Nephroprotective Mechanisms of SGLT2i: Beyond the Glucose-Lowering Effect
Abstract
1. Introduction
| Metabolic effects | |
| Antioxidant, anti-inflammatory, and antifibrotic effects | |
| Hemodynamic effects |
2. Metabolic Effects
2.1. Reduction in Body Weight
2.2. Effects on Dyslipidemia: Reduction in LDLc and Increase in HDLc
2.3. Metabolic Reprogramming: Ketogenesis
2.4. Reduction in Plasma Uric Acid Levels
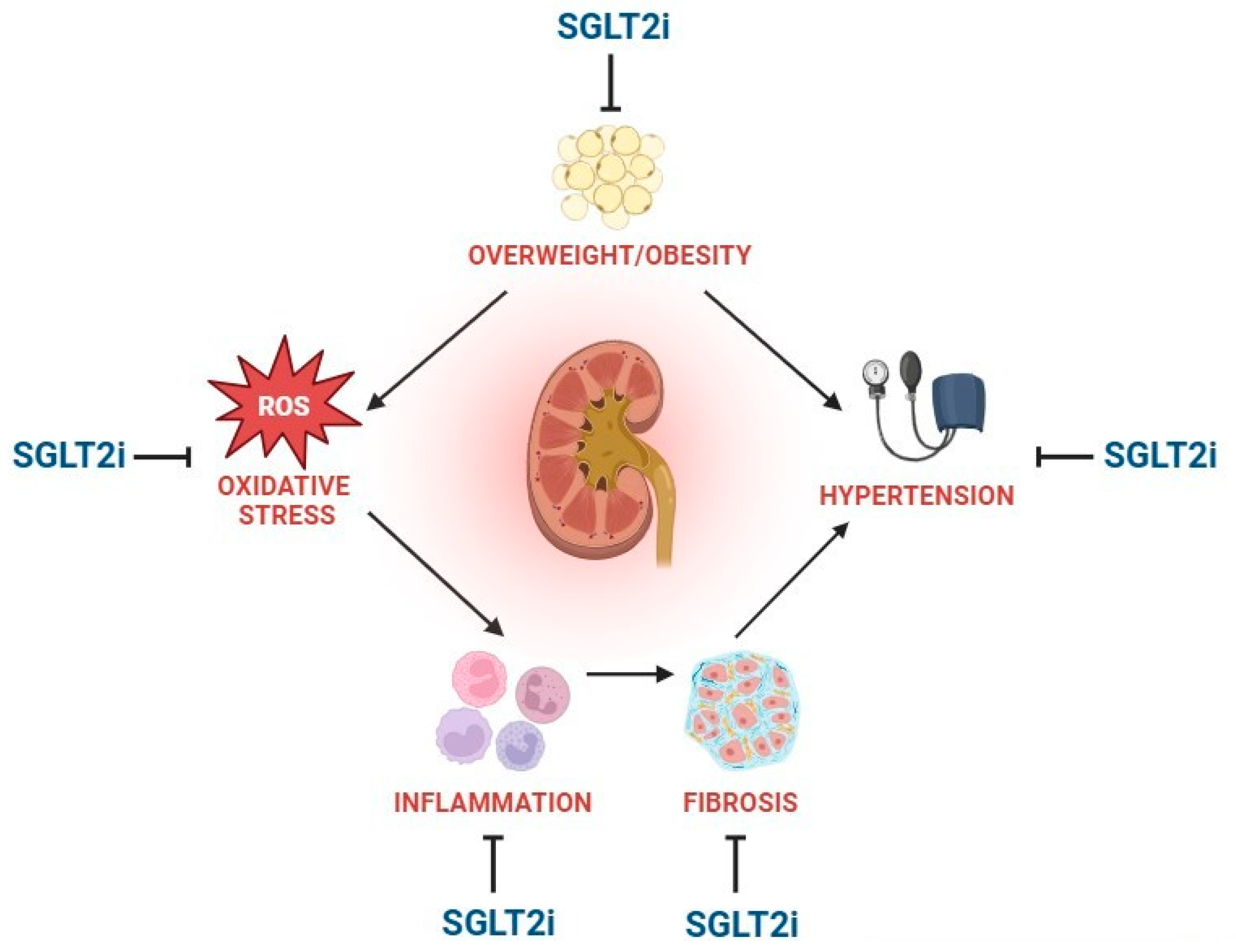
3. Antioxidant, Anti-Inflammatory, and Antifibrotic Effects
4. Hemodynamic Effects
4.1. Osmotic Diuresis and Natriuresis
4.2. Modulation of Tubuloglomerular Feedback
4.3. Neuroregulation of Blood Pressure
5. Safety Profile of SGLT2i
6. Drug Repurposing of SGLT2i for Nephroprotection
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Vallon, V.; Verma, S. Effects of SGLT2 Inhibitors on Kidney and Cardiovascular Function. Annu. Rev. Physiol. 2021, 83, 503–528. [Google Scholar] [CrossRef]
- Pop-Busui, R.; Januzzi, J.L.; Bruemmer, D.; Butalia, S.; Green, J.B.; Horton, W.B.; Knight, C.; Levi, M.; Rasouli, N.; Richardson, C.R. Heart Failure: An Underappreciated Complication of Diabetes. A Consensus Report of the American Diabetes Association. Diabetes Care 2022, 45, 1670–1690. [Google Scholar] [CrossRef]
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287. [Google Scholar] [CrossRef]
- Francis, A.; Harhay, M.N.; Ong, A.C.M.; Tummalapalli, S.L.; Ortiz, A.; Fogo, A.B.; Fliser, D.; Roy-Chaudhury, P.; Fontana, M.; Nangaku, M.; et al. Chronic kidney disease and the global public health agenda: An international consensus. Nat. Rev. Nephrol. 2024, 20, 473–485. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Matthews, D.R. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 2099. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefansson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Reeves, W.B.; Awad, A.S. Pathophysiology of diabetic kidney disease: Impact of SGLT2 inhibitors. Nat. Rev. Nephrol. 2021, 17, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Giaccari, A. Expanding the Use of SGLT2i in Diabetes Beyond Type 2. Diabetes Care 2024, 47, 50–51. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.A.; Norton, L.; DeFronzo, R.A. Efficacy and safety of SGLT2 inhibitors in the treatment of type 2 diabetes mellitus. Curr. Diab Rep. 2012, 12, 230–238. [Google Scholar] [CrossRef]
- Ferrannini, E.; Baldi, S.; Frascerra, S.; Astiarraga, B.; Heise, T.; Bizzotto, R.; Mari, A.; Pieber, T.R.; Muscelli, E. Shift to Fatty Substrate Utilization in Response to Sodium-Glucose Cotransporter 2 Inhibition in Subjects Without Diabetes and Patients With Type 2 Diabetes. Diabetes 2016, 65, 1190–1195. [Google Scholar] [CrossRef]
- Li, X.; Gao, L.; Li, X.; Xia, J.; Pan, Y.; Bai, C. Autophagy, Pyroptosis and Ferroptosis are Rising Stars in the Pathogenesis of Diabetic Nephropathy. Diabetes Metab. Syndr. Obes. 2024, 17, 1289–1299. [Google Scholar] [CrossRef]
- Szekeres, Z.; Toth, K.; Szabados, E. The Effects of SGLT2 Inhibitors on Lipid Metabolism. Metabolites 2021, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.J. Uric acid and the cardio-renal effects of SGLT2 inhibitors. Diabetes Obes. Metab. 2019, 21, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Uthman, L.; Li, X.; Baartscheer, A.; Schumacher, C.A.; Baumgart, P.; Hermanides, J.; Preckel, B.; Hollmann, M.W.; Coronel, R.; Zuurbier, C.J.; et al. Empagliflozin reduces oxidative stress through inhibition of the novel inflammation/NHE/[Na+](c)/ROS-pathway in human endothelial cells. Biomed. Pharmacother. 2022, 146, 112515. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, X.; Ilyas, I.; Zheng, X.; Luo, S.; Little, P.J.; Kamato, D.; Sahebkar, A.; Wu, W.; Weng, J.; et al. Impact of sodium glucose cotransporter 2 (SGLT2) inhibitors on atherosclerosis: From pharmacology to pre-clinical and clinical therapeutics. Theranostics 2021, 11, 4502–4515. [Google Scholar] [CrossRef]
- Afsar, B.; Hornum, M.; Afsar, R.E.; Ertuglu, L.A.; Ortiz, A.; Covic, A.; van Raalte, D.H.; Cherney, D.Z.I.; Kanbay, M. Mitochondrion-driven nephroprotective mechanisms of novel glucose lowering medications. Mitochondrion 2021, 58, 72–82. [Google Scholar] [CrossRef]
- Afsar, B.; Afsar, R.E. Sodium-glucose cotransporter inhibitors and kidney fibrosis: Review of the current evidence and related mechanisms. Pharmacol. Rep. 2023, 75, 44–68. [Google Scholar] [CrossRef] [PubMed]
- Lambers Heerspink, H.J.; de Zeeuw, D.; Wie, L.; Leslie, B.; List, J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes. Metab. 2013, 15, 853–862. [Google Scholar] [CrossRef]
- Vallon, V.; Thomson, S.C. The tubular hypothesis of nephron filtration and diabetic kidney disease. Nat. Rev. Nephrol. 2020, 16, 317–336. [Google Scholar] [CrossRef] [PubMed]
- Ahwin, P.; Martinez, D. The relationship between SGLT2 and systemic blood pressure regulation. Hypertens. Res. 2024, 47, 2094–2103. [Google Scholar] [CrossRef] [PubMed]
- Bolinder, J.; Ljunggren, O.; Johansson, L.; Wilding, J.; Langkilde, A.M.; Sjostrom, C.D.; Sugg, J.; Parikh, S. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes. Metab. 2014, 16, 159–169. [Google Scholar] [CrossRef]
- Zeng, Y.H.; Liu, S.C.; Lee, C.C.; Sun, F.J.; Liu, J.J. Effect of empagliflozin versus linagliptin on body composition in Asian patients with type 2 diabetes treated with premixed insulin. Sci. Rep. 2022, 12, 17065. [Google Scholar] [CrossRef] [PubMed]
- Schork, A.; Saynisch, J.; Vosseler, A.; Jaghutriz, B.A.; Heyne, N.; Peter, A.; Haring, H.U.; Stefan, N.; Fritsche, A.; Artunc, F. Effect of SGLT2 inhibitors on body composition, fluid status and renin-angiotensin-aldosterone system in type 2 diabetes: A prospective study using bioimpedance spectroscopy. Cardiovasc. Diabetol. 2019, 18, 46. [Google Scholar] [CrossRef] [PubMed]
- Storgaard, H.; Gluud, L.L.; Bennett, C.; Grondahl, M.F.; Christensen, M.B.; Knop, F.K.; Vilsboll, T. Benefits and Harms of Sodium-Glucose Co-Transporter 2 Inhibitors in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0166125. [Google Scholar] [CrossRef]
- Rajeev, S.P.; Cuthbertson, D.J.; Wilding, J.P. Energy balance and metabolic changes with sodium-glucose co-transporter 2 inhibition. Diabetes Obes. Metab. 2016, 18, 125–134. [Google Scholar] [CrossRef]
- Merovci, A.; Solis-Herrera, C.; Daniele, G.; Eldor, R.; Fiorentino, T.V.; Tripathy, D.; Xiong, J.; Perez, Z.; Norton, L.; Abdul-Ghani, M.A.; et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J. Clin. Investig. 2014, 124, 509–514. [Google Scholar] [CrossRef]
- Belosludtsev, K.N.; Starinets, V.S.; Belosludtsev, M.N.; Mikheeva, I.B.; Dubinin, M.V.; Belosludtseva, N.V. Chronic treatment with dapagliflozin protects against mitochondrial dysfunction in the liver of C57BL/6NCrl mice with high-fat diet/streptozotocin-induced diabetes mellitus. Mitochondrion 2021, 59, 246–254. [Google Scholar] [CrossRef]
- Xu, L.; Nagata, N.; Nagashimada, M.; Zhuge, F.; Ni, Y.; Chen, G.; Mayoux, E.; Kaneko, S.; Ota, T. SGLT2 Inhibition by Empagliflozin Promotes Fat Utilization and Browning and Attenuates Inflammation and Insulin Resistance by Polarizing M2 Macrophages in Diet-induced Obese Mice. EBioMedicine 2017, 20, 137–149. [Google Scholar] [CrossRef]
- Han, J.H.; Oh, T.J.; Lee, G.; Maeng, H.J.; Lee, D.H.; Kim, K.M.; Choi, S.H.; Jang, H.C.; Lee, H.S.; Park, K.S.; et al. The beneficial effects of empagliflozin, an SGLT2 inhibitor, on atherosclerosis in ApoE−/− mice fed a western diet. Diabetologia 2017, 60, 364–376. [Google Scholar] [CrossRef]
- Costa, J.; Moreira, A.; Moreira, P.; Delgado, L.; Silva, D. Effects of weight changes in the autonomic nervous system: A systematic review and meta-analysis. Clin. Nutr. 2019, 38, 110–126. [Google Scholar] [CrossRef]
- Onishi, T.; Shioda, Y.; Mori, Y. 1141-P: Effects of SGLT2 Inhibitors on Nighttime Sympathetic Nerve Activity, Heart Rate, Blood Pressure Variability, and Glycemic Variability in Type 2 Diabetic Patients. Diabetes 2020, 69, 1141-P. [Google Scholar] [CrossRef]
- Nishiyama, A.; Kitada, K. Possible renoprotective mechanisms of SGLT2 inhibitors. Front. Med. 2023, 10, 1115413. [Google Scholar] [CrossRef] [PubMed]
- Kogot-Levin, A.; Riahi, Y.; Abramovich, I.; Mosenzon, O.; Agranovich, B.; Kadosh, L.; Ben-Haroush Schyr, R.; Kleiman, D.; Hinden, L.; Cerasi, E.; et al. Mapping the metabolic reprogramming induced by sodium-glucose cotransporter 2 inhibition. JCI Insight 2023, 8, e164296. [Google Scholar] [CrossRef]
- Cai, T.; Ke, Q.; Fang, Y.; Wen, P.; Chen, H.; Yuan, Q.; Luo, J.; Zhang, Y.; Sun, Q.; Lv, Y.; et al. Sodium-glucose cotransporter 2 inhibition suppresses HIF-1alpha-mediated metabolic switch from lipid oxidation to glycolysis in kidney tubule cells of diabetic mice. Cell Death Dis. 2020, 11, 390. [Google Scholar] [CrossRef]
- Corral, P.; Nardelli, N.; Elbert, A.; Aranguren, F.; Schreier, L. Impact of SGLT2 Inhibitors on Lipoproteins in Type 2 Diabetes. Curr. Diab Rep. 2025, 25, 16. [Google Scholar] [CrossRef] [PubMed]
- Cahill, G.F., Jr. Fuel metabolism in starvation. Annu. Rev. Nutr. 2006, 26, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Defronzo, R.A. Banting Lecture. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009, 58, 773–795. [Google Scholar] [CrossRef]
- Garcia, E.; Shalaurova, I.; Matyus, S.P.; Oskardmay, D.N.; Otvos, J.D.; Dullaart, R.P.F.; Connelly, M.A. Ketone Bodies Are Mildly Elevated in Subjects with Type 2 Diabetes Mellitus and Are Inversely Associated with Insulin Resistance as Measured by the Lipoprotein Insulin Resistance Index. J. Clin. Med. 2020, 9, 321. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Q.; Jia, Z.; Guo, B. Ketone bodies in exercise, health and disease: Metabolic mechanisms, pathophysiology, and therapeutic implications. Adv. Exerc. Health Sci. 2025, 2, 83–93. [Google Scholar] [CrossRef]
- Gao, Y.M.; Feng, S.T.; Wen, Y.; Tang, T.T.; Wang, B.; Liu, B.C. Cardiorenal protection of SGLT2 inhibitors-Perspectives from metabolic reprogramming. EBioMedicine 2022, 83, 104215. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.J.; Schell, J.R.; Chocron, E.S.; Varmazyad, M.; Xu, G.; Chen, W.H.; Martinez, G.M.; Dong, F.F.; Sreenivas, P.; Trevino, R., Jr.; et al. Ketogenic diet induces p53-dependent cellular senescence in multiple organs. Sci. Adv. 2024, 10, eado1463. [Google Scholar] [CrossRef] [PubMed]
- Grodin, J.L.; Tang, W.H.W. Sodium-Glucose Cotransporter-2 Inhibitors and Loop Diuretics for Heart Failure: Priming the Natriuretic and Metabolic Reserve of the Kidney. Circulation 2020, 142, 1055–1058. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, L.; Tian, D.; Xia, P.; Zheng, H.; Wang, L.; Chen, L. Effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors on serum uric acid level: A meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 2018, 20, 458–462. [Google Scholar] [CrossRef]
- Feig, D.I.; Kang, D.H.; Johnson, R.J. Uric acid and cardiovascular risk. N. Engl. J. Med. 2008, 359, 1811–1821. [Google Scholar] [CrossRef]
- Inzucchi, S.E.; Zinman, B.; Fitchett, D.; Wanner, C.; Ferrannini, E.; Schumacher, M.; Schmoor, C.; Ohneberg, K.; Johansen, O.E.; George, J.T.; et al. How Does Empagliflozin Reduce Cardiovascular Mortality? Insights From a Mediation Analysis of the EMPA-REG OUTCOME Trial. Diabetes Care 2018, 41, 356–363. [Google Scholar] [CrossRef]
- Bonnet, F.; Scheen, A.J. Effects of SGLT2 inhibitors on systemic and tissue low-grade inflammation: The potential contribution to diabetes complications and cardiovascular disease. Diabetes Metab. 2018, 44, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Satirapoj, B. Sodium-Glucose Cotransporter 2 Inhibitors with Renoprotective Effects. Kidney Dis. 2017, 3, 24–32. [Google Scholar] [CrossRef]
- Ojima, A.; Matsui, T.; Nishino, Y.; Nakamura, N.; Yamagishi, S. Empagliflozin, an Inhibitor of Sodium-Glucose Cotransporter 2 Exerts Anti-Inflammatory and Antifibrotic Effects on Experimental Diabetic Nephropathy Partly by Suppressing AGEs-Receptor Axis. Horm. Metab. Res. 2015, 47, 686–692. [Google Scholar] [CrossRef]
- Yao, D.; Wang, S.; Wang, M.; Lu, W. Renoprotection of dapagliflozin in human renal proximal tubular cells via the inhibition of the high mobility group box 1-receptor for advanced glycation end products-nuclear factor-kappaB signaling pathway. Mol. Med. Rep. 2018, 18, 3625–3630. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Bussalino, E.; Maccio, L.; Verzola, D.; Saio, M.; Esposito, P.; Leoncini, G.; Pontremoli, R.; Viazzi, F. Non-Haemodynamic Mechanisms Underlying Hypertension-Associated Damage in Target Kidney Components. Int. J. Mol. Sci. 2023, 24, 9422. [Google Scholar] [CrossRef] [PubMed]
- Iordan, L.; Gaita, L.; Timar, R.; Avram, V.; Sturza, A.; Timar, B. The Renoprotective Mechanisms of Sodium-Glucose Cotransporter-2 Inhibitors (SGLT2i)-A Narrative Review. Int. J. Mol. Sci. 2024, 25, 7057. [Google Scholar] [CrossRef]
- Packer, M. Interplay of adenosine monophosphate-activated protein kinase/sirtuin-1 activation and sodium influx inhibition mediates the renal benefits of sodium-glucose co-transporter-2 inhibitors in type 2 diabetes: A novel conceptual framework. Diabetes Obes. Metab. 2020, 22, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Karg, M.V.; Bosch, A.; Kannenkeril, D.; Striepe, K.; Ott, C.; Schneider, M.P.; Boemke-Zelch, F.; Linz, P.; Nagel, A.M.; Titze, J.; et al. SGLT-2-inhibition with dapagliflozin reduces tissue sodium content: A randomised controlled trial. Cardiovasc. Diabetol. 2018, 17, 5. [Google Scholar] [CrossRef]
- Schneider, M.P.; Raff, U.; Kopp, C.; Scheppach, J.B.; Toncar, S.; Wanner, C.; Schlieper, G.; Saritas, T.; Floege, J.; Schmid, M.; et al. Skin Sodium Concentration Correlates with Left Ventricular Hypertrophy in CKD. J. Am. Soc. Nephrol. 2017, 28, 1867–1876. [Google Scholar] [CrossRef]
- Hallow, K.M.; Helmlinger, G.; Greasley, P.J.; McMurray, J.J.V.; Boulton, D.W. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes. Metab. 2018, 20, 479–487. [Google Scholar] [CrossRef]
- Verma, S.; McMurray, J.J.V. SGLT2 inhibitors and mechanisms of cardiovascular benefit: A state-of-the-art review. Diabetologia 2018, 61, 2108–2117. [Google Scholar] [CrossRef]
- Kohler, S.; Zeller, C.; Iliev, H.; Kaspers, S. Safety and Tolerability of Empagliflozin in Patients with Type 2 Diabetes: Pooled Analysis of Phase I-III Clinical Trials. Adv. Ther. 2017, 34, 1707–1726. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Kober, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Belohlavek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Zaccardi, F.; Webb, D.R.; Htike, Z.Z.; Youssef, D.; Khunti, K.; Davies, M.J. Efficacy and safety of sodium-glucose co-transporter-2 inhibitors in type 2 diabetes mellitus: Systematic review and network meta-analysis. Diabetes Obes. Metab. 2016, 18, 783–794. [Google Scholar] [CrossRef]
- Mazidi, M.; Rezaie, P.; Gao, H.K.; Kengne, A.P. Effect of Sodium-Glucose Cotransport-2 Inhibitors on Blood Pressure in People With Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of 43 Randomized Control Trials With 22 528 Patients. J. Am. Heart Assoc. 2017, 6, e004007. [Google Scholar] [CrossRef] [PubMed]
- Vestri, S.; Okamoto, M.M.; de Freitas, H.S.; Aparecida Dos Santos, R.; Nunes, M.T.; Morimatsu, M.; Heimann, J.C.; Machado, U.F. Changes in sodium or glucose filtration rate modulate expression of glucose transporters in renal proximal tubular cells of rat. J. Membr. Biol. 2001, 182, 105–112. [Google Scholar] [CrossRef]
- Vallon, V.; Richter, K.; Blantz, R.C.; Thomson, S.; Osswald, H. Glomerular hyperfiltration in experimental diabetes mellitus: Potential role of tubular reabsorption. J. Am. Soc. Nephrol. 1999, 10, 2569–2576. [Google Scholar] [CrossRef]
- Zatz, R.; Dunn, B.R.; Meyer, T.W.; Anderson, S.; Rennke, H.G.; Brenner, B.M. Prevention of diabetic glomerulopathy by pharmacological amelioration of glomerular capillary hypertension. J. Clin. Investig. 1986, 77, 1925–1930. [Google Scholar] [CrossRef] [PubMed]
- Thomson, S.C.; Rieg, T.; Miracle, C.; Mansoury, H.; Whaley, J.; Vallon, V.; Singh, P. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R75–R83. [Google Scholar] [CrossRef]
- Bailey, C.J.; Day, C.; Bellary, S. Renal Protection with SGLT2 Inhibitors: Effects in Acute and Chronic Kidney Disease. Curr. Diab Rep. 2022, 22, 39–52. [Google Scholar] [CrossRef]
- Sen, T.; Heerspink, H.J.L. A kidney perspective on the mechanism of action of sodium glucose co-transporter 2 inhibitors. Cell Metab. 2021, 33, 732–739. [Google Scholar] [CrossRef]
- Heerspink, H.J.; Perkins, B.A.; Fitchett, D.H.; Husain, M.; Cherney, D.Z. Sodium Glucose Cotransporter 2 Inhibitors in the Treatment of Diabetes Mellitus: Cardiovascular and Kidney Effects, Potential Mechanisms, and Clinical Applications. Circulation 2016, 134, 752–772. [Google Scholar] [CrossRef]
- Bae, J.H.; Park, E.G.; Kim, S.; Kim, S.G.; Hahn, S.; Kim, N.H. Effects of Sodium-Glucose Cotransporter 2 Inhibitors on Renal Outcomes in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Sci. Rep. 2019, 9, 13009. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Kim, N.H. Renoprotective Mechanism of Sodium-Glucose Cotransporter 2 Inhibitors: Focusing on Renal Hemodynamics. Diabetes Metab. J. 2022, 46, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Holtkamp, F.A.; de Zeeuw, D.; Thomas, M.C.; Cooper, M.E.; de Graeff, P.A.; Hillege, H.J.; Parving, H.H.; Brenner, B.M.; Shahinfar, S.; Lambers Heerspink, H.J. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int. 2011, 80, 282–287. [Google Scholar] [CrossRef]
- Scheen, A.J. An update on the safety of SGLT2 inhibitors. Expert. Opin. Drug Saf. 2019, 18, 295–311. [Google Scholar] [CrossRef]
- Lim, K.; Jackson, K.L.; Sata, Y.; Head, G.A. Factors Responsible for Obesity-Related Hypertension. Curr. Hypertens. Rep. 2017, 19, 53. [Google Scholar] [CrossRef]
- Kaur, J.; Young, B.E.; Fadel, P.J. Sympathetic Overactivity in Chronic Kidney Disease: Consequences and Mechanisms. Int. J. Mol. Sci. 2017, 18, 1682. [Google Scholar] [CrossRef]
- Matthews, V.B.; Elliot, R.H.; Rudnicka, C.; Hricova, J.; Herat, L.; Schlaich, M.P. Role of the sympathetic nervous system in regulation of the sodium glucose cotransporter 2. J. Hypertens. 2017, 35, 2059–2068. [Google Scholar] [CrossRef]
- Elliott, R.H.; Matthews, V.B.; Rudnicka, C.; Schlaich, M.P. Is it time to think about the sodium glucose co-transporter 2 sympathetically? Nephrology 2016, 21, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Briasoulis, A.; Al Dhaybi, O.; Bakris, G.L. SGLT2 Inhibitors and Mechanisms of Hypertension. Curr. Cardiol. Rep. 2018, 20, 1. [Google Scholar] [CrossRef]
- Vasilakou, D.; Karagiannis, T.; Athanasiadou, E.; Mainou, M.; Liakos, A.; Bekiari, E.; Sarigianni, M.; Matthews, D.R.; Tsapas, A. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: A systematic review and meta-analysis. Ann. Intern. Med. 2013, 159, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Mascolo, A.; Di Napoli, R.; Balzano, N.; Cappetta, D.; Urbanek, K.; De Angelis, A.; Scisciola, L.; Di Meo, I.; Sullo, M.G.; Rafaniello, C.; et al. Safety profile of sodium glucose co-transporter 2 (SGLT2) inhibitors: A brief summary. Front. Cardiovasc. Med. 2022, 9, 1010693. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes, C.K.D.W.G. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Gong, C.; Shen, S.C.; Zhang, K.; Zhou, L.; Shen, J.J.; Zhao, J.Y.; Ding, S.G.; Ma, L.K.; Gao, H. Association of sodium-glucose cotransporter 2 inhibitors with cardiovascular outcome and safety events: A meta-analysis of randomized controlled clinical trials. Front. Cardiovasc. Med. 2022, 9, 926979. [Google Scholar] [CrossRef]
- Cianciolo, G.; De Pascalis, A.; Capelli, I.; Gasperoni, L.; Di Lullo, L.; Bellasi, A.; La Manna, G. Mineral and Electrolyte Disorders With SGLT2i Therapy. J. Bone Miner. Res. Plus 2019, 3, e10242. [Google Scholar] [CrossRef] [PubMed]
- Tsimihodimos, V.; Filippatos, T.D.; Elisaf, M.S. Effects of sodium-glucose co-transporter 2 inhibitors on metabolism: Unanswered questions and controversies. Expert. Opin. Drug Metab. Toxicol. 2017, 13, 399–408. [Google Scholar] [CrossRef]
- Yavin, Y.; Mansfield, T.A.; Ptaszynska, A.; Johnsson, K.; Parikh, S.; Johnsson, E. Effect of the SGLT2 Inhibitor Dapagliflozin on Potassium Levels in Patients with Type 2 Diabetes Mellitus: A Pooled Analysis. Diabetes Ther. 2016, 7, 125–137. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, X.; Zhang, J.; Li, Y.; Del Gobbo, L.C.; Zhai, S.; Song, Y. Elevated serum magnesium associated with SGLT2 inhibitor use in type 2 diabetes patients: A meta-analysis of randomised controlled trials. Diabetologia 2016, 59, 2546–2551. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.; Del Prato, S.; Chilton, R.; DeFronzo, R.A. SGLT2 Inhibitors and Cardiovascular Risk: Lessons Learned From the EMPA-REG OUTCOME Study. Diabetes Care 2016, 39, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Weir, M.R.; Kline, I.; Xie, J.; Edwards, R.; Usiskin, K. Effect of canagliflozin on serum electrolytes in patients with type 2 diabetes in relation to estimated glomerular filtration rate (eGFR). Curr. Med. Res. Opin. 2014, 30, 1759–1768. [Google Scholar] [CrossRef]
- Tuttle, K.R.; Levin, A.; Nangaku, M.; Kadowaki, T.; Agarwal, R.; Hauske, S.J.; Elsasser, A.; Ritter, I.; Steubl, D.; Wanner, C.; et al. Safety of Empagliflozin in Patients With Type 2 Diabetes and Chronic Kidney Disease: Pooled Analysis of Placebo-Controlled Clinical Trials. Diabetes Care 2022, 45, 1445–1452. [Google Scholar] [CrossRef]
- Fioretto, P.; Del Prato, S.; Buse, J.B.; Goldenberg, R.; Giorgino, F.; Reyner, D.; Langkilde, A.M.; Sjostrom, C.D.; Sartipy, P.; Investigators, D.S. Efficacy and safety of dapagliflozin in patients with type 2 diabetes and moderate renal impairment (chronic kidney disease stage 3A): The DERIVE Study. Diabetes Obes. Metab. 2018, 20, 2532–2540. [Google Scholar] [CrossRef]
- Cianciolo, G.; De Pascalis, A.; Gasperoni, L.; Tondolo, F.; Zappulo, F.; Capelli, I.; Cappuccilli, M.; La Manna, G. The Off-Target Effects, Electrolyte and Mineral Disorders of SGLT2i. Molecules 2020, 25, 2757. [Google Scholar] [CrossRef]
- Jarman, P.R.; Mather, H.M. Diabetes may be independent risk factor for hyperkalaemia. BMJ 2003, 327, 812. [Google Scholar] [CrossRef]
- Nair, A.V.; Hocher, B.; Verkaart, S.; van Zeeland, F.; Pfab, T.; Slowinski, T.; Chen, Y.P.; Schlingmann, K.P.; Schaller, A.; Gallati, S.; et al. Loss of insulin-induced activation of TRPM6 magnesium channels results in impaired glucose tolerance during pregnancy. Proc. Natl. Acad. Sci. USA 2012, 109, 11324–11329. [Google Scholar] [CrossRef] [PubMed]
- Lacson, E., Jr.; Wang, W.; Ma, L.; Passlick-Deetjen, J. Serum Magnesium and Mortality in Hemodialysis Patients in the United States: A Cohort Study. Am. J. Kidney Dis. 2015, 66, 1056–1066. [Google Scholar] [CrossRef]
- Del Gobbo, L.C.; Imamura, F.; Wu, J.H.; de Oliveira Otto, M.C.; Chiuve, S.E.; Mozaffarian, D. Circulating and dietary magnesium and risk of cardiovascular disease: A systematic review and meta-analysis of prospective studies. Am. J. Clin. Nutr. 2013, 98, 160–173. [Google Scholar] [CrossRef]
- Abdelsayed, M.; Kort, E.J.; Jovinge, S.; Mercola, M. Repurposing drugs to treat cardiovascular disease in the era of precision medicine. Nat. Rev. Cardiol. 2022, 19, 751–764. [Google Scholar] [CrossRef]
- Bakris, G.L. Major Advancements in Slowing Diabetic Kidney Disease Progression: Focus on SGLT2 Inhibitors. Am. J. Kidney Dis. 2019, 74, 573–575. [Google Scholar] [CrossRef]
- Naaman, S.C.; Bakris, G.L. Diabetic Nephropathy: Update on Pillars of Therapy Slowing Progression. Diabetes Care 2023, 46, 1574–1586. [Google Scholar] [CrossRef] [PubMed]
- The, E.-K.C.G.; Herrington, W.G.; Staplin, N.; Wanner, C.; Green, J.B.; Hauske, S.J.; Emberson, J.R.; Preiss, D.; Judge, P.; Mayne, K.J.; et al. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar] [CrossRef]
- Eli, L. US FDA Approves Jardiance® for the Treatment of Adults with Chronic Kidney Disease.pdf. Available online: https://investor.lilly.com/news-releases/news-release-details/us-fda-approves-jardiancer-treatment-adults-chronic-kidney (accessed on 25 August 2025).
- American Diabetes Association Professional Practice, C. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2024. Diabetes Care 2024, 47, S20–S42. [Google Scholar] [CrossRef]
- Baigent, C.; Emberson, J.; Haynes, R.; Herrington, W.G.; Judge, P.; Landray, M.J.; Mayne, K.J.; Ng, S.Y.; Preiss, D.; Roddick, A.J.; et al. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: Collaborative meta-analysis of large placebo-controlled trials. Lancet 2022, 400, 1788–1801. [Google Scholar] [CrossRef]
- Kurki, S.; Halla-Aho, V.; Haussmann, M.; Lahdesmaki, H.; Leinonen, J.V.; Koskinen, M. A comparative study of clinical trial and real-world data in patients with diabetic kidney disease. Sci. Rep. 2024, 14, 1731. [Google Scholar] [CrossRef]
- Feng, Q.; Wu, M.; Mai, Z. Emerging horizons: Clinical applications and multifaceted benefits of SGLT-2 inhibitors beyond diabetes. Front. Cardiovasc. Med. 2025, 12, 1482918. [Google Scholar] [CrossRef]
- Gajewska, A.; Wasiak, J.; Sapeda, N.; Mlynarska, E.; Rysz, J.; Franczyk, B. SGLT2 Inhibitors in Kidney Diseases-A Narrative Review. Int. J. Mol. Sci. 2024, 25, 4959. [Google Scholar] [CrossRef]
- Vallon, V. Renoprotective Effects of SGLT2 Inhibitors. Heart Fail. Clin. 2022, 18, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Mazzieri, A.; Basta, G.; Calafiore, R.; Luca, G. GLP-1 RAs and SGLT2i: Two antidiabetic agents associated with immune and inflammation modulatory properties through the common AMPK pathway. Front. Immunol. 2023, 14, 1163288. [Google Scholar] [CrossRef] [PubMed]
- Marcon, L.M.R.; Mazzieri, A. Anti-Inflammatory and Anti-Oxidative Effects of GLP1-RAs and SGLT2i: The Guiding Star Towards Cardiovascular Protection in Type 2 Diabetes. Immuno 2025, 5, 11. [Google Scholar] [CrossRef]
- Frak, W.; Hajdys, J.; Radzioch, E.; Szlagor, M.; Mlynarska, E.; Rysz, J.; Franczyk, B. Cardiovascular Diseases: Therapeutic Potential of SGLT-2 Inhibitors. Biomedicines 2023, 11, 2085. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- Mariani, M.V.; Lavalle, C.; Palombi, M.; Pierucci, N.; Trivigno, S.; D’Amato, A.; Filomena, D.; Cipollone, P.; Laviola, D.; Piro, A.; et al. SGLT2i reduce arrhythmic events in heart failure patients with cardiac implantable electronic devices. ESC Heart Fail. 2025, 12, 2125–2133. [Google Scholar] [CrossRef]
- Milder, T.Y.; Stocker, S.L.; Day, R.O.; Greenfield, J.R. Potential Safety Issues with Use of Sodium-Glucose Cotransporter 2 Inhibitors, Particularly in People with Type 2 Diabetes and Chronic Kidney Disease. Drug Saf. 2020, 43, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Zhou, B.; Xu, G. SGLT2 inhibitors: A novel choice for the combination therapy in diabetic kidney disease. Cardiovasc. Diabetol. 2017, 16, 65. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.R.; Elkhatib, M.; El-Khashab, S.; Darwish, R.A.; Fayed, A.; Abdelaziz, T.S.; Hammad, H.; Ahmed, R.M.; Maamoun, H.A. Dual-faced guardians: SGLT2 inhibitors’ kidney protection and health challenges: A position statement by Kasralainy nephrology group (KANG). Diabetol. Metab. Syndr. 2025, 17, 214. [Google Scholar] [CrossRef] [PubMed]
- Alcantar-Vallin, L.; Zaragoza, J.J.; Diaz-Villavicencio, B.; Hernandez-Morales, K.; Camacho-Guerrero, J.R.; Perez-Venegas, M.A.; Carmona-Morales, E.J.; Oseguera-Gonzalez, A.N.; Murguia-Soto, C.; Chavez-Alonso, G.; et al. SGLT2i treatment during AKI and its association with major adverse kidney events. Front. Pharmacol. 2024, 15, 1356991. [Google Scholar] [CrossRef]
- Cannarella, R.; Rubulotta, M.; Cannarella, V.; La Vignera, S.; Calogero, A.E. A holistic view of SGLT2 inhibitors: From cardio-renal management to cognitive and andrological aspects. Eur. J. Intern. Med. 2025, 138, 6–28. [Google Scholar] [CrossRef]
| RCT, Year | Intervention | Kidney Outcome | Findings | Reference |
|---|---|---|---|---|
| CREDENCE, 2019 | Canagliflozin vs. Placebo | ESRD, doubling of the serum creatinine level, or death from renal or cardiovascular causes | Risk reduction of 30% | [61] |
| DAPA-CKD, 2020 | Dapagliflozin vs. Placebo | Sustained ≥ 50% reduction in eGFR, ESRD, or death from renal or cardiovascular cause | Risk reduction of 39% | [8] |
| EMPA-KIDNEY, 2023 | Empagliflozin vs. Placebo | ESRD, a sustained reduction in eGFR to <10 mL/min/1.73 m2, renal death, or a sustained decline ≥ 40% in eGFR | Risk reduction of 28% | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzieri, A.; Marcon, L.M.R. Nephroprotective Mechanisms of SGLT2i: Beyond the Glucose-Lowering Effect. Biomedicines 2025, 13, 2123. https://doi.org/10.3390/biomedicines13092123
Mazzieri A, Marcon LMR. Nephroprotective Mechanisms of SGLT2i: Beyond the Glucose-Lowering Effect. Biomedicines. 2025; 13(9):2123. https://doi.org/10.3390/biomedicines13092123
Chicago/Turabian StyleMazzieri, Alessio, and Livia Maria Rita Marcon. 2025. "Nephroprotective Mechanisms of SGLT2i: Beyond the Glucose-Lowering Effect" Biomedicines 13, no. 9: 2123. https://doi.org/10.3390/biomedicines13092123
APA StyleMazzieri, A., & Marcon, L. M. R. (2025). Nephroprotective Mechanisms of SGLT2i: Beyond the Glucose-Lowering Effect. Biomedicines, 13(9), 2123. https://doi.org/10.3390/biomedicines13092123







