Behavioral Phenotyping of WAG/Rij Rat Model of Absence Epilepsy: The Link to Anxiety and Sex Factors
Abstract
1. Introduction
- Neurobehavioral domains: Anxiety (the EPM test), anhedonia (sucrose preference), social function (preference, recognition, dominance), and associative learning (fear conditioning).
- Multidimensional metrics: Cognition, motor function, and exploration strategies derived from behavioral tests, with anxiety prioritized as a key influencing factor of other domains.
2. Materials and Methods
2.1. Animals
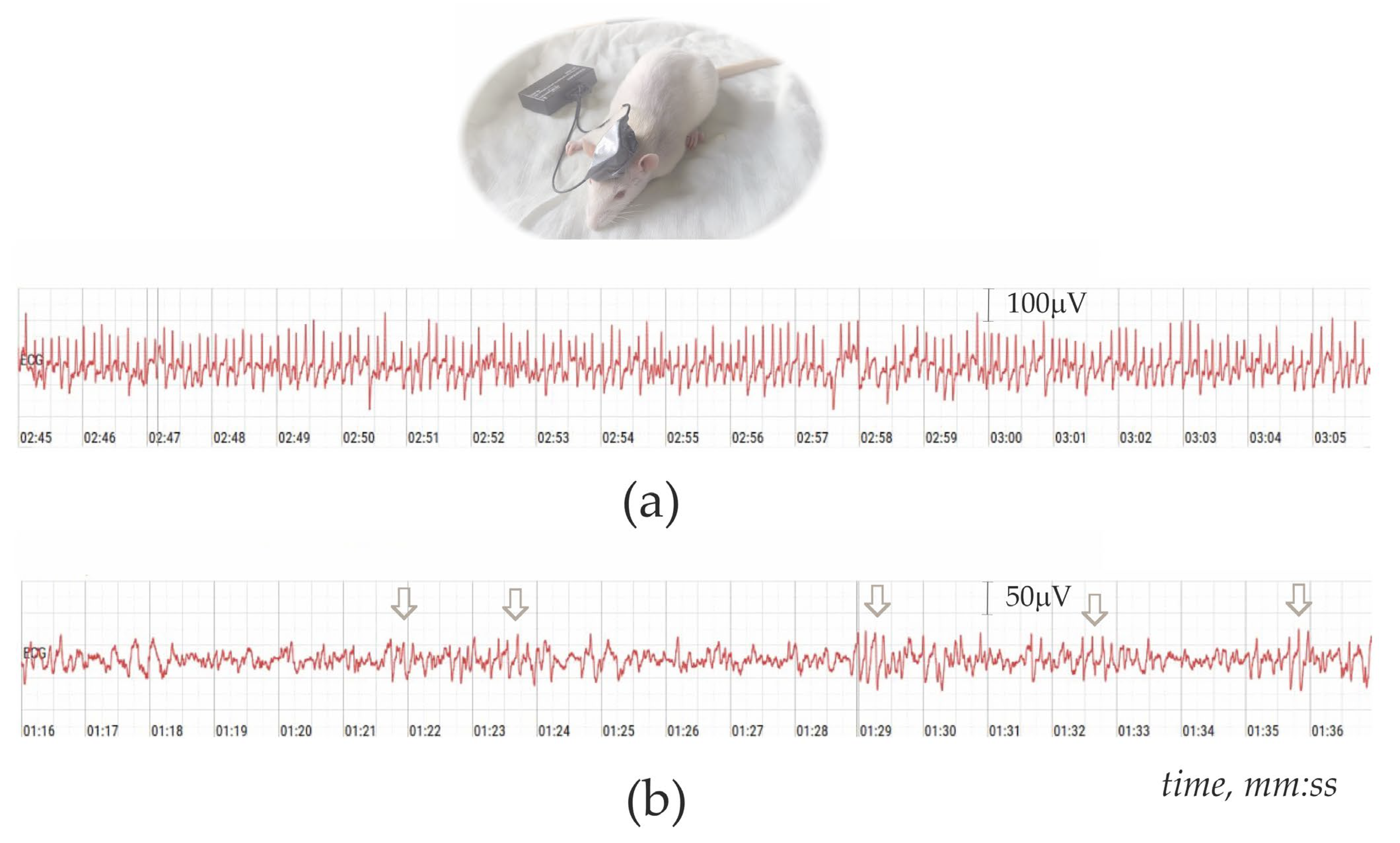
2.2. Non-Invasive Electroencephalography (EEG)-Based Diagnosing of Absence Epilepsy
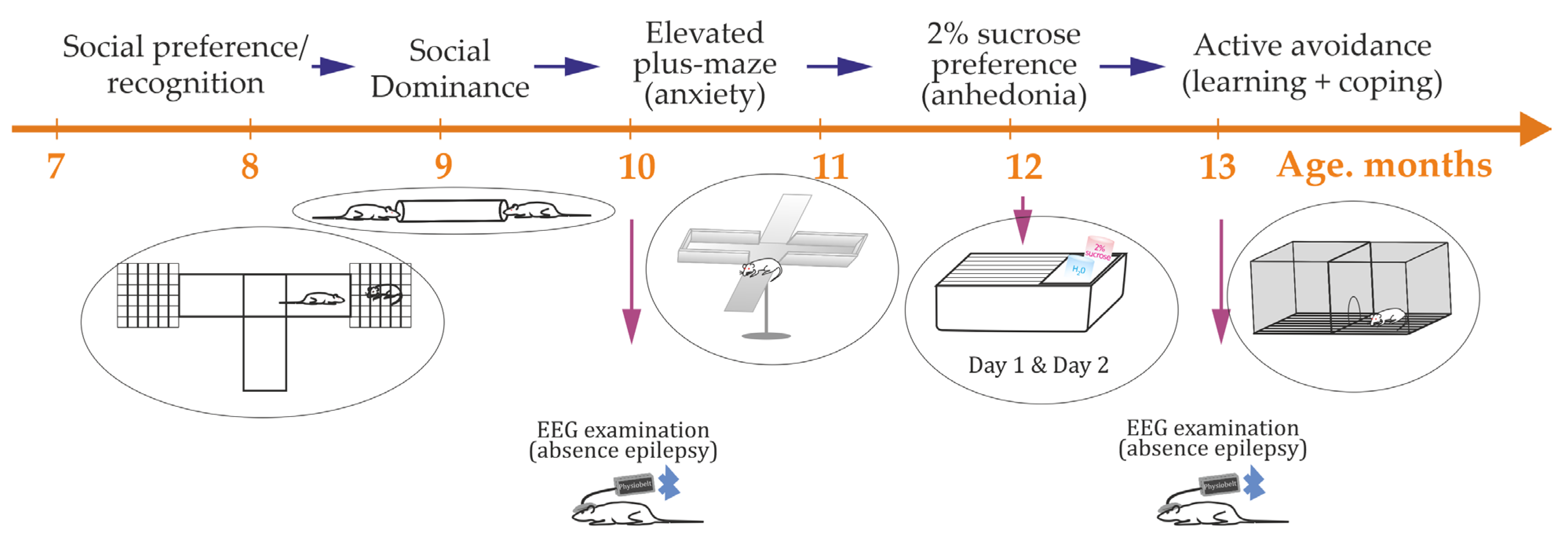
2.3. Behavioral Phenotyping Battery (Ordered to Minimize Stress Interference)
2.3.1. Social Preference and Recognition Test (Three-Chamber)
2.3.2. Social Dominance Test (Tube Test)
- A rat that maintained its position within the tube—Dominant (DS = 2 points);
- A rat that partially retreated, but subsequently re-engaged—Partial Submissive (DS = 1 point)
- A rat that fully withdraws from the tube—Complete Submissive (DS = 0 points).
2.3.3. The Elevated Plus Maze (EPM) Test for Anxiety
- Time spent in open arms (TOA in s)—primary indicator of anxiety.
- Defecation (fecal boli) and urination frequency (Nboli and Nuri)—physiological stress markers.
- Duration of self-grooming (Tgrooming in s)—displacement behavior under stressExploratory behaviors:
- The number of rearings (both wall-supported and unsupported, Nrearing)—vertical exploration.
- The number of head dips over open arm edges (Nheaddips)—risk assessment behavior.
2.3.4. Sucrose Preference Test
- Day 1 Water (left), sucrose (right).
- Day 2: Reweighed bottles with positions switched (water right, sucrose left).
2.3.5. Active Avoidance Fear Conditioning (Shuttle Box)
- The number of fails before 1st successful Avoidance indicating initial learning latency.
- The number of fails before 2nd successful Avoidance indicating consistency of initial learning.
- The number of trials needed to reach the learning criterion, i.e., 5 avoidance responses within a sequence of 6 consecutive trials. It indicated acquisition speed (Trials to criterion).
- The total number of avoidances (out of 50 trials, Total avoidances).
- The number of rearings (both wall-supported and unsupported, Nrearing)—vertical exploration
- The number of head dips over open arm edges (Nheaddips)—risk assessment behavior
2.3.6. Integrated Multidimensional Profiling Metrics
Domain-Specific Composite Metrics
Cross-Domain Composite Metrics
2.3.7. Statistical Analysis
3. Results
3.1. Direct Measures of Behavior in the EPM
- Time spent in open arms (a primary indicator of anxiety, Figure S1a):
- ○
- Epileptic males spent 16.4 s [0–36.6].
- ○
- Non-epileptic males spent 5.2 s [0–17.7].
- ○
- Epileptic females spent 20.6 s [0–49.8].
- ○
- Non-epileptic females spent 11.1 s [0–26.7].
- Duration of self-grooming, s (an indicator of displacement behavior under stress, Figure S1b):
- ○
- Epileptic males self-groomed for 57.9 s [34.2–97.6].
- ○
- Non-epileptic males—for 78.1 s [47.0–126.7].
- ○
- Epileptic females—for 61.7 s [21.0–94.6].
- ○
- Non-epileptic females—for 51.5 s [26.2–68.3].
- The number of head dips (an indicator of exploratory behavior):
- ○
- Epileptic males had 8.5 head dips [4.0–13.0].
- ○
- Non-epileptic males—6.0 head dips [4.0–9.0].
- ○
- Epileptic females—10.5 head dips [7.0–10.0].
- ○
- Non-epileptic females—8.5 head dips [6.5–13.5].
- The number of rearings (an indicator of risk assessment behavior):
- ○
- Epileptic males displayed 14.0 rearings [11.0–20.0].
- ○
- Non-epileptic males—15.0 [14.0–20.0].
- ○
- Epileptic females—15.0 [12.0–17.5].
- ○
- Non-epileptic females—18.5 [14.0–26.0].
- Low anxiety in 13 rats (11%) with median TOA = 5.2 s [0–21.4] and median Tgrooming = 46.8 s [25.5–68.5].
- Self-grooming strategy in 25 rats (21%) with median TOA = 21 s [0–29.5] and median Tgrooming 154 s [126.7–184].
- High anxiety in 81 rats (68%) with median TOA = 84 s [79–107] and median Tgrooming = 44 s [68–78].
3.2. Domain-Specific Composite Metrics
- Cognition. The median score for epileptic males was 29.0 [24.3–32.0], indicating a relatively high level of cognitive flexibility. The scores for non-epileptic males (27.3 [19.3–30.8]), epileptic females (35.0 [20.7–38.5]), and non-epileptic females (27.1 [26.5–33.2]) showed variability, but generally aligned with the high score indicating intact cognitive flexibility.
- Anxiety. The lowest median score was observed in non-epileptic males (0.69 [0.27–1.29]), followed by non-epileptic females (1.02 [0.37–2.55]), epileptic females (1.05 [1.00–2.86]), and epileptic males (1.47 [0.34–4.38]). The higher score of composite Anxiety metric for epileptic subjects suggested slightly higher anxiety levels, but overall, anxiety levels were low across all groups.
- Motor. The high median score for non-epileptic males (1.258 [0.845–1.322]) suggested strong motor capabilities and confidence. Epileptic males had a median score of 0.925 [0.700–1.318], epileptic females—0.894 [0.739–1.343]), and non-epileptic females—1.154 [0.772–1.324]. These scores indicate generally good motor performance across all groups, suggesting that absence epilepsy does not significantly impair motor function.
- Exploration. The scores for all groups were positive, indicating a tendency to explore, although the ranges varied. The median scores for epileptic males was 0.425 [−0.012–0.647]), for non-epileptic males—0.477 [0.229–0.606]), epileptic females—0.486 [0.035–0.834] and non-epileptic females—0.561 [0.416–0.800]. These scores suggested a generally consistent level of exploration tendencies across all groups.
3.3. Cross-Domain Composite Metrics
4. Discussion
Potential Clinical Applications
5. Conclusions
- Standard behavioral indices in the EPM (time in open arms, grooming, exploration) were unaffected by absence epilepsy, suggesting that core anxiety-like behaviors in this test are independent of the epileptic phenotype at the studied age (10–11 months). However, the Anxiety Composite Index, which incorporated physiological stress markers (defecation, urination), revealed a sex-specific effect: non-epileptic females exhibited higher ACI scores than epileptic females, indicating greater autonomic stress reactivity. This suggests that absence epilepsy may blunt physiological anxiety responses in females, possibly due to chronic neuroendocrine adaptations.
- Cognitive composite metrics showed no epilepsy- or sex-related effects, contrasting with previous reports. Females outperformed males in active avoidance learning (100% vs. 78%). Epilepsy exacerbated anxiety-related learning difficulties in males.
- The impact of absence epilepsy was modulated by baseline anxiety. In high-anxiety rats, epilepsy increased social-emotional competence (potentially reflecting heightened social sensitivity) and passive coping strategies (elevated anxiety-avoidance axis scores). This highlights that anxiety-like behavioral phenotype is a critical moderator of epilepsy-related behavioral changes, pointing to shared or interacting neural circuits.
- All WAG/Rij rats showed low exploration and high behavioral inhibition, as evidenced by negative EMI and EDP <1 across all rats, correspondingly. This may suggest a strong genetic influence on behavior, independent of epilepsy.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abarrategui, B.; Parejo-Carbonell, B.; García García, M.E.; Di Capua, D.; García-Morales, I. The Cognitive Phenotype of Idiopathic Generalized Epilepsy. Epilepsy Behav. 2018, 89, 99–104. [Google Scholar] [CrossRef]
- Gruenbaum, B.F.; Sandhu, M.R.S.; Bertasi, R.A.O.; Bertasi, T.G.O.; Schonwald, A.; Kurup, A.; Gruenbaum, S.E.; Freedman, I.G.; Funaro, M.C.; Blumenfeld, H.; et al. Absence Seizures and Their Relationship to Depression and Anxiety: Evidence for Bidirectionality. Epilepsia 2021, 62, 1041–1056. [Google Scholar] [CrossRef]
- Vega, C.; Guo, J.; Killory, B.; Danielson, N.; Vestal, M.; Berman, R.; Martin, L.; Gonzalez, J.L.; Blumenfeld, H.; Spann, M.N. Symptoms of Anxiety and Depression in Childhood Absence Epilepsy. Epilepsia 2011, 52, 70–74. [Google Scholar] [CrossRef]
- Caplan, R.; Siddarth, P.; Stahl, L.; Lanphier, E.; Vona, P.; Gurbani, S.; Koh, S.; Sankar, R.; Shields, W.D. Childhood Absence Epilepsy: Behavioral, Cognitive, and Linguistic Comorbidities. Epilepsia 2008, 49, 1838–1846. [Google Scholar] [CrossRef]
- Caplan, R.; Siddarth, P.; Gurbani, S.; Hanson, R.; Sankar, R.; Shields, W.D. Depression and Anxiety Disorders in Pediatric Epilepsy. Epilepsia 2005, 46, 720–730. [Google Scholar] [CrossRef]
- Tosun, D.; Siddarth, P.; Toga, A.W.; Hermann, B.; Caplan, R. Effects of Childhood Absence Epilepsy on Associations between Regional Cortical Morphometry and Aging and Cognitive Abilities. Hum. Brain Mapp. 2011, 32, 580–591. [Google Scholar] [CrossRef]
- Zhong, R.; Lin, W.; Chen, Q.; Zhang, X.; Li, G. Predictors of Comorbid Anxiety Symptoms After a New Diagnosis of Epilepsy: A Prospective 12-Month Follow-Up Observation. Front. Neurol. 2021, 12, 743251. [Google Scholar] [CrossRef]
- Gabriel, D.; Ventura, M.; Samões, R.; Freitas, J.; Lopes, J.; Ramalheira, J.; Martins da Silva, A.; Chaves, J. Social Impairment and Stigma in Genetic Generalized Epilepsies. Epilepsy Behav. 2020, 104, 106886. [Google Scholar] [CrossRef]
- van Luijtelaar, G.; van Oijen, G. Establishing Drug Effects on Electrocorticographic Activity in a Genetic Absence Epilepsy Model: Advances and Pitfalls. Front. Pharmacol. 2020, 11, 395. [Google Scholar] [CrossRef]
- Depaulis, A.; David, O.; Charpier, S. The Genetic Absence Epilepsy Rat from Strasbourg as a Model to Decipher the Neuronal and Network Mechanisms of Generalized Idiopathic Epilepsies. J. Neurosci. Methods 2016, 260, 159–174. [Google Scholar] [CrossRef]
- Marks, W.N.; Cavanagh, M.E.; Greba, Q.; Cain, S.M.; Snutch, T.P.; Howland, J.G. The Genetic Absence Epilepsy Rats from Strasbourg Model of Absence Epilepsy Exhibits Alterations in Fear Conditioning and Latent Inhibition Consistent with Psychiatric Comorbidities in Humans. Eur. J. Neurosci. 2016, 43, 25–40. [Google Scholar] [CrossRef]
- Marescaux, C.; Vergnes, M. Genetic Absence Epilepsy in Rats from Strasbourg (GAERS). Ital. J. Neurol. Sci. 1995, 16, 113–118. [Google Scholar] [CrossRef]
- van Luijtelaar, G.; Onat, F.Y.; Gallagher, M.J. Animal Models of Absence Epilepsies: What Do They Model and Do Sex and Sex Hormones Matter? Neurobiol. Dis. 2014, 72, 167–179. [Google Scholar] [CrossRef]
- Coenen, A.M.L.; van Luijtelaar, E.L.J.M. The WAG/Rij Rat Model for Absence Epilepsy: Age and Sex Factors. Epilepsy Res. 1987, 1, 297–301. [Google Scholar] [CrossRef]
- van Luijtelaar, E.L.J.M.; Coenen, A.M.L. Two Types of Electrocortical Paroxysms in an Inbred Strain of Rats. Neurosci. Lett. 1986, 70, 393–397. [Google Scholar] [CrossRef]
- Leo, A.; De Caro, C.; Nesci, V.; Tallarico, M.; Mangano, G.; Palma, E.; Iannone, M.; De Sarro, G.; Citraro, R.; Russo, E. WAG/Rij Rat Model: A Resource for the Pharmacology of Epileptogenesis and Related Neurological/Psychiatric Comorbidities. Neurosci. Res. Notes 2019, 1, 18–34. [Google Scholar] [CrossRef]
- Russo, E.; Citraro, R.; Constanti, A.; Leo, A.; Lüttjohann, A.; van Luijtelaar, G.; De Sarro, G. Upholding WAG/Rij Rats as a Model of Absence Epileptogenesis: Hidden Mechanisms and a New Theory on Seizure Development. Neurosci. Biobehav. Rev. 2016, 71, 388–408. [Google Scholar] [CrossRef]
- van Luijtelaar, G.; Sitnikova, E. Global and Focal Aspects of Absence Epilepsy: The Contribution of Genetic Models. Neurosci. Biobehav. Rev. 2006, 30, 983–1003. [Google Scholar] [CrossRef]
- Coenen, A.M.L.; van Luijtelaar, E.L.J.M. Genetic Animal Models for Absence Epilepsy: A Review of the WAG/Rij Strain of Rats. Behav. Genet. 2003, 33, 635–655. [Google Scholar] [CrossRef]
- van Luijtelaar, G.; Zobeiri, M.; Lüttjohann, A.; Depaulis, A. Experimental Treatment Options in Absence Epilepsy. Curr. Pharm. Des. 2018, 23, 5577–5592. [Google Scholar] [CrossRef]
- Blumenfeld, H.; Klein, J.P.; Schridde, U.; Vestal, M.; Rice, T.; Khera, D.S.; Bashyal, C.; Giblin, K.; Paul-Laughinghouse, C.; Wang, F.; et al. Early Treatment Suppresses the Development of Spike-Wave Epilepsy in a Rat Model. Epilepsia 2008, 49, 400–409. [Google Scholar] [CrossRef]
- Jones, N.C.; Salzberg, M.R.; Kumar, G.; Couper, A.; Morris, M.J.; O’Brien, T.J. Elevated Anxiety and Depressive-like Behavior in a Rat Model of Genetic Generalized Epilepsy Suggesting Common Causation. Exp. Neurol. 2008, 209, 254–260. [Google Scholar] [CrossRef]
- Marques-Carneiro, J.E.; Faure, J.-B.; Cosquer, B.; Koning, E.; Ferrandon, A.; de Vasconcelos, A.P.; Cassel, J.-C.; Nehlig, A. Anxiety and Locomotion in Genetic Absence Epilepsy Rats from Strasbourg (GAERS): Inclusion of Wistar Rats as a Second Control. Epilepsia 2014, 55, 1460–1468. [Google Scholar] [CrossRef]
- Neuparth-Sottomayor, M.; Pina, C.C.; Morais, T.P.; Farinha-Ferreira, M.; Abreu, D.S.; Solano, F.; Mouro, F.; Good, M.; Sebastião, A.M.; Di Giovanni, G.; et al. Cognitive Comorbidities of Experimental Absence Seizures Are Independent of Anxiety. Neurobiol. Dis. 2023, 186, 106275. [Google Scholar] [CrossRef]
- Sarkisova, K.; van Luijtelaar, G. The WAG/Rij Strain: A Genetic Animal Model of Absence Epilepsy with Comorbidity of Depressiony. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 854–876. [Google Scholar] [CrossRef]
- Sarkisova, K.Y.; Kulikov, M.A. Behavioral Characteristics of WAG/Rij Rats Susceptible and Non-Susceptible to Audiogenic Seizures. Behav. Brain Res. 2006, 166, 9–18. [Google Scholar] [CrossRef]
- Shaw, F.-Z.; Chuang, S.-H.; Shieh, K.-R.; Wang, Y.-J. Depression- and Anxiety-like Behaviors of a Rat Model with Absence Epileptic Discharges. Neuroscience 2009, 160, 382–393. [Google Scholar] [CrossRef]
- Karson, A.; Utkan, T.; Balcı, F.; Arıcıoğlu, F.; Ateş, N. Age-Dependent Decline in Learning and Memory Performances of WAG/Rij Rat Model of Absence Epilepsy. Behav. Brain Funct. 2012, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Carobrez, A.P.; Bertoglio, L.J. Ethological and Temporal Analyses of Anxiety-like Behavior: The Elevated plus-Maze Model 20 Years On. Neurosci. Biobehav. Rev. 2005, 29, 1193–1205. [Google Scholar] [CrossRef]
- Hogg, S. A Review of the Validity and Variability of the Elevated Plus-Maze as an Animal Model of Anxiety. Pharmacol. Biochem. Behav. 1996, 54, 21–30. [Google Scholar] [CrossRef]
- Nunes, E.J.; Rupprecht, L.E.; Foster, D.J.; Lindsley, C.W.; Conn, P.J.; Addy, N.A. Examining the Role of Muscarinic M5 Receptors in VTA Cholinergic Modulation of Depressive-like and Anxiety-Related Behaviors in Rats. Neuropharmacology 2020, 171, 108089. [Google Scholar] [CrossRef]
- Pupikina, M.; Sitnikova, E. Non-Invasive Electroencephalography-Based Technique for Rapid Diagnostics of Absence Epilepsy in Rats. Adv. Neurol. 2024, 3, 4464. [Google Scholar] [CrossRef]
- Crunelli, V.; Leresche, N. Childhood Absence Epilepsy: Genes, Channels, Neurons and Networks. Nat. Rev. Neurosci. 2002, 3, 371–382. [Google Scholar] [CrossRef]
- Scheffer, I.E.; Berkovic, S.; Capovilla, G.; Connolly, M.B.; French, J.; Guilhoto, L.; Hirsch, E.; Jain, S.; Mathern, G.W.; Moshé, S.L.; et al. ILAE Classification of the Epilepsies: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 512–521. [Google Scholar] [CrossRef]
- Guilhoto, L.M. Absence Epilepsy: Continuum of Clinical Presentation and Epigenetics? Seizure 2017, 44, 53–57. [Google Scholar] [CrossRef]
- Alexandrov, P.; Pupikina, M.; Adaeva, Z.; Sitnikova, E. The Difference between Male and Female Rats in Terms of Freezing and Aversive Ultrasonic Vocalization in an Active Avoidance Test. Physiologia 2023, 3, 406–420. [Google Scholar] [CrossRef]
- Sitnikova, E.; Rutskova, E.M.; Tsvetaeva, D.; Raevsky, V.V. Spike-Wave Seizures, Slow-Wave Sleep EEG and Morphology of Substantia Nigra Pars Compacta in WAG/Rij Rats with Genetic Predisposition to Absence Epilepsy. Brain Res. Bull. 2021, 174, 63–71. [Google Scholar] [CrossRef]
- Sitnikova, E.; Smirnov, K. Active Avoidance Learning in WAG/Rij Rats with Genetic Predisposition to Absence Epilepsy. Brain Res. Bull. 2020, 165, 198–208. [Google Scholar] [CrossRef]
- Smirnov, K.; Tsvetaeva, D.; Sitnikova, E. Neonatal Whisker Trimming in WAG/Rij Rat Pups Causes Developmental Delay, Encourages Maternal Care and Affects Exploratory Activity in Adulthood. Brain Res. Bull. 2018, 140, 120–131. [Google Scholar] [CrossRef]
- Sitnikova, E.; Rutskova, E.; Smirnov, K.; Runnova, A.; Zhuravlev, M. Intracortical Synchronization Pattern on the Preclinical and Clinical Stages of Absence Epilepsy (Analysis of Wavelet Bicoherence in WAG/Rij Rats). Eur. Phys. J. Spec. Top. 2022, 232, 583–594. [Google Scholar] [CrossRef]
- Estanislau, C. Cues to the Usefulness of Grooming Behavior in the Evaluation of Anxiety in the Elevated Plus-Maze. Psychol. Neurosci. 2012, 5, 105–112. [Google Scholar] [CrossRef]
- Song, C.; Berridge, K.C.; Kalueff, A.V. “Stressing” Rodent Self-Grooming for Neuroscience Research. Nat. Rev. Neurosci. 2016, 17, 591. [Google Scholar] [CrossRef]
- Leo, A.; Citraro, R.; Tallarico, M.; Iannone, M.; Fedosova, E.; Nesci, V.; De Sarro, G.; Sarkisova, K.; Russo, E. Cognitive Impairment in the WAG/Rij Rat Absence Model Is Secondary to Absence Seizures and Depressive-like Behavior. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 94, 109652. [Google Scholar] [CrossRef]
- Ewell, L.A. Assessing Levels of Awareness during Seizures in Animal Models. Epilepsy Curr. 2017, 17, 372–373. [Google Scholar] [CrossRef]
- Jafarian, M.; Karimzadeh, F.; Alipour, F.; Attari, F.; Lotfinia, A.A.; Speckmann, E.-J.; Zarrindast, M.-R.; Gorji, A. Cognitive Impairments and Neuronal Injury in Different Brain Regions of a Genetic Rat Model of Absence Epilepsy. Neuroscience 2015, 298, 161–170. [Google Scholar] [CrossRef]
- Akman, O.; Onat, F. Absence Seizures: Update on Signaling Mechanisms and Networks. Epilepsia Open 2025, 1–18. [Google Scholar] [CrossRef]
- Crunelli, V.; Lőrincz, M.L.; McCafferty, C.; Lambert, R.C.; Leresche, N.; Di Giovanni, G.; David, F. Clinical and Experimental Insight into Pathophysiology, Comorbidity and Therapy of Absence Seizures. Brain 2020, 143, 2341–2368. [Google Scholar] [CrossRef]
- Löscher, W.; Stafstrom, C.E. Epilepsy and Its Neurobehavioral Comorbidities: Insights Gained from Animal Models. Epilepsia 2023, 64, 54–91. [Google Scholar] [CrossRef]




| Strain | Sex | Age (Months) | Open Arm Time (%) | Open Arm Entries | Additional Behavioral Indices | Methodological Notes | Ref. |
|---|---|---|---|---|---|---|---|
| GAERS vs. NEC rats | Males, females | 1.6 and 3 | Lower % than NEC | No mention |
| Tested during light phase (inactive period); 5-min EPM session. | Jones et al., 2008 [22] |
| GAERS vs. NEC rats | Males, females | 1.2–2.1 | Lower % than NEC | Fewer than NEC |
| Combined EPM and ASR testing; SWD frequency inversely correlated with open arm time. | Marks et al., 2016 [11] |
| Long-Evans vs. Wistar | Males | 9–12 | Higher % than Wistar | Higher than Wistar |
| Nocturnal testing (active phase); SWD monitored via EEG. | Shaw et al., 2009 [27] |
| WAG/Rij vs. Wistar | Males | 5 and 13 | No difference | Higher than Wistar |
| Testing during light phase; 10-min EPM session. | Karson et al., 2012 [28] |
| WAG/Rij vs. Wistar | Males | 3–4 and 5–6 | No difference | No mention |
| Audiogenic priming used; EPM conducted after audiogenic seizure induction | Sarkisova & Kulikov, 2006 [26] |
| GAERS vs. NEC rats | Males, females | 1.6 and 3 | Lower % than NEC | No mention |
| Tested during light phase (inactive period); 5-min EPM session. | Jones et al., 2008 [22] |
| GAERS vs. NEC rats | Males, females | 1.2–2.1 | Lower % than NEC | Fewer than NEC |
| Combined EPM and ASR testing; SWD frequency inversely correlated with open arm time. | Marks et al., 2016 [11] |
| Long-Evans vs. Wistar | Males | 9–12 | Higher % than Wistar | Higher than Wistar |
| Nocturnal testing (active phase); SWD monitored via EEG. | Shaw et al., 2009 [27] |
| WAG/Rij vs. Wistar | Males | 5 and 13 | No difference | Higher than Wistar |
| Testing during light phase; 10-min EPM session. | Karson et al., 2012 [28] |
| WAG/Rij vs. Wistar | Males | 3–4 and 5–6 | No difference | No mention |
| Audiogenic priming used; EPM conducted after audiogenic seizure induction | Sarkisova & Kulikov, 2006 [26] |
| GAERS vs. NEC rats | Males, females | 1.6 and 3 | Lower % than NEC | No mention |
| Tested during light phase (inactive period); 5-min EPM session. | Jones et al., 2008 [22] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sitnikova, E.; Pupikina, M. Behavioral Phenotyping of WAG/Rij Rat Model of Absence Epilepsy: The Link to Anxiety and Sex Factors. Biomedicines 2025, 13, 2075. https://doi.org/10.3390/biomedicines13092075
Sitnikova E, Pupikina M. Behavioral Phenotyping of WAG/Rij Rat Model of Absence Epilepsy: The Link to Anxiety and Sex Factors. Biomedicines. 2025; 13(9):2075. https://doi.org/10.3390/biomedicines13092075
Chicago/Turabian StyleSitnikova, Evgenia, and Maria Pupikina. 2025. "Behavioral Phenotyping of WAG/Rij Rat Model of Absence Epilepsy: The Link to Anxiety and Sex Factors" Biomedicines 13, no. 9: 2075. https://doi.org/10.3390/biomedicines13092075
APA StyleSitnikova, E., & Pupikina, M. (2025). Behavioral Phenotyping of WAG/Rij Rat Model of Absence Epilepsy: The Link to Anxiety and Sex Factors. Biomedicines, 13(9), 2075. https://doi.org/10.3390/biomedicines13092075






