Congenital Thrombophilia in Chronic Thromboembolic Pulmonary Hypertension (CTEPH): A Systematic Review of Prevalence, Clinical Phenotype, and Surgical Outcomes
Abstract
1. Introduction
2. Materials and Methods
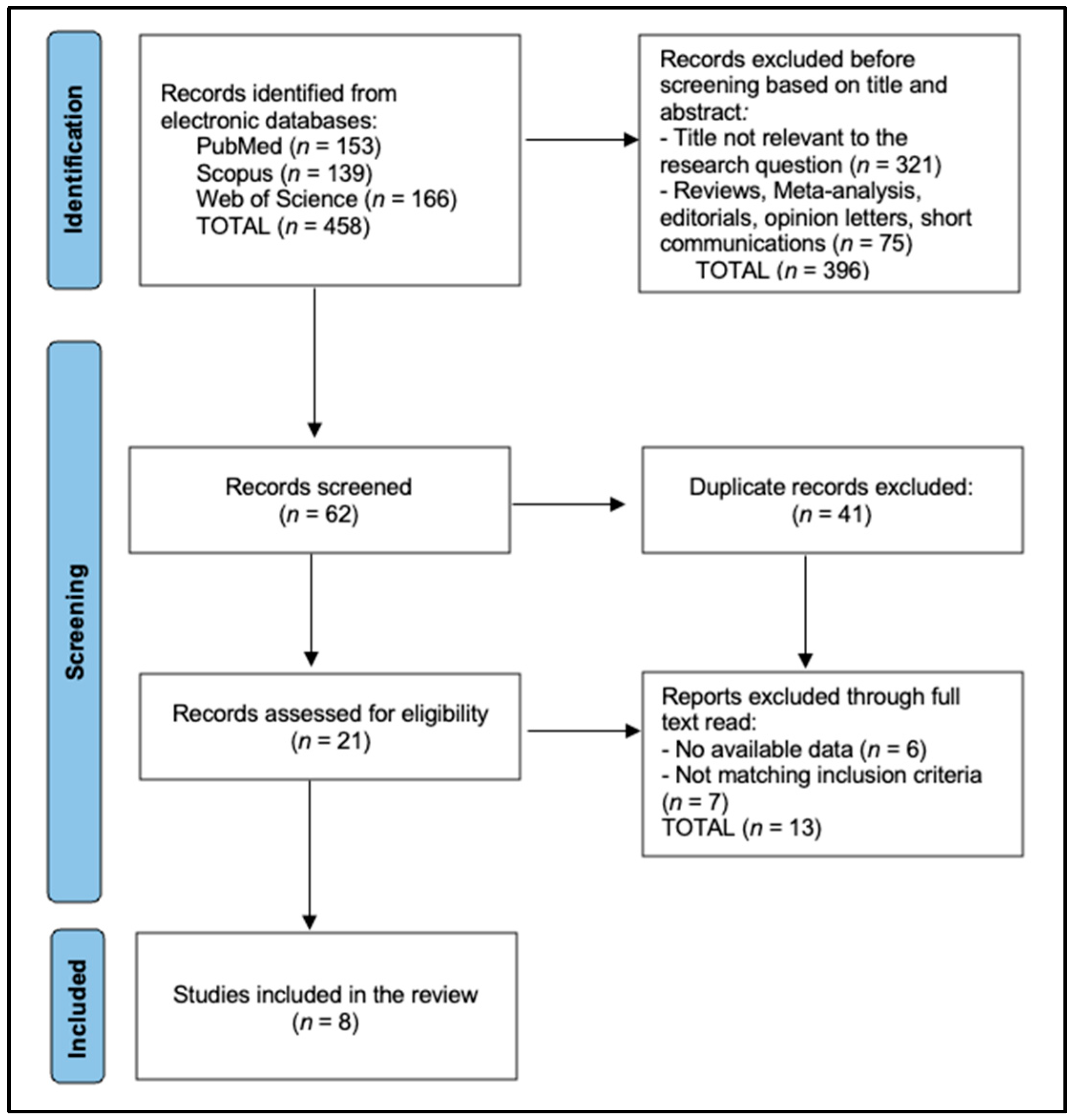
2.1. Protocol, Registration and Reporting Framework
2.2. Eligibility Criteria and Conceptual Framework
2.3. Information Sources and Exhaustive Search Strategy
2.4. Study Selection, Data-Extraction and Management
2.5. Risk-of-Bias Appraisal, Synthesis and Certainty Assessment
3. Results
4. Discussion
4.1. Summary of Evidence
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luijten, D.; Talerico, R.; Barco, S.; Cannegieter, S.C.; Delcroix, M.; Ende-Verhaar, Y.M.; Huisman, M.V.; Konstantinidis, S.; Mairuhu, A.T.A.; van Mens, T.E.; et al. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: An updated systematic review and meta-analysis. Eur. Respir. J. 2023, 62, 2300449. [Google Scholar] [CrossRef] [PubMed]
- Valerio, L.; Mavromanoli, A.C.; Barco, S.; Abele, C.; Becker, D.; Bruch, L.; Ewert, R.; Faehling, M.; Fistera, D.; Gerhardt, F.; et al. Chronic thromboembolic pulmonary hypertension and impairment after pulmonary embolism: The FOCUS study. Eur. Heart J. 2022, 43, 3387–3398. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morris, T.A.; Marsh, J.J.; Chiles, P.G.; Auger, W.R.; Fedullo, P.F.; Woods, V.L., Jr. Fibrin derived from patients with chronic thromboembolic pulmonary hypertension is resistant to lysis. Am. J. Respir. Crit. Care Med. 2006, 173, 1270–1275. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dicks, A.B.; Moussallem, E.; Stanbro, M.; Walls, J.; Gandhi, S.; Gray, B.H. A Comprehensive Review of Risk Factors and Thrombophilia Evaluation in Venous Thromboembolism. J. Clin. Med. 2024, 13, 362. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bonderman, D.; Turecek, P.L.; Jakowitsch, J.; Weltermann, A.; Adlbrecht, C.; Schneider, B.; Kneussl, M.; Rubin, L.J.; Kyrle, P.A.; Klepetko, W.; et al. High prevalence of elevated clotting factor VIII in chronic thromboembolic pulmonary hypertension. Thromb. Haemost. 2003, 90, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Kido, K.; Shimizu, M.; Shiga, T.; Hashiguchi, M.; Jalil, B.; Caccamo, M.; Sokos, G. Meta-Analysis Comparing Direct Oral Anticoagulants Versus Vitamin K Antagonists in Patients With Chronic Thromboembolic Pulmonary Hypertension. Am. J. Cardiol. 2024, 210, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Bonderman, D.; Wilkens, H.; Wakounig, S.; Schäfers, H.J.; Jansa, P.; Lindner, J.; Simkova, I.; Martischnig, A.M.; Dudczak, J.; Sadushi, R.; et al. Risk factors for chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2009, 33, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Barati, S.; Amini, H.; Ahmadi, Z.H.; Dastan, A.; Sharif Kashani, B.; Eskandari, R.; Dastan, F. Evaluating the efficacy and safety of rivaroxaban as a warfarin alternative in chronic thromboembolic pulmonary hypertension patients undergoing pulmonary endarterectomy: A randomized clinical trial. Rev. Port. Cardiol. 2023, 42, 139–144, (In English, Portuguese). [Google Scholar] [CrossRef] [PubMed]
- Benzidia, I.; Robitaille, C.; Abualsaud, A.; McDonald, L.; Lesenko, L.; Morin, J.F.; Langleben, D.; Kahn, S.R.; Hirsch, A. Safety and efficacy of direct oral anticoagulants in patients with chronic thromboembolic pulmonary hypertension. Thromb. Res. 2023, 229, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Yaoita, N.; Satoh, K.; Satoh, T.; Shimizu, T.; Saito, S.; Sugimura, K.; Tatebe, S.; Yamamoto, S.; Aoki, T.; Kikuchi, N.; et al. Identification of the Novel Variants in Patients With Chronic Thromboembolic Pulmonary Hypertension. J. Am. Heart Assoc. 2020, 9, e015902. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liley, J.; Newnham, M.; Bleda, M.; Bunclark, K.; Auger, W.; Barbera, J.A.; Bogaard, H.; Delcroix, M.; Fernandes, T.M.; Howard, L.; et al. Shared and Distinct Genomics of Chronic Thromboembolic Pulmonary Hypertension and Pulmonary Embolism. Am. J. Respir. Crit. Care Med. 2024, 209, 1477–1485. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Satoh, T.; Satoh, K.; Yaoita, N.; Kikuchi, N.; Omura, J.; Kurosawa, R.; Numano, K.; Al-Mamun, E.; Siddique, M.A.; Sunamura, S.; et al. Activated TAFI Promotes the Development of Chronic Thromboembolic Pulmonary Hypertension: A Possible Novel Therapeutic Target. Circ. Res. 2017, 120, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Jain, H.; Odat, R.M.; Ahmed, M.; Jain, J.; Goyal, A.; Idrees, M.; Passey, S.; Jha, J.; Shah, J.; Gole, S. Safety and Outcomes with Direct Oral Anticoagulants Versus Vitamin-K Antagonists in Chronic Thromboembolic Pulmonary Hypertension: A Systematic Review, Meta-Analysis, and Meta-Regression. Cardiol. Rev. 2024. [Google Scholar] [CrossRef] [PubMed]
- Salazar, A.M.; Panama, G.; Kim, A.G.; Rayamajhi, S.; Abela, G.S. Clinical outcomes between direct oral anticoagulants versus vitamin K antagonists in chronic thromboembolic pulmonary hypertension: A systematic review and meta-analysis. Curr. Probl. Cardiol. 2024, 49, 102377. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, S. 2022 ESC/ERS-Leitlinien zur Diagnostik und Therapie der pulmonalen Hypertonie: Ein fokussierter Überblick 2022 ESC/ERS guidelines on the diagnostics and treatment of pulmonary hypertension: A focussed review. Herz 2023, 48, 23–30. (In German) [Google Scholar] [CrossRef] [PubMed]
- Braams, N.J.; Kianzad, A.; van Wezenbeek, J.; Wessels, J.N.; Jansen, S.M.A.; Andersen, S.; Boonstra, A.; Nossent, E.J.; Marcus, J.T.; Bayoumy, A.A.; et al. Long-Term Effects of Pulmonary Endarterectomy on Right Ventricular Stiffness and Fibrosis in Chronic Thromboembolic Pulmonary Hypertension. Circ. Heart Fail. 2023, 16, e010336. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mayer, E.; Jenkins, D.; Lindner, J.; D’Armini, A.; Kloek, J.; Meyns, B.; Ilkjaer, L.B.; Klepetko, W.; Delcroix, M.; Lang, I.; et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: Results from an international prospective registry. J. Thorac. Cardiovasc. Surg. 2011, 141, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Alias, S.; Redwan, B.; Panzenboeck, A.; Winter, M.P.; Schubert, U.; Voswinckel, R.; Frey, M.K.; Jakowitsch, J.; Alimohammadi, A.; Hobohm, L.; et al. Defective angiogenesis delays thrombus resolution: A potential pathogenetic mechanism underlying chronic thromboembolic pulmonary hypertension. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 810–819. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pepke-Zaba, J.; Delcroix, M.; Lang, I.; Mayer, E.; Jansa, P.; Ambroz, D.; Treacy, C.; D’Armini, A.M.; Morsolini, M.; Snijder, R.; et al. Chronic thromboembolic pulmonary hypertension (CTEPH): Results from an international prospective registry. Circulation 2011, 124, 1973–1981. [Google Scholar] [CrossRef] [PubMed]
- Delcroix, M.; Pepke-Zaba, J.; D’Armini, A.M.; Fadel, E.; Guth, S.; Hoole, S.P.; Jenkins, D.P.; Kiely, D.G.; Kim, N.H.; Madani, M.M.; et al. Worldwide CTEPH Registry: Long-Term Outcomes With Pulmonary Endarterectomy, Balloon Pulmonary Angioplasty, and Medical Therapy. Circulation 2024, 150, 1354–1365. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lian, T.Y.; Liu, J.Z.; Guo, F.; Zhou, Y.P.; Wu, T.; Wang, H.; Li, J.Y.; Yan, X.X.; Peng, F.H.; Sun, K.; et al. Prevalence, Genetic Background, and Clinical Phenotype of Congenital Thrombophilia in Chronic Thromboembolic Pulmonary Hypertension. JACC Asia 2022, 2, 247–255. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kvasnička, J.; Jansa, P.; Cífková, R.; Dušková, D.; Bobčíková, P.; Ševčík, M.; Zenáhlíková, Z.; Kvasnička, T. The incidence of the thrombophilic SNPs rs6025, rs1799963, rs2066865, rs2289252, and rs8176719 in chronic thromboembolic pulmonary hypertension. Clin. Appl. Thromb. Hemost. 2024, 30, 10760296241271369. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dodson, M.W.; Sumner, K.; Carlsen, J.; Cirulis, M.M.; Wilson, E.L.; Gadre, A.; Fernandes, T.M.; Brown, L.M.; Best, D.H.; Elliott, C.G. The Factor V Leiden variant and risk of chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2020, 56, 2000774. [Google Scholar] [CrossRef] [PubMed]
- Colorio, C.C.; Martinuzzo, M.E.; Forastiero, R.R.; Pombo, G.; Adamczuk, Y.; Carreras, L.O. Thrombophilic factors in chronic thromboembolic pulmonary hypertension. Blood Coagul. Fibrinolysis 2001, 12, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.M.; Wang, J.; Zhang, S.; Wan, J.; Tao, X.C.; Gao, Q.; Zhai, Z.G.; Wang, C. Clinical characteristics of patients with chronic thromboembolic pulmonary hypertension. Zhonghua Yi Xue Za Zhi 2019, 99, 3461–3465. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Ando, M.; Takamoto, S.; Okita, Y.; Matsukawa, R.; Nakanishi, N.; Kyotani, S.; Satoh, T. Operation for chronic pulmonary thromboembolism accompanied by thrombophilia in 8 patients. Ann. Thorac. Surg. 1998, 66, 1919–1924. [Google Scholar] [CrossRef] [PubMed]
- Lang, I.M.; Klepetko, W.; Pabinger, I. No increased prevalence of the factor V Leiden mutation in chronic major vessel thromboembolic pulmonary hypertension (CTEPH). Thromb. Haemost. 1996, 76, 476–477. [Google Scholar] [CrossRef] [PubMed]
- Akbayrak, H.; Tekumit, H. Pulmonary thromboendarterectomy in a combined thrombophilia patient. Cardiovasc. J. Afr. 2019, 30, e4–e6. [Google Scholar] [CrossRef] [PubMed]
- Clapham, K.R.; Mesbah Uddin, M.; Honigberg, M.C.; Gilliland, T.; Ruan, Y.; Natarajan, P. Venous Thromboembolism Polygenic Risk Score Associates With Pulmonary Hypertension in the UK Biobank. Circ. Genom. Precis Med. 2022, 15, e003797. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Masaki, K.; Hosokawa, K.; Funakoshi, K.; Taniguchi, Y.; Adachi, S.; Inami, T.; Yamashita, J.; Ogino, H.; Tsujino, I.; Hatano, M.; et al. Outcomes of Chronic Thromboembolic Pulmonary Hypertension After Balloon Pulmonary Angioplasty and Pulmonary Endarterectomy. JACC Asia 2024, 4, 577–589. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lang, I.M.; Brenot, P.; Bouvaist, H.; Fadel, E.; Jaïs, X.; Madani, M.M.; Guth, S.; Kurzyna, M.; Simonneau, G.; Wiedenroth, C.B.; et al. Balloon Pulmonary Angioplasty for Chronic Thromboembolic Pulmonary Hypertension: Results of an International Multicenter Prospective Registry. J. Am. Coll. Cardiol. 2025, 85, 2270–2284. [Google Scholar] [CrossRef] [PubMed]
| Study (Year) | Country | Design | n (CTEPH) | AT Tested (n) | PC Tested (n) | PS Tested (n) | FVL Tested (n) | F2 G20210A Tested (n) |
|---|---|---|---|---|---|---|---|---|
| Lian et al. [21] | China | Cross-sectional | 367 | 367 | 367 | 367 | 367 | 367 |
| Kvasnička et al. [22] | Czech Rep. | Case–control | 129 | NT | NT | NT | 129 | 129 |
| Dodson et al. [23] | USA | Case–control | NR | NT | NT | NT | 200 | NT |
| Colorio et al. [24] | Argentina | Prospective cohort | 24 | 24 | 13 | 10 | 22 | 18 |
| Lang et al. [27] | Austria | Case series | NR | NT | NT | NT | 38 | NT |
| Ando et al. [26] | Japan | Surgical series | 8 | 8 | 8 | NT | NT | NT |
| Akbayrak et al. [28] | Turkey | Case report | 1 | 1 | 1 | 1 | 1 | 1 |
| Xie et al. [25] | China | Prospective cohort | 148 | 148 | 148 | 148 | NT | NT |
| Study | PC Deficiency n/N (%) | PS Deficiency n/N (%) | AT Deficiency n/N (%) | FVL Carriers n/N (%) | F2 G20210A n/N (%) |
|---|---|---|---|---|---|
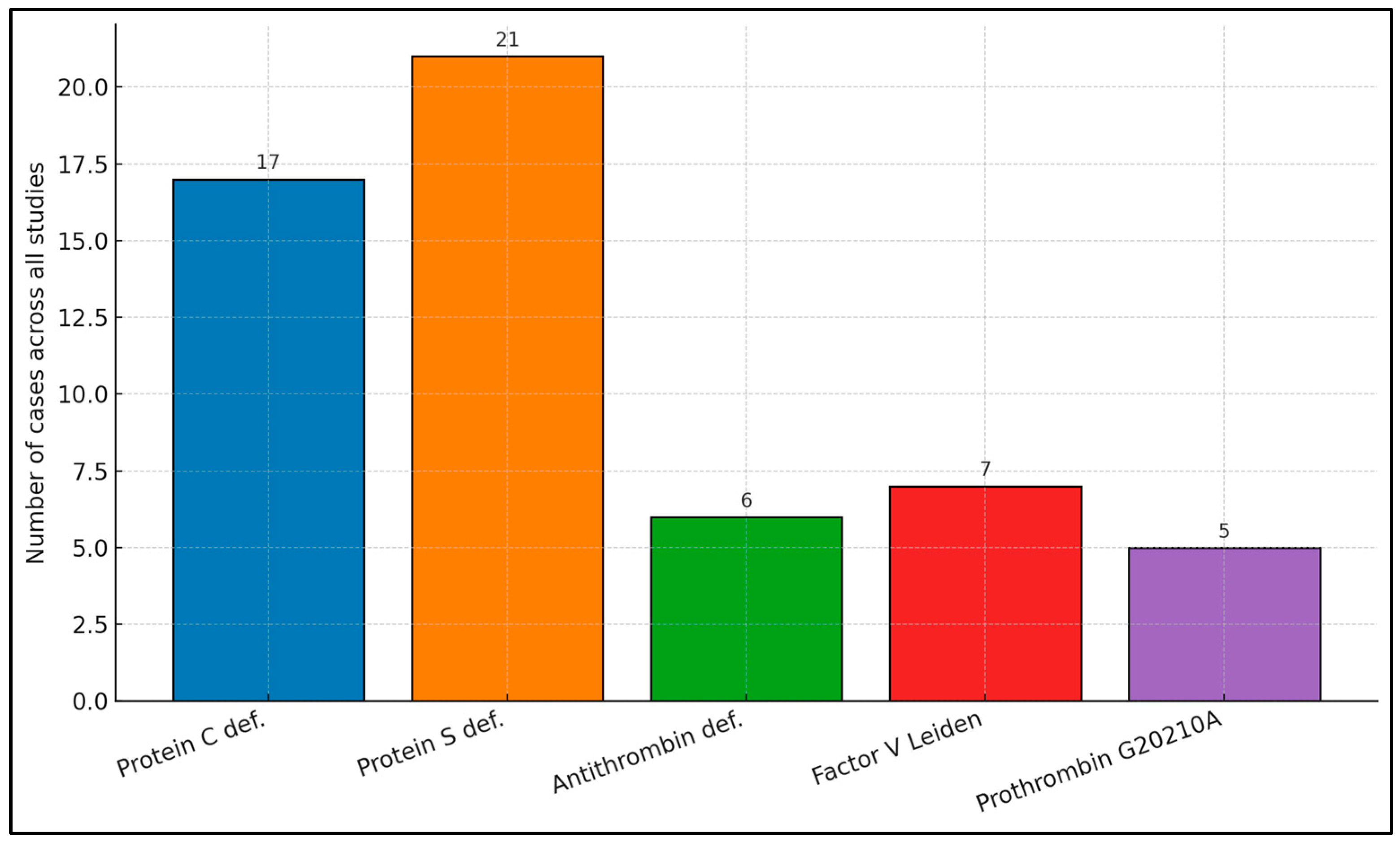
| Lian et al. [21] | 13/367 (3.5%) | 19/367 (5.2%) | 4/367 (1.1%) | 0/367 (0%) | 0/367 (0%) |
| Kvasnička et al. [22] | NR | NR | NR | 5/129 (3.9%) | 4/129 (3.1%) |
| Dodson et al. [23] | NR | NR | NR | 2/200 (1.0%) | — |
| Colorio et al. [24] | 1/13 (7.7%) | 1/10 (10.0%) | 1/24 (4.2%) | 0/22 (0%) | 1/18 (5.6%) |
| Lang et al. [27] | NR | NR | NR | 0/38 (0%) | NR |
| Ando et al. [26] | 2/8 (25.0%) | NR | 1/8 (12.5%) | NR | NR |
| Akbayrak et al. [28] | 1/1 (100%) | 1/1 (100%) | 0/1 (0%) | 0/1 (0%) | 0/1 (0%) |
| Xie et al. [25] | NR | NR | NR | NR | NR |
| Study | Baseline mPAP (mmHg) | Intervention (PEA/BPA %) | Post-Intervention mPAP (mmHg) | 1-Year Survival % |
|---|---|---|---|---|
| Lian et al. [21] | 43 ± 11 | PEA 48% / BPA 22% | 22 ± 8 | 96 |
| Kvasnička et al. [22] | 44 ± 12 | PEA 55% / BPA 19% | 23 ± 9 | 95 |
| Dodson et al. [23] | NR | NR | NR | NR |
| Colorio et al. [24] | 47 ± 7 | PEA 33% | 18 ± 5 | 92 |
| Lang et al. [27] | NR | NR | NR | NR |
| Ando et al. [26] | 47 ± 6 | PEA 100% | 16 ± 6 | 100 |
| Akbayrak et al. [28] | 54 | PEA 100% | 20 | 100 |
| Xie et al. [25] | 45 ± 11 | PEA 42% / BPA 15% | 24 ± 9 | 94 |
| Study | n | Mean Age (Years) | Male (%) | Baseline mPAP (mm Hg) | PVR (WU) | Cardiac Index (L min-1 m-2) |
|---|---|---|---|---|---|---|
| Lian et al. [21] | 367 | 54.4 ± 14.8 | 46 | 49.7 ± 12.4 | 9.4 ± 4.5 | 2.5 ± 0.6 |
| Kvasnička et al. [22] | 129 | 65.5 ± 9.5 | 54 | 47.6 ± 12.3 | 8.8 ± 4.4 | 2.2 ± 0.5 |
| Group | n | Male, n (%) | Mean Age, Years (±SD) |
|---|---|---|---|
| Carriers (any congenital defect) | 56 | 32 (57%) | 57.8 ± 12.1 |
| Non-carriers | 122 | 57 (47%) | 56.9 ± 12.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borsi, E.; Potre, C.; Ionita, I.; Samfireag, M.; Secosan, C.; Potre, O. Congenital Thrombophilia in Chronic Thromboembolic Pulmonary Hypertension (CTEPH): A Systematic Review of Prevalence, Clinical Phenotype, and Surgical Outcomes. Biomedicines 2025, 13, 2215. https://doi.org/10.3390/biomedicines13092215
Borsi E, Potre C, Ionita I, Samfireag M, Secosan C, Potre O. Congenital Thrombophilia in Chronic Thromboembolic Pulmonary Hypertension (CTEPH): A Systematic Review of Prevalence, Clinical Phenotype, and Surgical Outcomes. Biomedicines. 2025; 13(9):2215. https://doi.org/10.3390/biomedicines13092215
Chicago/Turabian StyleBorsi, Ema, Cristina Potre, Ioana Ionita, Miruna Samfireag, Cristina Secosan, and Ovidiu Potre. 2025. "Congenital Thrombophilia in Chronic Thromboembolic Pulmonary Hypertension (CTEPH): A Systematic Review of Prevalence, Clinical Phenotype, and Surgical Outcomes" Biomedicines 13, no. 9: 2215. https://doi.org/10.3390/biomedicines13092215
APA StyleBorsi, E., Potre, C., Ionita, I., Samfireag, M., Secosan, C., & Potre, O. (2025). Congenital Thrombophilia in Chronic Thromboembolic Pulmonary Hypertension (CTEPH): A Systematic Review of Prevalence, Clinical Phenotype, and Surgical Outcomes. Biomedicines, 13(9), 2215. https://doi.org/10.3390/biomedicines13092215







