Atherogenic Index of Plasma Predicts Futile Reperfusion and Early Deterioration After Successful Recanalization: A Multicenter Study of EVT-Treated LAA Stroke
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Data Collection and Definition of Parameters
2.3. Statistical Analysis
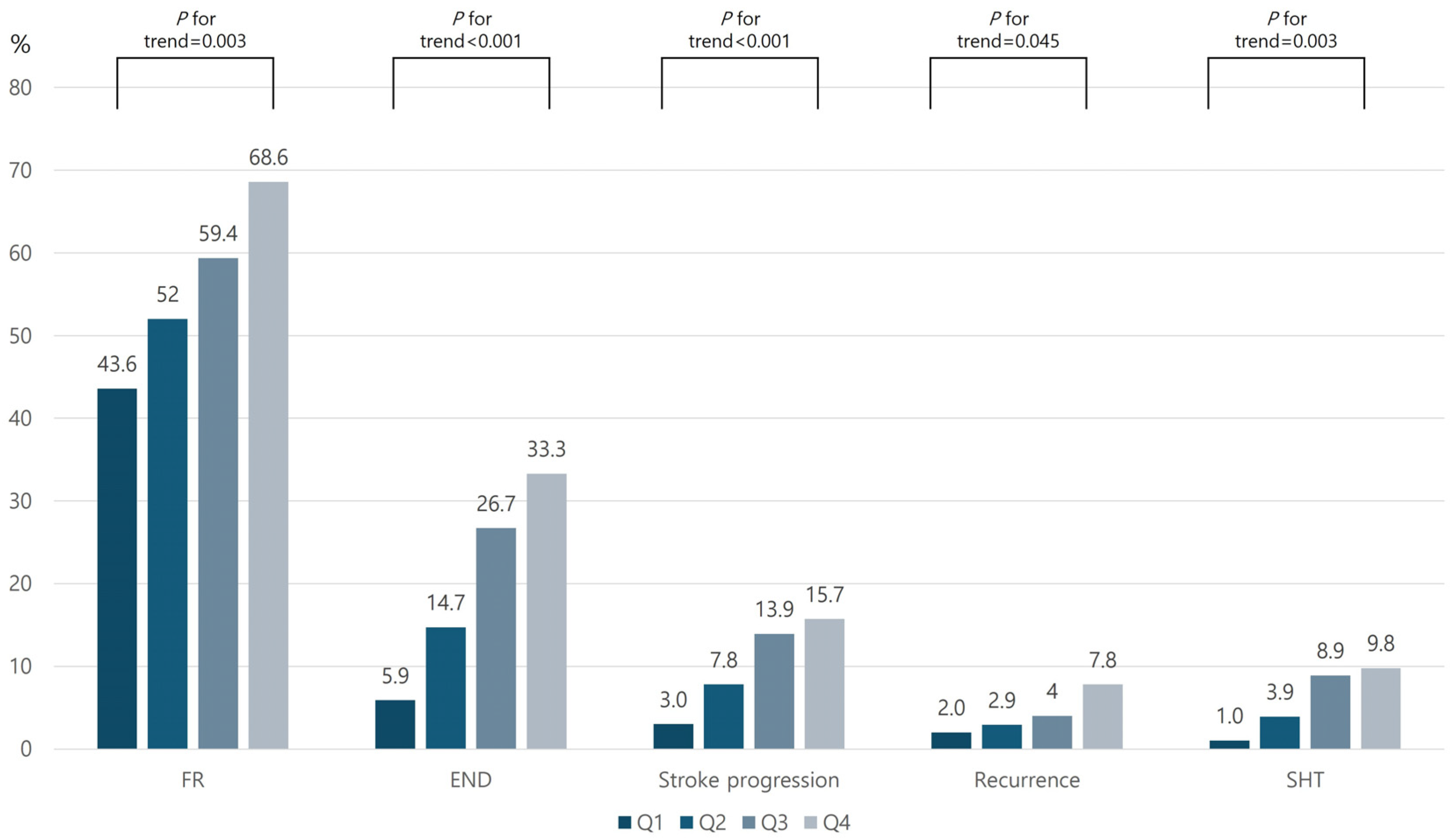
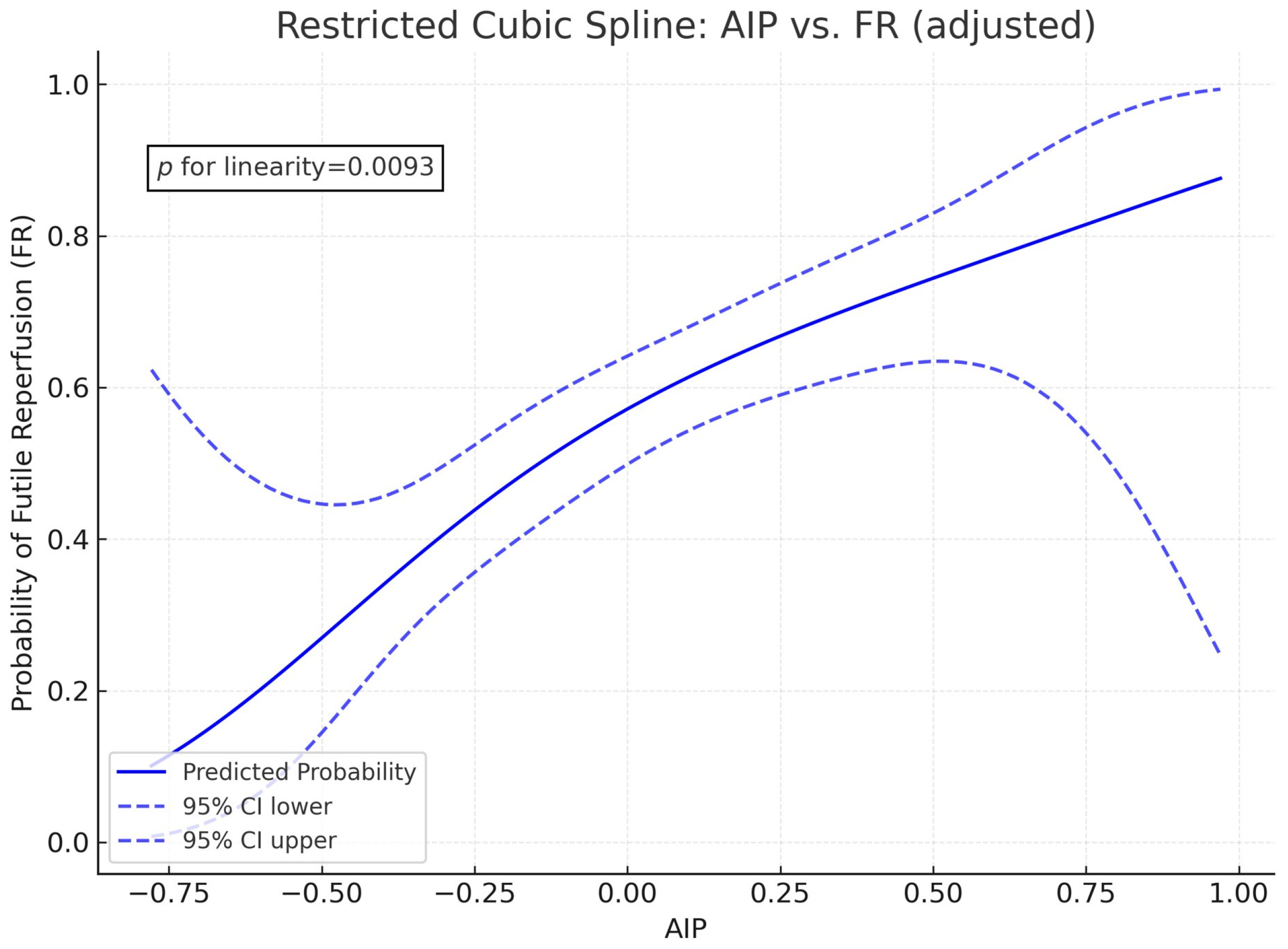
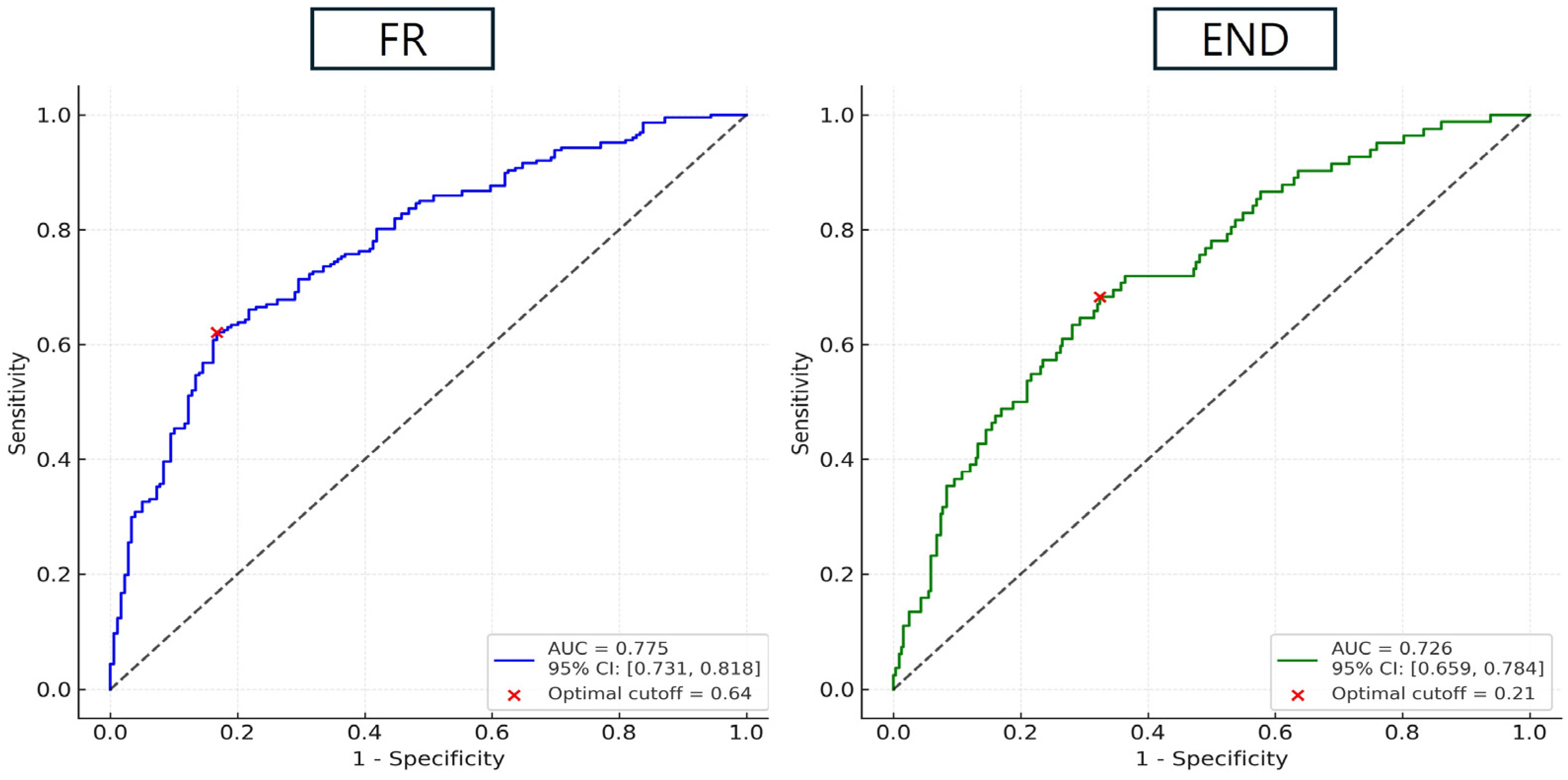
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [PubMed]
- Goyal, M.; Demchuk, A.M.; Menon, B.K.; Eesa, M.; Rempel, J.L.; Thornton, J.; Roy, D.; Jovin, T.G.; Willinsky, R.A.; Sapkota, B.L.; et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N. Engl. J. Med. 2015, 372, 1019–1030. [Google Scholar] [CrossRef]
- Campbell, B.C.; Mitchell, P.J.; Kleinig, T.J.; Dewey, H.M.; Churilov, L.; Yassi, N.; Yan, B.; Dowling, R.J.; Parsons, M.W.; Oxley, T.J.; et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N. Engl. J. Med. 2015, 372, 1009–1018. [Google Scholar] [CrossRef]
- Berkhemer, O.A.; Fransen, P.S.; Beumer, D.; van den Berg, L.A.; Lingsma, H.F.; Yoo, A.J.; Schonewille, W.J.; Vos, J.A.; Nederkoorn, P.J.; Wermer, M.J.; et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N. Engl. J. Med. 2015, 372, 11–20. [Google Scholar] [CrossRef]
- Saver, J.L.; Goyal, M.; Bonafe, A.; Diener, H.C.; Levy, E.I.; Pereira, V.M.; Albers, G.W.; Cognard, C.; Cohen, D.J.; Hacke, W.; et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N. Engl. J. Med. 2015, 372, 2285–2295. [Google Scholar] [CrossRef]
- Jovin, T.G.; Chamorro, A.; Cobo, E.; de Miquel, M.A.; Molina, C.A.; Rovira, A.; San Roman, L.; Serena, J.; Abilleira, S.; Ribo, M.; et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N. Engl. J. Med. 2015, 372, 2296–2306. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, B.J.; Han, M.K.; Park, T.H.; Lee, K.B.; Lee, B.C.; Yu, K.H.; Oh, M.S.; Cha, J.K.; Kim, D.H.; et al. Futile reperfusion and predicted therapeutic benefits after successful endovascular treatment according to initial stroke severity. BMC Neurol. 2019, 19, 11. [Google Scholar] [CrossRef]
- Xu, H.; Jia, B.; Huo, X.; Mo, D.; Ma, N.; Gao, F.; Yang, M.; Miao, Z. Predictors of Futile Recanalization After Endovascular Treatment in Patients with Acute Ischemic Stroke in a Multicenter Registry Study. J. Stroke Cerebrovasc. Dis. 2020, 29, 105067. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Lin, C.; Chen, L.; Qiao, Y.; Huang, P.; Liu, B.; Zhu, Y.; Su, J.; Liu, J. Multiple-Factor Analyses of Futile Recanalization in Acute Ischemic Stroke Patients Treated With Mechanical Thrombectomy. Front. Neurol. 2021, 12, 704088. [Google Scholar] [CrossRef] [PubMed]
- Pedraza, M.I.; de Lera, M.; Bos, D.; Calleja, A.I.; Cortijo, E.; Gomez-Vicente, B.; Reyes, J.; Coco-Martin, M.B.; Calonge, T.; Agulla, J.; et al. Brain Atrophy and the Risk of Futile Endovascular Reperfusion in Acute Ischemic Stroke. Stroke 2020, 51, 1514–1521. [Google Scholar] [CrossRef]
- Rabinstein, A.A.; Albers, G.W.; Brinjikji, W.; Koch, S. Factors that may contribute to poor outcome despite good reperfusion after acute endovascular stroke therapy. Int. J. Stroke 2019, 14, 23–31. [Google Scholar] [CrossRef]
- Shah, S.; Wood, S.; Logue, L.; Meyer, J.; Pikel, K.; Germroth, M.; Peethamber, G.; Kodumuri, N.; Lowe, F.J.; Kothari, R.; et al. Cerebral collateral flow state in acute ischemic stroke correlates with clinical functional outcomes in non-thrombectomy patients. J. Stroke Cerebrovasc. Dis. 2025, 34, 108211. [Google Scholar] [CrossRef]
- Maguida, G.; Shuaib, A. Collateral Circulation in Ischemic Stroke: An Updated Review. J. Stroke 2023, 25, 179–198. [Google Scholar] [CrossRef] [PubMed]
- Uniken Venema, S.M.; Dankbaar, J.W.; van der Lugt, A.; Dippel, D.W.J.; van der Worp, H.B. Cerebral Collateral Circulation in the Era of Reperfusion Therapies for Acute Ischemic Stroke. Stroke 2022, 53, 3222–3234. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, W.; Chen, S.; Yan, E.; Liu, L.; Chen, J.; Qian, M. Association between the atherogenic index of plasma and early neurological deterioration in mechanical thrombectomy patients. J. Stroke Cerebrovasc. Dis. 2024, 33, 107993. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, L.; Wang, L.; Li, K.; Fan, F.; Jia, J.; Li, J.; Zhang, Y. The predictive value of cumulative atherogenic index of plasma (AIP) for cardiovascular outcomes: A prospective community-based cohort study. Cardiovasc. Diabetol. 2024, 23, 264. [Google Scholar] [CrossRef]
- Liu, H.; Liu, K.; Pei, L.; Li, S.; Zhao, J.; Zhang, K.; Zong, C.; Zhao, L.; Fang, H.; Wu, J.; et al. Atherogenic Index of Plasma Predicts Outcomes in Acute Ischemic Stroke. Front. Neurol. 2021, 12, 741754. [Google Scholar] [CrossRef]
- Li, J.; Hou, D.; Li, J.; Li, R.; Sun, M. Association between the atherogenic index of plasma and the systemic immuno-inflammatory index using NHANES data from 2005 to 2018. Sci. Rep. 2025, 15, 11245. [Google Scholar] [CrossRef]
- Huang, X.; Wen, S.; Huang, Z.; Qin, G.; Zhou, H.; Xia, Z. A U-shaped relationship between the atherogenic index of plasma and repeated target vessel revascularization in patients undergoing percutaneous coronary intervention: A retrospective study. Front. Endocrinol. 2024, 15, 1428830. [Google Scholar] [CrossRef]
- Sun, F.; Zhou, J.; Chen, X.; Yang, T.; Wang, G.; Ge, J.; Zhang, Z.; Mei, Z. No-reflow after recanalization in ischemic stroke: From pathomechanisms to therapeutic strategies. J. Cereb. Blood Flow Metab. 2024, 44, 857–880. [Google Scholar] [CrossRef]
- Ko, Y.; Lee, S.; Chung, J.W.; Han, M.K.; Park, J.M.; Kang, K.; Park, T.H.; Park, S.S.; Cho, Y.J.; Hong, K.S.; et al. MRI-based Algorithm for Acute Ischemic Stroke Subtype Classification. J. Stroke 2014, 16, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Menon, B.K.; D’eSterre, C.D.; Qazi, E.M.; Almekhlafi, M.; Hahn, L.; Demchuk, A.M.; Goyal, M. Multiphase CT Angiography: A New Tool for the Imaging Triage of Patients with Acute Ischemic Stroke. Radiology 2015, 275, 510–520. [Google Scholar] [CrossRef]
- Deng, J.; Tang, X.; Tang, R.; Chen, J.; Guo, H.; Zhou, Q.; Zhan, X.; Long, H.; Peng, F.; Wang, X.; et al. Atherogenic index predicts all-cause and cardiovascular mortality in incident peritoneal dialysis patients. Atherosclerosis 2023, 387, 117389. [Google Scholar] [CrossRef]
- Sohn, J.H.; Kim, C.; Lee, M.; Kim, Y.; Jung Mo, H.; Yu, K.H.; Lee, S.H. Effects of prior antiplatelet use on futile reperfusion in patients with acute ischemic stroke receiving endovascular treatment. Eur. Stroke J. 2023, 8, 208–214. [Google Scholar] [CrossRef]
- Park, T.H.; Lee, J.K.; Park, M.S.; Park, S.S.; Hong, K.S.; Ryu, W.S.; Kim, D.E.; Park, M.S.; Choi, K.H.; Kim, J.T.; et al. Neurologic deterioration in patients with acute ischemic stroke or transient ischemic attack. Neurology 2020, 95, e2178–e2191. [Google Scholar] [CrossRef]
- Wang, X.; Wen, P.; Liao, Y.; Wu, T.; Zeng, L.; Huang, Y.; Song, X.; Xiong, Z.; Deng, L.; Li, D.; et al. Association of atherogenic index of plasma and its modified indices with stroke risk in individuals with cardiovascular-kidney-metabolic syndrome stages 0-3: A longitudinal analysis based on CHARLS. Cardiovasc. Diabetol. 2025, 24, 254. [Google Scholar] [CrossRef]
- Dobiasova, M. Atherogenic index of plasma [log(triglycerides/HDL-cholesterol)]: Theoretical and practical implications. Clin. Chem. 2004, 50, 1113–1115. [Google Scholar] [CrossRef]
- Higashi, Y. Endothelial Function in Dyslipidemia: Roles of LDL-Cholesterol, HDL-Cholesterol and Triglycerides. Cells 2023, 12, 1293. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.W.; Kwon, H.M.; Lee, Y.S. Effect of atherogenic index of plasma and triglyceride-glucose index on early neurological deterioration of patients with large artery atherosclerotic ischemic stroke. Diabetol. Metab. Syndr. 2025, 17, 123. [Google Scholar] [CrossRef] [PubMed]
- Bang, O.Y.; Saver, J.L.; Kim, S.J.; Kim, G.M.; Chung, C.S.; Ovbiagele, B.; Lee, K.H.; Liebeskind, D.S. Collateral flow predicts response to endovascular therapy for acute ischemic stroke. Stroke 2011, 42, 693–699. [Google Scholar] [CrossRef]
- Lakhan, S.E.; Kirchgessner, A.; Hofer, M. Inflammatory mechanisms in ischemic stroke: Therapeutic approaches. J. Transl. Med. 2009, 7, 97. [Google Scholar] [CrossRef]
- Hall, C.N.; Reynell, C.; Gesslein, B.; Hamilton, N.B.; Mishra, A.; Sutherland, B.A.; O’Farrell, F.M.; Buchan, A.M.; Lauritzen, M.; Attwell, D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014, 508, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Chen, J.; Zhang, J. Meta-analysis of the correlation between inflammatory response indices and no-reflow after PCI in patients with acute STEMI. Am. J. Transl. Res. 2024, 16, 5168–5181. [Google Scholar] [CrossRef] [PubMed]
- Strambo, D.; Michel, P.; Nguyen, T.N.; Abdalkader, M.; Qureshi, M.M.; Strbian, D.; Herweh, C.; Mohlenbruch, M.A.; Raty, S.; Olive-Gadea, M.; et al. Endovascular Versus Medical Therapy in Posterior Cerebral Artery Stroke: Role of Baseline NIHSS Score and Occlusion Site. Stroke 2024, 55, 1787–1797. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.C.; Chan, S.L.; Edwards, N.J.; Rao, V.A.; Klingman, J.G.; Nguyen-Huynh, M.N.; Yan, B.; Mitchell, P.J.; Davis, S.M.; Campbell, B.C.; et al. Outcome prediction in large vessel occlusion ischemic stroke with or without endovascular stroke treatment: THRIVE-EVT. Int. J. Stroke 2023, 18, 331–337. [Google Scholar] [CrossRef]
- Kim, J.S. Role of Blood Lipid Levels and Lipid-Lowering Therapy in Stroke Patients with Different Levels of Cerebral Artery Diseases: Reconsidering Recent Stroke Guidelines. J. Stroke 2021, 23, 149–161. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, G.; Yan, L.; Chen, R.; Liu, Y.; Liu, L.; Zhang, X.; Wang, M.; Zhao, L. Association of atherogenic index of plasma with early neurological deterioration in patients with acute ischemic stroke. Clin. Neurol. Neurosurg. 2023, 234, 108014. [Google Scholar] [CrossRef]
- Ois, A.; Cuadrado-Godia, E.; Giralt-Steinhauer, E.; Jimenez-Conde, J.; Soriano-Tarraga, C.; Rodriguez-Campello, A.; Avellaneda, C.; Cascales, D.; Fernandez-Perez, I.; Roquer, J. Long-Term Stroke Recurrence after Transient Ischemic Attack: Implications of Etiology. J. Stroke 2019, 21, 184–189. [Google Scholar] [CrossRef]
- Yaghi, S.; Elkind, M.S. Lipids and Cerebrovascular Disease: Research and Practice. Stroke 2015, 46, 3322–3328. [Google Scholar] [CrossRef]
- Ke, D.; Zhou, F.; Liang, H.; Xu, Y.; Lou, H. Hypertriglyceridemia Is Associated with Reduced Leukoaraiosis Severity in Patients with a Small Vessel Stroke. Behav. Neurol. 2018, 2018, 1361780. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Z.; Wang, M.; Mo, J.; Jing, J.; Yalkun, G.; Dai, L.; Meng, X.; Li, H.; Li, Z.; et al. Low LDL-C level and intracranial haemorrhage risk after ischaemic stroke: A prospective cohort study. Stroke Vasc. Neurol. 2023, 8, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Sperring, C.P.; Savage, W.M.; Argenziano, M.G.; Leifer, V.P.; Alexander, J.; Echlov, N.; Spinazzi, E.F.; Connolly, E.S., Jr. No-Reflow Post-Recanalization in Acute Ischemic Stroke: Mechanisms, Measurements, and Molecular Markers. Stroke 2023, 54, 2472–2480. [Google Scholar] [CrossRef] [PubMed]
- Barter, P.J.; Nicholls, S.; Rye, K.A.; Anantharamaiah, G.M.; Navab, M.; Fogelman, A.M. Antiinflammatory properties of HDL. Circ. Res. 2004, 95, 764–772. [Google Scholar] [CrossRef] [PubMed]

| Q1 (n = 101) | Q2 (n = 102) | Q3 (n = 101) | Q4 (n = 102) | p-Value | |
|---|---|---|---|---|---|
| Age, year (SD) | 69.2 (1.4) | 69.7 (12.9) | 66.2 (14.4) | 66.6 (12.7) | 0.15 |
| Male, % (SD) | 61 (60.4) | 62 (60.8) | 67 (66.3) | 58 (56.9) | 0.58 |
| BMI, kg/cm2 (IQR) | 23 (21–25) | 23 (21–25) | 23 (22–26) | 23 (22–26) | 0.14 |
| Initial NIHSS, score (IQR) | 14 (9–18) | 14 (10–17) | 14 (9–18) | 13 (8–17) | 0.28 |
| Time Interval from stroke onset to arrival, h (IQR) | 1.38 (0.73–3.97) | 1.29 (0.63–4.06) | 1.92 (0.96–4.87) | 1.46 (0.67–3.70) | 0.08 |
| Time Interval from arrival to groin puncture, min (IQR) | 116 (90–144) | 105 (76–141) | 104 (77–147) | 106 (81–153) | 0.64 |
| Prior stroke, % (SD) | 20 (19.8) | 26 (25.5) | 22 (21.8) | 17 (16.7) | 0.48 |
| Hypertension, % (SD) | 61 (60.4) | 61 (59.8) | 54 (53.5) | 61 (59.8) | 0.72 |
| Diabetes mellitus, % (SD) | 22 (21.8) | 22 (21.6) | 28 (27.7) | 36 (35.3) | 0.09 |
| Hyperlipidemia, % (SD) | 26 (25.7) | 17 (16.7) | 19 (18.8) | 22 (21.6) | 0.42 |
| Current smoking, % (SD) | 9 (8.9) | 3 (2.9) | 14 (13.9) | 22 (21.6) | <0.001 |
| Prior antithrombotics, % (SD) | 18 (17.8) | 25 (24.5) | 25 (24.8) | 25 (24.5) | 0.58 |
| Reperfusion therapy, % (SD) | 0.14 | ||||
| EVT only | 60 (59.4) | 44 (43.1) | 51 (50.5) | 50 (49.0) | |
| IVT + EVT | 41 (40.6) | 58 (56.9) | 50 (49.5) | 52 (51.0) | |
| ASPECTS, score (IQR) | 8 (7–9) | 8 (7–9) | 8 (7–9) | 8 (7–9) | |
| Collateral status, % (SD) | 0.75 | ||||
| Good | 58 (57.4) | 61 (59.8) | 60 (59.4) | 66 (64.70) | |
| Intermediate | 43 (42.6) | 41 (4.02) | 41 (40.6) | 36 (35.3) | |
| Medications at discharge | |||||
| Antiplatelet agents | 92 (91.1) | 84 (82.4) | 82 (81.2) | 83 (81.5) | 0.17 |
| Antihypertensive agents | 89 (88.1) | 85 (83.3) | 82 (81.2) | 80 (78.4) | 0.31 |
| Antidiabetic agents | 50 (49.5) | 44 (43.1) | 46 (45.5) | 47 (46.1) | 0.85 |
| Statin | 84 (83.2) | 78 (76.5) | 74 (73.3) | 81 (79.4) | 0.37 |
| Platelet count, ×1000/μL (SD) | 214 (23.1) | 148 (26.6) | 148 (24.0) | 148 (25.6) | 0.18 |
| Creatinine, mg/dL (SD) | 1.08 (0.69) | 1.18 (1.64) | 1.03 (0.29) | 1.11 (0.81) | 0.76 |
| LDL, mmol (SD) | 2.2 (0.8) | 2.6 (0.9) | 2.8 (0.9) | 2.7 (1.1) | <0.001 |
| Glycated hemoglobin, % (SD) | 5.9 (1.1) | 5.9 (0.9) | 6.3 (1.4) | 6.5 (1.6) | 0.002 |
| CRP, mg/dL (SD) | 9.4 (16.9) | 9.9 (12.9) | 13.4 (28.6) | 16.1 (31.1) | 0.15 |
| Initial glucose, mg/dL (SD) | 143 (62.1) | 139 (51.9) | 154 (66.3) | 154 (66.3) | 0.32 |
| SBP, mmHg (SD) | 144 (23.1) | 148 (26.6) | 148 (25.6) | 147 (24.8) | 0.47 |
| FR | END | Stroke Progression | Stroke Recurrence | SHT | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR * | 95% CI | OR * | 95% CI | OR * | 95% CI | OR * | 95% CI | OR * | 95% CI | |
| AIP in Quartiles | ||||||||||
| Q1 | reference | reference | reference | reference | reference | |||||
| Q2 | 1.48 | 0.80–2.74 | 2.96 | 1.08–8.11 | 2.78 | 0.71–10.92 | 1.52 | 0.23–10.09 | 4.87 | 0.52–37.45 |
| Q3 | 2.74 | 1.43–5.26 | 7.25 | 2.75–19.14 | 5.92 | 1.61–21.87 | 1.38 | 0.22–8.78 | 16.01 | 1.90–134.96 |
| Q4 | 4.31 | 2.16–8.61 | 9.08 | 3.48–23.67 | 6.34 | 1.73–23.27 | 4.62 | 0.80–25.02 | 11.87 | 1.43–98.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sohn, J.-H.; In, Y.-H.; Kim, C.; Sung, J.H.; Lee, M.; Kim, Y.; Lee, J.J.; Lee, S.-H. Atherogenic Index of Plasma Predicts Futile Reperfusion and Early Deterioration After Successful Recanalization: A Multicenter Study of EVT-Treated LAA Stroke. Biomedicines 2025, 13, 2127. https://doi.org/10.3390/biomedicines13092127
Sohn J-H, In Y-H, Kim C, Sung JH, Lee M, Kim Y, Lee JJ, Lee S-H. Atherogenic Index of Plasma Predicts Futile Reperfusion and Early Deterioration After Successful Recanalization: A Multicenter Study of EVT-Treated LAA Stroke. Biomedicines. 2025; 13(9):2127. https://doi.org/10.3390/biomedicines13092127
Chicago/Turabian StyleSohn, Jong-Hee, Yong-Ho In, Chulho Kim, Joo Hye Sung, Minwoo Lee, Yerim Kim, Jae Jun Lee, and Sang-Hwa Lee. 2025. "Atherogenic Index of Plasma Predicts Futile Reperfusion and Early Deterioration After Successful Recanalization: A Multicenter Study of EVT-Treated LAA Stroke" Biomedicines 13, no. 9: 2127. https://doi.org/10.3390/biomedicines13092127
APA StyleSohn, J.-H., In, Y.-H., Kim, C., Sung, J. H., Lee, M., Kim, Y., Lee, J. J., & Lee, S.-H. (2025). Atherogenic Index of Plasma Predicts Futile Reperfusion and Early Deterioration After Successful Recanalization: A Multicenter Study of EVT-Treated LAA Stroke. Biomedicines, 13(9), 2127. https://doi.org/10.3390/biomedicines13092127






