Chemerin Is the Adipokine Linked with Endothelin-Dependent Vasoconstriction in Human Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Recruited Subjects
2.2. Laboratory Analyses
2.3. Measurement of ET-1-Dependent Vascular Tone
2.4. Statistical Analyses
3. Results
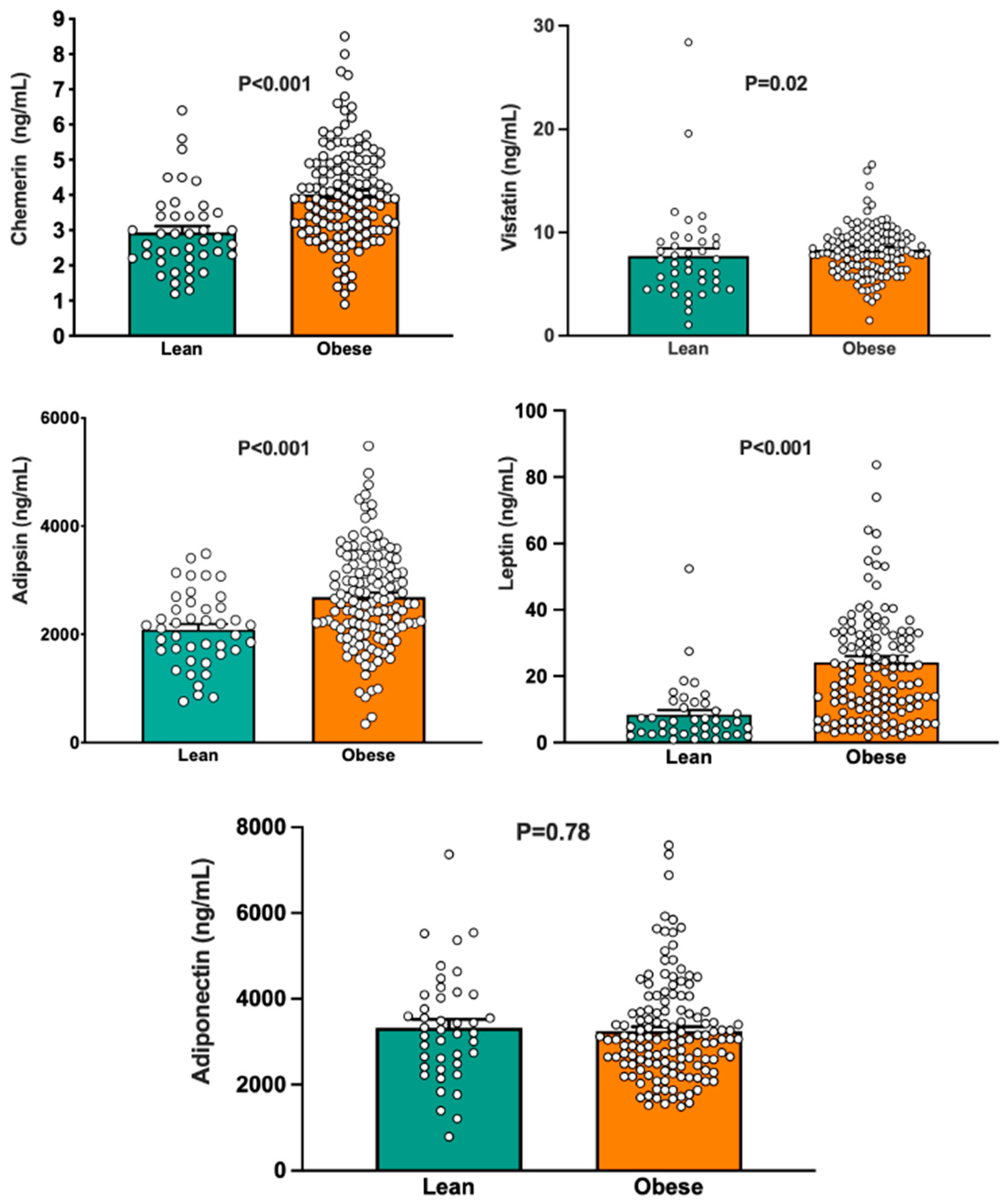
3.1. Group Differences in Circulating Adipokines
3.2. Group Differences in Endothelin-Mediated Vasoconstrictor Tone
3.3. Relationships of the Vascular Response to BQ-123 with Circulating Adipokines and with Anthropometric, Hemodynamic, and Biochemical Variables
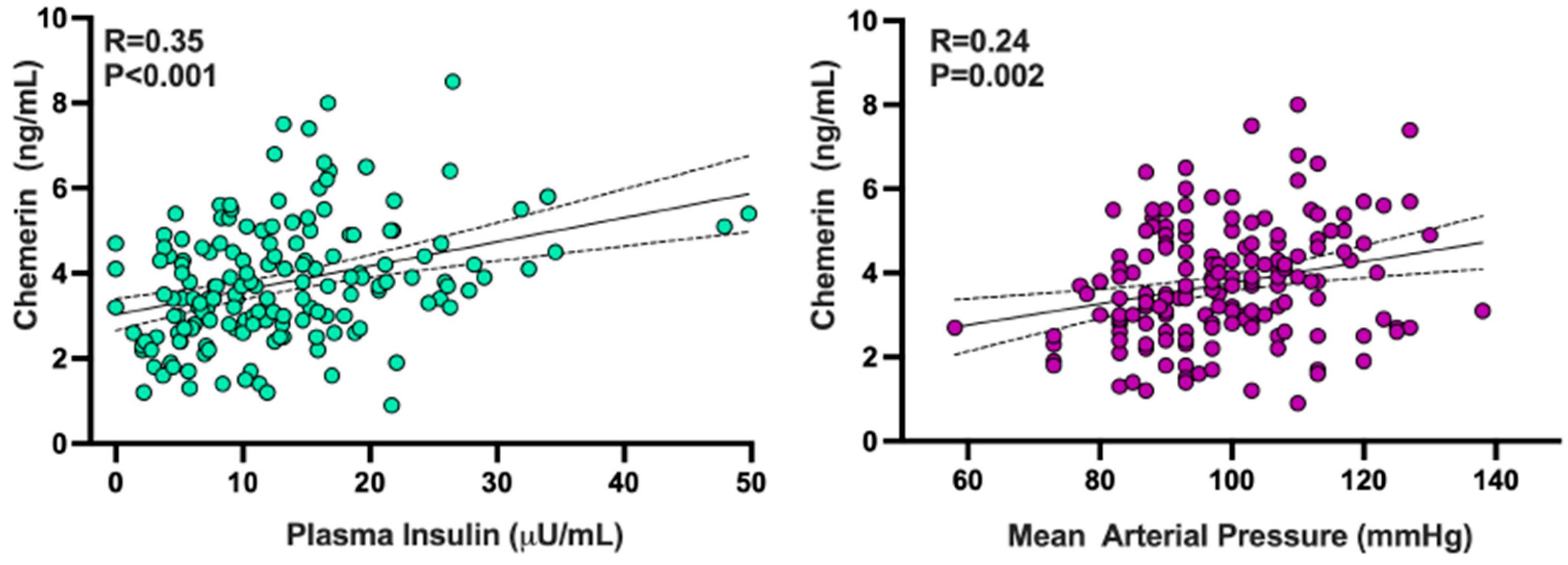
3.4. Relationships of Chemerin with Other Adipokines and with Demographic, Hemodynamic, and Biochemical Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taylor, E.B. The complex role of adipokines in obesity, inflammation, and autoimmunity. Clin. Sci. 2021, 135, 731–752. [Google Scholar] [CrossRef]
- Unamuno, X.; Gomez-Ambrosi, J.; Rodriguez, A.; Becerril, S.; Fruhbeck, G.; Catalan, V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur. J. Clin. Investig. 2018, 48, e12997. [Google Scholar] [CrossRef]
- Akoumianakis, I.; Antoniades, C. The interplay between adipose tissue and the cardiovascular system: Is fat always bad? Cardiovasc. Res. 2017, 113, 999–1008. [Google Scholar] [CrossRef]
- Guzik, T.J.; Skiba, D.S.; Touyz, R.M.; Harrison, D.G. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc. Res. 2017, 113, 1009–1023. [Google Scholar] [CrossRef] [PubMed]
- Koenen, M.; Hill, M.A.; Cohen, P.; Sowers, J.R. Obesity, adipose tissue and vascular dysfunction. Circ. Res. 2021, 128, 951–968. [Google Scholar] [CrossRef] [PubMed]
- Galley, J.C.; Singh, S.; Awata, W.M.C.; Alves, J.V.; Bruder-Nascimento, T. Adipokines: Deciphering the cardiovascular signature of adipose tissue. Biochem. Pharmacol. 2022, 206, 115324. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, L.; Zhong, N.; Wen, D.; Liu, L. Multifaced roles of adipokines in endothelial cell function. Front. Endocrinol. 2024, 15, 1490143. [Google Scholar] [CrossRef]
- Helfer, G.; Wu, Q.F. Chemerin: A multifaceted adipokine involved in metabolic disorders. J. Endocrinol. 2018, 238, R79–R94. [Google Scholar] [CrossRef]
- Neves, K.B.; Lobato, N.S.; Lopes, R.A.; Filgueira, F.P.; Zanotto, C.Z.; Oliveira, A.M.; Tostes, R.C. Chemerin reduces vascular nitric oxide/cGMP signalling in rat aorta: A link to vascular dysfunction in obesity? Clin. Sci. 2014, 127, 111–122. [Google Scholar] [CrossRef]
- Tan, L.; Lu, X.; Danser, A.H.J.; Verdonk, K. The role of chemerin in metabolic and cardiovascular disease: A literature review of its physiology and pathology from a nutritional perspective. Nutrients 2023, 15, 2878. [Google Scholar] [CrossRef]
- Romacho, T.; Valencia, I.; Ramos-Gonzalez, M.; Vallejo, S.; Lopez-Esteban, M.; Lorenzo, O.; Cannata, P.; Romero, A.; San Hipolito-Luengo, A.; Gomez-Cerezo, J.F.; et al. Visfatin/eNampt induces endothelial dysfunction in vivo: A role for Toll-Like Receptor 4 and NLRP3 inflammasome. Sci. Rep. 2020, 10, 5386. [Google Scholar] [CrossRef] [PubMed]
- Dare, A.; Chen, S.Y. Adipsin in the pathogenesis of cardiovascular diseases. Vascul. Pharmacol. 2024, 154, 107270. [Google Scholar] [CrossRef]
- Milek, M.; Moulla, Y.; Kern, M.; Stroh, C.; Dietrich, A.; Schon, M.R.; Gartner, D.; Lohmann, T.; Dressler, M.; Kovacs, P.; et al. Adipsin serum concentrations and adipose tissue expression in people with obesity and type 2 diabetes. Int. J. Mol. Sci. 2022, 23, 2222. [Google Scholar] [CrossRef]
- Jin, S.; Eussen, S.; Schalkwijk, C.G.; Stehouwer, C.D.A.; van Greevenbroek, M.M.J. Plasma factor D is cross-sectionally associated with low-grade inflammation, endothelial dysfunction and cardiovascular disease: The Maastricht study. Atherosclerosis 2023, 377, 60–67. [Google Scholar] [CrossRef]
- Kang, K.W.; Ok, M.; Lee, S.K. Leptin as a key between obesity and cardiovascular disease. J. Obes. Metab. Syndr. 2020, 29, 248–259. [Google Scholar] [CrossRef]
- Zhao, S.; Kusminski, C.M.; Scherer, P.E. Adiponectin, leptin and cardiovascular disorders. Circ. Res. 2021, 128, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Bohm, F.; Pernow, J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc. Res. 2007, 76, 8–18. [Google Scholar] [CrossRef]
- Schinzari, F.; Iantorno, M.; Campia, U.; Mores, N.; Rovella, V.; Tesauro, M.; Di Daniele, N.; Cardillo, C. Vasodilator responses and endothelin-dependent vasoconstriction in metabolically healthy obesity and the metabolic syndrome. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E787–E792. [Google Scholar] [CrossRef]
- Weil, B.R.; Westby, C.M.; Van Guilder, G.P.; Greiner, J.J.; Stauffer, B.L.; DeSouza, C.A. Enhanced endothelin-1 system activity with overweight and obesity. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H689–H695. [Google Scholar] [CrossRef]
- Sutton, G.; Pugh, D.; Dhaun, N. Developments in the role of endothelin-1 in atherosclerosis: A potential therapeutic target? Am. J. Hypertens. 2019, 32, 813–815. [Google Scholar] [CrossRef] [PubMed]
- Jankowich, M.; Choudhary, G. Endothelin-1 levels and cardiovascular events. Trends Cardiovasc. Med. 2020, 30, 1–8. [Google Scholar] [CrossRef]
- Yang, C.; Zhu, C.G.; Guo, Y.L.; Wu, N.Q.; Dong, Q.; Xu, R.X.; Wu, Y.J.; Qian, J.; Li, J.J. Prognostic value of plasma endothelin-1 in predicting worse outcomes in patients with prediabetes and diabetes and stable coronary artery diseases. Diabetes Metab. J. 2024, 48, 993–1002. [Google Scholar] [CrossRef]
- Tesauro, M.; Schinzari, F.; Rovella, V.; Di Daniele, N.; Lauro, D.; Mores, N.; Veneziani, A.; Cardillo, C. Ghrelin restores the endothelin 1/nitric oxide balance in patients with obesity-related metabolic syndrome. Hypertension 2009, 54, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Cardillo, C.; Campia, U.; Bryant, M.B.; Panza, J.A. Increased activity of endogenous endothelin in patients with type II diabetes mellitus. Circulation 2002, 106, 1783–1787. [Google Scholar] [CrossRef]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef]
- Silha, J.V.; Krsek, M.; Skrha, J.V.; Sucharda, P.; Nyomba, B.L.; Murphy, L.J. Plasma resistin, adiponectin and leptin levels in lean and obese subjects: Correlations with insulin resistance. Eur. J. Endocrinol. 2003, 149, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Hoffstedt, J.; Arvidsson, E.; Sjolin, E.; Wahlen, K.; Arner, P. Adipose tissue adiponectin production and adiponectin serum concentration in human obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2004, 89, 1391–1396. [Google Scholar] [CrossRef]
- Doumatey, A.P.; Bentley, A.R.; Zhou, J.; Huang, H.; Adeyemo, A.; Rotimi, C.N. Paradoxical hyperadiponectinemia is associated with the metabolically healthy obese (MHO) phenotype in African Americans. J. Endocrinol. Metab. 2012, 2, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Turer, A.T.; Khera, A.; Ayers, C.R.; Turer, C.B.; Grundy, S.M.; Vega, G.L.; Scherer, P.E. Adipose tissue mass and location affect circulating adiponectin levels. Diabetologia 2011, 54, 2515–2524. [Google Scholar] [CrossRef]
- Guenther, M.; James, R.; Marks, J.; Zhao, S.; Szabo, A.; Kidambi, S. Adiposity distribution influences circulating adiponectin levels. Transl. Res. 2014, 164, 270–277. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, J.; Abbasi, F.; Fathzadeh, M.; Knowles, J.W.; Leung, L.L.K.; Morser, J. Chemerin in participants with or without insulin resistance and diabetes. Biomedicines 2024, 12, 924. [Google Scholar] [CrossRef]
- Leniz, A.; Gonzalez, M.; Besne, I.; Carr-Ugarte, H.; Gomez-Garcia, I.; Portillo, M.P. Role of chemerin in the control of glucose homeostasis. Mol. Cell. Endocrinol. 2022, 541, 111504. [Google Scholar] [CrossRef]
- Rourke, J.L.; Dranse, H.J.; Sinal, C.J. Towards an integrative approach to understanding the role of chemerin in human health and disease. Obes. Rev. 2013, 14, 245–262. [Google Scholar] [CrossRef]
- Lobato, N.S.; Neves, K.B.; Filgueira, F.P.; Fortes, Z.B.; Carvalho, M.H.; Webb, R.C.; Oliveira, A.M.; Tostes, R.C. The adipokine chemerin augments vascular reactivity to contractile stimuli via activation of the MEK-ERK1/2 pathway. Life Sci. 2012, 91, 600–606. [Google Scholar] [CrossRef]
- Neves, K.B.; Nguyen Dinh Cat, A.; Lopes, R.A.; Rios, F.J.; Anagnostopoulou, A.; Lobato, N.S.; de Oliveira, A.M.; Tostes, R.C.; Montezano, A.C.; Touyz, R.M. Chemerin regulates crosstalk between adipocytes and vascular cells through Nox. Hypertension 2015, 66, 657–666. [Google Scholar] [CrossRef]
- Hanthazi, A.; Jespers, P.; Vegh, G.; Degroot, G.N.; Springael, J.Y.; Lybaert, P.; Dewachter, L.; Mc Entee, K. Chemerin influences endothelin- and serotonin-induced pulmonary artery vasoconstriction in rats. Life Sci. 2019, 231, 116580. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.J.; Yang, P.; Read, C.; Kuc, R.E.; Yang, L.; Taylor, E.J.; Taylor, C.W.; Maguire, J.J.; Davenport, A.P. Chemerin elicits potent constrictor actions via chemokine-like receptor 1 (CMKLR1), not G-protein-coupled receptor 1 (GPR1), in human and rat vasculature. J. Am. Heart Assoc. 2016, 5, e004421. [Google Scholar] [CrossRef] [PubMed]
- Ferland, D.J.; Darios, E.S.; Neubig, R.R.; Sjogren, B.; Truong, N.; Torres, R.; Dexheimer, T.S.; Thompson, J.M.; Watts, S.W. Chemerin-induced arterial contraction is G(i)- and calcium-dependent. Vascul. Pharmacol. 2017, 88, 30–41. [Google Scholar] [CrossRef]
- Eichelmann, F.; Schulze, M.B.; Wittenbecher, C.; Menzel, J.; Weikert, C.; di Giuseppe, R.; Biemann, R.; Isermann, B.; Fritsche, A.; Boeing, H.; et al. Chemerin as a biomarker linking inflammation and cardiovascular diseases. J. Am. Coll. Cardiol. 2019, 73, 378–379. [Google Scholar] [CrossRef] [PubMed]


| Lean Subjects (n = 42) | Obese Patients (n = 134) | p Value | |
|---|---|---|---|
| Sex, m/f | 19/23 | 80/64 | - |
| Age, yr | 42 ± 2 | 41 ± 1 | >0.05 |
| BMI, kg/m2 | 23 ± 1 | 38 ± 1 | <0.001 |
| Waist, cm | 118 ± 2 | ||
| MAP, mmHg | 86 ± 1 | 95 ± 1 | <0.001 |
| Glucose, mg/dL | 86 ± 1 | 101 ± 1 | <0.001 |
| Total Cholesterol, mg/dL | 173 ± 4 | 196 ± 4 | 0.005 |
| LDL Cholesterol, mg/dL | 109 ± 5 | 125 ± 4 | 0.002 |
| HDL Cholesterol, mg/dL | 51 ± 2 | 45 ± 1 | 0.004 |
| Triglycerides, mg/dL | 92 ± 8 | 152 ± 8 | <0.001 |
| Insulin, μU/mL | 7 ± 1 | 14 ± 1 | <0.001 |
| HOMA | 1.5 ± 0.1 | 3.2 ± 0.3 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schinzari, F.; Montenero, R.; Cardillo, C.; Tesauro, M. Chemerin Is the Adipokine Linked with Endothelin-Dependent Vasoconstriction in Human Obesity. Biomedicines 2025, 13, 2131. https://doi.org/10.3390/biomedicines13092131
Schinzari F, Montenero R, Cardillo C, Tesauro M. Chemerin Is the Adipokine Linked with Endothelin-Dependent Vasoconstriction in Human Obesity. Biomedicines. 2025; 13(9):2131. https://doi.org/10.3390/biomedicines13092131
Chicago/Turabian StyleSchinzari, Francesca, Rossella Montenero, Carmine Cardillo, and Manfredi Tesauro. 2025. "Chemerin Is the Adipokine Linked with Endothelin-Dependent Vasoconstriction in Human Obesity" Biomedicines 13, no. 9: 2131. https://doi.org/10.3390/biomedicines13092131
APA StyleSchinzari, F., Montenero, R., Cardillo, C., & Tesauro, M. (2025). Chemerin Is the Adipokine Linked with Endothelin-Dependent Vasoconstriction in Human Obesity. Biomedicines, 13(9), 2131. https://doi.org/10.3390/biomedicines13092131







