Exploring the Cellular and Molecular Landscape of Idiopathic Pulmonary Fibrosis: Integrative Multi-Omics and Single-Cell Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Mendelian Randomization (MR) and Functional Enrichment Analysis
2.3. Workflow for Identification of Differentially Expressed Genes (DEGs)
2.4. Identification and Validation of Diagnostic Gene Signatures
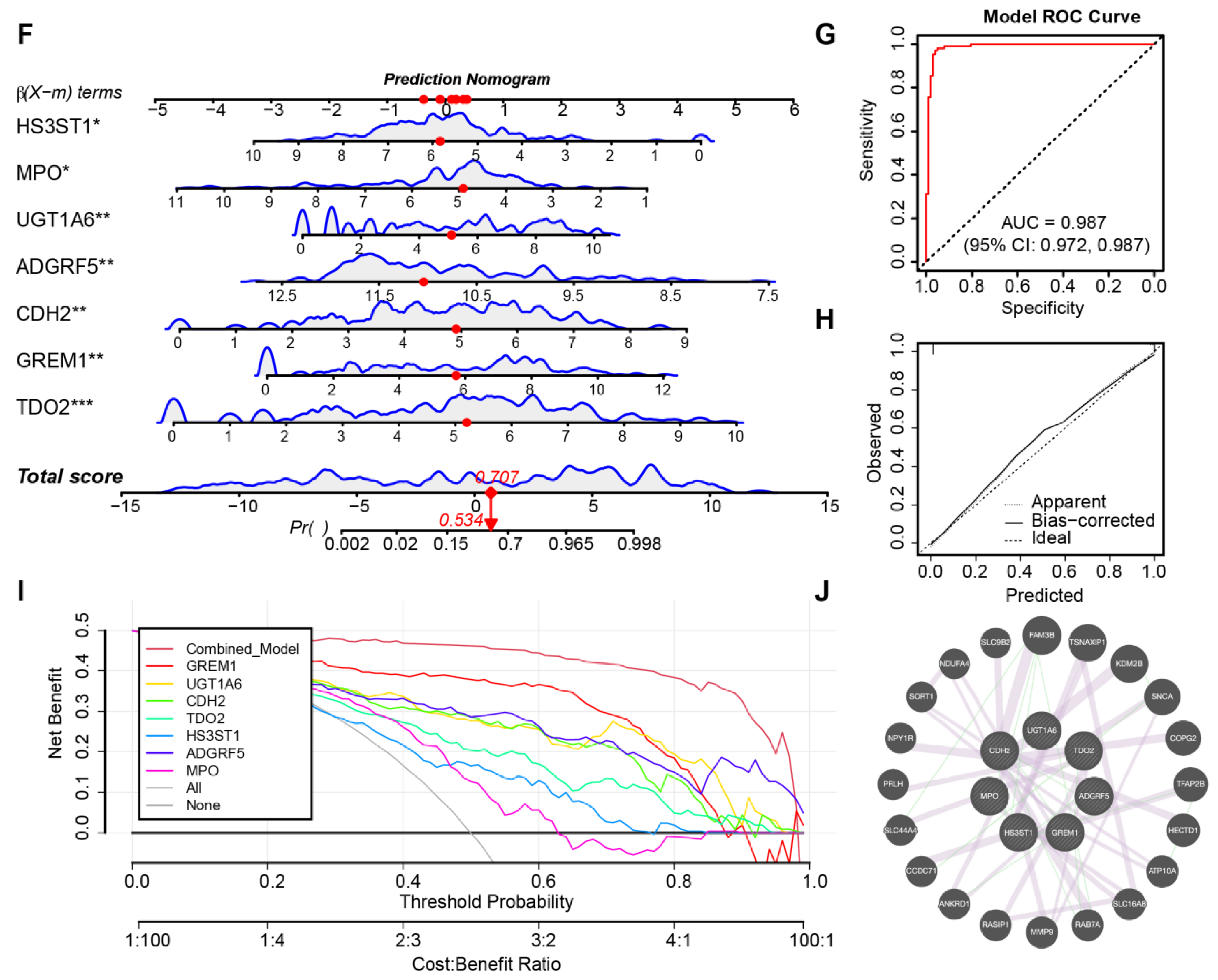
2.5. Development of a Predictive Nomogram and Gene Interaction Network Analysis
2.6. Single-Cell RNA Sequencing (ScRNA-Seq) Data Processing
3. Results
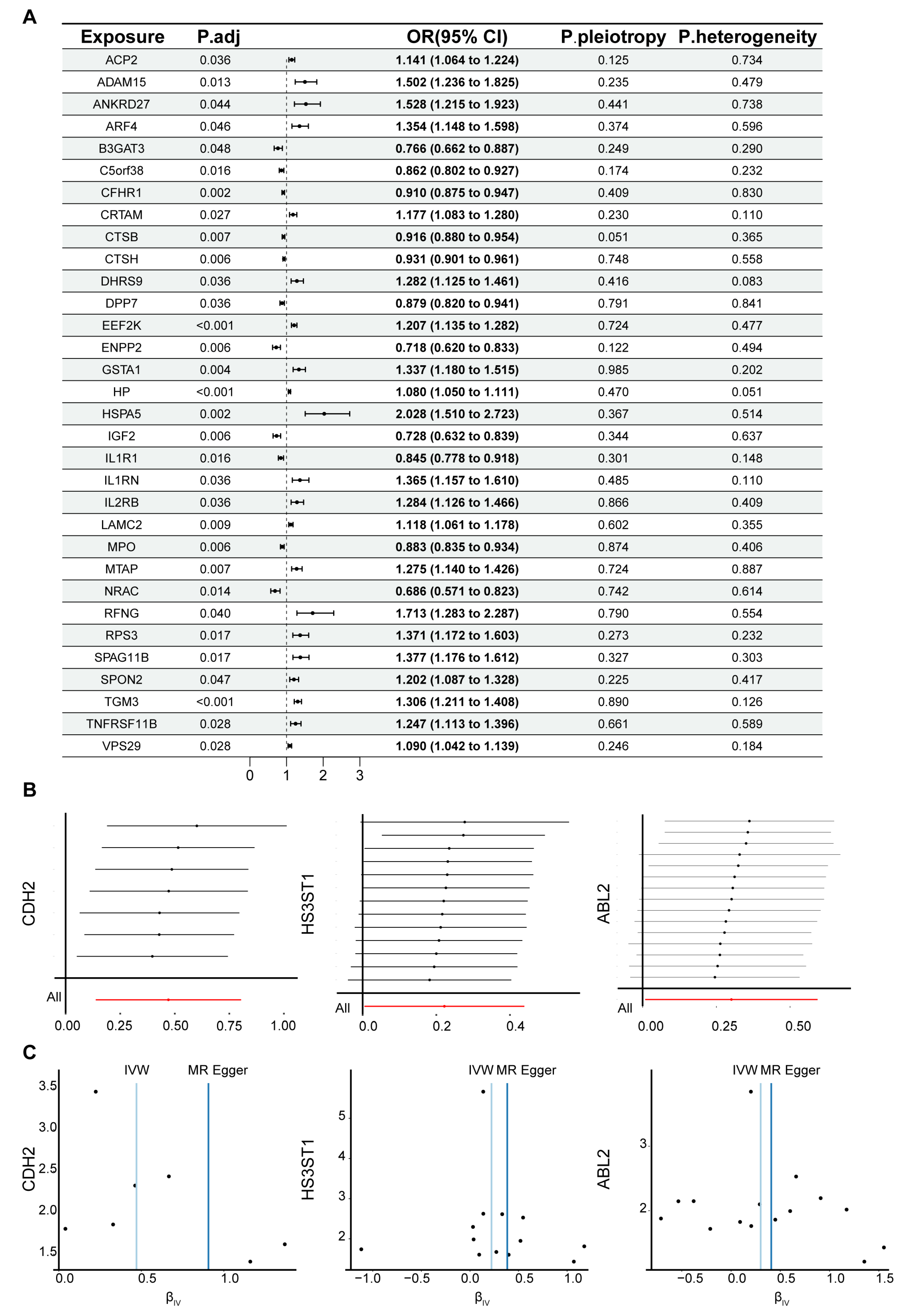
3.1. MR Analysis of the Relationship Between Plasma Circulating Proteins and IPF
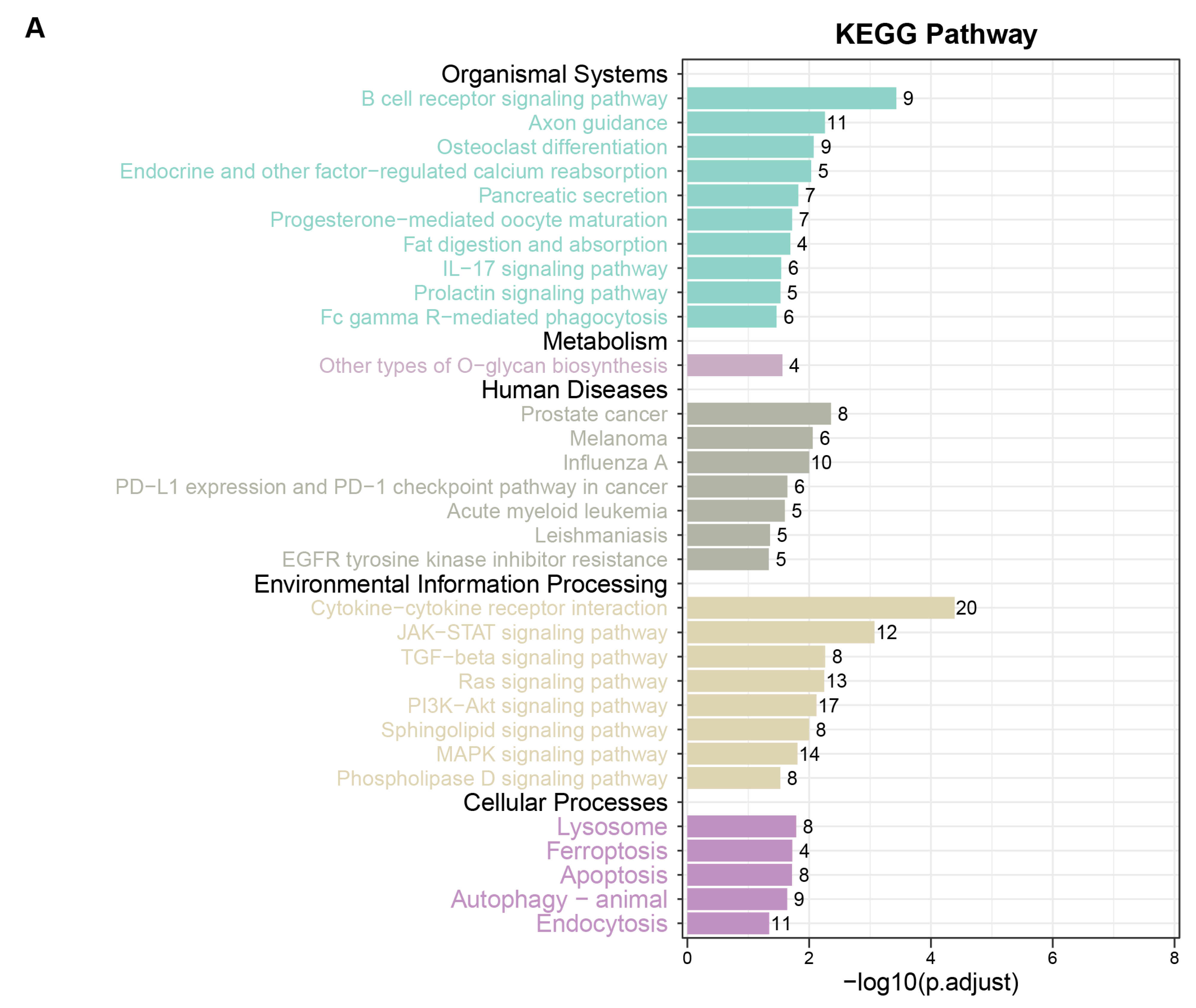
3.2. Functional Enrichment Analysis for MR-Identified Plasma Circulating Proteins
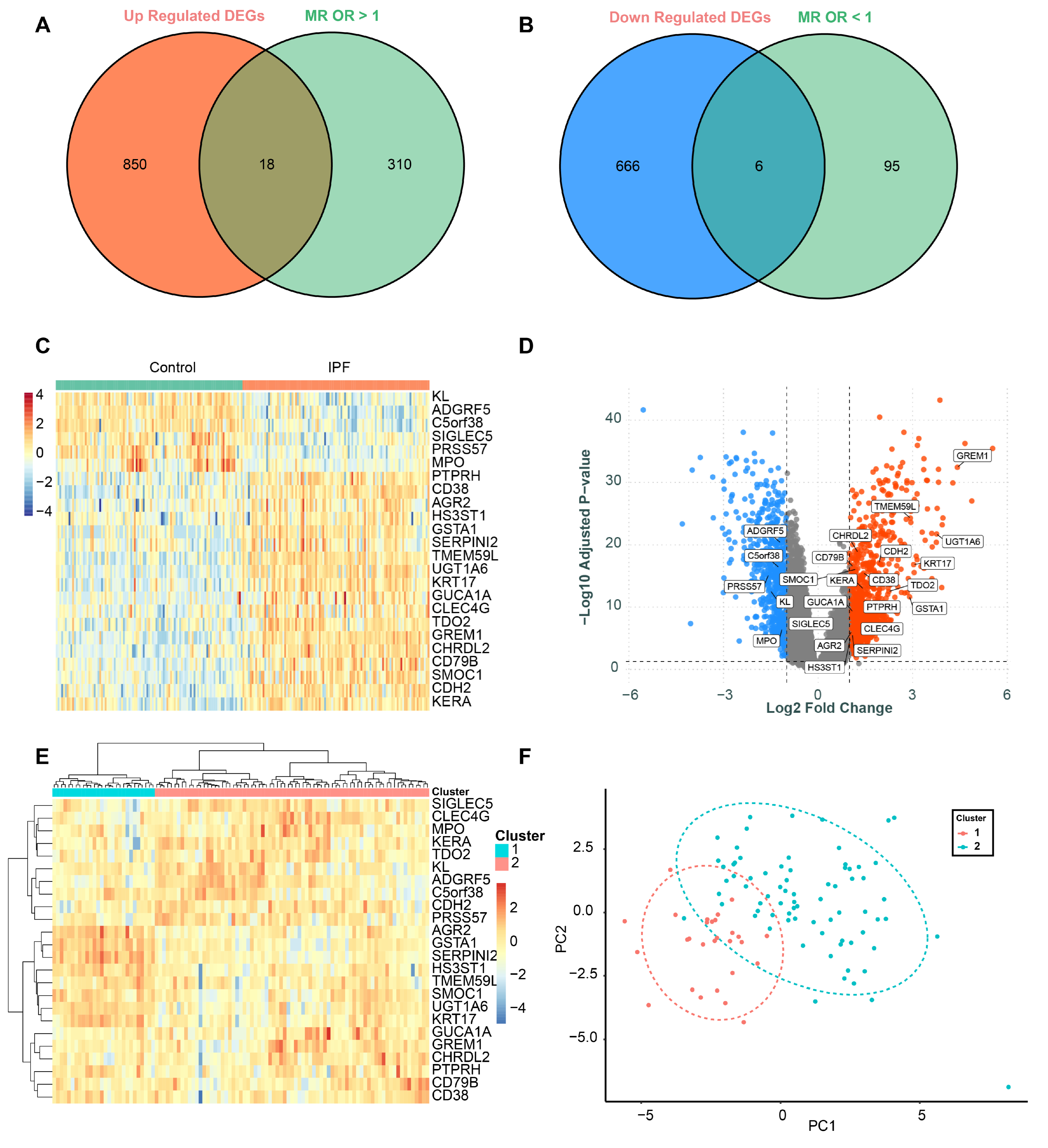
3.3. Identification of Differentially Expressed Genes (DEGs)
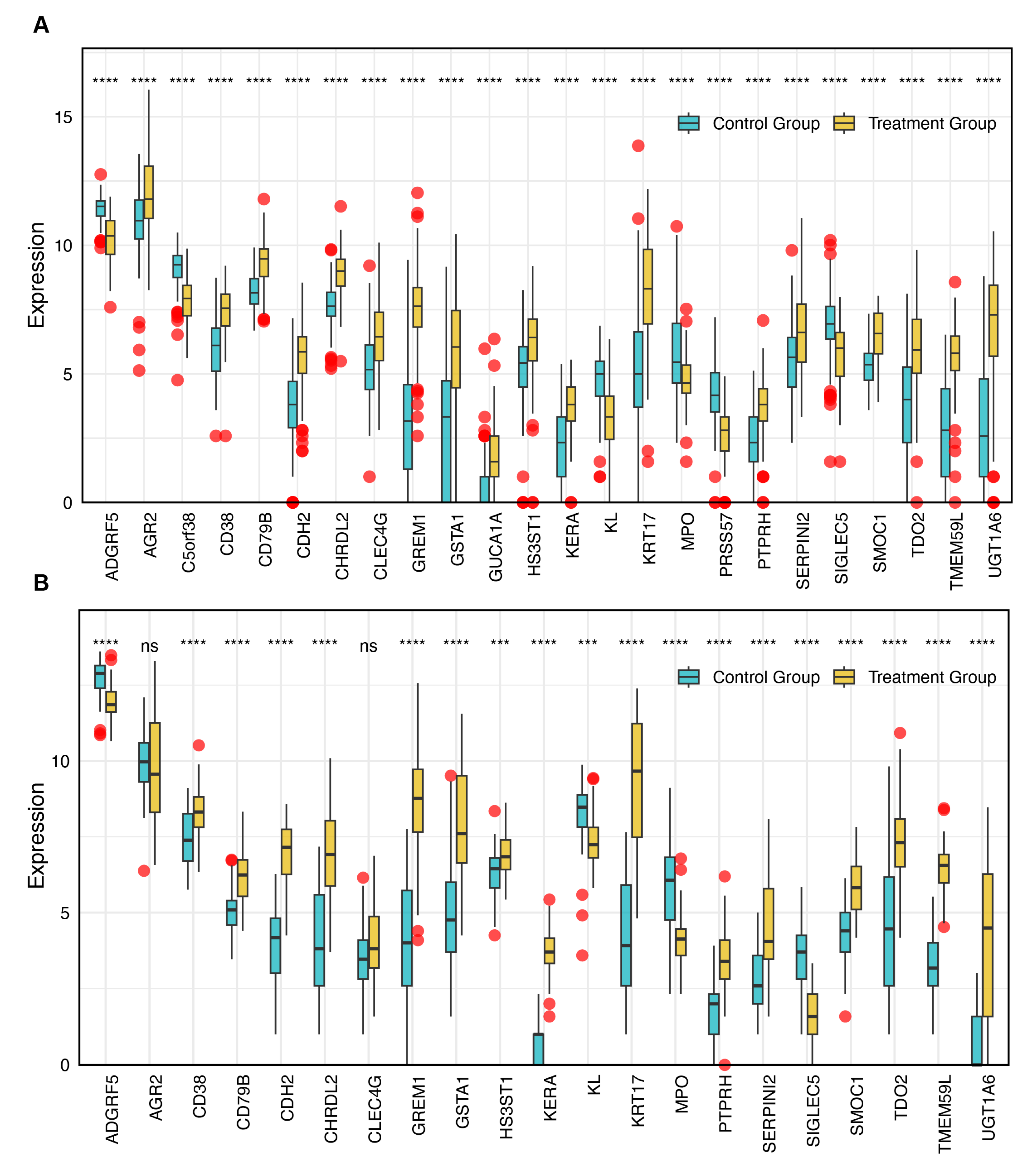
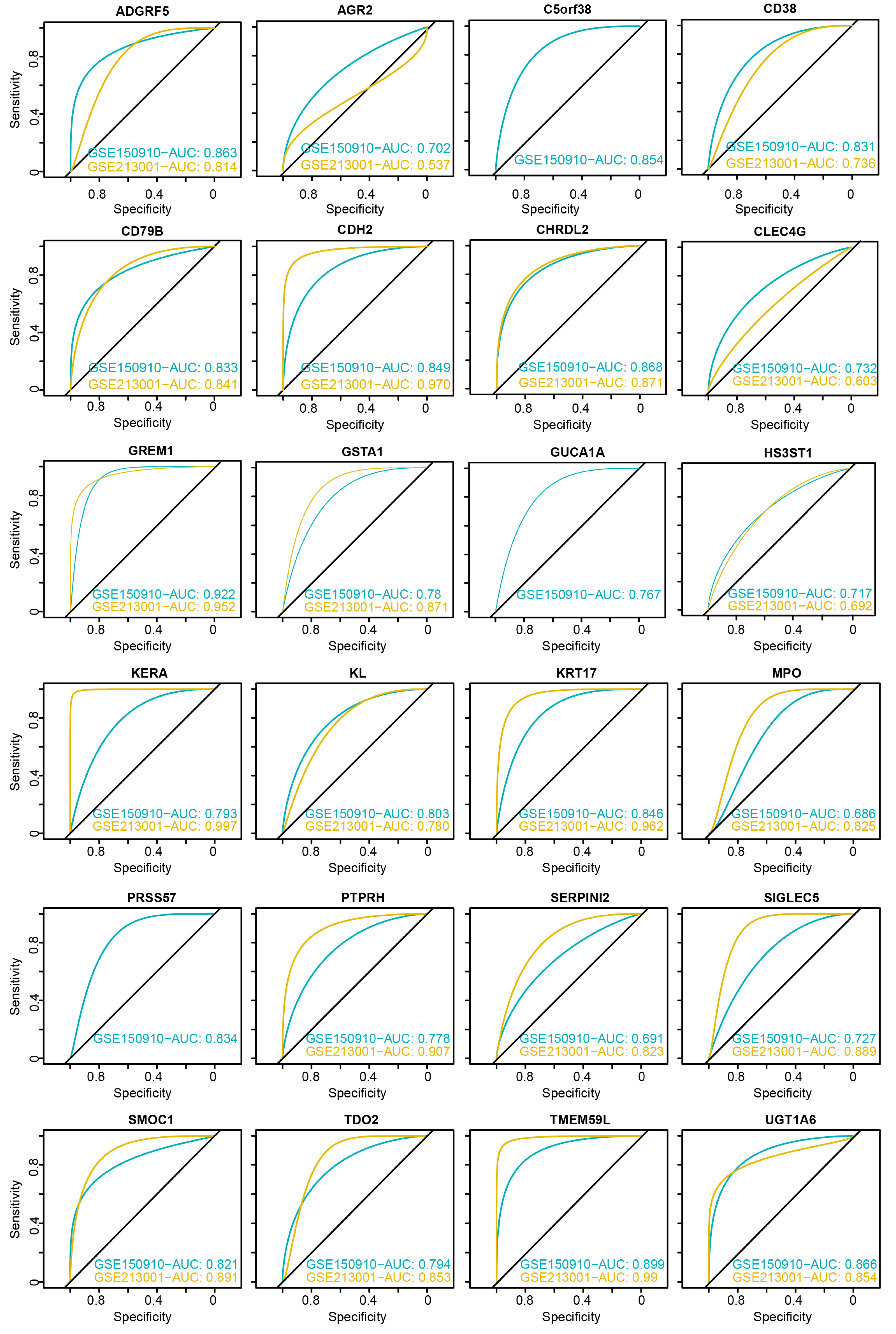
3.4. Evaluation of Diagnostic Performance for DEGs
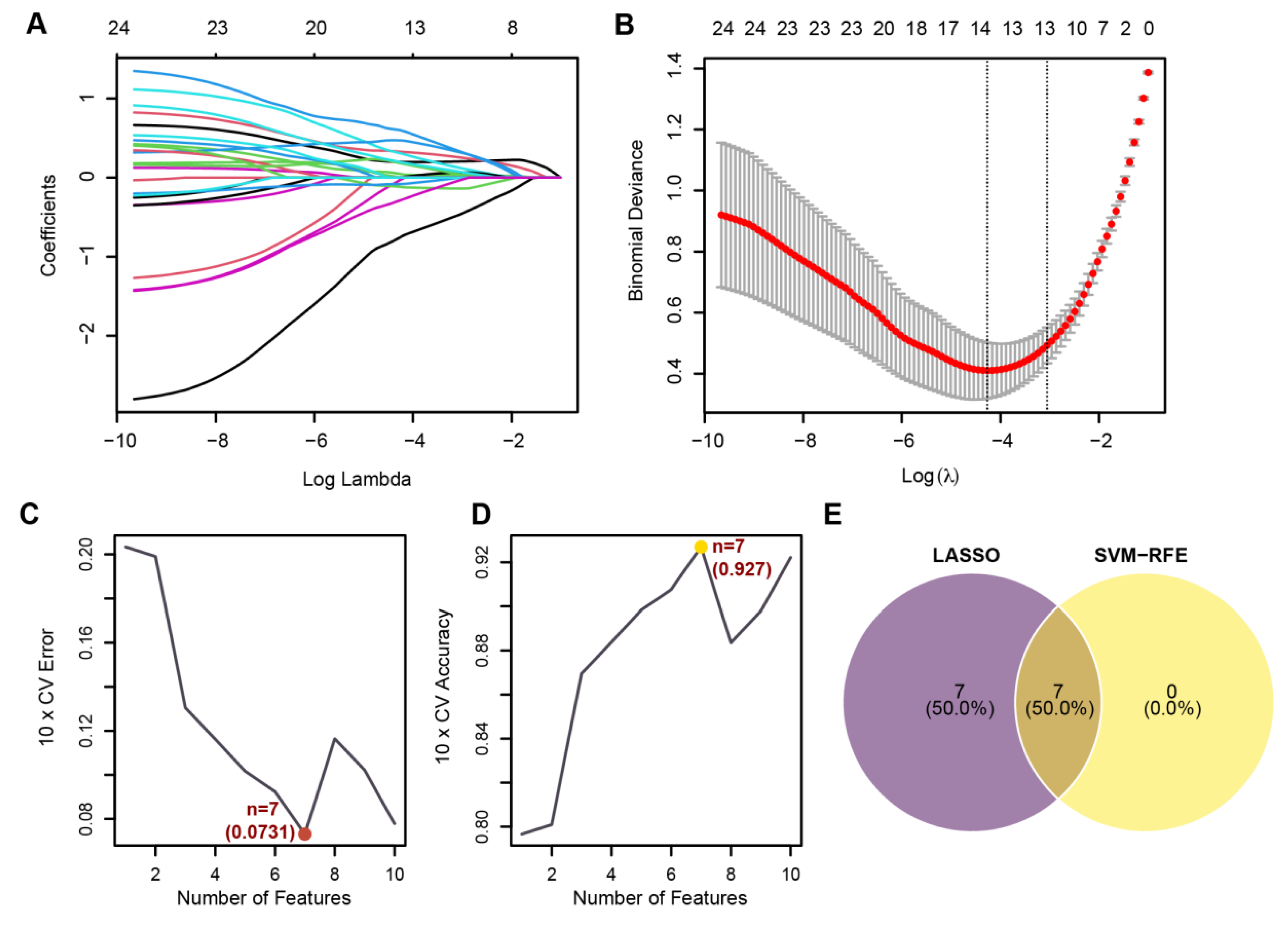
3.5. Identification of Core Genes and Construction of a Robust Diagnostic Model
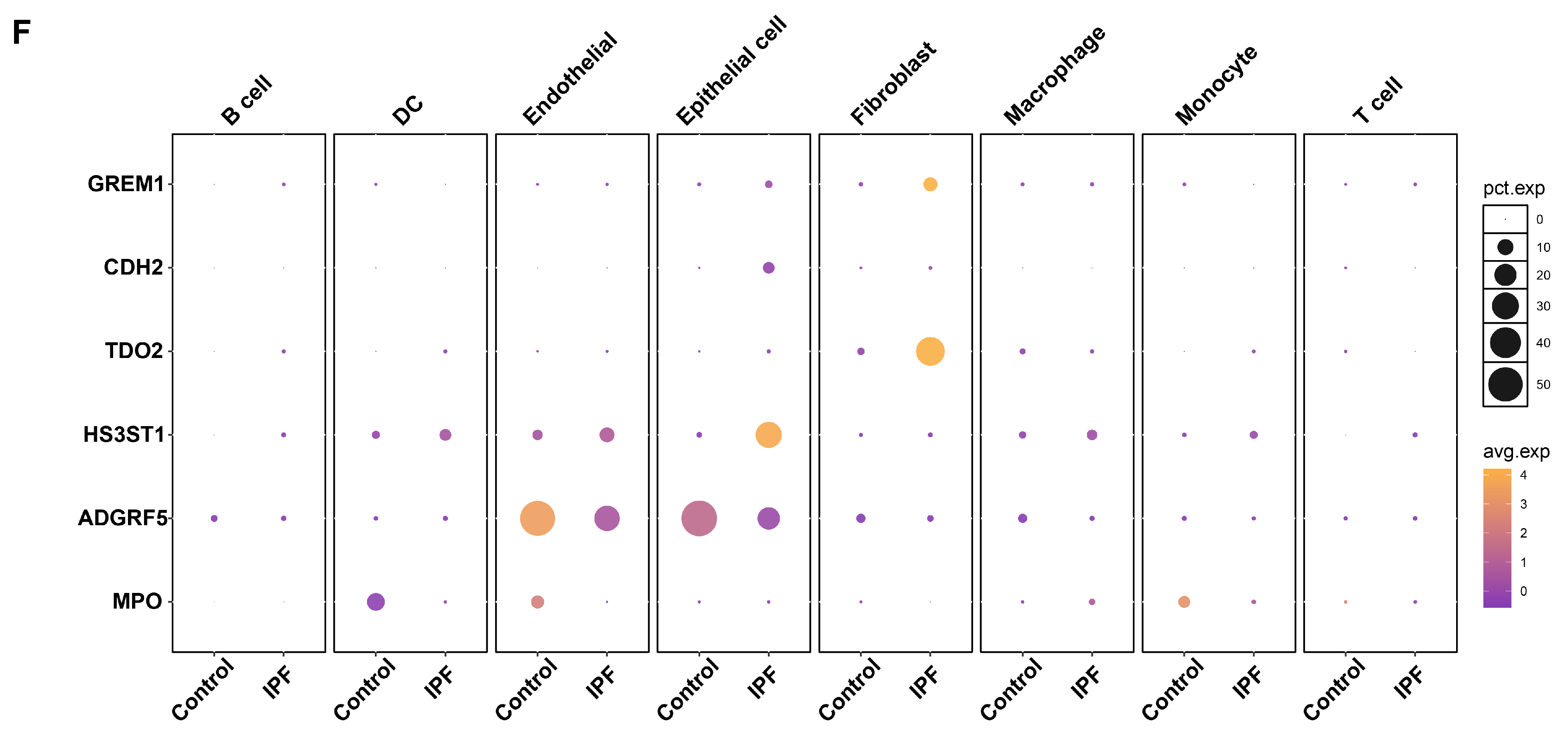
3.6. ScRNA-Seq Analysis Reveals Distinct Expression Patterns of Core Genes in Normal and Fibrotic Lung Tissues
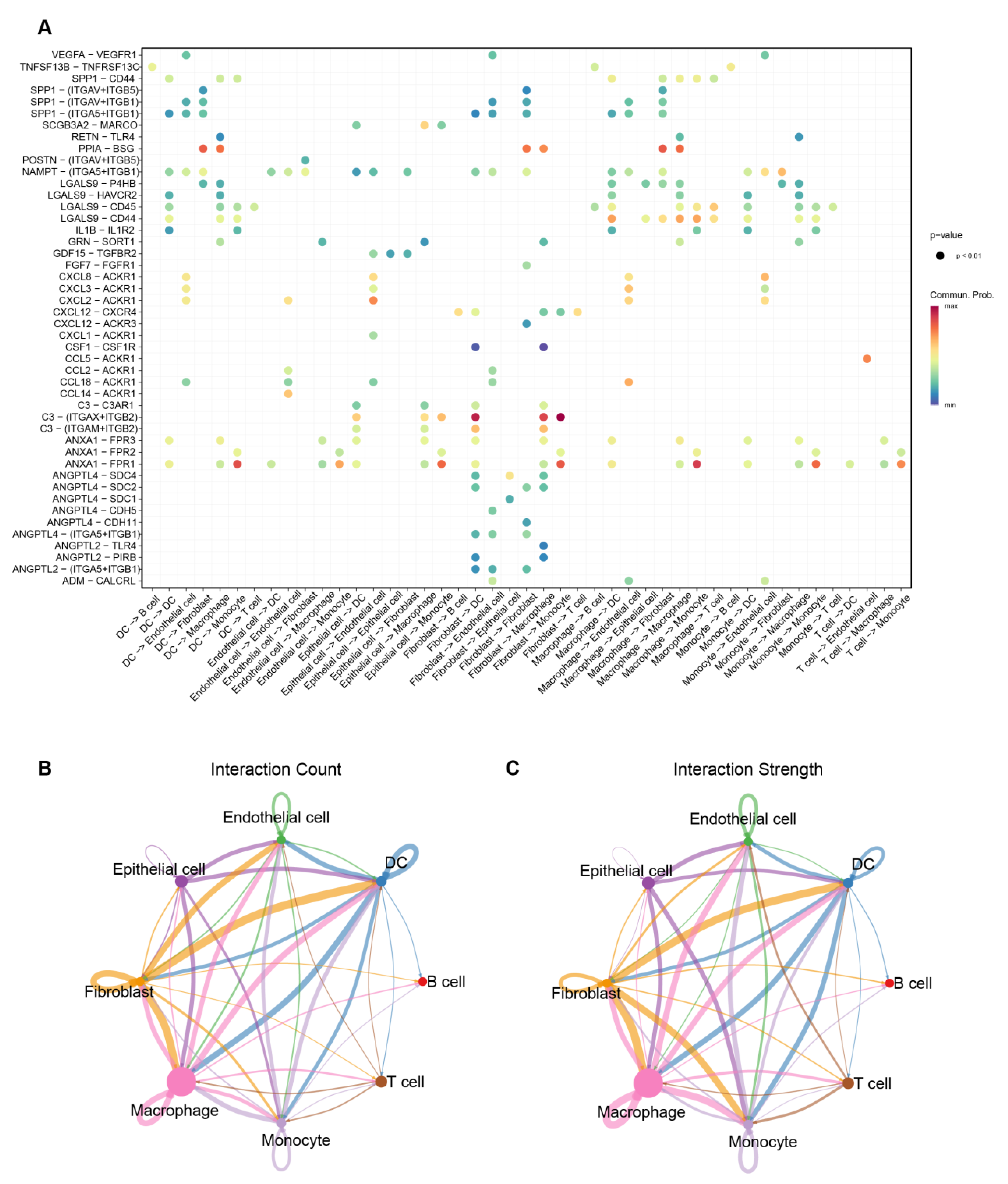
3.7. Cell–Cell Interaction Patterns Highlight Macrophage- and Fibroblast-Associated Communication in IPF
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IPF | Idiopathic pulmonary fibrosis |
| ILD | Interstitial lung diseases |
| ECM | Extracellular matrix |
| GWAS | Genome-wide association studies |
| IVW | Inverse variance weighted |
| IV | Instrumental variable |
| MR | Mendelian randomization |
| FDR | False discovery rate |
| DEGs | Differentially expressed genes |
| iLISI | Local inverse Simpson’s index |
References
- Lederer, D.J.; Martinez, F.J. Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2018, 378, 1811–1823. [Google Scholar] [CrossRef]
- Ley, B.; Collard, H.R.; King, T.E. Clinical Course and Prediction of Survival in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2011, 183, 431–440. [Google Scholar] [CrossRef]
- Konigsberg, I.R.; Borie, R.; Walts, A.D.; Cardwell, J.; Rojas, M.; Metzger, F.; Hauck, S.M.; Fingerlin, T.E.; Yang, I.V.; Schwartz, D.A. Molecular Signatures of Idiopathic Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2021, 65, 430–441. [Google Scholar] [CrossRef]
- Sisson, T.H.; Mendez, M.; Choi, K.; Subbotina, N.; Courey, A.; Cunningham, A.; Dave, A.; Engelhardt, J.F.; Liu, X.; White, E.S.; et al. Targeted Injury of Type II Alveolar Epithelial Cells Induces Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2010, 181, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhang, Y.; Xie, T.; Liu, N.; Chen, H.; Geng, Y.; Kurkciyan, A.; Mena, J.M.; Stripp, B.R.; Jiang, D.; et al. Hyaluronan and TLR4 Promote Surfactant-Protein-C-Positive Alveolar Progenitor Cell Renewal and Prevent Severe Pulmonary Fibrosis in Mice. Nat. Med. 2016, 22, 1285–1293. [Google Scholar] [CrossRef]
- Korfei, M.; Ruppert, C.; Mahavadi, P.; Henneke, I.; Markart, P.; Koch, M.; Lang, G.; Fink, L.; Bohle, R.-M.; Seeger, W.; et al. Epithelial Endoplasmic Reticulum Stress and Apoptosis in Sporadic Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2008, 178, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Pardo, A.; Selman, M. The Interplay of the Genetic Architecture, Aging, and Environmental Factors in the Pathogenesis of Idiopathic Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2021, 64, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Nance, T.; Smith, K.S.; Anaya, V.; Richardson, R.; Ho, L.; Pala, M.; Mostafavi, S.; Battle, A.; Feghali-Bostwick, C.; Rosen, G.; et al. Transcriptome Analysis Reveals Differential Splicing Events in IPF Lung Tissue. PLoS ONE 2014, 9, e92111. [Google Scholar] [CrossRef]
- Pan, Y.; Lei, X.; Zhang, Y. Association Predictions of Genomics, Proteinomics, Transcriptomics, Microbiome, Metabolomics, Pathomics, Radiomics, Drug, Symptoms, Environment Factor, and Disease Networks: A Comprehensive Approach. Med. Res. Rev. 2022, 42, 441–461. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, Y.; Yang, J.; Dong, L.; Zhang, R.; Tian, S.; Yu, Y.; Ren, L.; Hou, W.; Zhu, F.; et al. Multi-Omics Data Integration Using Ratio-Based Quantitative Profiling with Quartet Reference Materials. Nat. Biotechnol. 2024, 42, 1133–1149. [Google Scholar] [CrossRef]
- Shao, M.; Chen, K.; Zhang, S.; Tian, M.; Shen, Y.; Cao, C.; Gu, N. Multiome-Wide Association Studies: Novel Approaches for Understanding Diseases. Genom. Proteom. Bioinform. 2024, 22, qzae077. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-S.; You, J.; Chen, S.-D.; Zhang, Y.; Feng, J.-F.; Xu, Y.-M.; Yu, J.-T.; Cheng, W. Plasma Proteomics Identify Biomarkers and Undulating Changes of Brain Aging. Nat. Aging 2025, 5, 99–112. [Google Scholar] [CrossRef]
- Chin, D.; Hernandez-Beeftink, T.; Donoghue, L.; Guillen-Uio, B.; Leavy, O.C.; Adegunsoye, A.; Booth, H.L.; CleanUP-IPF Investigators of the Pulmonary Trials Cooperative; Fahy, W.A.; Fingerlin, T.E.; et al. Genome-Wide Association Study of Idiopathic Pulmonary Fibrosis Susceptibility Using Clinically-Curated European-Ancestry Datasets. medRxiv 2025. [Google Scholar] [CrossRef]
- Ferkingstad, E.; Sulem, P.; Atlason, B.A.; Sveinbjornsson, G.; Magnusson, M.I.; Styrmisdottir, E.L.; Gunnarsdottir, K.; Helgason, A.; Oddsson, A.; Halldorsson, B.V.; et al. Large-Scale Integration of the Plasma Proteome with Genetics and Disease. Nat. Genet. 2021, 53, 1712–1721. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, Y.; Zhou, R.; Hao, Y.; Wu, X.; Li, G.; Du, Q. Deciphering the Molecular Mechanism of Bu Yang Huan Wu Decoction in Interference with Diabetic Pulmonary Fibrosis via Regulating Oxidative Stress and Lipid Metabolism Disorder. J. Pharm. Biomed. Anal. 2024, 243, 116061. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Wu, G.; Xiong, W.; Gu, W.; Wang, C.-Y. Macrophages: Friend or Foe in Idiopathic Pulmonary Fibrosis? Respir. Res. 2018, 19, 170. [Google Scholar] [CrossRef]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Wynn, T.A. Obstacles and Opportunities for Understanding Macrophage Polarization. J. Leukoc. Biol. 2011, 89, 557–563. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, Y.; Zhang, Z.; He, L.; Zhu, J.; Zhang, M.; He, X.; Cheng, Z.; Ao, Q.; Cao, Y.; et al. Chop Deficiency Protects Mice Against Bleomycin-Induced Pulmonary Fibrosis by Attenuating M2 Macrophage Production. Mol. Ther. 2016, 24, 915–925. [Google Scholar] [CrossRef]
- Duffield, J.S.; Lupher, M.; Thannickal, V.J.; Wynn, T.A. Host Responses in Tissue Repair and Fibrosis. Annu. Rev. Pathol. 2013, 8, 241–276. [Google Scholar] [CrossRef] [PubMed]
- Van Dyken, S.J.; Locksley, R.M. Interleukin-4- and Interleukin-13-Mediated Alternatively Activated Macrophages: Roles in Homeostasis and Disease. Annu. Rev. Immunol. 2013, 31, 317–343. [Google Scholar] [CrossRef] [PubMed]
- Prasse, A.; Pechkovsky, D.V.; Toews, G.B.; Jungraithmayr, W.; Kollert, F.; Goldmann, T.; Vollmer, E.; Müller-Quernheim, J.; Zissel, G. A Vicious Circle of Alveolar Macrophages and Fibroblasts Perpetuates Pulmonary Fibrosis via CCL18. Am. J. Respir. Crit. Care Med. 2006, 173, 781–792. [Google Scholar] [CrossRef]
- Hou, J.; Yang, Y.; Han, X. Machine Learning and Single-Cell Analysis Identify Molecular Features of IPF-Associated Fibroblast Subtypes and Their Implications on IPF Prognosis. Int. J. Mol. Sci. 2023, 25, 94. [Google Scholar] [CrossRef]
- Gervasi, M.; Bianchi-Smiraglia, A.; Cummings, M.; Zheng, Q.; Wang, D.; Liu, S.; Bakin, A.V. JunB Contributes to Id2 Repression and the Epithelial-Mesenchymal Transition in Response to Transforming Growth Factor-β. J. Cell Biol. 2012, 196, 589–603. [Google Scholar] [CrossRef]
- Liu, T.; Liu, Y.; Miller, M.; Cao, L.; Zhao, J.; Wu, J.; Wang, J.; Liu, L.; Li, S.; Zou, M.; et al. Autophagy Plays a Role in FSTL1-Induced Epithelial Mesenchymal Transition and Airway Remodeling in Asthma. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 313, L27–L40. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Kang, Q.; Qin, L.; Cui, L.; Pei, C. TGF-Β2 Induces Transdifferentiation and Fibrosis in Human Lens Epithelial Cells via Regulating Gremlin and CTGF. Biochem. Biophys. Res. Commun. 2014, 447, 689–695. [Google Scholar] [CrossRef]
- Rodrigues-Diez, R.R.; Lavoz, C.; Carvajal, G.; Droguett, A.; Garcia-Redondo, A.B.; Rodriguez, I.; Ortiz, A.; Egido, J.; Mezzano, S.; Ruiz-Ortega, M. Gremlin Activates the Smad Pathway Linked to Epithelial Mesenchymal Transdifferentiation in Cultured Tubular Epithelial Cells. BioMed Res. Int. 2014, 2014, 802841. [Google Scholar] [CrossRef]
- Sun, Z.; Cai, S.; Liu, C.; Cui, Y.; Ji, J.; Jiang, W.G.; Ye, L. Increased Expression of Gremlin1 Promotes Proliferation and Epithelial Mesenchymal Transition in Gastric Cancer Cells and Correlates With Poor Prognosis of Patients With Gastric Cancer. Cancer Genom. Proteom. 2020, 17, 49–60. [Google Scholar] [CrossRef]
- Mulvihill, M.S.; Kwon, Y.-W.; Lee, S.; Fang, L.T.; Choi, H.; Ray, R.; Kang, H.C.; Mao, J.-H.; Jablons, D.; Kim, I.-J.; et al. Gremlin Is Overexpressed in Lung Adenocarcinoma and Increases Cell Growth and Proliferation in Normal Lung Cells. PLoS ONE 2012, 7, e42264. [Google Scholar] [CrossRef]
- Rodrigues-Diez, R.; Lavoz, C.; Carvajal, G.; Rayego-Mateos, S.; Diez, R.R.R.; Ortiz, A.; Egido, J.; Mezzano, S.; Ruiz-Ortega, M. Gremlin Is a Downstream Profibrotic Mediator of Transforming Growth Factor-Beta in Cultured Renal Cells. Nephron Exp. Nephrol. 2012, 122, 62–74. [Google Scholar] [CrossRef]
- Shi, X.; Wang, J.; Zhang, X.; Yang, S.; Luo, W.; Wang, S.; Huang, J.; Chen, M.; Cheng, Y.; Chao, J. GREM1/PPP2R3A Expression in Heterogeneous Fibroblasts Initiates Pulmonary Fibrosis. Cell Biosci. 2022, 12, 123. [Google Scholar] [CrossRef] [PubMed]
- Aoshima, Y.; Enomoto, Y.; Muto, S.; Meguro, S.; Kawasaki, H.; Kosugi, I.; Fujisawa, T.; Enomoto, N.; Inui, N.; Nakamura, Y.; et al. Gremlin-1 for the Differential Diagnosis of Idiopathic Pulmonary Fibrosis Versus Other Interstitial Lung Diseases: A Clinical and Pathophysiological Analysis. Lung 2021, 199, 289–298. [Google Scholar] [CrossRef]
- Liberty Mthunzi, L.; Rowan, S.C.; Kostyunina, D.S.; Baugh, J.A.; Knaus, U.G.; McLoughlin, P. Gremlin 1 Is Required for Macrophage M2 Polarization. Am. J. Physiol. Lung Cell. Mol. Physiol. 2023, 325, L270–L276. [Google Scholar] [CrossRef]
- Ge, C.; Huang, M.; Han, Y.; Shou, C.; Li, D.; Zhang, Y. Demethyleneberberine Alleviates Pulmonary Fibrosis through Disruption of USP11 Deubiquitinating GREM1. Pharmaceuticals 2024, 17, 279. [Google Scholar] [CrossRef]
- Meyer, K.C.; Arend, R.A.; Kalayoglu, M.V.; Rosenthal, N.S.; Byrne, G.I.; Brown, R.R. Tryptophan Metabolism in Chronic Inflammatory Lung Disease. J. Lab. Clin. Med. 1995, 126, 530–540. [Google Scholar] [PubMed]
- Carter, H.; Costa, R.M.; Adams, T.S.; Gilchrist, T.M.; Emch, C.E.; Bame, M.; Oldham, J.M.; Huang, S.K.; Linderholm, A.L.; Noth, I.; et al. CD103+ Dendritic Cell-Fibroblast Crosstalk via TLR9, TDO2, and AHR Signaling Drives Lung Fibrogenesis. JCI Insight 2025, 10, e177072. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, M.; Zhang, M.; Ye, X.; Gu, H.; Jiang, C.; Zhu, H.; Ye, X.; Li, Q.; Huang, X.; et al. The Gene Expression of CALD1, CDH2, and POSTN in Fibroblast Are Related to Idiopathic Pulmonary Fibrosis. Front. Immunol. 2024, 15, 1275064. [Google Scholar] [CrossRef]
- Liu, P.; Luo, G.; Dodson, M.; Schmidlin, C.J.; Wei, Y.; Kerimoglu, B.; Ooi, A.; Chapman, E.; Garcia, J.G.; Zhang, D.D. The NRF2-LOC344887 Signaling Axis Suppresses Pulmonary Fibrosis. Redox Biol. 2021, 38, 101766. [Google Scholar] [CrossRef]
- Black, M.; Milewski, D.; Le, T.; Ren, X.; Xu, Y.; Kalinichenko, V.V.; Kalin, T.V. FOXF1 Inhibits Pulmonary Fibrosis by Preventing CDH2-CDH11 Cadherin Switch in Myofibroblasts. Cell Rep. 2018, 23, 442–458. [Google Scholar] [CrossRef] [PubMed]
- Maillet, T.; Goletto, T.; Beltramo, G.; Dupuy, H.; Jouneau, S.; Borie, R.; Crestani, B.; Cottin, V.; Blockmans, D.; Lazaro, E.; et al. Usual Interstitial Pneumonia in ANCA-Associated Vasculitis: A Poor Prognostic Factor. J. Autoimmun. 2020, 106, 102338. [Google Scholar] [CrossRef]
- Kagiyama, N.; Takayanagi, N.; Kanauchi, T.; Ishiguro, T.; Yanagisawa, T.; Sugita, Y. Antineutrophil Cytoplasmic Antibody-Positive Conversion and Microscopic Polyangiitis Development in Patients with Idiopathic Pulmonary Fibrosis. BMJ Open Respir. Res. 2015, 2, e000058. [Google Scholar] [CrossRef]
- Cassone, G.; Dei, G.; Sambataro, G.; Manfredi, A.; Cerri, S.; Vacchi, C.; Faverio, P.; Sambataro, D.; Gozzi, F.; Vancheri, C.; et al. AB0528 characterization of anti-mpo positive interstitial lung disease. clinical-serologic and radiologic features and survival. Ann. Rheum. Dis. 2020, 79, 1561–1562. [Google Scholar] [CrossRef]
- Kubo, F.; Ariestanti, D.M.; Oki, S.; Fukuzawa, T.; Demizu, R.; Sato, T.; Sabirin, R.M.; Hirose, S.; Nakamura, N. Loss of the Adhesion G-Protein Coupled Receptor ADGRF5 in Mice Induces Airway Inflammation and the Expression of CCL2 in Lung Endothelial Cells. Respir. Res. 2019, 20, 11. [Google Scholar] [CrossRef]
- Bridges, J.P.; Safina, C.; Pirard, B.; Brown, K.; Filuta, A.; Panchanathan, R.; Bouhelal, R.; Reymann, N.; Patel, S.; Seuwen, K.; et al. Regulation of Pulmonary Surfactant by the Adhesion GPCR GPR116/ADGRF5 Requires a Tethered Agonist-Mediated Activation Mechanism. eLife 2022, 11, e69061. [Google Scholar] [CrossRef] [PubMed]
- Jacenik, D.; Hikisz, P.; Beswick, E.J.; Fichna, J. The Clinical Relevance of the Adhesion G Protein-Coupled Receptor F5 for Human Diseases and Cancers. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2023, 1869, 166683. [Google Scholar] [CrossRef]
- Kang, J.; Yeo, H.J.; Kim, Y.H.; Cho, W.H. Molecular Differences between Stable Idiopathic Pulmonary Fibrosis and Its Acute Exacerbation. Front. Biosci. 2021, 26, 1444–1452. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, H.; Wang, S.; Zhong, F.; Shen, T. Exploring the Cellular and Molecular Landscape of Idiopathic Pulmonary Fibrosis: Integrative Multi-Omics and Single-Cell Analysis. Biomedicines 2025, 13, 2135. https://doi.org/10.3390/biomedicines13092135
Jiang H, Wang S, Zhong F, Shen T. Exploring the Cellular and Molecular Landscape of Idiopathic Pulmonary Fibrosis: Integrative Multi-Omics and Single-Cell Analysis. Biomedicines. 2025; 13(9):2135. https://doi.org/10.3390/biomedicines13092135
Chicago/Turabian StyleJiang, Huanyu, Shujie Wang, Fanghui Zhong, and Tao Shen. 2025. "Exploring the Cellular and Molecular Landscape of Idiopathic Pulmonary Fibrosis: Integrative Multi-Omics and Single-Cell Analysis" Biomedicines 13, no. 9: 2135. https://doi.org/10.3390/biomedicines13092135
APA StyleJiang, H., Wang, S., Zhong, F., & Shen, T. (2025). Exploring the Cellular and Molecular Landscape of Idiopathic Pulmonary Fibrosis: Integrative Multi-Omics and Single-Cell Analysis. Biomedicines, 13(9), 2135. https://doi.org/10.3390/biomedicines13092135




