Distinct Markers of Discordant Treatment Response to Lifestyle Intervention in MASLD, Independent of Weight Loss
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Clinical and Metabolic Evaluation
2.3. Hepatic Steatosis Assessment with MRI-PDFF
2.4. Liver Stiffness Measurements with 2D-SWE
2.5. Lifestyle Intervention and Follow-Up
2.6. Study Endpoints
2.7. Statistical Analysis
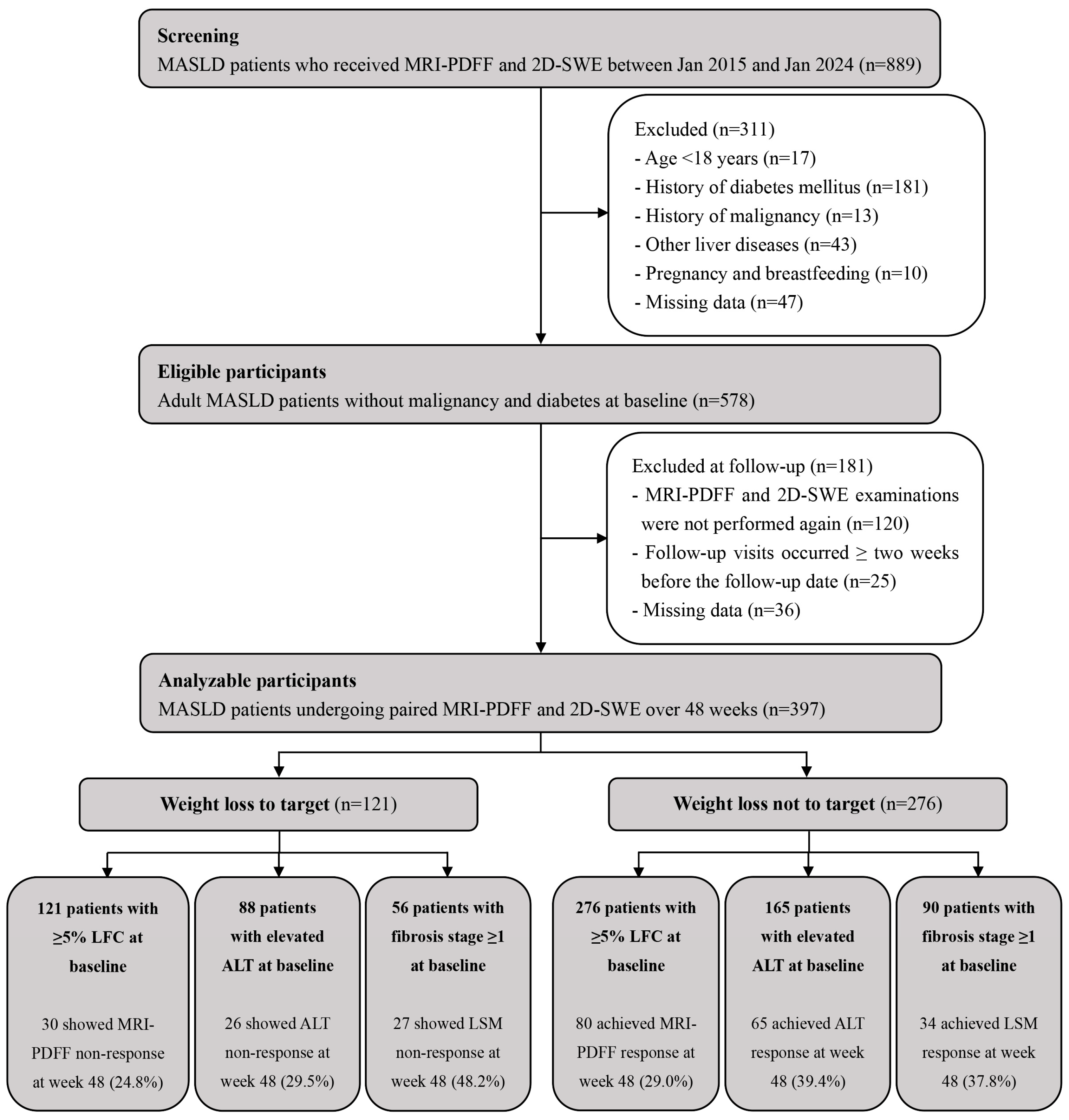
3. Results
3.1. Study Cohort Characteristics at Baseline and During Follow-Up
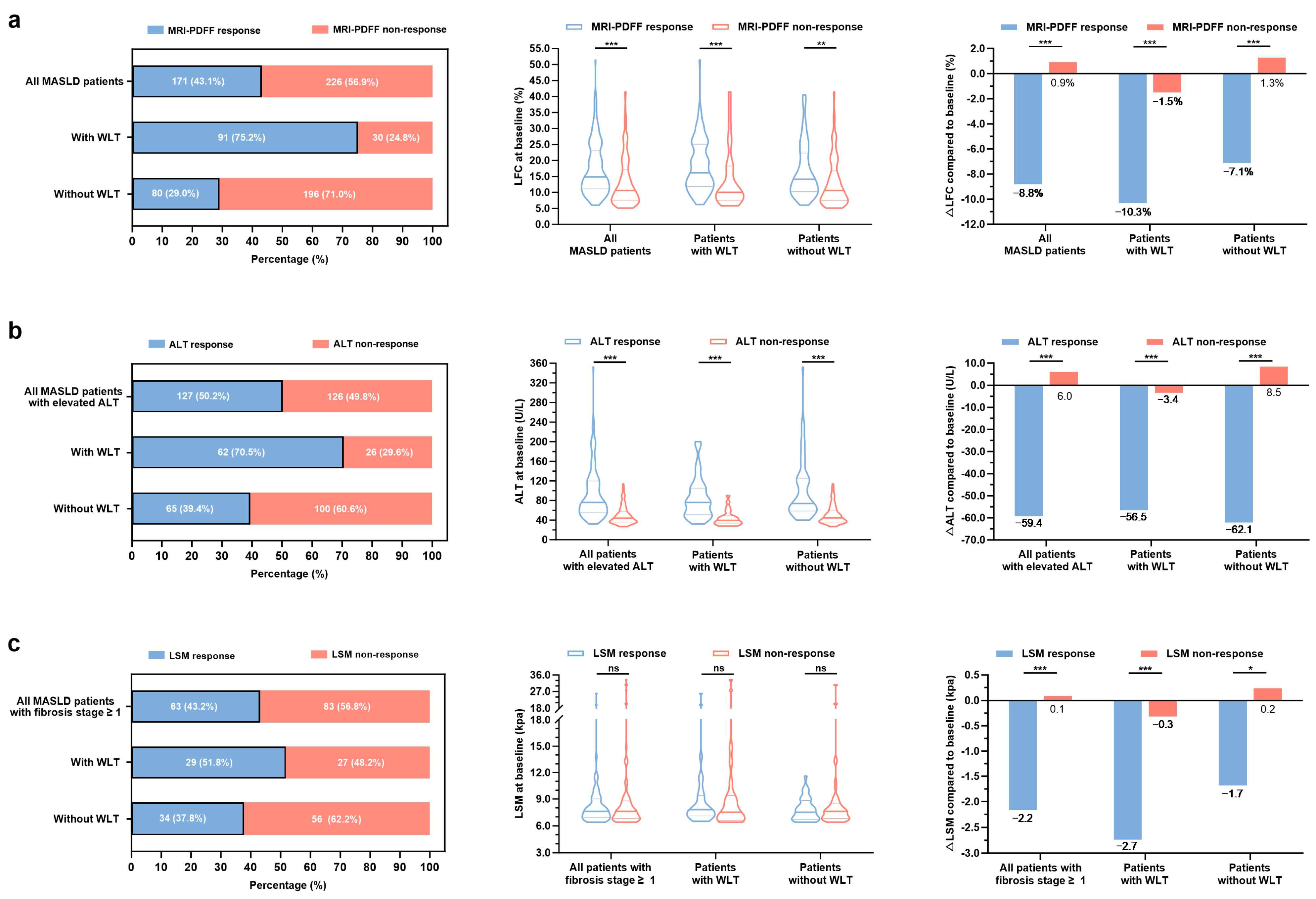
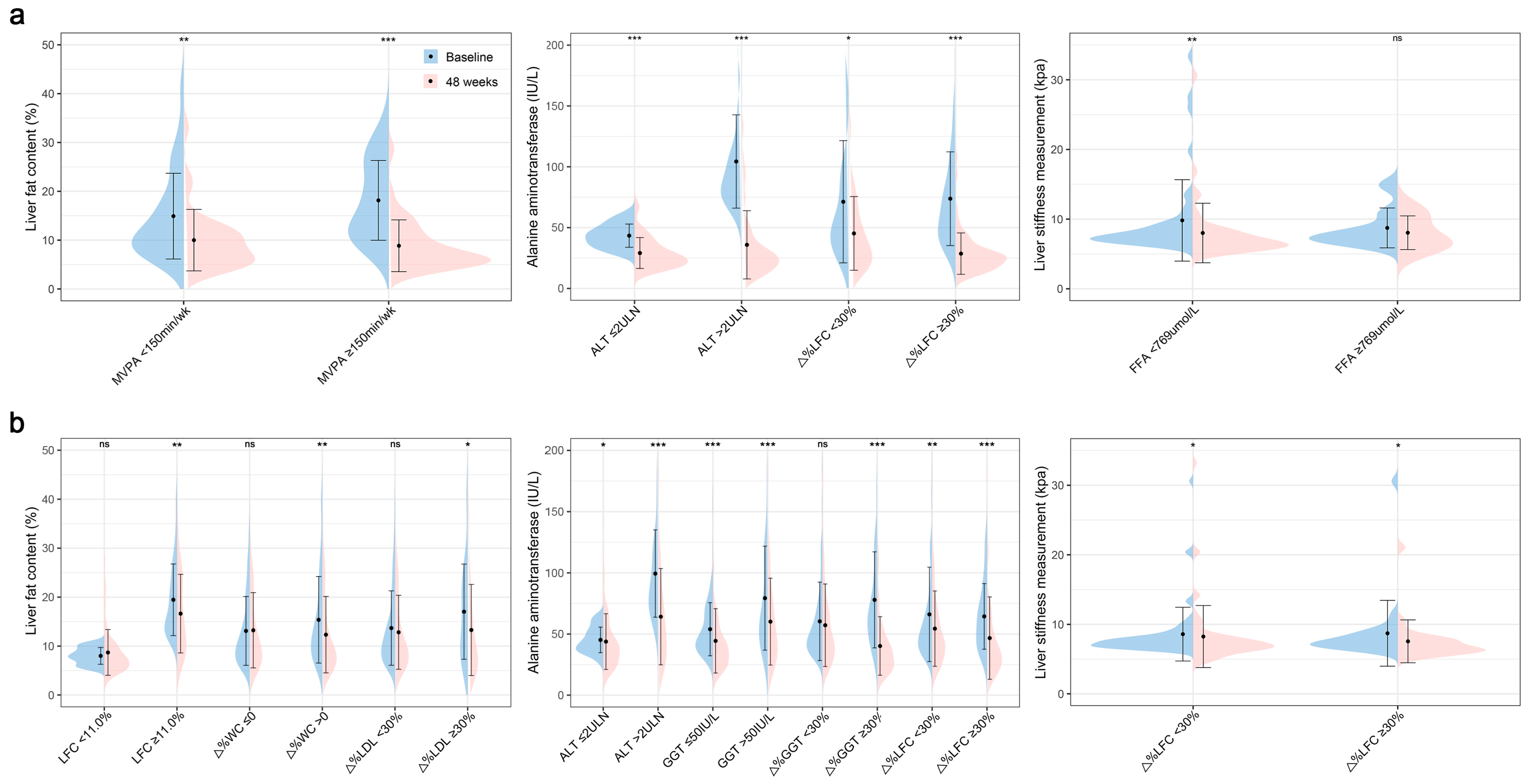
3.2. Clinical Characteristic Patterns of Treatment Response in Patients with MASLD
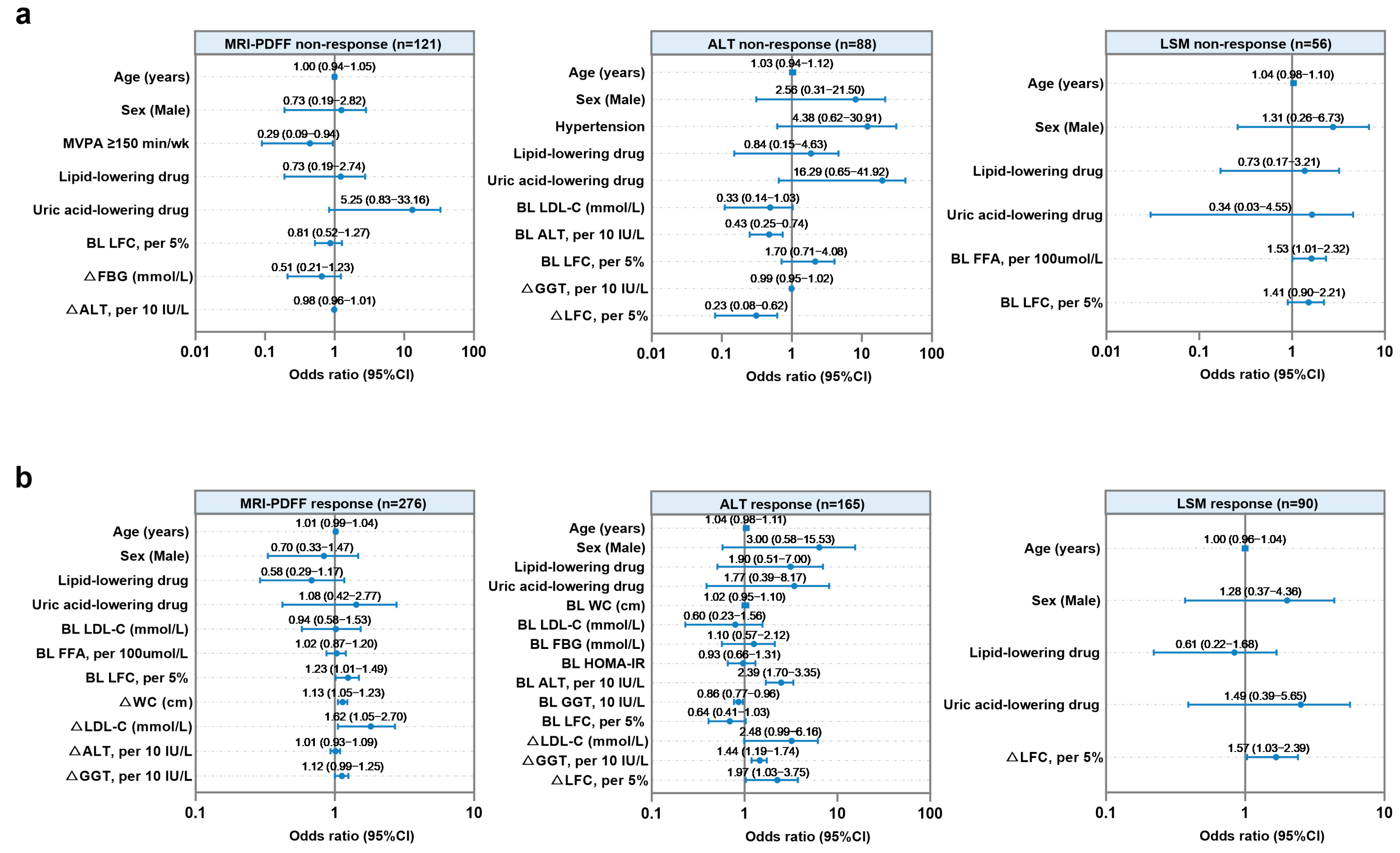
3.3. Factors Associated with Treatment Non-Response to Hepatic Steatosis, Injury, or Fibrosis in Patients with MASLD Undergoing WLT
3.4. Factors Associated with Treatment Response to Hepatic Steatosis, Injury, or Fibrosis in Patients with MASLD Without WLT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| MASH | Metabolic dysfunction-associated steatohepatitis |
| WLT | Weight loss target |
| CI | Confidence interval |
| ALT | Alanine aminotransferase |
| MRI | Magnetic resonance imaging |
| MRI-PDFF | Magnetic resonance imaging-based proton density fat fraction |
| LFC | Liver fat content |
| 2D-SWE | Two-dimensional shear wave elastography |
| WC | Waist circumference |
| BMI | Body mass index |
| FFA | Free fatty acid |
| FBG | Fasting glucose |
| FINS | Fasting insulin |
| UA | Uric acid |
| HOMA-IR | Homeostasis model assessment of insulin resistance |
| GGT | γ-glutamyl transpeptidase |
| LSM | Liver stiffness measurements |
| MVPA | Moderate-vigorous physical activity |
| LDL | Low-density lipoprotein cholesterol |
| TG | Triglyceride |
References
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef]
- Chan, W.K.; Chuah, K.H.; Rajaram, R.B.; Lim, L.L.; Ratnasingam, J.; Vethakkan, S.R. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review. J. Obes. Metab. Syndr. 2023, 32, 197–213. [Google Scholar] [CrossRef]
- Harrison, S.A.; Bedossa, P.; Guy, C.D.; Schattenberg, J.M.; Loomba, R.; Taub, R.; Labriola, D.; Moussa, S.E.; Neff, G.W.; Rinella, M.E.; et al. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. N. Engl. J. Med. 2024, 390, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Chitturi, S.; Wong, V.W.; Chan, W.K.; Wong, G.L.; Wong, S.K.; Sollano, J.; Ni, Y.H.; Liu, C.J.; Lin, Y.C.; Lesmana, L.A.; et al. The Asia-Pacific Working Party on Non-alcoholic Fatty Liver Disease guidelines 2017-Part 2: Management and special groups. J. Gastroenterol. Hepatol. 2018, 33, 86–98. [Google Scholar] [CrossRef]
- Cusi, K.; Isaacs, S.; Barb, D.; Basu, R.; Caprio, S.; Garvey, W.T.; Kashyap, S.; Mechanick, J.I.; Mouzaki, M.; Nadolsky, K.; et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr. Pract. 2022, 28, 528–562. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef]
- Long, M.T.; Noureddin, M.; Lim, J.K. AGA Clinical Practice Update: Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Lean Individuals: Expert Review. Gastroenterology 2022, 163, 764–774. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Corey, K.E.; Lim, J.K. AGA Clinical Practice Update on Lifestyle Modification Using Diet and Exercise to Achieve Weight Loss in the Management of Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology 2021, 160, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Koutoukidis, D.A.; Koshiaris, C.; Henry, J.A.; Noreik, M.; Morris, E.; Manoharan, I.; Tudor, K.; Bodenham, E.; Dunnigan, A.; Jebb, S.A.; et al. The effect of the magnitude of weight loss on non-alcoholic fatty liver disease: A systematic review and meta-analysis. Metabolism 2021, 115, 154455. [Google Scholar] [CrossRef] [PubMed]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015, 149, 367–378. [Google Scholar] [CrossRef]
- Malespin, M.H.; Barritt, A.S., 4th; Watkins, S.E.; Schoen, C.; Tincopa, M.A.; Corbin, K.D.; Mospan, A.R.; Munoz, B.; Trinh, H.N.; Weiss, L.M.; et al. Weight Loss and Weight Regain in Usual Clinical Practice: Results from the TARGET-NASH Observational Cohort. Clin. Gastroenterol. Hepatol. 2022, 20, 2393–2395. [Google Scholar] [CrossRef]
- Katsagoni, C.N.; Papatheodoridis, G.V.; Ioannidou, P.; Deutsch, M.; Alexopoulou, A.; Papadopoulos, N.; Papageorgiou, M.V.; Fragopoulou, E.; Kontogianni, M.D. Improvements in clinical characteristics of patients with non-alcoholic fatty liver disease, after an intervention based on the Mediterranean lifestyle: A randomised controlled clinical trial. Br. J. Nutr. 2018, 120, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Stine, J.G.; Munaganuru, N.; Barnard, A.; Wang, J.L.; Kaulback, K.; Argo, C.K.; Singh, S.; Fowler, K.J.; Sirlin, C.B.; Loomba, R. Change in MRI-PDFF and Histologic Response in Patients with Nonalcoholic Steatohepatitis: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2021, 9, 2274–2283. [Google Scholar] [CrossRef] [PubMed]
- Imajo, K.; Honda, Y.; Kobayashi, T.; Nagai, K.; Ozaki, A.; Iwaki, M.; Kessoku, T.; Ogawa, Y.; Takahashi, H.; Saigusa, Y.; et al. Direct Comparison of US and MR Elastography for Staging Liver Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2022, 20, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Christou, G.A.; Katsiki, N.; Blundell, J.; Fruhbeck, G.; Kiortsis, D.N. Semaglutide as a promising antiobesity drug. Obes. Rev. 2019, 20, 805–815. [Google Scholar] [CrossRef]
- Park, S.; Buranakitjaroen, P.; Chen, C.H.; Chia, Y.C.; Divinagracia, R.; Hoshide, S.; Shin, J.; Siddique, S.; Sison, J.; Soenarta, A.A.; et al. Expert panel consensus recommendations for home blood pressure monitoring in Asia: The Hope Asia Network. J. Hum. Hypertens. 2018, 32, 249–258. [Google Scholar] [CrossRef]
- Li, F.; Ye, J.; Sun, Y.; Lin, Y.; Wu, T.; Shao, C.; Ma, Q.; Liao, X.; Feng, S.; Zhong, B. Distinct Dose-Dependent Association of Free Fatty Acids with Diabetes Development in Nonalcoholic Fatty Liver Disease Patients. Diabetes Metab. J. 2021, 45, 417–429. [Google Scholar] [CrossRef]
- Yuwaki, K.; Shimazu, T.; Yamagiwa, Y.; Inoue, M.; Goto, A.; Yamaji, T.; Iwasaki, M.; Sawada, N.; Tsugane, S. Association between serum liver enzymes and all-cause mortality: The Japan Public Health Center-based Prospective Study. Liver Int. 2019, 39, 1566–1576. [Google Scholar] [CrossRef]
- Luo, L.; Ye, J.; Zhuo, S.; Ma, B.; Mai, W.; Cao, X.; Liang, L.; Wang, W.; Feng, S.; Dong, Z.; et al. Specific metabolic impairments indicate loss of sustained liver improvements in metabolic dysfunction-associated steatotic liver disease treatment. Hepatobiliary Surg. Nutr. 2024, 13, 632–649. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Luo, Y.; Zhang, Z.; Cai, H.; Li, Y.; Chan, T.; Wu, L.; Li, Z.; Feng, S. MR quantification of total liver fat in patients with impaired glucose tolerance and healthy subjects. PLoS ONE 2014, 9, e111283. [Google Scholar] [CrossRef]
- Loprinzi, P.D. Frequency of moderate-to-vigorous physical activity (MVPA) is a greater predictor of systemic inflammation than total weekly volume of MVPA: Implications for physical activity promotion. Physiol. Behav. 2015, 141, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; He, J.; Chen, N.; Lu, J.; Shen, S.; Xiao, W.; Hu, F.; Xiao, H.; Wu, Y.; Xia, X.; et al. Validity and Reproducibility of a Dietary Questionnaire for Consumption Frequencies of Foods during Pregnancy in the Born in Guangzhou Cohort Study (BIGCS). Nutrients 2016, 8, 454. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z. China Food Composition Tables: Standard Edition, 6th ed.; Peking University Medical Press: Beijing, China, 2018. [Google Scholar]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, e285–e350. [Google Scholar] [CrossRef]
- Multidisciplinary Expert Task Force on Hyperuricemia and Related Diseases. Chinese Multidisciplinary Expert Consensus on the Diagnosis and Treatment of Hyperuricemia and Related Diseases. Chin. Med. J. 2017, 130, 2473–2488. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Sanyal, A.J.; Kowdley, K.V.; Terrault, N.; Chalasani, N.P.; Abdelmalek, M.F.; McCullough, A.J.; Shringarpure, R.; Ferguson, B.; Lee, L.; et al. Factors Associated With Histologic Response in Adult Patients With Nonalcoholic Steatohepatitis. Gastroenterology 2019, 156, 88–95. [Google Scholar] [CrossRef] [PubMed]
- James, W.P.; Davies, H.L.; Bailes, J.; Dauncey, M.J. Elevated metabolic rates in obesity. Lancet 1978, 1, 1122–1125. [Google Scholar] [CrossRef]
- Leibel, R.L.; Rosenbaum, M.; Hirsch, J. Changes in energy expenditure resulting from altered body weight. N. Engl. J. Med. 1995, 332, 621–628. [Google Scholar] [CrossRef]
- Stine, J.G.; DiJoseph, K.; Pattison, Z.; Harrington, A.; Chinchilli, V.M.; Schmitz, K.H.; Loomba, R. Exercise Training Is Associated With Treatment Response in Liver Fat Content by Magnetic Resonance Imaging Independent of Clinically Significant Body Weight Loss in Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2023, 118, 1204–1213. [Google Scholar] [CrossRef]
- Thorp, A.; Stine, J.G. Exercise as Medicine: The Impact of Exercise Training on Nonalcoholic Fatty Liver Disease. Curr. Hepatol. Rep. 2020, 19, 402–411. [Google Scholar] [CrossRef]
- Claypool, K.; Long, M.T.; Patel, C.J. Waist Circumference and Insulin Resistance Are the Most Predictive Metabolic Factors for Steatosis and Fibrosis. Clin. Gastroenterol. Hepatol. 2023, 21, 1950–1954. [Google Scholar] [CrossRef]
- Saponaro, C.; Sabatini, S.; Gaggini, M.; Carli, F.; Rosso, C.; Positano, V.; Armandi, A.; Caviglia, G.P.; Faletti, R.; Bugianesi, E.; et al. Adipose tissue dysfunction and visceral fat are associated with hepatic insulin resistance and severity of NASH even in lean individuals. Liver Int. 2022, 42, 2418–2427. [Google Scholar] [CrossRef] [PubMed]
- Shiri-Sverdlov, R.; Wouters, K.; van Gorp, P.J.; Gijbels, M.J.; Noel, B.; Buffat, L.; Staels, B.; Maeda, N.; van Bilsen, M.; Hofker, M.H. Early diet-induced non-alcoholic steatohepatitis in APOE2 knock-in mice and its prevention by fibrates. J. Hepatol. 2006, 44, 732–741. [Google Scholar] [CrossRef]
- Fernández-Miranda, C.; Pérez-Carreras, M.; Colina, F.; López-Alonso, G.; Vargas, C.; Solís-Herruzo, J.A. A pilot trial of fenofibrate for the treatment of non-alcoholic fatty liver disease. Dig. Liver Dis. 2008, 40, 200–205. [Google Scholar] [CrossRef]
- Ahsan, F.; Oliveri, F.; Goud, H.K.; Mehkari, Z.; Mohammed, L.; Javed, M.; Althwanay, A.; Rutkofsky, I.H. Pleiotropic Effects of Statins in the Light of Non-Alcoholic Fatty Liver Disease and Non-Alcoholic Steatohepatitis. Cureus 2020, 12, e10446. [Google Scholar] [CrossRef]
- Huttasch, M.; Roden, M.; Kahl, S. Obesity and MASLD: Is weight loss the (only) key to treat metabolic liver disease? Metabolism 2024, 157, 155937. [Google Scholar] [CrossRef] [PubMed]
- Malhi, H.; Gores, G.J. Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin. Liver Dis. 2008, 28, 360–369. [Google Scholar] [CrossRef]
- Xiao, J.; Ho, C.T.; Liong, E.C.; Nanji, A.A.; Leung, T.M.; Lau, T.Y.; Fung, M.L.; Tipoe, G.L. Epigallocatechin gallate attenuates fibrosis, oxidative stress, and inflammation in non-alcoholic fatty liver disease rat model through TGF/SMAD, PI3 K/Akt/FoxO1, and NF-kappa B pathways. Eur. J. Nutr. 2014, 53, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, Y.; Xu, C.; Hong, Y.; Lu, H.; Wu, J.; Chen, Y. Association between serum free fatty acid levels and nonalcoholic fatty liver disease: A cross-sectional study. Sci. Rep. 2014, 4, 5832. [Google Scholar] [CrossRef]
| Characteristics | Baseline (n = 397) | 48 Weeks (n = 397) | Change | p |
|---|---|---|---|---|
| Body weight (kg) | 74.6 ± 12.1 | 72.8 ± 11.9 | 1.8 ± 4.0 | 0.04 |
| Body mass index (kg/m2) | 26.8 ± 3.4 | 26.2 ± 3.4 | 0.6 ± 1.4 | 0.009 |
| Waist circumference (cm) | 90.0 ± 8.2 | 88.6 ± 7.8 | 1.5 ± 4.8 | 0.01 |
| Waist-to-hip ratio | 0.90 ± 0.05 | 0.90 ± 0.05 | 0.00 ± 0.04 | 0.46 |
| Systolic blood pressure (mmHg) | 131.8 ± 16.5 | 129.3 ± 17.0 | 2.5 ± 15.5 | 0.04 |
| Diastolic blood pressure (mmHg) | 86.1 ± 11.7 | 83.2 ± 11.4 | 2.9 ± 12.3 | 0.001 |
| Total cholesterol (mmol/L) | 5.06 ± 1.04 | 4.78 ± 0.98 | 0.28 ± 1.04 | <0.001 |
| Triglyceride (mmol/L) | 1.55 (1.08, 2.16) | 1.44 (0.99, 1.99) | 0.09 (−0.26, 0.56) | 0.02 |
| HDL cholesterol (mmol/L) | 1.17 ± 0.25 | 1.17 ± 0.29 | 0.00 ± 0.21 | 0.71 |
| LDL cholesterol (mmol/L) | 3.17 ± 0.78 | 2.96 ± 0.73 | 0.21 ± 0.79 | <0.001 |
| Free fatty acid (μmol/L) | 517 (418, 643) | 500 (400, 609) | 6.0 (−108, 149) | 0.09 |
| Fasting glucose (mmol/L) | 4.9 (4.5, 5.4) | 4.9 (4.5, 5.4) | 0.0 (−0.4, 0.4) | 0.81 |
| Fasting insulin (uU/mL) | 10.1 (7.3, 14.7) | 9.2 (6.7, 12.5) | 1.1 (−1.2, 3.7) | 0.002 |
| HOMA-IR | 2.34 (1.65, 3.46) | 2.09 (1.39, 2.89) | 0.29 (−0.39, 0.90) | 0.003 |
| Uric acid (μmol/L) | 420.6 ± 103.1 | 413.1 ± 107.6 | 7.2 ± 102.4 | 0.32 |
| Alanine aminotransferase (IU/L) | 40.0 (25.0, 67.5) | 30.0 (21.0, 45.0) | 5.0 (−5.0, 25.0) | <0.001 |
| Aspartate aminotransferase (IU/L) | 31.0 (23.0, 42.5) | 25.0 (21.0, 34.0) | 3.0 (−2.0, 13.0) | <0.001 |
| γ-glutamyl transpeptidase (IU/L) | 41.0 (27.0, 62.0) | 32.0 (22.0, 49.5) | 4.0 (−3.0, 22.0) | <0.001 |
| Alkaline phosphatase (IU/L) | 76.0 (66.0, 86.0) | 73.0 (64.0, 84.8) | 2.0 (−4.0, 9.0) | 0.04 |
| Total bilirubin (μmol/L) | 12.6 (9.9, 16.5) | 12.8 (10.3, 16.5) | 0.0 (−2.3, 2.0) | 0.64 |
| Albumin (g/L) | 45.7 ± 3.1 | 45.4 ± 3.0 | 0.4 ± 2.8 | 0.09 |
| Total bile acid (μmol/L) | 2.8 (1.8, 3.9) | 2.5 (1.6, 3.9) | 0.1 (−0.8, 1.3) | 0.04 |
| Liver fat content (%) | 12.4 (8.7, 20.0) | 9.1 (6.1, 14.5) | 2.9 (−0.4, 6.3) | <0.001 |
| Liver stiffness measurement (kpa) | 5.9 (5.1, 7.1) | 5.8 (5.0, 6.7) | 0.0 (−0.3, 0.8) | 0.18 |
| Characteristics | Total (n = 397) | With WLT (n = 121) | Without WLT (n = 276) | p |
|---|---|---|---|---|
| Age (years) | 42.4 ± 13.6 | 42.9 ± 13.7 | 42.1 ± 13.6 | 0.59 |
| Male, n (%) | 282 (71.0%) | 79 (65.3%) | 203 (73.6%) | 0.10 |
| Smoking, n (%) | 44 (11.1%) | 12 (9.9%) | 32 (11.6%) | 0.62 |
| Body weight (kg) | 74.6 ± 12.1 | 76.6 ± 12.5 | 73.7 ± 11.9 | 0.03 |
| Body mass index (kg/m2) | 26.8 ± 3.4 | 27.6 ± 3.5 | 26.5 ± 3.4 | 0.005 |
| Waist circumference (cm) | 90.0 ± 8.2 | 91.4 ± 8.7 | 89.4 ± 7.9 | 0.03 |
| Waist-to-hip ratio | 0.90 ± 0.05 | 0.90 ± 0.05 | 0.90 ± 0.05 | 0.37 |
| Systolic blood pressure (mmHg) | 131.8 ± 16.5 | 131.0 ± 16.2 | 132.2 ± 16.6 | 0.50 |
| Diastolic blood pressure (mmHg) | 86.1 ± 11.7 | 86.4 ± 11.8 | 86.0 ± 11.7 | 0.74 |
| Total cholesterol (mmol/L) | 5.06 ± 1.04 | 5.21 ± 1.13 | 5.00 ± 1.00 | 0.07 |
| Triglyceride (mmol/L) | 1.55 (1.08, 2.16) | 1.54 (1.02, 2.14) | 1.58 (1.12, 2.19) | 0.47 |
| HDL cholesterol (mmol/L) | 1.17 ± 0.25 | 1.17 ± 0.22 | 1.17 ± 0.27 | 0.95 |
| LDL cholesterol (mmol/L) | 3.17 ± 0.78 | 3.32 ± 0.85 | 3.10 ± 0.74 | 0.02 |
| Free fatty acid (μmol/L) | 517 (418, 643) | 563 (461, 690) | 501 (412, 616) | 0.02 |
| Fasting glucose (mmol/L) | 4.9 (4.5, 5.4) | 4.9 (4.5, 5.5) | 4.9 (4.5, 5.3) | 0.73 |
| Fasting insulin (uU/mL) | 10.1 (7.3, 14.7) | 11.1 (7.8, 16.5) | 10.0 (7.2, 13.9) | 0.04 |
| HOMA-IR | 2.34 (1.65, 3.46) | 2.51 (1.79, 3.80) | 2.22 (1.55, 3.32) | 0.03 |
| Uric acid (μmol/L) | 420.6 ± 103.1 | 421.6 ± 106.8 | 420.1 ± 101.6 | 0.90 |
| Alanine aminotransferase (IU/L) | 40.0 (25.0, 67.5) | 46.0 (29.5, 78.5) | 37.0 (23.0, 62.8) | 0.01 |
| Aspartate aminotransferase (IU/L) | 31.0 (23.0, 42.5) | 34.0 (24.5, 48.5) | 29.5 (22.0, 40.0) | 0.008 |
| γ-glutamyl transpeptidase (IU/L) | 41.0 (27.0, 62.0) | 45.0 (28.0, 66.5) | 39.0 (26.0, 60.8) | 0.17 |
| Alkaline phosphatase (IU/L) | 76.0 (66.0, 86.0) | 77.0 (68.0, 87.0) | 76.0 (64.0, 86.3) | 0.22 |
| Total bilirubin (μmol/L) | 12.6 (9.9, 16.5) | 13.2 (10.3, 16.6) | 12.3 (9.7, 16.4) | 0.18 |
| Albumin (g/L) | 45.7 ± 3.1 | 45.5 ± 3.1 | 45.8 ± 3.1 | 0.34 |
| Total bile acid (μmol/L) | 2.8 (1.8, 3.9) | 2.7 (2.0, 3.8) | 2.9 (1.8, 3.9) | 0.60 |
| Liver fat content (%) | 12.4 (8.7, 20.0) | 14.9 (10.2, 24.1) | 11.4 (8.1, 18.2) | <0.001 |
| Liver stiffness measurement (kpa) | 5.9 (5.1, 7.1) | 6.2 (5.2, 7.7) | 5.8 (5.0, 6.8) | 0.01 |
| Elevated ALT 1, n (%) | 253 (63.7%) | 88 (72.3%) | 165 (59.8%) | 0.01 |
| Fibrosis ≥ stage 1, n (%) | 146 (36.8%) | 56 (46.3%) | 90 (32.6%) | 0.009 |
| MVPA ≥ 150 min/wk, n (%) | 251 (63.2%) | 82 (67.8%) | 169 (61.2%) | 0.31 |
| Reduced energy intake ≥ 500 kcal/d, n (%) | 186 (46.9%) | 73 (60.3%) | 113 (40.9%) | 0.002 |
| Lipid-lowering drug, n (%) | 129 (32.5%) | 36 (29.8%) | 93 (33.7%) | 0.44 |
| Uric acid-lowering drug, n (%) | 39 (9.8%) | 11 (9.1%) | 28 (10.1%) | 0.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, L.; Shao, C.; Dong, Z.; Zhuo, S.; Feng, S.; Wang, W.; Ye, J.; Zhong, B. Distinct Markers of Discordant Treatment Response to Lifestyle Intervention in MASLD, Independent of Weight Loss. Biomedicines 2025, 13, 2161. https://doi.org/10.3390/biomedicines13092161
Luo L, Shao C, Dong Z, Zhuo S, Feng S, Wang W, Ye J, Zhong B. Distinct Markers of Discordant Treatment Response to Lifestyle Intervention in MASLD, Independent of Weight Loss. Biomedicines. 2025; 13(9):2161. https://doi.org/10.3390/biomedicines13092161
Chicago/Turabian StyleLuo, Ling, Congxiang Shao, Zhi Dong, Shuyu Zhuo, Shiting Feng, Wei Wang, Junzhao Ye, and Bihui Zhong. 2025. "Distinct Markers of Discordant Treatment Response to Lifestyle Intervention in MASLD, Independent of Weight Loss" Biomedicines 13, no. 9: 2161. https://doi.org/10.3390/biomedicines13092161
APA StyleLuo, L., Shao, C., Dong, Z., Zhuo, S., Feng, S., Wang, W., Ye, J., & Zhong, B. (2025). Distinct Markers of Discordant Treatment Response to Lifestyle Intervention in MASLD, Independent of Weight Loss. Biomedicines, 13(9), 2161. https://doi.org/10.3390/biomedicines13092161






