Contemporary and Emerging Therapeutics in Cardiovascular-Kidney-Metabolic (CKM) Syndrome: In Memory of Professor Akira Endo
Abstract
1. Introduction
2. Materials and Methods
3. Current and Emerging Therapeutics
3.1. Sodium Glucose Co-Transport 2 Inhibitors
3.2. Glucagon-like-Peptide-1 Receptor Agonists
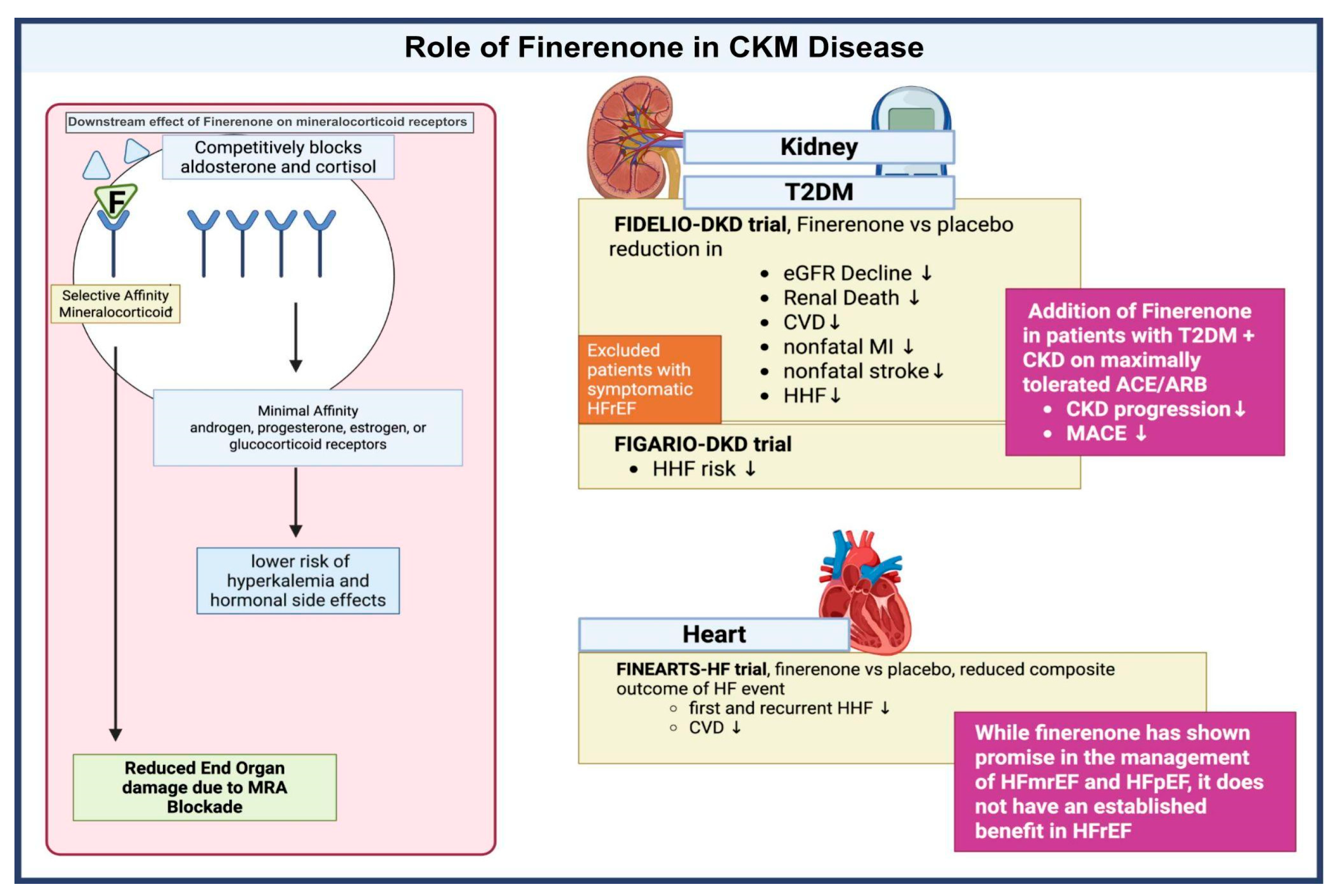
3.3. Mineralocorticoid Receptor Antagonists
3.4. Thyroid Receptor Beta Agonists
3.5. Lipoprotein-a (Lp(a))
3.6. Phase 3 Trial Drugs for CKM Syndrome
3.7. Role of Artificial Intelligence
| Drugs | Phase 3 Trials | Principal Investigator | Indication | MOA | Outcome | Adverse Effects |
|---|---|---|---|---|---|---|
| Olezarsen | NCT05681351 | Ionis Pharmaceuticals, Inc. [96] | Severe hypertriglyceridemia | Gal- NAc3 conjugated ASO targeting ApoC-III | Recruiting | Abdominal pain, and diarrhea. |
| Pelacarsen | NCT05305664 | Novartis Pharmaceuticals [97] | Acute coronary syndrome (ACS), Hyperlipoproteinemia | ASO targeting Lp(a) | Pending results | Mild injection site reactions. |
| Plozasiran | NCT06347016 | Arrowhead Pharmaceuticals [98] | Mixed dyslipidemia, Hypertriglyceridemia, Familial chylomicronemia syndrome (FCS) | siRNA targeting apoC-III mRNA | Currently recruiting | Worsening glycemic control, diarrhea, and urinary tract infection. |
| Inclisiran | NCT05399992 | Novartis Pharmaceuticals [99] | Elevated low density lipoprotein (LDL),Atherosclerotic cardiovascular disease (ASCVD) | siRNA targeting PCSK9 | Recruitment complete | Injection site reactions. |
| Lepodisiran | NCT06292013 | Ferdinand et al. [100] | Cardiovascular disorders (CVD), Metabolic disorders | siRNA targeting ApoA | Currently recruiting | Injection site reactions, hypersensitivity reactions, and hepatobiliary adverse events. |
| Olpasiran | NCT05581303 | UCSD Health [101] | Coronary artery disease (CAD), elevated Lp(a) | siRNA targeting Lp(a) | Pre-recruitment stage | Injection-site reactions. |
| Ziltivekimab | NCT05021835 | Ridker et al. [102] | CVD, Chronic kidney disease (CKD) | IL-6 Blocker | Currently recruiting | Injection-site reactions. |
| Obicetrapib | NCT05142722 | Ditmarsch et al. [103] | Heterozygous FHS, CAD | CETP Inhibitors | Completed, pending publication of results | Nausea, urinary tract infection, and headache. |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC | American College of Cardiology |
| ADA | American Diabetes Association |
| AGA | American Gastroenterology Association |
| AHA | American Heart Association |
| AI | Artificial intelligence |
| ACEi | Angiotensin converting enzyme inhibitor |
| ARB | Angiotensin receptor blocker |
| ASO | Antisense Oligonucleotides |
| ANGPTL3 | Angiopoietin-like 3 |
| ASCVD | Atherosclerotic cardiovascular disease |
| CAD | Coronary artery disease |
| CETP | Cholesteryl ester transfer protein |
| CKM | Cardiovascular-kidney-metabolic syndrome |
| CVD | Cardiovascular disease |
| CKD | Chronic kidney disease |
| EASD | European Association for the Study of Diabetes |
| ESC | European Society of Cardiology |
| GLP1-RA | Glucagon-like peptide-1 receptor agonists |
| HFMREF | Heart failure with mildly reduced ejection fraction |
| HFPEF | Heart failure with preserved ejection fraction |
| HFREF | Heart failure with reduced ejection fraction |
| KDIGO | Kidney Disease: Improving Global Outcomes |
| KDOQI | National Kidney Foundation Kidney Disease Outcomes Quality Initiative |
| LDL | Low density lipoprotein |
| MACE | Major adverse cardiovascular events |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| MASH | Metabolic dysfunction associated steatohepatitis |
| NAFLD | Nonalcoholic fatty liver disease |
| NASH | Nonalcoholic steatohepatitis |
| PCSK9 | Proprotein convertase subtilisin–kexin type 9 |
| RAS | Renin angiotensin system |
| SGLT2i | Sodium-glucose cotransporter 2 inhibitors |
| TRβ | Thyroid receptor beta |
References
- Ndumele, C.E.; Rangaswami, J.; Chow, S.L.; Neeland, I.J.; Tuttle, K.R.; Khan, S.S.; Coresh, J.; Mathew, R.O.; Baker-Smith, C.M.; Carnethon, M.R.; et al. Cardiovascular-Kidney-Metabolic Health: A Presidential Advisory from the American Heart Association. Circulation 2023, 148, 1606–1635. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Li, T. Social Determinants of Health in the Development of Cardiovascular-kidney-metabolic Syndrome. Rev. Cardiovasc. Med. 2025, 26, 26580. [Google Scholar] [CrossRef] [PubMed]
- Bharaj, I.S.; Brar, A.S.; Kahlon, J.; Singh, A.; Hotwani, P.; Kumar, V.; Sohal, A.; Batta, A. Metabolic-dysfunction associated steatotic liver disease and atrial fibrillation: A review of pathogenesis. World J. Cardiol. 2025, 17, 106147. [Google Scholar] [CrossRef] [PubMed]
- Schnell, O.; Almandoz, J.; Anderson, L.; Barnard-Kelly, K.; Battelino, T.; Blüher, M.; Busetto, L.; Catrinou, D.; Ceriello, A.; Cos, X.; et al. CVOT summit report 2024: New cardiovascular, kidney, and metabolic outcomes. Cardiovasc. Diabetol. 2025, 24, 187. [Google Scholar] [CrossRef]
- Minhas, A.M.K.; Mathew, R.O.; Sperling, L.S.; Nambi, V.; Virani, S.S.; Navaneethan, S.D.; Shapiro, M.D.; Abramov, D. Prevalence of the Cardiovascular-Kidney-Metabolic Syndrome in the United States. J. Am. Coll. Cardiol. 2024, 83, 1824–1826. [Google Scholar] [CrossRef] [PubMed]
- Ndumele, C.E.; Neeland, I.J.; Tuttle, K.R.; Chow, S.L.; Mathew, R.O.; Khan, S.S.; Coresh, J.; Baker-Smith, C.M.; Carnethon, M.R.; Després, J.-P.; et al. A Synopsis of the Evidence for the Science and Clinical Management of Cardiovascular-Kidney-Metabolic (CKM) Syndrome: A Scientific Statement from the American Heart Association. Circulation 2023, 148, 1636–1664. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, X.; Long, T.; Jiang, Y.; Wang, M.; Lv, Z.; Hou, H.; Li, Z.; Liu, M. Prevalence and Mortality Association of Different Stages of Cardiovascular-Kidney-Metabolic Syndrome. JACC Adv. 2025, 4, 101843. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Ostrominski, J.W.; Vaduganathan, M. Prevalence of Cardiovascular-Kidney-Metabolic Syndrome Stages in US Adults, 2011–2020. JAMA 2024, 331, 1858–1860. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Wang, R.; He, J.; Wang, L.; Chen, H.; Niu, X.; Sun, Y.; Guan, Y.; Gong, Y.; Zhang, L.; et al. Prevalence of Cardiovascular-Kidney-Metabolic Syndrome Stages by Social Determinants of Health. JAMA Netw. Open 2024, 7, e2445309. [Google Scholar] [CrossRef]
- Li, J.; Lei, L.; Wang, W.; Ding, W.; Yu, Y.; Pu, B.; Peng, Y.; Li, Y.; Zhang, L.; Guo, Y. Social Risk Profile and Cardiovascular-Kidney-Metabolic Syndrome in US Adults. J. Am. Heart Assoc. 2024, 13, e034996. [Google Scholar] [CrossRef]
- Lee, H.; Jeong, J.; Yim, Y.; Smith, L.; Pizzol, D.; Hwang, J.; Yon, D.K. Family history of non-communicable diseases and the risk of cardiovascular-kidney-metabolic syndrome. Sci. Rep. 2025, 15, 20710. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Hill-Briggs, F.; Adler, N.E.; Berkowitz, S.A.; Chin, M.H.; Gary-Webb, T.L.; Navas-Acien, A.; Thornton, P.L.; Haire-Joshu, D. Social Determinants of Health and Diabetes: A Scientific Review. Diabetes Care 2021, 44, 258–279. [Google Scholar] [CrossRef]
- Sebastian, S.A.; Padda, I.; Johal, G. Cardiovascular-Kidney-Metabolic (CKM) syndrome: A state-of-the-art review. Curr. Probl. Cardiol. 2024, 49, 102344. [Google Scholar] [CrossRef] [PubMed]
- Warrens, H.; Banerjee, D.; Herzog, C.A. Cardiovascular Complications of Chronic Kidney Disease: An Introduction. Eur. Cardiol. Rev. 2022, 17, e13. [Google Scholar] [CrossRef]
- Deferrari, G.; Cipriani, A.; La Porta, E. Renal dysfunction in cardiovascular diseases and its consequences. J. Nephrol. 2021, 34, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, J.; Shen, J.; Chen, Y.; He, L.; Li, M.; Xie, X. Association of lipoprotein (a) and 1 year prognosis in patients with heart failure with reduced ejection fraction. ESC Heart Fail. 2022, 9, 2399–2406. [Google Scholar] [CrossRef]
- Mohammadi, A.; Karimian, A.; Shokri, K.; Mohammadi, A.; Hazhir-Karzar, N.; Bahar, R.; Radfar, A.; Pakyari, M.; Tehrani, B. RNA Therapies in Cardio-Kidney-Metabolic Syndrome: Advancing Disease Management. J. Cardiovasc. Transl. Res. 2025. ahead of print. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; De Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef]
- Witztum, J.L.; Gaudet, D.; Freedman, S.D.; Alexander, V.J.; Digenio, A.; Williams, K.R.; Yang, Q.; Hughes, S.G.; Geary, R.S.; Arca, M.; et al. Volanesorsen and Triglyceride Levels in Familial Chylomicronemia Syndrome. N. Engl. J. Med. 2019, 381, 531–542. [Google Scholar] [CrossRef]
- Stitziel, N.O. Reducing the Risk of Pancreatitis by Inhibiting APOC3. N. Engl. J. Med. 2025, 392, 197–199. [Google Scholar] [CrossRef]
- The EMPA-KIDNEY Collaborative Group. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar] [CrossRef]
- Raal, F.J.; Kallend, D.; Ray, K.K.; Turner, T.; Koenig, W.; Wright, R.S.; Wijngaard, P.L.J.; Curcio, D.; Jaros, M.J.; Leiter, L.A.; et al. Inclisiran for the Treatment of Heterozygous Familial Hypercholesterolemia. N. Engl. J. Med. 2020, 382, 1520–1530. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Perkins, B.A.; Fitchett, D.H.; Husain, M.; Cherney, D.Z.I. Sodium Glucose Cotransporter 2 Inhibitors in the Treatment of Diabetes Mellitus: Cardiovascular and Kidney Effects, Potential Mechanisms, and Clinical Applications. Circulation 2016, 134, 752–772. [Google Scholar] [CrossRef] [PubMed]
- Di Costanzo, A.; Esposito, G.; Indolfi, C.; Spaccarotella, C.A.M. SGLT2 Inhibitors: A New Therapeutical Strategy to Improve Clinical Outcomes in Patients with Chronic Kidney Diseases. Int. J. Mol. Sci. 2023, 24, 8732. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Correa, J.I.; Correa-Rotter, R. Sodium-Glucose Cotransporter 2 Inhibitors Mechanisms of Action: A Review. Front. Med. 2021, 8, 777861. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V.; Verma, S. Effects of SGLT2 Inhibitors on Kidney and Cardiovascular Function. Annu. Rev. Physiol. 2021, 83, 503–528. [Google Scholar] [CrossRef]
- Chen, X.; Hocher, C.-F.; Shen, L.; Krämer, B.K.; Hocher, B. Reno- and cardioprotective molecular mechanisms of SGLT2 inhibitors beyond glycemic control: From bedside to bench. Am. J. Physiol.-Cell Physiol. 2023, 325, C661–C681. [Google Scholar] [CrossRef]
- Salvatore, T.; Galiero, R.; Caturano, A.; Rinaldi, L.; Di Martino, A.; Albanese, G.; Di Salvo, J.; Epifani, R.; Marfella, R.; Docimo, G. An Overview of the Cardiorenal Protective Mechanisms of SGLT2 Inhibitors. Int. J. Mol. Sci. 2022, 23, 3651. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Rangaswami, J.; Bhalla, V.; De Boer, I.H.; Staruschenko, A.; Sharp, J.A.; Singh, R.R.; Lo, K.B.; Tuttle, K.; Vaduganathan, M.; Ventura, H.; et al. Cardiorenal Protection with the Newer Antidiabetic Agents in Patients with Diabetes and Chronic Kidney Disease: A Scientific Statement from the American Heart Association. Circulation 2020, 142, e265–e286. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner–La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Neuen, B.L.; Ohkuma, T.; Neal, B.; Matthews, D.R.; De Zeeuw, D.; Mahaffey, K.W.; Fulcher, G.; Li, Q.; Jardine, M.; Oh, R.; et al. Effect of Canagliflozin on Renal and Cardiovascular Outcomes across Different Levels of Albuminuria: Data from the CANVAS Program. J. Am. Soc. Nephrol. 2019, 30, 2229–2242. [Google Scholar] [CrossRef]
- Patel, S.M.; Kang, Y.M.; Im, K.; Neuen, B.L.; Anker, S.D.; Bhatt, D.L.; Butler, J.; Cherney, D.Z.I.; Claggett, B.L.; Fletcher, R.A.; et al. Sodium-Glucose Cotransporter-2 Inhibitors and Major Adverse Cardiovascular Outcomes: A SMART-C Collaborative Meta-Analysis. Circulation 2024, 149, 1789–1801. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.-F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [CrossRef]
- Beghini, A.; Sammartino, A.M.; Papp, Z.; Von Haehling, S.; Biegus, J.; Ponikowski, P.; Adamo, M.; Falco, L.; Lombardi, C.M.; Pagnesi, M.; et al. 2024 update in heart failure. ESC Heart Fail. 2025, 12, 8–42. [Google Scholar] [CrossRef]
- Blonde, L.; Umpierrez, G.E.; Reddy, S.S.; McGill, J.B.; Berga, S.L.; Bush, M.; Chandrasekaran, S.; DeFronzo, R.A.; Einhorn, D.; Galindo, R.J.; et al. American Association of Clinical Endocrinology Clinical Practice Guideline: Developing a Diabetes Mellitus Comprehensive Care Plan—2022 Update. Endocr. Pract. 2022, 28, 923–1049. [Google Scholar] [CrossRef]
- Abdelmalek, M.F.; Harrison, S.A.; Sanyal, A.J. The role of glucagon-like peptide-1 receptor agonists in metabolic dysfunction-associated steatohepatitis. Diabetes Obes. Metab. 2024, 26, 2001–2016. [Google Scholar] [CrossRef] [PubMed]
- De Boer, I.H.; Khunti, K.; Sadusky, T.; Tuttle, K.R.; Neumiller, J.J.; Rhee, C.M.; Rosas, S.E.; Rossing, P.; Bakris, G. Diabetes management in chronic kidney disease: A consensus report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2022, 102, 974–989. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee; ElSayed, N.A.; McCoy, R.G.; Aleppo, G.; Bajaj, M.; Balapattabi, K.; Beverly, E.A.; Briggs Early, K.; Bruemmer, D.; Cusi, K.; et al. 4. Comprehensive Medical Evaluation and Assessment of Comorbidities: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48, S59–S85. [Google Scholar] [CrossRef]
- Loomba, R.; Hartman, M.L.; Lawitz, E.J.; Vuppalanchi, R.; Boursier, J.; Bugianesi, E.; Yoneda, M.; Behling, C.; Cummings, O.W.; Tang, Y.; et al. Tirzepatide for Metabolic Dysfunction–Associated Steatohepatitis with Liver Fibrosis. N. Engl. J. Med. 2024, 391, 299–310. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Bedossa, P.; Fraessdorf, M.; Neff, G.W.; Lawitz, E.; Bugianesi, E.; Anstee, Q.M.; Hussain, S.A.; Newsome, P.N.; Ratziu, V.; et al. A Phase 2 Randomized Trial of Survodutide in MASH and Fibrosis. N. Engl. J. Med. 2024, 391, 311–319. [Google Scholar] [CrossRef]
- Newsome, P.N.; Buchholtz, K.; Cusi, K.; Linder, M.; Okanoue, T.; Ratziu, V.; Sanyal, A.J.; Sejling, A.-S.; Harrison, S.A. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2021, 384, 1113–1124. [Google Scholar] [CrossRef]
- Alkhouri, N.; Charlton, M.; Gray, M.; Alkhouri, N.M.; Naim; Charlton, M.; Gray, M.; Noureddin, M. The Pleiotropic Effects of Glucagon-like Peptide-1 Receptor Agonists in Patients with Metabolic Dysfunction-Associated Steatohepatitis: A Review for Gastroenterologists. Expert Opin. Investig. Drugs 2025, 34, 169–195. [Google Scholar] [CrossRef]
- Yabut, J.M.; Drucker, D.J. Glucagon-like Peptide-1 Receptor-based Therapeutics for Metabolic Liver Disease. Endocr. Rev. 2023, 44, 14–32. [Google Scholar] [CrossRef]
- Trevella, P.; Ekinci, E.I.; MacIsaac, R.J. Potential kidney protective effects of glucagon-like peptide-1 receptor agonists. Nephrol. Carlton Vic. 2024, 29, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Sourris, K.C.; Ding, Y.; Maxwell, S.S.; Al-Sharea, A.; Kantharidis, P.; Mohan, M.; Rosado, C.J.; Penfold, S.A.; Haase, C.; Xu, Y.; et al. Glucagon-like peptide-1 receptor signaling modifies the extent of diabetic kidney disease through dampening the receptor for advanced glycation end products-induced inflammation. Kidney Int. 2024, 105, 132–149. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-Y.; Hsu, T.-W.; Liu, J.-H.; Pan, H.-C.; Lai, C.-F.; Yang, S.-Y.; Wu, V.-C. Kidney and Cardiovascular Outcomes Among Patients With CKD Receiving GLP-1 Receptor Agonists: A Systematic Review and Meta-Analysis of Randomized Trials. Am. J. Kidney Dis. 2025, 85, 555–569.e1. [Google Scholar] [CrossRef]
- Grunvald, E.; Shah, R.; Hernaez, R.; Chandar, A.K.; Pickett-Blakely, O.; Teigen, L.M.; Harindhanavudhi, T.; Sultan, S.; Singh, S.; Davitkov, P. AGA Clinical Practice Guideline on Pharmacological Interventions for Adults with Obesity. Gastroenterology 2022, 163, 1198–1225. [Google Scholar] [CrossRef]
- Moiz, A.; Filion, K.B.; Tsoukas, M.A.; Yu OHy Peters, T.M.; Eisenberg, M.J. Mechanisms of GLP-1 Receptor Agonist-Induced Weight Loss: A Review of Central and Peripheral Pathways in Appetite and Energy Regulation. Am. J. Med. 2025, 138, 934–940. [Google Scholar] [CrossRef]
- Taha, M.B.; Yahya, T.; Satish, P.; Laird, R.; Agatston, A.S.; Cainzos-Achirica, M.; Patel, K.V.; Nasir, K. Glucagon-Like Peptide 1 Receptor Agonists: A Medication for Obesity Management. Curr. Atheroscler. Rep. 2022, 24, 643–654. [Google Scholar] [CrossRef]
- Ansari, H.U.H.; Qazi, S.U.; Sajid, F.; Altaf, Z.; Ghazanfar, S.; Naveed, N.; Ashfaq, A.S.; Siddiqui, A.H.; Iqbal, H.; Qazi, S. Efficacy and Safety of Glucagon-Like Peptide-1 Receptor Agonists on Body Weight and Cardiometabolic Parameters in Individuals with Obesity and Without Diabetes: A Systematic Review and Meta-Analysis. Endocr. Pract. 2024, 30, 160–171. [Google Scholar] [CrossRef]
- Xie, W.; Hong, Z.; Li, B.; Huang, B.; Dong, S.; Cai, Y.; Ruan, L.; Xu, Q.; Mou, L.; Zhang, Y. Influence of glucagon-like peptide-1 receptor agonists on fat accumulation in patients with diabetes mellitus and non-alcoholic fatty liver disease or obesity: A systematic review and meta-analysis of randomized control trials. J. Diabetes Complicat. 2024, 38, 108743. [Google Scholar] [CrossRef]
- Pandey, A.K.; Bhatt, D.L.; Cosentino, F.; Marx, N.; Rotstein, O.; Pitt, B.; Pandey, A.; Butler, J.; Verma, S. Non-steroidal mineralocorticoid receptor antagonists in cardiorenal disease. Eur. Heart J. 2022, 43, 2931–2945. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Kolkhof, P.; Bakris, G.; Bauersachs, J.; Haller, H.; Wada, T.; Zannad, F. Steroidal and Non-Steroidal Mineralocorticoid Receptor Antagonists in Cardiorenal Medicine. Eur. Heart J. 2021, 42, 152–161. [Google Scholar] [CrossRef] [PubMed]
- González-Juanatey, J.R.; Górriz, J.L.; Ortiz, A.; Valle, A.; Soler, M.J.; Facila, L. Cardiorenal benefits of finerenone: Protecting kidney and heart. Ann. Med. 2023, 55, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Georgianos, P.I.; Agarwal, R. The Nonsteroidal Mineralocorticoid-Receptor-Antagonist Finerenone in Cardiorenal Medicine: A State-of-the-Art Review of the Literature. Am. J. Hypertens. 2023, 36, 135–143. [Google Scholar] [CrossRef]
- Yuan, C.-Y.; Gao, Y.-C.; Lin, Y.; Liu, L.; Shen, X.-G.; Zou, W.-L.; Wang, M.-M.; Shen, Q.-Q.; Shao, L.-N.; Liu, Y.-M.; et al. Effects of Mineralocorticoid Receptor Antagonists for Chronic Kidney Disease: A Systemic Review and Meta-Analysis. Am. J. Nephrol. 2024, 55, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Nishiwaki, H.; Ota, E.; Levack, W.M.; Noma, H. Aldosterone antagonists for people with chronic kidney disease requiring dialysis. Cochrane Database Syst. Rev. 2021, 2021, CD013109. [Google Scholar] [CrossRef]
- Chung, E.Y.; Ruospo, M.; Natale, P.; Bolignano, D.; Navaneethan, S.D.; Palmer, S.C.; Strippoli, G.F. Aldosterone antagonists in addition to renin angiotensin system antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst. Rev. 2020, 2020, CD007004. [Google Scholar] [CrossRef] [PubMed]
- Pitt, B.; Filippatos, G.; Agarwal, R.; Anker, S.D.; Bakris, G.L.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Schloemer, P.; et al. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 2252–2263. [Google Scholar] [CrossRef]
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 2219–2229. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Vaduganathan, M.; Claggett, B.; Jhund, P.S.; Desai, A.S.; Henderson, A.D.; Lam, C.S.P.; Pitt, B.; Senni, M.; et al. Finerenone in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2024, 391, 1475–1485. [Google Scholar] [CrossRef]
- Ismahel, H.; Docherty, K.F. The role of finerenone in heart failure. Trends Cardiovasc. Med. 2025, 35, 468–476. [Google Scholar] [CrossRef]
- Yang, M.; Henderson, A.D.; Talebi, A.; Atherton, J.J.; Chiang, C.-E.; Chopra, V.; Comin-Colet, J.; Kosiborod, M.N.; Kerr Saraiva, J.F.; Claggett, B.L.; et al. Effect of Finerenone on the KCCQ in Patients With HFmrEF/HFpEF. J. Am. Coll. Cardiol. 2025, 85, 120–136. [Google Scholar] [CrossRef]
- Navaneethan, S.D.; Bansal, N.; Cavanaugh, K.L.; Chang, A.; Crowley, S.; Delgado, C.; Estrella, M.M.; Ghossein, C.; Ikizler, T.A.; Koncicki, H.; et al. KDOQI US Commentary on the KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of CKD. Am. J. Kidney Dis. 2025, 85, 135–176. [Google Scholar] [CrossRef]
- Petta, S.; Targher, G.; Romeo, S.; Pajvani, U.B.; Zheng, M.; Aghemo, A.; Valenti, L.V.C. The first MASH drug therapy on the horizon: Current perspectives of resmetirom. Liver Int. 2024, 44, 1526–1536. [Google Scholar] [CrossRef]
- Li, C.; Wang, T.; Song, J. A review regarding the article ‘Electrocardiographic abnormalities in patients with metabolic dysfunction-associated Steatotic liver disease: A systematic review and meta-analysis. Curr. Probl. Cardiol. 2024, 49, 102626. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.W. Selective Agonists of Thyroid Hormone Receptor Beta: Promising Tools for the Treatment of Nonalcoholic Fatty Liver Disease. Endocrinol. Metab. 2024, 39, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Bedossa, P.; Guy, C.D.; Schattenberg, J.M.; Loomba, R.; Taub, R.; Labriola, D.; Moussa, S.E.; Neff, G.W.; Rinella, M.E.; et al. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. N. Engl. J. Med. 2024, 390, 497–509. [Google Scholar] [CrossRef]
- Harrison, S.A.; Bashir, M.R.; Guy, C.D.; Zhou, R.; Moylan, C.A.; Frias, J.P.; Alkhouri, N.; Bansal, M.B.; Baum, S.; Neuschwander-Tetri, B.A.; et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2019, 394, 2012–2024. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Stepanova, M.; Taub, R.A.; Barbone, J.M.; Harrison, S.A. Hepatic Fat Reduction Due to Resmetirom in Patients With Nonalcoholic Steatohepatitis Is Associated With Improvement of Quality of Life. Clin. Gastroenterol. Hepatol. 2022, 20, 1354–1361.e7. [Google Scholar] [CrossRef]
- Karim, G.; Bansal, M.B. Resmetirom: An Orally Administered, Small-molecule, Liver-directed, β-selective THR Agonist for the Treatment of Non-alcoholic Fatty Liver Disease and Non-alcoholic Steatohepatitis. Eur. Endocrinol. 2023, 19, 60. [Google Scholar] [CrossRef]
- A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Study of ISIS 304801 Administered Subcutaneously to Patients with Familial Chylomicronemia Syndrome (FCS). 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT02211209 (accessed on 2 June 2025).
- Marino, L.; Kim, A.; Ni, B.; Celi, F.S. Thyroid hormone action and liver disease, a complex interplay. Hepatology 2025, 81, 651–669. [Google Scholar] [CrossRef]
- Reyes-Soffer, G.; Ginsberg, H.N.; Berglund, L.; Duell, P.B.; Heffron, S.P.; Kamstrup, P.R.; Lloyd-Jones, D.M.; Marcovina, S.M.; Yeang, C.; Koschinsky, M.L.; et al. Lipoprotein(a): A Genetically Determined, Causal, and Prevalent Risk Factor for Atherosclerotic Cardiovascular Disease: A Scientific Statement From the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 2022, 42, e48–e60. [Google Scholar] [CrossRef]
- Kamstrup, P.R.; Nordestgaard, B.G. Elevated Lipoprotein(a) Levels, LPA Risk Genotypes, and Increased Risk of Heart Failure in the General Population. JACC Heart Fail. 2016, 4, 78–87. [Google Scholar] [CrossRef]
- Januzzi, J.L.; Van Kimmenade, R.R.J.; Liu, Y.; Hu, X.; Browne, A.; Plutzky, J.; Tsimikas, S.; Blankstein, R.; Natarajan, P. Lipoprotein(a), Oxidized Phospholipids, and Progression to Symptomatic Heart Failure: The CASABLANCA Study. J. Am. Heart Assoc. 2024, 13, e034774. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Baars, D.P.; Aggarwal, K.; Desai, R.; Singh, D.; Pinto-Sietsma, S.-J. Association between lipoprotein (a) and risk of heart failure: A systematic review and meta-analysis of Mendelian randomization studies. Curr. Probl. Cardiol. 2024, 49, 102439. [Google Scholar] [CrossRef] [PubMed]
- Steffen, B.T.; Duprez, D.; Bertoni, A.G.; Guan, W.; Tsai, M.Y. Lp(a) [Lipoprotein(a)]-Related Risk of Heart Failure Is Evident in Whites but Not in Other Racial/Ethnic Groups: The Multi-Ethnic Study of Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Zhang, Z.; Long, J.; Zhao, H.; Zeng, F. Association between lipoprotein (a) and heart failure with reduced ejection fraction development. J. Clin. Lab. Anal. 2022, 36, e24083. [Google Scholar] [CrossRef]
- Khera, A.V.; Everett, B.M.; Caulfield, M.P.; Hantash, F.M.; Wohlgemuth, J.; Ridker, P.M.; Mora, S. Lipoprotein(a) Concentrations, Rosuvastatin Therapy, and Residual Vascular Risk: An Analysis From the JUPITER Trial (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin). Circulation 2014, 129, 635–642. [Google Scholar] [CrossRef]
- Tsimikas, S.; Viney, N.J.; Hughes, S.G.; Singleton, W.; Graham, M.J.; Baker, B.F.; Burkey, J.L.; Yang, Q.; Marcovina, S.M.; Geary, R.S.; et al. Antisense therapy targeting apolipoprotein(a): A randomised, double-blind, placebo-controlled phase 1 study. Lancet 2015, 386, 1472–1483. [Google Scholar] [CrossRef]
- O’Donoghue, M.L.; Rosenson, R.S.; López, J.A.G.; Lepor, N.E.; Baum, S.J.; Stout, E.; Gaudet, D.; Knusel, B.; Kuder, J.F.; Murphy, S.A.; et al. The Off-Treatment Effects of Olpasiran on Lipoprotein(a) Lowering. J. Am. Coll. Cardiol. 2024, 84, 790–797. [Google Scholar] [CrossRef]
- Ridker, P.M.; Devalaraja, M.; Baeres, F.M.M.; Engelmann, M.D.M.; Hovingh, G.K.; Ivkovic, M.; Lo, L.; Kling, D.; Pergola, P.; Raj, D.; et al. IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet 2021, 397, 2060–2069. [Google Scholar] [CrossRef]
- Chang, B.; Laffin, L.J.; Sarraju, A.; Nissen, S.E. Obicetrapib—The Rebirth of CETP Inhibitors? Curr. Atheroscler. Rep. 2024, 26, 603–608. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Ditmarsch, M.; Kastelein, J.J.; Rigby, S.P.; Kling, D.; Curcio, D.L.; Alp, N.J.; Davidson, M.H. Lipid lowering effects of the CETP inhibitor obicetrapib in combination with high-intensity statins: A randomized phase 2 trial. Nat. Med. 2022, 28, 1672–1678. [Google Scholar] [CrossRef]
- Kersten, S. ANGPTL3 as therapeutic target. Curr. Opin. Lipidol. 2021, 32, 335–341. [Google Scholar] [CrossRef]
- Loftus, T.J.; Shickel, B.; Ozrazgat-Baslanti, T.; Ren, Y.; Glicksberg, B.S.; Cao, J.; Singh, K.; Chan, L.; Nadkarni, G.N.; Bihorac, A. Artificial intelligence-enabled decision support in nephrology. Nat. Rev. Nephrol. 2022, 18, 452–465. [Google Scholar] [CrossRef]
- Gill, S.K.; Karwath, A.; Uh, H.-W.; Cardoso, V.R.; Gu, Z.; Barsky, A.; Slater, L.; Acharjee, A.; Duan, J.; Dall’Olio, L.; et al. Artificial intelligence to enhance clinical value across the spectrum of cardiovascular healthcare. Eur. Heart J. 2023, 44, 713–725. [Google Scholar] [CrossRef]
- Khan, S.S.; Coresh, J.; Pencina, M.J.; Ndumele, C.E.; Rangaswami, J.; Chow, S.L.; Palaniappan, L.P.; Sperling, L.S.; Virani, S.S.; Ho, J.E.; et al. Novel Prediction Equations for Absolute Risk Assessment of Total Cardiovascular Disease Incorporating Cardiovascular-Kidney-Metabolic Health: A Scientific Statement from the American Heart Association. Circulation 2023, 148, 1982–2004. [Google Scholar] [CrossRef]
- CORE-OLE: A Study of Olezarsen (ISIS 678354) Administered Subcutaneously to Participants with Severe Hypertriglyceridemia (SHTG). 2022. Available online: https://clinicaltrials.gov/study/NCT05681351 (accessed on 13 June 2025).
- A Multicenter Trial Assessing the Impact of Lipoprotein(a) Lowering with Pelacarsen (TQJ230) on the Rate of Weekly Lipoprotein Apheresis Sessions in Patients with Hyperlipoproteinemia(a) and Established Cardiovascular Disease in Germany. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05305664 (accessed on 11 June 2025).
- Double-Blind, Placebo-Controlled, Phase 3 Study to Evaluate the Efficacy and Safety of Plozasiran in Adults with Severe Hypertriglyceridemia (SHTG). 2024. Available online: https://clinicaltrials.gov/ct2/show/NCT06347016 (accessed on 13 June 2025).
- Study Evaluating LDL-C Change and Adherence to Inclisiran Lipid-Lowering Therapy in ASCVD (VICTORION REAL). 2022. Available online: https://clinicaltrials.gov/study/NCT05399992 (accessed on 11 August 2025).
- A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study to Investigate the Effect of Lepodisiran on the Reduction of Major Adverse Cardiovascular Events in Adults with Elevated Lipoprotein(a) Who Have Established Atherosclerotic Cardiovascular Disease or Are at Risk for a First Cardiovascular Event—ACCLAIM-Lp(a). 2024. Available online: https://clinicaltrials.gov/ct2/show/NCT06292013 (accessed on 13 June 2025).
- Olpasiran Trials of Cardiovascular Events and Lipoprotein(a) Reduction (OCEAN(a))—Outcomes Trial. 2022. Available online: https://clinicaltrials.ucsd.edu/trial/NCT05581303 (accessed on 13 June 2025).
- ZEUS—Effects of Ziltivekimab Versus Placebo on Cardiovascular Outcomes in Participants with Established Atherosclerotic Cardiovascular Disease, Chronic Kidney Disease and Systemic Inflammation. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT05021835 (accessed on 13 June 2025).
- A Placebo-Controlled, Double-Blind, Randomized Phase 3 Study to Evaluate the Effect of 10 mg Obicetrapib in Participants with HeFH and/or ASCVD Who Are Not Adequately Controlled by Their Lipid Modifying Therapies (BROADWAY). 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT05142722 (accessed on 13 June 2025).


| Paper Name | Authors | Year | Study Type | Population Studied | Key Findings | Inclusion Criteria/Indication | Outcome Trial Results (with p-Value if Available) | Side Effect Profile |
|---|---|---|---|---|---|---|---|---|
| Defining CKM Syndrome | ||||||||
| Cardiovascular-Kidney-Metabolic (CKM) syndrome: A state-of-the-art review | Sebastian S.A. et al. [3] | 2024 | Review | Epidemiological data from NHANES and AHA reports, highlighting prevalence across different demographics | CKM syndrome involves interconnected metabolic, cardiovascular, and renal diseases. Key mechanisms include insulin resistance, RAAS activation, oxidative stress, chronic inflammation, and lipotoxicity. The syndrome progresses through five stages, from no risk factors to symptomatic cardiovascular disease with kidney failure. Management focuses on screening, early intervention, and multidisciplinary care to reduce adverse outcomes. | N/A (Review) | N/A (Review) | 1. GLP-1 RA: Primarily causes gastrointestinal issues like nausea, vomiting, and diarrhea. 2. SGLT2 inhibitors: Increase the risk of genital and urinary tract infections 3. Finerenone: May lead to hyperkalemia |
| Cardiovascular-Kidney-Metabolic Health: A Presidential Advisory From the American Heart Association | AHA (Ndumele C.E. et al.) [17] | 2023 | Presidential Advisory/Scientific Statement | General US population; focus on individuals with/at risk for CVD, CKD, T2D, Obesity. | Defines CKM syndrome as a health disorder linking obesity, diabetes, CKD, and CVD. Proposes staging (0–4) based on risk factors and disease presence. Emphasizes prevention, integrated care, and addressing social determinants of health (SDOH). | N/A (Definitional document) | N/A (Definitional document) | N/A (Recommends therapies like SGLT2i/GLP-1 RA for appropriate stages) |
| An Overview of Cardiovascular-Kidney-Metabolic Syndrome | Ferdinand K.C. et al. [18] | 2024 | Review | General overview of CKM syndrome patients. | Reinforces CKM definition, staging. Highlights role of excess/dysfunctional adipose tissue, inflammation, oxidative stress. Notes impact of SDOH and additional risk factors (chronic inflammation, family history, sleep/mental health). | N/A (Review) | N/A (Review) | N/A (Review) |
| SGLT2 Inhibitor Trials (Meta-Analyses) | ||||||||
| Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction | Solomon et al. [19] | 2022 | Phase 3, multicenter, randomized, double-blind, placebo-controlled trial | 6263 patients with heart failure and left ventricular ejection fraction (LVEF) > 40%, with or without type 2 diabetes mellitus | Dapagliflozin significantly reduced the risk of worsening heart failure or cardiovascular death in patients with mildly reduced or preserved LVEF. Benefits were consistent across subgroups, including those with LVEF ≥ 60%, diabetes, and recent hospitalization. | Age ≥ 40 years, heart failure with LVEF > 40%, structural heart disease, elevated natriuretic peptides; included patients with previously reduced LVEF now >40% and those recently hospitalized | 16.4% in dapagliflozin vs. 19.5% in placebo (HR 0.82; 95% CI 0.73–0.92; p < 0.001); Worsening HF: HR 0.79; 95% CI 0.69–0.91; Cardiovascular death: HR 0.88; 95% CI 0.74–1.05 | Similar rates of serious adverse events in both groups (43.5% vs. 45.5%), no significant increase in hypoglycemia, ketoacidosis, or volume depletion; no cases of Fournier’s gangrene reported |
| Effects of SGLT2 inhibitors on cardiovascular outcomes in patients with stage 3/4 CKD: A meta-analysis | Li N. et al. [20] | 2022 | Meta-analysis | 11 RCTs; 27,823 patients with stage 3/4 CKD. | SGLT2i significantly reduced primary CV outcomes (CV death/HHF) across stage 3a, 3b, and 4 CKD, irrespective of T2D or HF status. | Patients with stage 3/4 CKD included in RCTs comparing SGLT2i vs. placebo | Reduced primary CV outcome risk by 26% (HR 0.74, 95% CI 0.69–0.80, p < 0.001 inferred). Consistent benefit across CKD stages (p interaction = 0.71). | General Class Effects: Genitourinary infections, potential for volume depletion/hypotension, rare risk of DKA. |
| Effect of SGLT2 Inhibitors on Cardiovascular Outcomes Across Various Patient Populations | Usman, et al. [21] | 2023 | Meta-analysis | 13 RCTs; >90,000 patients with HF, T2D, CKD or combinations. | SGLT2i consistently reduced the composite of first HHF or CV death (~23–24%) across HF, T2D, and CKD populations and combinations. Also reduced CV death (~12–16%) and HHF (~29–32%) separately. | Patients with HF, T2D, or CKD in large RCTs comparing SGLT2i vs. placebo | Reduced HHF/CV Death by ~24% (HR ~0.76–0.77, p < 0.001 inferred). Reduced CV Death by ~12–16% (p < 0.001 inferred). Reduced HHF by ~29–32% (p < 0.001 inferred) | General Class Effects: Genitourinary infections, potential for volume depletion/hypotension, rare risk of DKA. |
| GLP-1 Receptor Agonist Trials (Meta-Analyses) | ||||||||
| Kidney and Cardiovascular Outcomes Among Patients With CKD Receiving GLP-1 Receptor Agonists: A Systematic Review and Meta-Analysis of Randomized Trials | Chen et al. [22] | 2024 | Meta-analysis | 12 RCTs; 17,996 participants with baseline eGFR < 60 mL/min/1.73 m2. | GLP-1 RAs significantly reduced composite kidney outcome, risk of >30/40/50% eGFR decline, all-cause mortality, and composite CV outcomes in patients with CKD. | Adults with varying kidney function (incl. CKD eGFR < 60) in RCTs comparing GLP-1 RA vs. control | Reduced composite kidney outcome (OR 0.85, 95% CI 0.77–0.94, p = 0.001). Reduced all-cause mortality (OR 0.77, 95% CI 0.60–0.98, p = 0.03). Reduced composite CV outcomes (OR 0.86, 95% CI 0.74–0.99, p = 0.03) | General Class Effects: Gastrointestinal side effects (nausea, vomiting, diarrhea), injection site reactions, rare risk of pancreatitis/thyroid tumors. |
| Effects of GLP-1 receptor agonists on kidney and cardiovascular disease outcomes: a meta-analysis of randomized controlled trials | Badve et al. [23] | 2024 | Meta-analysis (incl. SELECT trial) | 11 RCTs; 85,373 participants (mostly T2D, one trial non-diabetic obesity/CVD). | GLP-1 RAs reduced composite kidney outcome, kidney failure, MACE, and all-cause death in T2D patients. Similar effects when non-diabetic SELECT trial included. | Participants (mostly T2D, one non-diabetic obesity/CVD trial) in large RCTs comparing GLP-1 RA vs. placebo | Reduced composite kidney outcome by 18% (HR 0.82, 95% CI 0.73–0.93). Reduced kidney failure by 16% (HR 0.84, 95% CI 0.72–0.99). Reduced MACE by 13% (HR 0.87, 95% CI 0.81–0.93). Reduced all-cause death by 12% (HR 0.88, 95% CI 0.83–0.93) | Higher treatment discontinuation due to AEs (RR 1.51). No difference in serious AEs vs. placebo. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bharaj, I.S.; Brar, A.; Kacheria, A.; Purewal, K.; Simister, A.; Thirupathy, U.; Gupta, P.; Kahlon, J.; Munaim, J.; Thwe, E.E.; et al. Contemporary and Emerging Therapeutics in Cardiovascular-Kidney-Metabolic (CKM) Syndrome: In Memory of Professor Akira Endo. Biomedicines 2025, 13, 2192. https://doi.org/10.3390/biomedicines13092192
Bharaj IS, Brar A, Kacheria A, Purewal K, Simister A, Thirupathy U, Gupta P, Kahlon J, Munaim J, Thwe EE, et al. Contemporary and Emerging Therapeutics in Cardiovascular-Kidney-Metabolic (CKM) Syndrome: In Memory of Professor Akira Endo. Biomedicines. 2025; 13(9):2192. https://doi.org/10.3390/biomedicines13092192
Chicago/Turabian StyleBharaj, Inderjeet Singh, Ajit Brar, Aayushi Kacheria, Karen Purewal, Austin Simister, Umabalan Thirupathy, Palak Gupta, Jasraj Kahlon, Juzer Munaim, Ei Ei Thwe, and et al. 2025. "Contemporary and Emerging Therapeutics in Cardiovascular-Kidney-Metabolic (CKM) Syndrome: In Memory of Professor Akira Endo" Biomedicines 13, no. 9: 2192. https://doi.org/10.3390/biomedicines13092192
APA StyleBharaj, I. S., Brar, A., Kacheria, A., Purewal, K., Simister, A., Thirupathy, U., Gupta, P., Kahlon, J., Munaim, J., Thwe, E. E., Ibrahim, S., Martinez Vargas, V., & Vijayaraghavan, K. (2025). Contemporary and Emerging Therapeutics in Cardiovascular-Kidney-Metabolic (CKM) Syndrome: In Memory of Professor Akira Endo. Biomedicines, 13(9), 2192. https://doi.org/10.3390/biomedicines13092192








