Long-Term Osteoporosis Risk in Colorectal Cancer Survivors: A Nationwide Longitudinal Cohort with up to 16 Years of Follow-Up
Abstract
1. Introduction
2. Materials and Methods
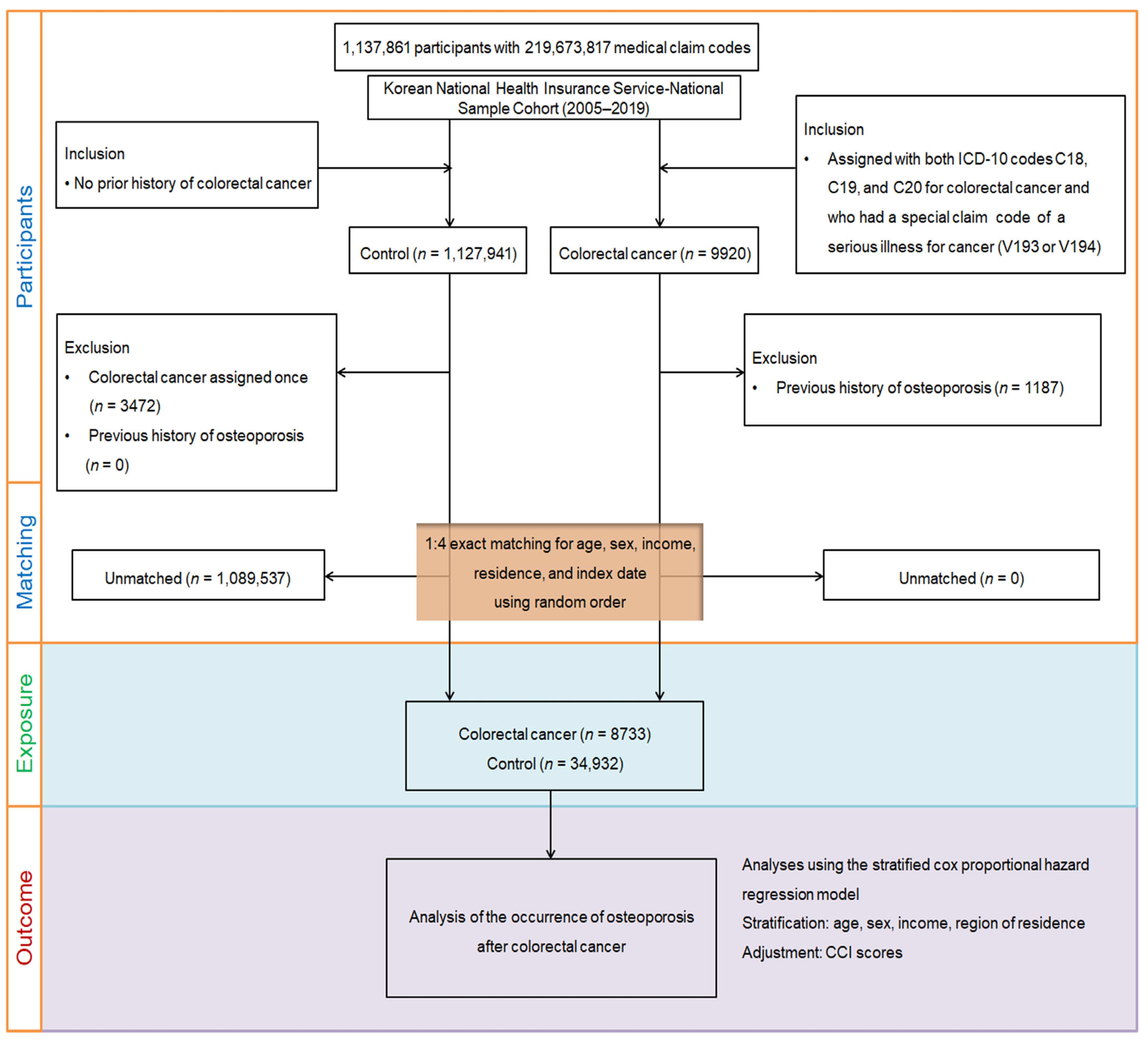
2.1. Research Design, Data Resource, and Cohort Selection
2.2. Covariates and Comorbidity Adjustment
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef]
- Khil, H.; Kim, S.M.; Hong, S.; Gil, H.M.; Cheon, E.; Lee, D.H.; Kim, Y.A.; Keum, N. Time trends of colorectal cancer incidence and associated lifestyle factors in South Korea. Sci. Rep. 2021, 11, 2413. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.U.; Kim, H.O.; Kim, H. Epidemiology, Risk Factors, and Prevention of Colorectal Cancer-An English Version. J. Anus Rectum Colon 2022, 6, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Vera, R.; Aparicio, J.; Carballo, F.; Esteva, M.; González-Flores, E.; Santianes, J.; Santolaya, F.; Fernández-Cebrián, J.M. Recommendations for follow-up of colorectal cancer survivors. Clin. Transl. Oncol. 2019, 21, 1302–1311. [Google Scholar] [CrossRef] [PubMed]
- Sonkin, D.; Thomas, A.; Teicher, B.A. Cancer treatments: Past, present, and future. Cancer Genet. 2024, 286, 18–24. [Google Scholar] [CrossRef]
- Barzi, A.; Hershman, D.L.; Till, C.; Barlow, W.E.; Ramsey, S.; Lenz, H.J.; Hochster, H.S.; Unger, J.M. Osteoporosis in colorectal cancer survivors: Analysis of the linkage between SWOG trial enrollees and Medicare claims. Arch. Osteoporos. 2019, 14, 83. [Google Scholar] [CrossRef]
- Han, K.M.; Kwon, M.J.; Kim, J.H.; Kim, J.H.; Bang, W.J.; Choi, H.G.; Yoo, D.M.; Lee, N.E.; Kim, N.Y.; Kang, H.S. Association between Gastric Cancer and Osteoporosis: A Longitudinal Follow-Up Study Using a National Health Sample Cohort. Cancers 2024, 16, 2291. [Google Scholar] [CrossRef]
- VanderWalde, A.; Hurria, A. Aging and osteoporosis in breast and prostate cancer. CA Cancer J. Clin. 2011, 61, 139–156. [Google Scholar] [CrossRef]
- Khan, N.F.; Mant, D.; Carpenter, L.; Forman, D.; Rose, P.W. Long-term health outcomes in a British cohort of breast, colorectal and prostate cancer survivors: A database study. Br. J. Cancer 2011, 105 (Suppl. S1), S29–S37. [Google Scholar] [CrossRef]
- Rino, Y.; Oshima, T.; Yoshikawa, T. Changes in fat-soluble vitamin levels after gastrectomy for gastric cancer. Surg. Today 2017, 47, 145–150. [Google Scholar] [CrossRef]
- Utrilla Fornals, A.; Costas-Batlle, C.; Medlin, S.; Menjón-Lajusticia, E.; Cisneros-González, J.; Saura-Carmona, P.; Montoro-Huguet, M.A. Metabolic and Nutritional Issues after Lower Digestive Tract Surgery: The Important Role of the Dietitian in a Multidisciplinary Setting. Nutrients 2024, 16, 246. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.H.; Koh, M.; Kang, H.W.; Ryu, K.H.; Lee, D.S.; Kim, S.H.; Jang, D.K.; Jeong, J.B.; Kim, J.W.; Lee, K.L.; et al. Osteoporosis Is Associated with an Increased Risk of Colorectal Adenoma and High-Risk Adenoma: A Retrospective, Multicenter, Cross-Sectional, Case-Control Study. Gut Liver 2022, 16, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, J.S.; Park, S.H.; Shin, S.A.; Kim, K. Cohort Profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. 2017, 46, e15. [Google Scholar] [CrossRef]
- Kyoung, D.S.; Kim, H.S. Understanding and Utilizing Claim Data from the Korean National Health Insurance Service (NHIS) and Health Insurance Review & Assessment (HIRA) Database for Research. J. Lipid Atheroscler. 2022, 11, 103–110. [Google Scholar] [CrossRef]
- Kwon, M.J.; Kang, H.S.; Kim, J.H.; Kim, J.H.; Kim, S.H.; Kim, N.Y.; Nam, E.S.; Min, K.W.; Choi, H.G. Association between Statin Use and Gastric Cancer: A Nested Case-Control Study Using a National Health Screening Cohort in Korea. Pharmaceuticals 2021, 14, 1283. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, H.J.; Min, C.; Choi, H.G. Association between benign paroxysmal positional vertigo and osteoporosis: Two nested case-control studies. Osteoporos. Int. 2020, 31, 2017–2024. [Google Scholar] [CrossRef]
- Kwon, M.J.; Kim, J.H.; Kim, J.H.; Cho, S.J.; Nam, E.S.; Choi, H.G. The Occurrence of Alzheimer’s Disease and Parkinson’s Disease in Individuals with Osteoporosis: A Longitudinal Follow-Up Study Using a National Health Screening Database in Korea. Front. Aging Neurosci. 2021, 13, 786337. [Google Scholar] [CrossRef]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef]
- Bone, H.G.; Hosking, D.; Devogelaer, J.P.; Tucci, J.R.; Emkey, R.D.; Tonino, R.P.; Rodriguez-Portales, J.A.; Downs, R.W.; Gupta, J.; Santora, A.C.; et al. Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N. Engl. J. Med. 2004, 350, 1189–1199. [Google Scholar] [CrossRef]
- Mellström, D.D.; Sörensen, O.H.; Goemaere, S.; Roux, C.; Johnson, T.D.; Chines, A.A. Seven years of treatment with risedronate in women with postmenopausal osteoporosis. Calcif. Tissue Int. 2004, 75, 462–468. [Google Scholar] [CrossRef]
- Black, D.M.; Schwartz, A.V.; Ensrud, K.E.; Cauley, J.A.; Levis, S.; Quandt, S.A.; Satterfield, S.; Wallace, R.B.; Bauer, D.C.; Palermo, L.; et al. Effects of continuing or stopping alendronate after 5 years of treatment: The Fracture Intervention Trial Long-term Extension (FLEX): A randomized trial. JAMA 2006, 296, 2927–2938. [Google Scholar] [CrossRef]
- Morales, J.S.; Valenzuela, P.L.; Rincón-Castanedo, C.; Santos-Lozano, A.; Fiuza-Luces, C.; Lucia, A. Is health status impaired in childhood cancer survivors? A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2019, 142, 94–118. [Google Scholar] [CrossRef]
- Shapiro, C.L.; Poznak, C.V.; Lacchetti, C.; Kirshner, J.; Eastell, R.; Gagel, R.; Smith, S.; Edwards, B.J.; Frank, E.; Lyman, G.H.; et al. Management of Osteoporosis in Survivors of Adult Cancers with Nonmetastatic Disease: ASCO Clinical Practice Guideline. J. Clin. Oncol. 2019, 37, 2916–2946. [Google Scholar] [CrossRef]
- Li, F.; Morgan, K.L.; Zaslavsky, A.M. Balancing covariates via propensity score weighting. J. Am. Stat. Assoc. 2017, 113, 390–400. [Google Scholar] [CrossRef]
- Song, J.W.; Chung, K.C. Observational studies: Cohort and case-control studies. Plast. Reconstr. Surg. 2010, 126, 2234–2242. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.F.; Yu, K.H.; See, L.C.; Chou, I.J.; Ko, Y.S.; Chang, H.C.; Chiou, M.J.; Luo, S.F. Risk of myocardial infarction among patients with gout: A nationwide population-based study. Rheumatology 2013, 52, 111–117. [Google Scholar] [CrossRef]
- Thomas, L.E.; Li, F.; Pencina, M.J. Overlap Weighting: A Propensity Score Method That Mimics Attributes of a Randomized Clinical Trial. JAMA 2020, 323, 2417–2418. [Google Scholar] [CrossRef]
- Denlinger, C.S.; Barsevick, A.M. The challenges of colorectal cancer survivorship. J. Natl. Compr. Cancer Netw. 2009, 7, 883–893. [Google Scholar] [CrossRef]
- Hirschey, R.; Nyrop, K.A.; Mayer, D.K. Healthy Behaviors: Prevalence of Uptake Among Cancer Survivors. Clin. J. Oncol. Nurs. 2020, 24, 19–29. [Google Scholar] [CrossRef]
- Ramin, C.; May, B.J.; Roden, R.B.S.; Orellana, M.M.; Hogan, B.C.; McCullough, M.S.; Petry, D.; Armstrong, D.K.; Visvanathan, K. Evaluation of osteopenia and osteoporosis in younger breast cancer survivors compared with cancer-free women: A prospective cohort study. Breast Cancer Res. 2018, 20, 134. [Google Scholar] [CrossRef]
- Van Hellemond, I.E.G.; Smorenburg, C.H.; Peer, P.G.M.; Swinkels, A.C.P.; Seynaeve, C.M.; van der Sangen, M.J.C.; Kroep, J.R.; de Graaf, H.; Honkoop, A.H.; Erdkamp, F.L.G.; et al. Assessment and management of bone health in women with early breast cancer receiving endocrine treatment in the DATA study. Int. J. Cancer 2019, 145, 1325–1333. [Google Scholar] [CrossRef]
- Park, E.J.; Joo, I.W.; Jang, M.J.; Kim, Y.T.; Oh, K.; Oh, H.J. Prevalence of osteoporosis in the Korean population based on Korea National Health and Nutrition Examination Survey (KNHANES), 2008–2011. Yonsei Med. J. 2014, 55, 1049–1057. [Google Scholar] [CrossRef]
- Wu, X.P.; Liao, E.Y.; Huang, G.; Dai, R.C.; Zhang, H. A comparison study of the reference curves of bone mineral density at different skeletal sites in native Chinese, Japanese, and American Caucasian women. Calcif. Tissue Int. 2003, 73, 122–132. [Google Scholar] [CrossRef]

| Characteristics | Total Participants | |||
|---|---|---|---|---|
| Colorectal Cancer | Control | Standardized Difference | ||
| Age (y), n (%) | 0.00 | |||
| 0–4 | 1 (0.01) | 4 (0.01) | ||
| 5–9 | N/A | N/A | ||
| 10–14 | 3 (0.03) | 12 (0.03) | ||
| 15–19 | 1 (0.01) | 4 (0.01) | ||
| 20–24 | 8 (0.09) | 32 (0.09) | ||
| 25–29 | 26 (0.30) | 104 (0.30) | ||
| 30–34 | 93 (1.06) | 372 (1.06) | ||
| 35–39 | 178 (2.04) | 712 (2.04) | ||
| 40–44 | 354 (4.05) | 1416 (4.05) | ||
| 45–49 | 570 (6.53) | 2280 (6.53) | ||
| 50–54 | 940 (10.76) | 3760 (10.76) | ||
| 55–59 | 1182 (13.53) | 4728 (13.53) | ||
| 60–64 | 1280 (14.66) | 5120 (14.66) | ||
| 65–69 | 1310 (15.00) | 5240 (15.00) | ||
| 70–74 | 1228 (14.06) | 4912 (14.06) | ||
| 75–79 | 801 (9.17) | 3204 (9.17) | ||
| 80–84 | 491 (5.62) | 1964 (5.62) | ||
| 85+ | 267 (3.06) | 1068 (3.06) | ||
| Sex, n (%) | 0.00 | |||
| Male | 5782 (66.21) | 23,128 (66.21) | ||
| Female | 2951 (33.79) | 11,804 (33.79) | ||
| Income, n (%) | 0.00 | |||
| 1 (lowest) | 1702 (19.49) | 6808 (19.49) | ||
| 2 | 1141 (13.07) | 4564 (13.07) | ||
| 3 | 1416 (16.21) | 5664 (16.21) | ||
| 4 | 1849 (21.17) | 7396 (21.17) | ||
| 5 (highest) | 2625 (30.06) | 10,500 (30.06) | ||
| Region of residence, n (%) | 0.00 | |||
| Urban | 3964 (45.39) | 15,856 (45.39) | ||
| Rural | 4769 (54.61) | 19,076 (54.61) | ||
| CCI score, mean (Sd) | 0.77 (1.17) | 0.66 (1.16) | 0.10 | |
| Osteoporosis, n (%) | 579 (6.63) | 2909 (8.33) | 0.07 | |
| N of Event/ N of Total (%) | Follow-Up Duration (PY) | IR per 1000 (PY) | IRD (95% CI) | HR for Osteoporosis (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Crude † | p | Adjusted ‡ | p | ||||||
| Total participants (n = 43,665) | |||||||||
| CRC | 579/8733 (6.63) | 41,967 | 13.80 | −0.50 (−1.77–0.74) | 0.96 (0.87–1.05) | 0.33 | 0.95 (0.87–1.04) | 0.302 | |
| Control | 2909/34,932 (8.33) | 203,280 | 14.30 | 1 | 1 | ||||
| Age < 65 years old (n = 23,180) | |||||||||
| CRC | 254/4636 (5.48) | 25,573 | 9.93 | 1.17 (−0.10–2.45) | 1.15 (1.00–1.32) | 0.043 * | 1.14 (0.99–1.31) | 0.064 | |
| Control | 1065/18,544 (5.74) | 121,590 | 8.76 | 1 | 1 | ||||
| Age ≥ 65 years old (n = 20,485) | |||||||||
| CRC | 325/4097 (7.93) | 16,394 | 19.80 | −2.80 (−5.24–−0.25) | 0.84 (0.75–0.95) | 0.005 * | 0.84 (0.75–0.95) | 0.005 * | |
| Control | 1844/16,388 (11.25) | 81,690 | 22.60 | 1 | 1 | ||||
| Male (n = 28,910) | |||||||||
| CRC | 141/5782 (2.44) | 28,553 | 4.94 | −0.33 (−1.25–0.59) | 0.94 (0.78–1.12) | 0.493 | 0.92 (0.77–1.11) | 0.397 | |
| Control | 715/23,128 (3.09) | 135,772 | 5.27 | 1 | 1 | ||||
| Female (n = 14,755) | |||||||||
| CRC | 438/2951 (14.84) | 13,414 | 32.70 | 0.20 (−3.19–3.49) | 0.96 (0.87–1.07) | 0.472 | 0.96 (0.87–1.07) | 0.468 | |
| Control | 2194/11,804 (18.59) | 67,508 | 32.50 | 1 | 1 | ||||
| Low-income group (n = 21,295) | |||||||||
| CRC | 281/4259 (6.60) | 19,337 | 14.50 | 0.40 (−1.39–2.28) | 1.01 (0.89–1.15) | 0.844 | 1.00 (0.88–1.14) | 0.949 | |
| Control | 1354/17,036 (7.95) | 96,120 | 14.10 | 1 | 1 | ||||
| High-income group (n = 22,370) | |||||||||
| CRC | 298/4474 (6.66) | 22,630 | 13.20 | −1.30 (−3.06–0.37) | 0.91 (0.80–1.03) | 0.131 | 0.91 (0.80–1.03) | 0.130 | |
| Control | 1555/17,896 (8.69) | 107,160 | 14.50 | 1 | 1 | ||||
| Urban resident (n = 19,820) | |||||||||
| CRC | 247/3964 (6.23) | 19,971 | 12.40 | −0.40 (−2.13–1.31) | 0.96 (0.83–1.10) | 0.515 | 0.95 (0.83–1.09) | 0.502 | |
| Control | 1233/15,856 (7.78) | 96,492 | 12.80 | 1 | 1 | ||||
| Rural resident (n = 23,845) | |||||||||
| CRC | 332/4769 (6.96) | 21,996 | 15.10 | −0.60 (−2.41–1.21) | 0.96 (0.85–1.08) | 0.469 | 0.95 (0.85–1.07) | 0.432 | |
| Control | 1676/19,076 (8.79) | 106,788 | 15.70 | 1 | 1 | ||||
| CCI scores = 0 (n = 27,426) | |||||||||
| CRC | 284/4887 (5.81) | 23,703 | 12.00 | −0.40 (−1.92–1.15) | 0.95 (0.83–1.07) | 0.397 | 0.96 (0.84–1.08) | 0.485 | |
| Control | 1638/22,539 (7.27) | 132,416 | 12.40 | 1 | 1 | ||||
| CCI scores = 1 (n = 9119) | |||||||||
| CRC | 164/2262 (7.25) | 10,812 | 15.20 | −2.30 (−5.11–0.43) | 0.84 (0.71–1.00) | 0.048 * | 0.90 (0.76–1.06) | 0.215 | |
| Control | 700/6857 (10.21) | 39,976 | 17.50 | 1 | 1 | ||||
| CCI scores ≥ 2 (n = 7120) | |||||||||
| CRC | 131/1584 (8.27) | 7452 | 17.60 | −0.90 (−4.33–2.52) | 0.92 (0.76–1.12) | 0.421 | 1.01 (0.84–1.23) | 0.880 | |
| Control | 571/5536 (10.31) | 30,888 | 18.50 | 1 | 1 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, H.S.; Kim, J.-H.; Kim, E.S.; Yoo, D.M.; Han, K.M.; Kim, N.Y.; Choi, H.G.; Park, H.Y.; Kwon, M.J. Long-Term Osteoporosis Risk in Colorectal Cancer Survivors: A Nationwide Longitudinal Cohort with up to 16 Years of Follow-Up. Biomedicines 2025, 13, 2159. https://doi.org/10.3390/biomedicines13092159
Kang HS, Kim J-H, Kim ES, Yoo DM, Han KM, Kim NY, Choi HG, Park HY, Kwon MJ. Long-Term Osteoporosis Risk in Colorectal Cancer Survivors: A Nationwide Longitudinal Cohort with up to 16 Years of Follow-Up. Biomedicines. 2025; 13(9):2159. https://doi.org/10.3390/biomedicines13092159
Chicago/Turabian StyleKang, Ho Suk, Joo-Hee Kim, Eun Soo Kim, Dae Myoung Yoo, Kyeong Min Han, Nan Young Kim, Hyo Geun Choi, Ha Young Park, and Mi Jung Kwon. 2025. "Long-Term Osteoporosis Risk in Colorectal Cancer Survivors: A Nationwide Longitudinal Cohort with up to 16 Years of Follow-Up" Biomedicines 13, no. 9: 2159. https://doi.org/10.3390/biomedicines13092159
APA StyleKang, H. S., Kim, J.-H., Kim, E. S., Yoo, D. M., Han, K. M., Kim, N. Y., Choi, H. G., Park, H. Y., & Kwon, M. J. (2025). Long-Term Osteoporosis Risk in Colorectal Cancer Survivors: A Nationwide Longitudinal Cohort with up to 16 Years of Follow-Up. Biomedicines, 13(9), 2159. https://doi.org/10.3390/biomedicines13092159






