Effects of Novel Nutraceutical Combination on Lipid Pattern of Subjects with Sub-Optimal Blood Cholesterol Levels
Abstract
1. Introduction
- (1)
- aqueous extract of Berberis aristata (cortex ex ramis), titrated at 85% in berberine, an alkaloid known for the treatment of hypercholesterolemia, known for its action on the increased expression in the membrane of a receptor protein capable of internalizing LDL-C [8];
- (2)
- aqueous extract of Olea europea titrated in hydroxytyrosol (SelectSIEVE® OptiChol) which has demonstrated, at a daily dosage of 100 mg, a significant improvement in dyslipidemia in subjects with high cholesterol (115–190 mg/dL) after 1 month of treatment, with a reduction in LDL by 24% [9];
- (3)
- fenugreek seed extract (Trigonella foenum-graecum L.), an ingredient that may improve dyslipidemia, even in type II diabetic patients [10];
- (4)
- water/ethanol extract of artichoke leaf (Cynara scolymus L.) titrated at 0.5% in chlorogenic acid, capable of inhibiting the HMGCoA-reductase 16 enzyme and which represents an ingredient with high potential for lowering hypercholesterolemia [11];
- (5)
- phytosterols from sunflower seeds (Helianthus annuus L.) titrated at 95%, of which 40–50% β-sitosterols, known for their ability to reduce hypercholesterolemia demonstrated in several clinical studies and also recommended by the European Society of Atherosclerosis [12].
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 5 May 2025).
- Jain, K.S.; Kathiravan, M.K.; Somani, R.S.; Shishoo, C.J. The biology and chemistry of hyperlipidemia. Bioorg. Med. Chem. 2007, 15, 4674–4699. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Borén, J.; Chapman, M.J.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020, 41, 2313–2330. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Kleiman, N.S. Statin related muscle symptoms: Is it time to move on. BMJ 2022, 379, o2939. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Colletti, A.; Bajraktari, G.; Descamps, O.; Djuric, D.M.; Ezhov, M.; Fras, Z.; Katsiki, N.; Langlois, M.; Latkovskis, G.; et al. Lipid lowering nutraceuticals in clinical practice: Position paper from an International Lipid Expert Panel. Arch. Med. Sci. 2017, 13, 965–1005. [Google Scholar] [CrossRef] [PubMed]
- Amarowicz, R.; Pegg, R.B. Protection of natural antioxidants against low-density lipoprotein oxidation. Adv. Food Nutr. Res. 2020, 93, 251–291. [Google Scholar] [CrossRef]
- Derosa, G.; Romano, D.; D’Angelo, A.; Maffioli, P. Berberis aristata combined with Silybum marianum on lipid profile in patients not tolerating statins at high doses. Atherosclerosis 2015, 239, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Di Micoli, A.; Veronesi, M.; Grandi, E.; Borghi, C. Hydroxytyrosol-Rich Olive Extract for Plasma Cholesterol Control. Appl. Sci. 2022, 12, 10086. [Google Scholar] [CrossRef]
- Geberemeskel, G.A.; Debebe, Y.G.; Nguse, N.A. Antidiabetic Effect of Fenugreek Seed Powder Solution (Trigonella foenum-graecum L.) on Hyperlipidemia in Diabetic Patients. J. Diabetes Res. 2019, 2019, 8507453. [Google Scholar] [CrossRef]
- Gebhardt, R. Inhibition of cholesterol biosynthesis in primary cultured rat hepatocytes by artichoke (Cynara scolymus L.) extracts. J. Pharmacol. Exp. Ther. 1998, 286, 1122–1128. [Google Scholar] [CrossRef]
- Cabral, C.E.; Klein, M.R.S.T. Phytosterols in the Treatment of Hypercholesterolemia and Prevention of Cardiovascular Diseases. Arq. Bras. Cardiol. 2017, 109, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; D’Addato, S.; Borghi, C. A Randomized, Double-Blinded, Placebo-Controlled, Clinical Study of the Effects of a Nutraceutical Combination (LEVELIP DUO®) on LDL Cholesterol Levels and Lipid Pattern in Subjects with Sub-Optimal Blood Cholesterol Levels (NATCOL Study). Nutrients 2020, 12, 3127. [Google Scholar] [CrossRef]
- D’Agostino, R.B., Sr.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Townsend, N.; Nichols, M.; Scarborough, P.; Rayner, M. Cardiovascular disease in Europe--epidemiological update 2015. Eur. Heart J. 2015, 36, 2696–2705. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef]
- Banach, M.; Reiner, Ž.; Surma, S.; Bajraktari, G.; Bielecka-Dabrowa, A.; Bunc, M.; Bytyçi, I.; Ceska, R.; Cicero, A.F.G.; Dudek, D.; et al. 2024 Recommendations on the Optimal Use of Lipid-Lowering Therapy in Established Atherosclerotic Cardiovascular Disease and Following Acute Coronary Syndromes: A Position Paper of the International Lipid Expert Panel (ILEP). Drugs 2024, 84, 1541–1577. [Google Scholar] [CrossRef] [PubMed]
- Makover, M.E.; Shapiro, M.D.; Toth, P.P. There is urgent need to treat atherosclerotic cardiovascular disease risk earlier, more intensively, and with greater precision: A review of current practice and recommendations for improved effectiveness. Am. J. Prev. Cardiol. 2022, 12, 100371. [Google Scholar] [CrossRef] [PubMed]
- Newman, W.P., 3rd; Freedman, D.S.; Voors, A.W.; Gard, P.D.; Srinivasan, S.R.; Cresanta, J.L.; Williamson, G.D.; Webber, L.S.; Berenson, G.S. Relation of serum lipoprotein levels and systolic blood pressure to early atherosclerosis. The Bogalusa Heart Study. N. Engl. J. Med. 1986, 314, 138–144. [Google Scholar] [CrossRef]
- Tabas, I.; Williams, K.J.; Borén, J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: Update and therapeutic implications. Circulation 2007, 116, 1832–1844. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Brown, M.S. A century of cholesterol and coronaries: From plaques to genes to statins. Cell 2015, 161, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Lütjohann, D.; Klör, H.U.; Stellaard, F. Measurement of Serum Low Density Lipoprotein Cholesterol and Triglyceride-Rich Remnant Cholesterol as Independent Predictors of Atherosclerotic Cardiovascular Disease: Possibilities and Limitations. Nutrients 2023, 15, 2202. [Google Scholar] [CrossRef]
- Burger, P.M.; Dorresteijn, J.A.N.; Koudstaal, S.; Holtrop, J.; Kastelein, J.J.P.; Jukema, J.W.; Ridker, P.M.; Mosterd, A.; Visseren, F.L.J. Course of the effects of LDL-cholesterol reduction on cardiovascular risk over time: A meta-analysis of 60 randomized controlled trials. Atherosclerosis 2024, 396, 118540. [Google Scholar] [CrossRef]
- Ray, K.K.; Ference, B.A.; Séverin, T.; Blom, D.; Nicholls, S.J.; Shiba, M.H.; Almahmeed, W.; Alonso, R.; Daccord, M.; Ezhov, M.; et al. World Heart Federation Cholesterol Roadmap 2022. Glob. Heart 2022, 17, 75. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.D.; Panza, G.; Zaleski, A.; Taylor, B. Statin-Associated Side Effects. J. Am. Coll. Cardiol. 2016, 67, 2395–2410. [Google Scholar] [CrossRef]
- Ray, K.K.; Haq, I.; Bilitou, A.; Manu, M.C.; Burden, A.; Aguiar, C.; Arca, M.; Connolly, D.L.; Eriksson, M.; Ferrières, J.; et al. Treatment gaps in the implementation of LDL cholesterol control among high- and very high-risk patients in Europe between 2020 and 2021: The multinational observational SANTORINI study. Lancet Reg. Health Eur. 2023, 29, 100624. [Google Scholar] [CrossRef]
- Derosa, G.; Colletti, A.; Maffioli, P.; D’Angelo, A.; Lupi, A.; Zito, G.B.; Mureddu, G.F.; Raddino, R.; Fedele, F.; Cicero, A.F.G. Lipid-lowering nutraceuticals update on scientific evidence. J. Cardiovasc. Med. 2020, 21, 845–859. [Google Scholar] [CrossRef]
- Giglio, R.V.; Pantea Stoian, A.; Al-Rasadi, K.; Banach, M.; Patti, A.M.; Ciaccio, M.; Rizvi, A.A.; Rizzo, M. Novel Therapeutical Approaches to Managing Atherosclerotic Risk. Int. J. Mol. Sci. 2021, 22, 4633. [Google Scholar] [CrossRef]
- Patti, A.M.; Toth, P.P.; Giglio, R.V.; Banach, M.; Noto, M.; Nikolic, D.; Montalto, G.; Rizzo, M. Nutraceuticals as an Important Part of Combination Therapy in Dyslipidaemia. Curr. Pharm. Des. 2017, 23, 2496–2503. [Google Scholar] [CrossRef]
- Ikeda, I.; Sugano, M. Some aspects of mechanism of inhibition of cholesterol absorption by beta-sitosterol. Biochim. Biophys. Acta 1983, 732, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Ras, R.T.; Geleijnse, J.M.; Trautwein, E.A. LDL-cholesterol-lowering effect of plant sterols and stanols across different dose ranges: A meta-analysis of randomised controlled studies. Br. J. Nutr. 2014, 112, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Bosco, G.; Di Giacomo Barbagallo, F.; Spampinato, S.; Lanzafame, L.; Di Pino, A.; Piro, S.; Purrello, F.; Scicali, R. Management of Statin Intolerant Patients in the Era of Novel Lipid Lowering Therapies: A Critical Approach in Clinical Practice. J. Clin. Med. 2023, 12, 2444. [Google Scholar] [CrossRef] [PubMed]
- Fuller, S.; Stephens, J.M. Diosgenin, 4-hydroxyisoleucine, and fiber from fenugreek: Mechanisms of actions and potential effects on metabolic syndrome. Adv. Nutr. 2015, 6, 189–197. [Google Scholar] [CrossRef]
- Yao, D.; Zhang, B.; Zhu, J.; Zhang, Q.; Hu, Y.; Wang, S.; Wang, Y.; Cao, H.; Xiao, J. Advances on application of fenugreek seeds as functional foods: Pharmacology, clinical application, products, patents and market. Crit. Rev. Food Sci. Nutr. 2020, 60, 2342–2352. [Google Scholar] [CrossRef] [PubMed]
- Fakhr, L.; Chehregosha, F.; Zarezadeh, M.; Chaboksafar, M.; Tarighat-Esfanjani, A. Effects of fenugreek supplementation on the components of metabolic syndrome: A systematic review and dose-response meta-analysis of randomized clinical trials. Pharmacol. Res. 2023, 187, 106594. [Google Scholar] [CrossRef] [PubMed]
- Porro, C.; Benameur, T.; Cianciulli, A.; Vacca, M.; Chiarini, M.; De Angelis, M.; Panaro, M.A. Functional and Therapeutic Potential of Cynara scolymus in Health Benefits. Nutrients 2024, 16, 872. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.; Daia, A.M. Exploring the Cardiovascular Potential of Artichoke-A Comprehensive Review. Biology 2025, 14, 397. [Google Scholar] [CrossRef]
- Ataei, S.; Kesharwani, P.; Sahebkar, A. Berberine: Ins and outs of a nature-made PCSK9 inhibitor. EXCLI J. 2022, 21, 1099–1110. [Google Scholar] [CrossRef]
- Pisciotta, L.; Bellocchio, A.; Bertolini, S. Nutraceutical pill containing berberine versus ezetimibe on plasma lipid pattern in hypercholesterolemic subjects and its additive effect in patients with familial hypercholesterolemia on stable cholesterol-lowering treatment. Lipids Health Dis. 2012, 11, 123. [Google Scholar] [CrossRef]
- Spigoni, V.; Aldigeri, R.; Antonini, M.; Micheli, M.M.; Fantuzzi, F.; Fratter, A.; Pellizzato, M.; Derlindati, E.; Zavaroni, I.; Bonadonna, R.C.; et al. Effects of a New Nutraceutical Formulation (Berberine, Red Yeast Rice and Chitosan) on Non-HDL Cholesterol Levels in Individuals with Dyslipidemia: Results from a Randomized, Double Blind, Placebo-Controlled Study. Int. J. Mol. Sci. 2017, 18, 1498. [Google Scholar] [CrossRef] [PubMed]
- Bucciantini, M.; Leri, M.; Nardiello, P.; Casamenti, F.; Stefani, M. Olive Polyphenols: Antioxidant and Anti-Inflammatory Properties. Antioxidants 2021, 10, 1044. [Google Scholar] [CrossRef]

| N = 44 | |
|---|---|
| Age (years) | 54 (43;58) |
| <50 years | 18 (41%) |
| 50–60 years | 21 (48%) |
| >60 years | 5 (11%) |
| Male | 33 (75%) |
| Current smoker | 19 (43%) |
| Weight (Kg) | 76 (68;85) |
| Body Mass Index (Kg/m2) | 26.0 ± 3.7 |
| Waist circumference (cm) | 87 (80;95) |
| Heart rate (b/min) | 68 (60;75) |
| Systolic blood pressure (mm Hg) | 126 ± 16 |
| Diastolic blood pressure (mm Hg) | 75 ± 8 |
| Total cholesterol (mg/dL) | 223 ± 24 |
| HDL cholesterol (mg/dL) | 52 ± 14 |
| LDL cholesterol (mg/dL) | 151 ± 21 |
| LDL/HDL ratio | 3.2 ± 0.9 |
| Triglycerides (mg/dL) | 124 ± 58 |
| D-Dimers (ng/mL) | 283 (170;442) |
| Creatinine (mg/dL) | 0.86 ± 0.12 |
| Estimated GFR (mL/min/1.73 m2) | 100 ± 11 |
| Azotemia (mg/dL) | 33 ± 9 |
| Aspartate transaminase (U/L) | 22 ± 5 |
| Alanine transaminase (U/L) | 24 ± 13 |
| C-Reactive Protein (mg/L) | 0.20 ± 0.18 |
| Total bilirubin (mg/dL) | 0.81 ± 0.36 |
| Direct bilirubin (mg/dL) | 0.240 ± 0.089 |
| 1 Month | 3 Month | 6 Month | |
|---|---|---|---|
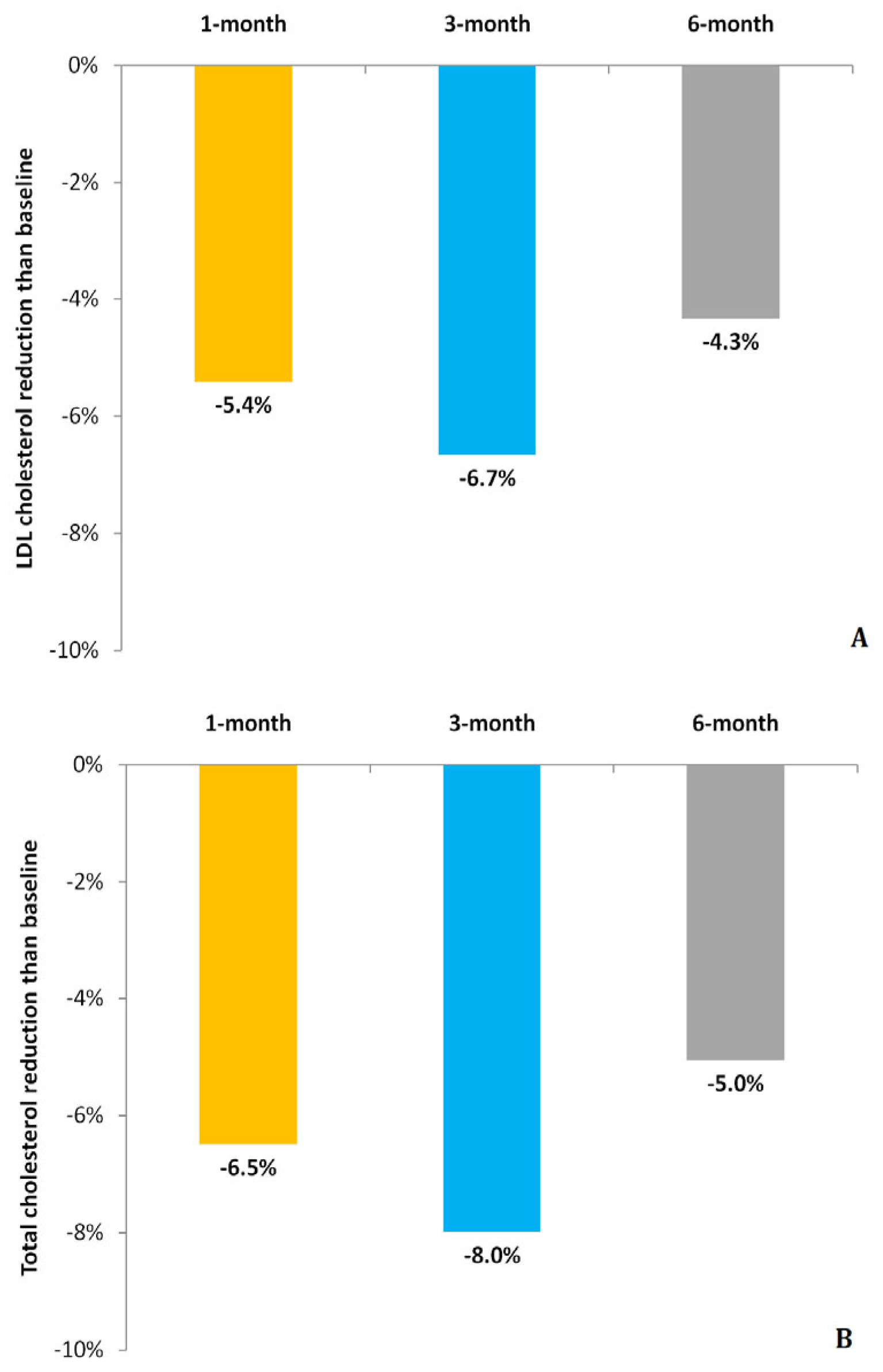
| Total cholesterol (mg/dL) | 208 ± 28 | 205 ± 32 | 210 ± 27 |
| Change than baseline (mg/dL) | −15 ± 22 | −18 ± 28 | −12 ± 29 |
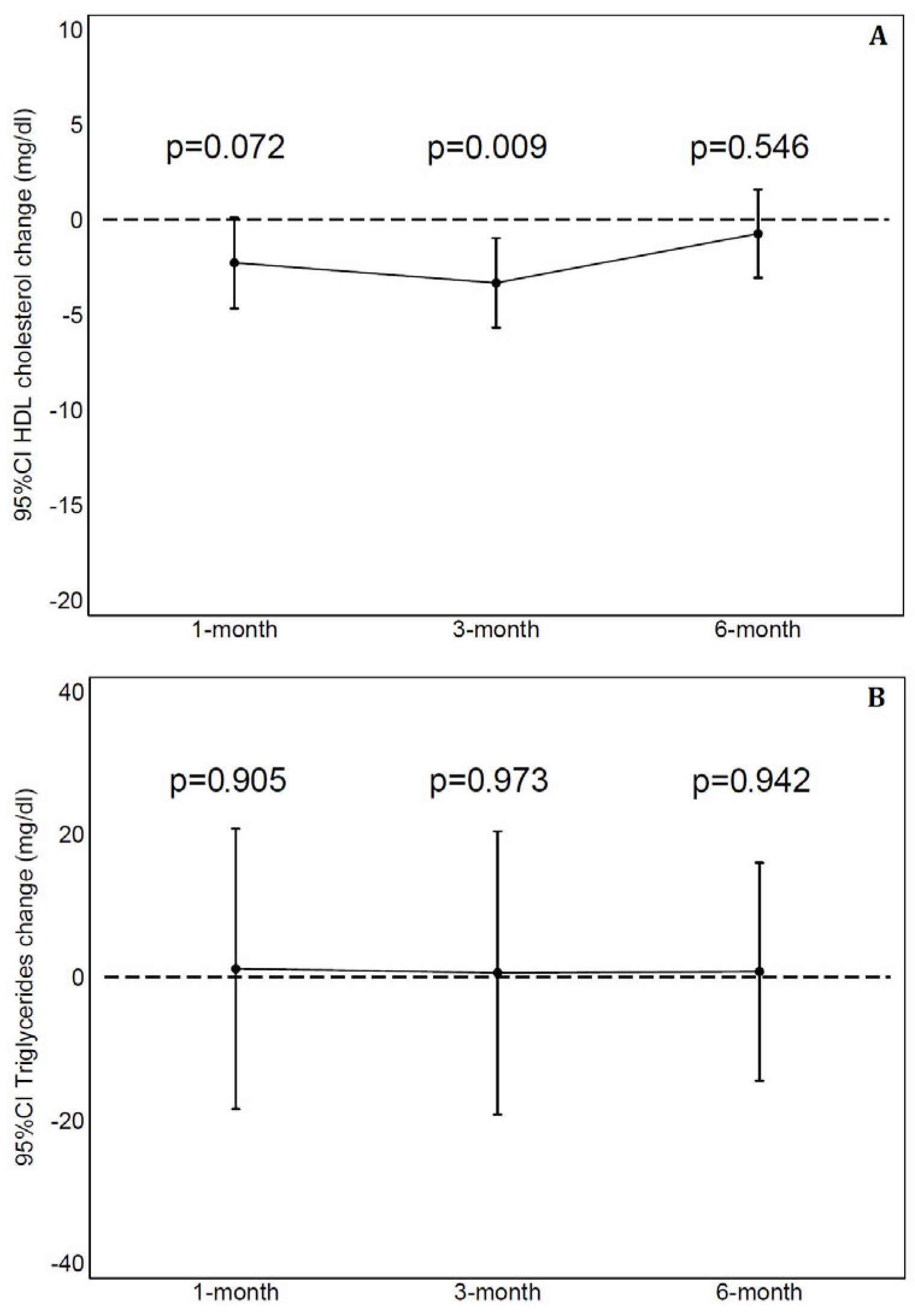
| HDL cholesterol (mg/dL) | 49 ± 12 | 49 ± 11 | 51 ± 11 |
| Change than baseline (mg/dL) | −2 ± 8 | −3 ± 8 | −1 ± 7 |
| LDL cholesterol (mg/dL) | 142 ± 24 | 141 ± 27 | 144 ± 25 |
| Change than baseline (mg/dL) | −9 ± 19 | −10 ± 21 | −7 ± 22 |
| LDL/HDL ratio | 3.0 ± 0.8 | 3.0 ± 0.9 | 3.0 ± 0.9 |
| Change than baseline | −0.1 ± 0.4 | −0.1 ± 0.6 | −0.2 ± 0.5 |
| Triglycerides (mg/dL) | 122 ± 72 | 120 ± 66 | 114 ± 59 |
| Change than baseline (mg/dL) | 1 ± 68 | 0 ± 68 | 1 ± 49 |
| D-Dimers (ng/mL) | 256 (127;404) | 283 (163;478) | 307 (162;561) |
| Change than baseline (ng/mL) | −43 (−110;17) | 10 (−78;89) | −22 (−128;163) |
| Creatinine (mg/dL) | 0.87 ± 0.12 | 0.84 ± 0.12 | 0.83 ± 0.13 |
| Change than baseline (mg/dL) | 0.009 ± 0.073 | −0.018 ± 0.081 | −0.033 ± 0.085 |
| Estimated GFR (mL/min/1.73 m2) | 100 ± 10 | 102 ± 10 | 102 ± 9 |
| Change than baseline (mL/min/1.73 m2) | 0 ± 7 | 2 ± 8 | 3 ± 8 |
| Azotemia (mg/dL) | 35 ± 8 | 34 ± 9 | 35 ± 9 |
| Change than baseline (mg/dL) | 2 ± 8 | 1 ± 8 | 2 ± 8 |
| Aspartate transaminase (U/L) | 21 ± 5 | 23 ± 5 | 31 ± 52 |
| Change than baseline (U/L) | −1 ± 6 | 0 ± 5 | 9 ± 53 |
| Alanine transaminase (U/L) | 22 ± 11 | 20 ± 11 | 25 ± 21 |
| Change than baseline (U/L) | −2 ± 9 | −3 ± 9 | 2 ± 21 |
| C-reactive protein (mg/L) | 0.22 ± 0.22 | 0.31 ± 0.66 | 0.22 ± 0.31 |
| Change than baseline in (mg/L) | 0.03 ± 0.20 | 0.13 ± 0.72 | 0.02 ± 0.24 |
| Total bilirubin (mg/dL) | 0.77 ± 0.35 | 0.76 ± 0.42 | 0.81 ± 0.53 |
| Change than baseline (mg/dL) | −0.03 ± 0.32 | −0.06 ± 0.34 | 0.01 ± 0.39 |
| Direct bilirubin (mg/dL) | 0.25 ± 0.100 | 0.27 ± 0.11 | 0.26 ± 0.13 |
| Change than baseline (mg/dL) | 0.01 ± 0.10 | 0.022 ± 0.096 | 0.02 ± 0.10 |
| <10 mg/dL | ≥10 mg/dL | ||
|---|---|---|---|
| n = 22 | n = 22 | p | |
| Age (years) | 50 ± 8 | 50 ± 14 | 0.606 |
| Males | 77% | 73% | 0.728 |
| Weight (Kg) | 77 ± 13 | 78 ± 17 | 0.752 |
| Body Mass Index (Kg/m2) | 26.0 ± 3.1 | 25.9 ± 4.2 | 0.976 |
| Waist circumference (cm) | 89 ± 11 | 87 ± 12 | 0.654 |
| Heart rate (b/min) | 69 ± 11 | 68 ± 6 | 0.612 |
| Systolic blood pressure (mm Hg) | 127 ± 19 | 124 ± 11 | 0.759 |
| Diastolic blood pressure (mm Hg) | 76 ± 9 | 75 ± 8 | 0.574 |
| Total cholesterol (mg/dL) | 220 ± 23 | 225 ± 25 | 0.557 |
| LDL cholesterol (mg/dL) | 147 ± 21 | 156 ± 20 | 0.159 |
| HDL cholesterol (mg/dL) | 51 ± 14 | 52 ± 15 | 0.833 |
| LDL/HDL ratio | 3.1 ± 1.0 | 3.2 ± 0.9 | 0.700 |
| Triglycerides (mg/dL) | 119 ± 48 | 129 ± 67 | 0.880 |
| D-Dimers (ng/mL) | 259 (147;580) | 285 (198;442) | 0.766 |
| Creatinine (mg/dL) | 0.87 ± 0.13 | 0.85 ± 0.12 | 0.620 |
| Estimated GFR (mL/min/1.73 m2) | 100 ± 10 | 101 ± 12 | 0.786 |
| Azotemia (mg/dL) | 34 ± 9 | 32 ± 8 | 0.429 |
| Aspartate transaminase (U/L) | 20 ± 4 | 25 ± 5 | 0.002 |
| Alanine transaminase (U/L) | 20 ± 5 | 28 ± 16 | 0.089 |
| C-reactive protein (mg/L) | 0.21 ± 0.21 | 0.20 ± 0.15 | 0.187 |
| Total bilirubin (mg/dL) | 0.75 ± 0.30 | 0.87 ± 0.42 | 0.510 |
| Direct bilirubin (mg/dL) | 0.227 ± 0.077 | 0.255 ± 0.100 | 0.317 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitulano, N.; Guida, P.; Abrusci, V.; Ceci, E.; De Nicolò, E.V.; Martinotti, S.; Duni, N.; Troisi, F.; Quadrini, F.; di Monaco, A.; et al. Effects of Novel Nutraceutical Combination on Lipid Pattern of Subjects with Sub-Optimal Blood Cholesterol Levels. Biomedicines 2025, 13, 1948. https://doi.org/10.3390/biomedicines13081948
Vitulano N, Guida P, Abrusci V, Ceci E, De Nicolò EV, Martinotti S, Duni N, Troisi F, Quadrini F, di Monaco A, et al. Effects of Novel Nutraceutical Combination on Lipid Pattern of Subjects with Sub-Optimal Blood Cholesterol Levels. Biomedicines. 2025; 13(8):1948. https://doi.org/10.3390/biomedicines13081948
Chicago/Turabian StyleVitulano, Nicola, Pietro Guida, Vito Abrusci, Edmondo Ceci, Edy Valentina De Nicolò, Stefano Martinotti, Nicola Duni, Federica Troisi, Federico Quadrini, Antonio di Monaco, and et al. 2025. "Effects of Novel Nutraceutical Combination on Lipid Pattern of Subjects with Sub-Optimal Blood Cholesterol Levels" Biomedicines 13, no. 8: 1948. https://doi.org/10.3390/biomedicines13081948
APA StyleVitulano, N., Guida, P., Abrusci, V., Ceci, E., De Nicolò, E. V., Martinotti, S., Duni, N., Troisi, F., Quadrini, F., di Monaco, A., Iacoviello, M., Passantino, A., & Grimaldi, M. (2025). Effects of Novel Nutraceutical Combination on Lipid Pattern of Subjects with Sub-Optimal Blood Cholesterol Levels. Biomedicines, 13(8), 1948. https://doi.org/10.3390/biomedicines13081948







