sci. 2023, 24, 11903. [Google Scholar] [CrossRef]
- Gupta, N.; Geethika, L.S.; Sneha, P. Antibody-drug Conjugates in Cancer Treatment: An Overview. J. Cancer Tumor Int. 2024, 14, 33–45. [Google Scholar] [CrossRef]
- Dean, A.Q.; Luo, S.; Twomey, J.D.; Zhang, B. Targeting cancer with antibody-drug conjugates: Promises and challenges. MAbs 2021, 13, 1951427. [Google Scholar] [CrossRef] [PubMed]
- Paz-Manrique, R.; Pinto, J.A.; Gomez Moreno, H.L. Antibody-Drug Conjugates in Breast Cancer: Toward a Molecular Perspective Into Clinical Practice. JCO Precis. Oncol. 2024, 8, e2400173. [Google Scholar] [CrossRef] [PubMed]
- Khongorzul, P.; Ling, C.J.; Khan, F.U.; Ihsan, A.U.; Zhang, J. Antibody–drug conjugates: A comprehensive review. Mol. Cancer Res. 2020, 18, 3–19. [Google Scholar] [CrossRef]
- Peters, C.; Brown, S. Antibody-drug conjugates as novel anti-cancer chemotherapeutics. Biosci. Rep. 2015, 35, e00225. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, J. Therapeutic antibodies for precise cancer immunotherapy: Current and future perspectives. Med. Rev. 2022, 2, 555–569. [Google Scholar] [CrossRef]
- Riccardi, F.; Dal Bo, M.; Macor, P.; Toffoli, G. A comprehensive overview on antibody-drug conjugates: From the conceptualization to cancer therapy. Front. Pharmacol. 2023, 14, 1274088. [Google Scholar] [CrossRef]
- Kovtun, Y.V.; Audette, C.A.; Ye, Y.; Xie, H.; Ruberti, M.F.; Phinney, S.J.; Leece, B.A.; Chittenden, T.; Blättler, W.A.; Goldmacher, V.S. Antibody-Drug Conjugates Designed to Eradicate Tumors with Homogeneous and Heterogeneous Expression of the Target Antigen. Cancer Res. 2006, 66, 3214–3221. [Google Scholar] [CrossRef]
- Selvakumar, P.; Prabakaran, M.; Bhattacharya, S. Antibody-Drug Conjugates (ADCs). In Spatially Variable Genes in Cancer: Development, Progression, and Treatment Response; Raghavan, R., Ed.; IGI Global Scientific Publishing: Hershey, PA, USA, 2025; pp. 429–446. [Google Scholar]
- Bargh, J.D.; Isidro-Llobet, A.; Parker, J.S.; Spring, D.R. Cleavable linkers in antibody-drug conjugates. Chem. Soc. Rev. 2019, 48, 4361–4374. [Google Scholar] [CrossRef]
- Erickson, H.K.; Park, P.U.; Widdison, W.C.; Kovtun, Y.V.; Garrett, L.M.; Hoffman, K.; Lutz, R.J.; Goldmacher, V.S.; Blättler, W.A. Antibody-Maytansinoid Conjugates Are Activated in Targeted Cancer Cells by Lysosomal Degradation and Linker-Dependent Intracellular Processing. Cancer Res. 2006, 66, 4426–4433. [Google Scholar] [CrossRef]
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody drug conjugate: The “biological missile” for targeted cancer therapy. Signal Transduct. Target. Ther. 2022, 7, 93. [Google Scholar] [CrossRef]
- Izzo, D.; Ascione, L.; Guidi, L.; Marsicano, R.M.; Koukoutzeli, C.; Trapani, D.; Curigliano, G. Innovative payloads for ADCs in cancer treatment: Moving beyond the selective delivery of chemotherapy. Ther. Adv. Med. Oncol. 2025, 17, 17588359241309461. [Google Scholar] [CrossRef]
- Park, M.H.; Lee, B.I.; Byeon, J.J.; Shin, S.H.; Choi, J.; Park, Y.; Shin, Y.G. Pharmacokinetic and Metabolism Studies of Monomethyl Auristatin F via Liquid Chromatography-Quadrupole-Time-of-Flight Mass Spectrometry. Molecules 2019, 24, 2754. [Google Scholar] [CrossRef] [PubMed]
- McKertish, C.M.; Kayser, V. Advances and Limitations of Antibody Drug Conjugates for Cancer. Biomedicines 2021, 9, 872. [Google Scholar] [CrossRef] [PubMed]
- Amani, N.; Dorkoosh, F.A.; Mobedi, H. ADCs, as novel revolutionary weapons for providing a step forward in targeted therapy of malignancies. Curr. Drug Deliv. 2020, 17, 23–51. [Google Scholar] [CrossRef]
- Larose, E.A.; Hua, X.; Yu, S.; Pillai, A.T.; Yi, Z.; Yu, H. Antibody-drug conjugates in breast cancer treatment: Resistance mechanisms and the role of therapeutic sequencing. Cancer Drug Resist. 2025, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.L.; Schwettmann, B.; McArthur, H.L.; Chan, I.S. Antibody-drug conjugates in breast cancer: Overcoming resistance and boosting immune response. J. Clin. Invest. 2023, 133, e172156. [Google Scholar] [CrossRef]
- Abuqayyas, L.; Zhang, X.; Balthasar, J.P. Application of knockout mouse models to investigate the influence of FcγR on the pharmacokinetics and anti-platelet effects of MWReg30, a monoclonal anti-GPIIb antibody. Int. J. Pharm. 2013, 444, 185–192. [Google Scholar] [CrossRef]
- Carrasco-Triguero, M.; Dere, R.C.; Milojic-Blair, M.; Saad, O.M.; Nazzal, D.; Hong, K.; Kaur, S. Immunogenicity of antibody-drug conjugates: Observations across 8 molecules in 11 clinical trials. Bioanalysis 2019, 11, 1555–1568. [Google Scholar] [CrossRef]
- Schäffler, H.; Jakob, D.; Huesmann, S.; Pfister, K.; Veselinovic, K.; Schochter, F.; Leinert, E.; Fink, V.; Rack, B.; Englisch, A.; et al. Novel Antibody-Drug-Conjugates in Routine Clinical Practice for the Treatment of Metastatic Breast Cancer: Adherence, Efficacy and Tolerability—Real-World Data from German Breast Centers. Geburtshilfe Frauenheilkd. 2024, 84, 855–865. [Google Scholar] [CrossRef]
- Pizano-Martinez, O.; Mendieta-Condado, E.; Vazquez-Del Mercado, M.; Martinez-Garcia, E.A.; Chavarria-Avila, E.; Ortuno-Sahagun, D.; Marquez-Aguirre, A.L. Anti-Drug Antibodies in the Biological Therapy of Autoimmune Rheumatic Diseases. J. Clin. Med. 2023, 12, 3271. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Adhikari, D.; Rojo, A.L.; Kelly, L.; Ibdah, A.; Orbegoso, I.; Eak, G.; Merriman, C.; Feng, J.; Trexler, K.; et al. Detection of Anti-drug Antibodies (ADAs) to an Antibody-drug Conjugate (ADC) PYX-201 in Human Plasma Using a Novel Electrochemiluminescence (ECL) Immunoassay. Curr. Anal. Chem. 2025, 21, 1573–4110. [Google Scholar] [CrossRef]
- Yin, F.; Adhikari, D.; Liu, X.F.; Xu, T.; Liao, L.; Landauer, T.; Ke, Y.; Yang, W.; Simon, T.; Lei, W.; et al. A Novel Electrochemiluminescence (ECL) Immunoassay for Detection of Anti-drug Antibodies (ADAs) to a Monoclonal Antibody (mAb) PYX-106 in Human Serum. Curr. Anal. Chem. 2024, 21, 702–715. [Google Scholar] [CrossRef]
- Partridge, M.A.; Purushothama, S.; Elango, C.; Lu, Y. Emerging Technologies and Generic Assays for the Detection of Anti-Drug Antibodies. J. Immunol. Res. 2016, 2016, 6262383. [Google Scholar] [CrossRef]
- Chen, L.Z.; Roos, D.; Philip, E. Development of Immunocapture-LC/MS Assay for Simultaneous ADA Isotyping and Semiquantitation. J. Immunol. Res. 2016, 2016, 7682472. [Google Scholar] [CrossRef]
- Suh, K.; Kyei, I.; Hage, D.S. Approaches for the detection and analysis of antidrug antibodies to biopharmaceuticals: A review. J. Sep. Sci. 2022, 45, 2077–2092. [Google Scholar] [CrossRef]
- Chen, N.; Michaels, E.; Howard, F.; Nanda, R. The evolving therapeutic landscape of antibody–drug conjugates in breast cancer. Expert Rev. Anticancer Ther. 2022, 22, 1325–1331. [Google Scholar] [CrossRef]
- Ali, M.A.; Aiman, W.; Afzal, F.; Zahoor, H.; Kazmi, S.H.; Anwar, A.; Bajwa, A.R.; Maroules, M.; Guron, G.K.; Shaaban, H.S. Efficacy of antibody-drug conjugates in breast cancer: A systematic review and meta-analysis of randomized clinical trials. J. Clin. Oncol. 2023, 41, e13113. [Google Scholar] [CrossRef]
- Deslandes, A. Comparative clinical pharmacokinetics of antibody-drug conjugates in first-in-human Phase 1 studies. MAbs 2014, 6, 859–870. [Google Scholar] [CrossRef]
- Michelon, I.; Dacoregio, M.I.; Vilbert, M.N.; Priantti, J.; Castro, C.; Vian, L.; Cavalcante, L. Antibody-drug conjugates (ADC) in patients with advanced/metastatic HER2-low expressing breast cancer: A systematic review and meta-analysis. J. Clin. Oncol. 2024, 42, e13174. [Google Scholar] [CrossRef]
- Lotz, G.P.; Benstein, K.; Bloem, K.; Buddiger, H.; Calonder, C.; Elm, S.; Fernandez, E.; Goodman, J.; Gorovits, B.; Grudzinska-Goebel, J.; et al. When to Extend Monitoring of Anti-drug Antibodies for High-risk Biotherapeutics in Clinical Trials: An Opinion from the European Immunogenicity Platform. AAPS J. 2022, 24, 68. [Google Scholar] [CrossRef]
- Trukhin, D.; Poddubskaya, E.; Andric, Z.; Makharadze, T.; Bellala, R.S.; Charoentum, C.; Yanez Ruiz, E.P.; Fulop, A.; Hyder Ali, I.A.; Syrigos, K.; et al. Efficacy, Safety and Immunogenicity of MB02 (Bevacizumab Biosimilar) versus Reference Bevacizumab in Advanced Non-Small Cell Lung Cancer: A Randomized, Double-Blind, Phase III Study (STELLA). BioDrugs 2021, 35, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Jena, D.; Bhatta, P.; Mishra, K. Efficacy, Pharmacokinetics, Pharmacodynamics, Immunogenicity, and Safety of Rituximab (Test Product, Zydus) vs Rituximab (Reference Product, Roche/Genentech) in Patients with Diffuse Large B Cell Lymphoma (DLBCL). Asian J. Res. Pharm. Sci. 2024, 14, 203–210. [Google Scholar] [CrossRef]
- Harris, C.T.; Cohen, S. Reducing Immunogenicity by Design: Approaches to Minimize Immunogenicity of Monoclonal Antibodies. BioDrugs 2024, 38, 205–226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ou, C.; Liu, H.; Prabhu, S.K.; Li, C.; Yang, Q.; Wang, L.-X. General and Robust Chemoenzymatic Method for Glycan-Mediated Site-Specific Labeling and Conjugation of Antibodies: Facile Synthesis of Homogeneous Antibody–Drug Conjugates. ACS Chem. Biol. 2021, 16, 2502–2514. [Google Scholar] [CrossRef]
- Liu, J.; Yang, J.; Sun, Y.; Gong, J.; Yue, J.; Pan, Y.; Sun, M.; Song, R.; Xiao, X.; Tazbirkova, A.; et al. CLDN18.2–targeting antibody–drug conjugate IBI343 in advanced gastric or gastroesophageal junction adenocarcinoma: A phase 1 trial. Nat. Med. 2025. [Google Scholar] [CrossRef]
- Yan, J.; Wang, K.; Liu, H.; Wang, L.; Li, Y.; Zhang, G.; Deng, L. Construction of electrochemical biosensors based on MoSe2@1T-MoS2 heterojunction for the sensitive and rapid detection of miRNA-155 biomarker in breast cancer. Bioelectrochemistry 2023, 154, 108541. [Google Scholar] [CrossRef]
- Shashkova, T.I.; Umerenkov, D.; Salnikov, M.; Strashnov, P.V.; Konstantinova, A.V.; Lebed, I.; Shcherbinin, D.N.; Asatryan, M.N.; Kardymon, O.L.; Ivanisenko, N.V. SEMA: Antigen B-cell conformational epitope prediction using deep transfer learning. Front. Immunol. 2022, 13, 960985. [Google Scholar] [CrossRef] [PubMed]
- Ruffolo, J.A.; Chu, L.-S.; Mahajan, S.P.; Gray, J.J. Fast, accurate antibody structure prediction from deep learning on massive set of natural antibodies. Nat. Commun. 2023, 14, 2389. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.M.; Selvarajoo, K. Advancing drug-response prediction using multi-modal and -omics machine learning integration (MOMLIN): A case study on breast cancer clinical data. Brief. Bioinform. 2024, 25, bbae300. [Google Scholar] [CrossRef] [PubMed]
- Saka, K.; Kakuzaki, T.; Metsugi, S.; Kashiwagi, D.; Yoshida, K.; Wada, M.; Tsunoda, H.; Teramoto, R. Antibody design using LSTM based deep generative model from phage display library for affinity maturation. Sci. Rep. 2021, 11, 5852. [Google Scholar] [CrossRef]
- Schneider, C.; Buchanan, A.; Taddese, B.; Deane, C.M. DLAB: Deep learning methods for structure-based virtual screening of antibodies. Bioinformatics 2021, 38, 377–383. [Google Scholar] [CrossRef]
- Kang, Y.; Leng, D.; Guo, J.; Pan, L. Sequence-based deep learning antibody design for in silico antibody affinity maturation. arXiv 2021, arXiv:2103.03724. [Google Scholar]
- Bardia, A.; Hu, X.; Dent, R.; Yonemori, K.; Barrios, C.H.; O’Shaughnessy, J.A.; Wildiers, H.; Pierga, J.-Y.; Zhang, Q.; Saura, C.; et al. Trastuzumab Deruxtecan after Endocrine Therapy in Metastatic Breast Cancer. N. Engl. J. Med. 2024, 391, 2110–2122. [Google Scholar] [CrossRef]
- Arruda Navarro Albuquerque, D.; Trotta Vianna, M.; Alencar Fernandes Sampaio, L.; Vasiliu, A.; Cunha Neves Filho, E.H. Diagnostic Accuracy of Artificial Intelligence in Classifying HER2 Status in Breast Cancer Immunohistochemistry Slides and Implications for HER2-Low Cases: A Systematic Review and Meta-Analysis. medRxiv 2024. [Google Scholar] [CrossRef]
- EBCTCG. Trastuzumab for early-stage, HER2-positive breast cancer: A meta-analysis of 13,864 women in seven randomised trials. Lancet Oncol. 2021, 22, 1139–1150. [Google Scholar] [CrossRef]
- Garrido, C.; Manoogian, M.; Ghambire, D.; Lucas, S.; Karnoub, M.; Olson, M.T.; Hicks, D.G.; Tozbikian, G.; Prat, A.; Ueno, N.T.; et al. Analytical and clinical validation of PATHWAY Anti-HER-2/neu (4B5) antibody to assess HER2-low status for trastuzumab deruxtecan treatment in breast cancer. Virchows Arch. 2024, 484, 1005–1014. [Google Scholar] [CrossRef]
- Pillow, T.H.; Adhikari, P.; Blake, R.A.; Chen, J.; Del Rosario, G.; Deshmukh, G.; Figueroa, I.; Gascoigne, K.E.; Kamath, A.V.; Kaufman, S.; et al. Antibody Conjugation of a Chimeric BET Degrader Enables in vivo Activity. ChemMedChem 2020, 15, 17–25. [Google Scholar] [CrossRef]
- Shuai, R.W.; Ruffolo, J.A.; Gray, J.J. IgLM: Infilling language modeling for antibody sequence design. Cell Syst. 2023, 14, 979–989.e974. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabit, H.; Abbas, S.; El-Safoury, M.T.; Madkour, E.M.; Mahmoud, S.; Abdel-Ghany, S.; Albrahim, Y.; Al-Dhuayan, I.S.; Rashwan, S.; El-Hashash, A.; et al. Antibody–Drug Conjugates in Breast Cancer: Navigating Innovations, Overcoming Resistance, and Shaping Future Therapies. Biomedicines 2025, 13, 2227. https://doi.org/10.3390/biomedicines13092227
Sabit H, Abbas S, El-Safoury MT, Madkour EM, Mahmoud S, Abdel-Ghany S, Albrahim Y, Al-Dhuayan IS, Rashwan S, El-Hashash A, et al. Antibody–Drug Conjugates in Breast Cancer: Navigating Innovations, Overcoming Resistance, and Shaping Future Therapies. Biomedicines. 2025; 13(9):2227. https://doi.org/10.3390/biomedicines13092227
Chicago/Turabian StyleSabit, Hussein, Salma Abbas, Moataz T. El-Safoury, Engy M. Madkour, Sahar Mahmoud, Shaimaa Abdel-Ghany, Yasser Albrahim, Ibtesam S. Al-Dhuayan, Sanaa Rashwan, Ahmed El-Hashash, and et al. 2025. "Antibody–Drug Conjugates in Breast Cancer: Navigating Innovations, Overcoming Resistance, and Shaping Future Therapies" Biomedicines 13, no. 9: 2227. https://doi.org/10.3390/biomedicines13092227
APA StyleSabit, H., Abbas, S., El-Safoury, M. T., Madkour, E. M., Mahmoud, S., Abdel-Ghany, S., Albrahim, Y., Al-Dhuayan, I. S., Rashwan, S., El-Hashash, A., & Arneth, B. (2025). Antibody–Drug Conjugates in Breast Cancer: Navigating Innovations, Overcoming Resistance, and Shaping Future Therapies. Biomedicines, 13(9), 2227. https://doi.org/10.3390/biomedicines13092227









